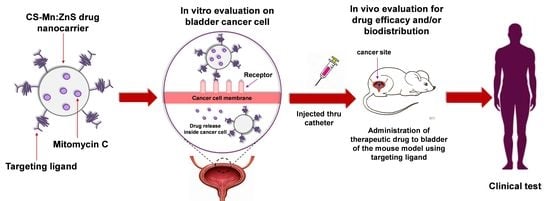
Drug Release Profiles of Mitomycin C Encapsulated Quantum Dots–Chitosan Nanocarrier System for the Possible Treatment of Non-Muscle Invasive Bladder Cancer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of MMC@CS-Mn:ZnS Nanocarriers
2.3. Reaction Yield, Drug Loading, and Encapsulation Efficiency
chitosan (mg) + mass of Mn:ZnS (mg) + mass of MMC (mg)) × 100
(mg)]/[Mass of nanocarriers used (mg)] × 100
(mg)]/[Initial mass of MMC in the system (mg)] × 100
2.4. MMC Drug Release Studies
2.5. Characterization of MMC@CS-Mn:ZnS Nanocarriers
2.6. Statistical Analysis
3. Results and Discussion
3.1. Characterization of ZnS and Mn:ZnS QDs
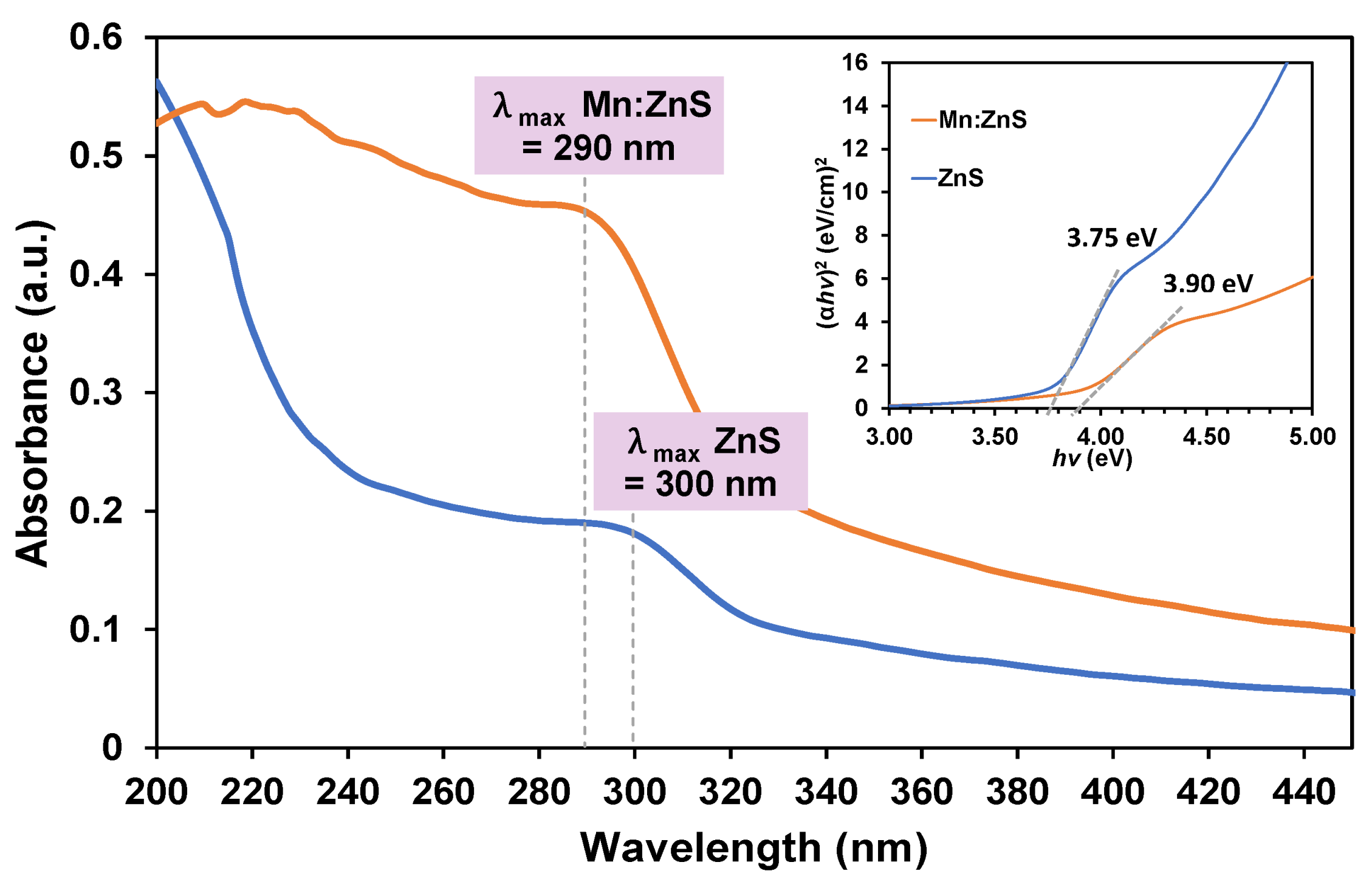
3.1.1. UV–Vis Spectroscopy
3.1.2. PL Spectroscopy
3.1.3. HRTEM Analysis
3.1.4. FTIR Spectroscopy
3.1.5. XRD Analysis
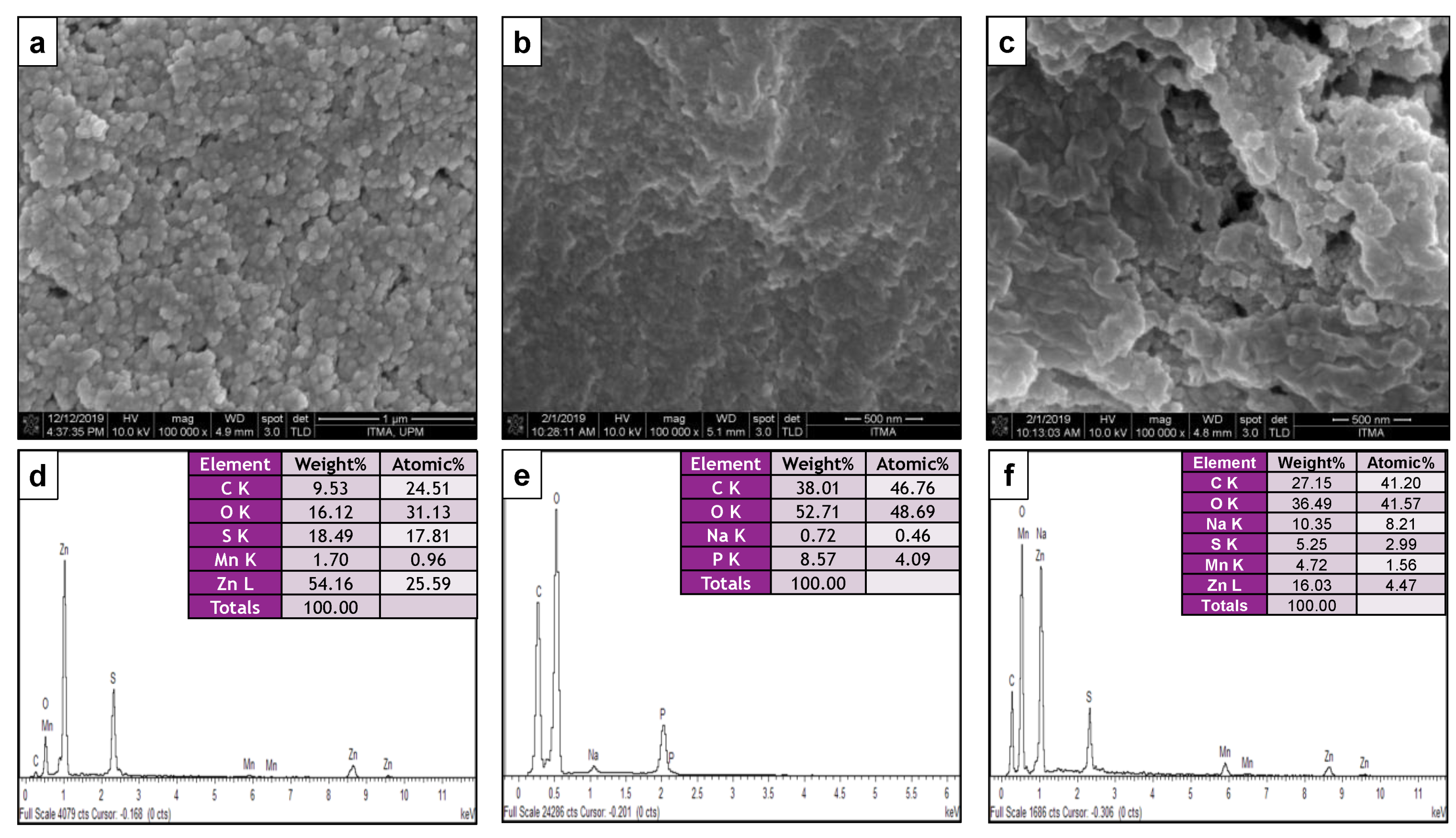
3.1.6. Morphology and Particle Size Distribution
3.2. Reaction Yield, Drug Loading Capacity, and Encapsulation Efficiency
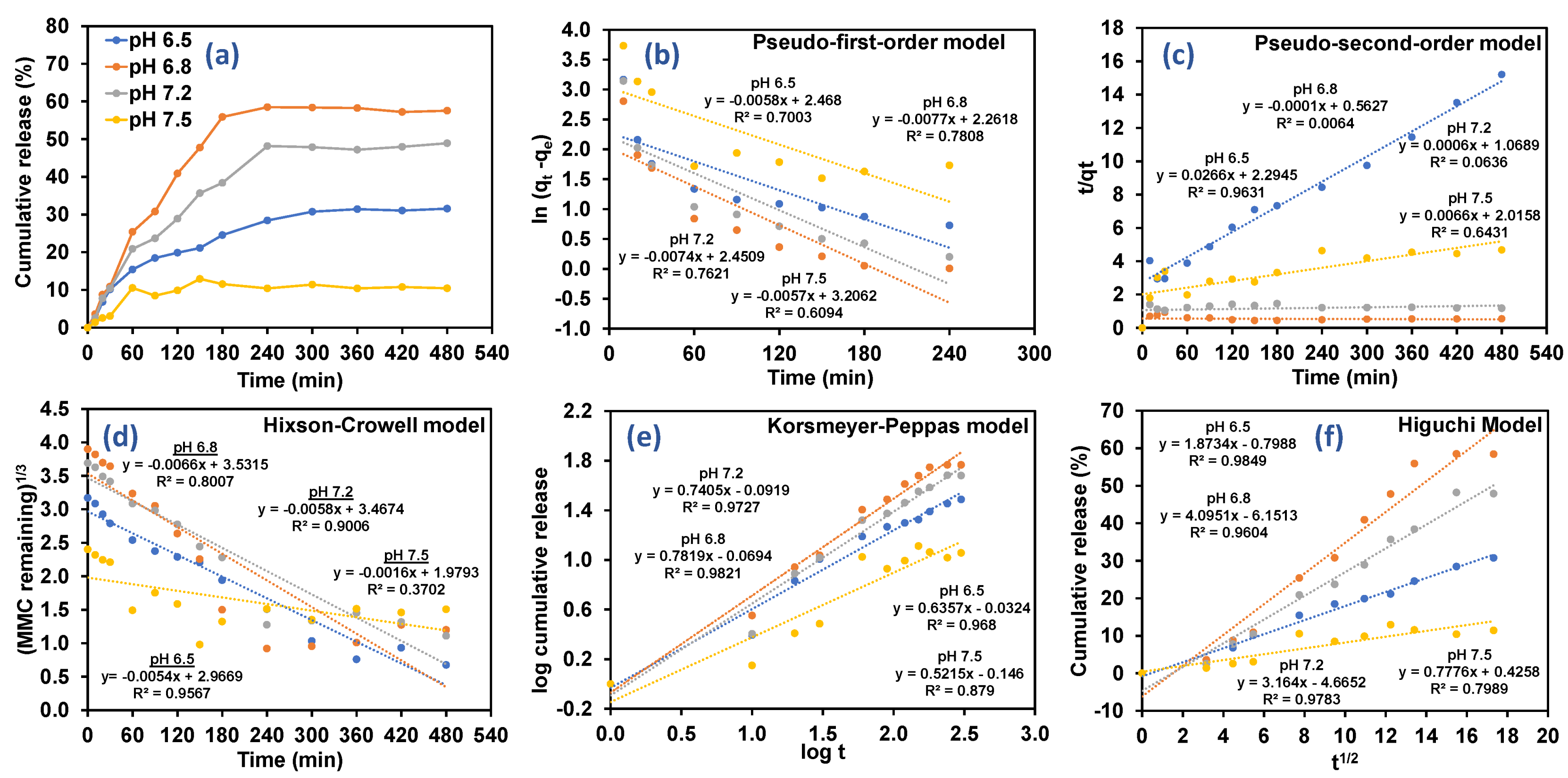
3.3. Drug Release Kinetics Based on Different Types of Pharmacokinetics Models
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Amreddy, N.; Babu, A.; Muralidharan, R.; Panneerselvam, J.; Srivastava, A.; Ahmed, R.; Mehta, M.; Munshi, A.; Ramesh, R. Recent advances in nanoparticle-based cancer drug and gene delivery. Adv. Cancer Res. 2018, 137, 115–170. [Google Scholar]
- Kumar, H.; Kumari, N.; Sharma, R. Nanocomposites (conducting polymer and nanoparticles) based electrochemical biosensor for the detection of environment pollutant: Its issues and challenges. Environ. Impact Assess. Rev. 2020, 85, 106438. [Google Scholar] [CrossRef]
- Khiste, S.K.; Liu, Z.; Roy, K.R.; Uddin, M.; Hosain, S.B.; Gu, X.; Nazzal, S.; Hill, R.A.; Liu, Y.-Y. Ceramide–rubusoside nanomicelles, a potential therapeutic approach to target cancers carrying p53 missense mutations. Mol. Cancer Ther. 2020, 19, 564–574. [Google Scholar] [CrossRef] [Green Version]
- Maria, G.F.C.; De Annunzio, S.R.; Victorelli, F.D.; Frade, M.L.; Ferreira, P.S.; Chorilli, M.; Fontana, C.R. Chitosan-based drug delivery systems for optimization of photodynamic therapy: A review. AAPS PharmSciTech 2019, 20, 1–17. [Google Scholar]
- Tang, L.; Li, J.; Zhao, Q.; Pan, T.; Zhong, H.; Wang, W. Advanced and innovative nano-systems for anticancer targeted drug delivery. Pharmaceutics 2021, 13, 1151. [Google Scholar] [CrossRef]
- Kukkar, D.; Kukkar, P.; Kumar, V.; Hong, J.; Kim, K.H.; Deep, A. Recent advances in nanoscale materials for antibody-based cancer theranostics. Biosens. Bioelectron. 2021, 173, 112787. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Giottonini, K.Y.; Rosalva, J.R.; Alejandra, C.G.; Omar, P.M.; Zavala-Rivera, P.; Patricia, G.; Armando, L. PLGA nanoparticle preparations by emulsification and nanoprecipitation techniques: Effects of formulation parameters. RSC Adv. 2020, 10, 4218–4231. [Google Scholar] [CrossRef] [Green Version]
- Divya, K.; Jisha, M.S. Chitosan nanoparticles preparation and applications. Environ. Chem. Lett. 2018, 16, 101–112. [Google Scholar] [CrossRef]
- Hasheminejad, N.; Khodaiyan, F.; Safari, M. Improving the antifungal activity of clove essential oil encapsulated by chitosan nanoparticles. Food Chem. 2018, 275, 113–122. [Google Scholar] [CrossRef]
- Ruman, U.; Fakurazi, S.; Masarudin, M.J.; Hussein, M.Z. Nanocarrier-Based Therapeutics and Theranostics Drug Delivery Systems for Next Generation of Liver Cancer Nanodrug Modalities. Int. J. Nanomed. 2020, 15, 1437–1456. [Google Scholar] [CrossRef] [Green Version]
- Quiñones, J.P.; Peniche, H.; Peniche, C. Chitosan Based Self-Assembled Nanoparticles in Drug Delivery. Polymers 2018, 10, 235. [Google Scholar] [CrossRef] [Green Version]
- Musalli, A.H.; Talukdar, P.D.; Royd, P.; Kumar, P.; Wong, T.W. Folate-induced nanostructural changes of oligochitosan nanoparticles and their fate of cellular internalization by melanoma. Carbohydr. Polym. 2020, 244, 116488. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Sun, M.; Wang, T.; Chen, X.; Wang, H. Chitosan-based self-assembled nanomaterials: Their application in drug delivery. Polymers 2020, 10, 1–13. [Google Scholar] [CrossRef]
- Sun, X.; Liu, C.; Omer, A.M.; Yang, L.; Ouyang, X. Dual-layered pH-sensitive alginate/chitosan/kappa-carrageenan microbeads for colon-targeted release of 5-fluorouracil. Int. J. Biol. Macromol. 2019, 132, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Susanna Monti, S.T.; Jose, J.; Sahajan, A.; Kalarikkal, N. Structure and dynamics of gold nanoparticles decorated with chitosan—Gentamicin conjugates: ReaxFF molecular dynamics simulations to disclose drug delivery. Phys. Chem. Chem. Phys. 2019, 21, 13099–13108. [Google Scholar] [CrossRef] [PubMed]
- Sepahi, H.; Pourmadadi, M.; Moradi, A.; Yazdian, F.; Omidi, M. Chitosan/carbon quantum dot/aptamer complex as a potential anticancer drug delivery system towards the release of 5-fluorouracil. Int. J. Biol. Macromol. 2020, 165, 1422–1430. [Google Scholar]
- Chinnathambi, S.; Chen, S.; Ganesan, S.; Hanagata, N. Silicon quantum dots for biological applications. Adv. Healthc. Mater. 2014, 3, 10–29. [Google Scholar] [CrossRef]
- Chinnathambi, S.; Shirahata, N. Recent advances on fluorescent biomarkers of near-infrared quantum dots for in vitro and in vivo imaging. Sci. Technol. Adv. Mater. 2019, 20, 337–355. [Google Scholar] [CrossRef] [Green Version]
- Manan, F.A.A.; Hong, W.W.; Abdullah, J.; Yusof, N.A.; Ahmad, I. Nanocrystalline cellulose decorated quantum dots based tyrosinase biosensor for phenol determination. Mater. Sci. Eng. C 2019, 99, 37–46. [Google Scholar] [CrossRef]
- Abdelhamid, H.N. Nanoparticles Assisted Laser Desorption/Ionization Mass Spectrom. In Handbook of Smart Materials in Analytical Chemistry; de la Guardia, M., Esteve-Turrillas, F.A., Eds.; John Wiley Sons: Hoboken, NJ, USA, 2019; Chapter 23; pp. 725–755. [Google Scholar]
- Matea, C.T.; Mocan, T.; Tabaran, F.; Pop, T.; Mosteanu, O.; Puia, C.; Iancu, C.; Mocan, L. Quantum dots in imaging, drug delivery and sensor applications. Int. J. Nanomed. 2017, 12, 5421–5431. [Google Scholar] [CrossRef] [Green Version]
- Desai, M.L.; Deshmukh, B.; Lenka, N.; Haran, V.; Jha, S.; Basu, H.; Singhal, R.K.; Sharma, P.; Kailasa, S.K.; Kim, K.-H. Inflence of doping ion, capping agent and pH on the fluorescence properties of zinc sulfide quantum dots: Sensing of Cu2+ and Hg2+ ions and their biocompatibility with cancer and fungal cells. Spectrochim. Acta A Mol. Biomol. Spec. 2019, 210, 212–221. [Google Scholar] [CrossRef]
- Ribeiro, E.F.; de Barros-Alexandrino, T.T.; Assis, O.B.G.; Cruz Junior, A.; Quiles, A.; Hernando, I.; Nicoletti, V.R. Chitosan and crosslinked chitosan nanoparticles: Synthesis, characterization and their role as pickering emulsifiers. Carbohydr. Polym. 2020, 250, 116878. [Google Scholar] [CrossRef]
- Ghobashy, M.M.; Elbarbary, A.M.; Hegazy, D.E. Gamma Radiation Synthesis of a Novel Amphiphilic Terpolymer Hydrogel PH-Responsive Based Chitosan for Colon Cancer Drug Delivery. Carbohydr. Polym. 2021, 263, 117975. [Google Scholar] [CrossRef]
- De Carvalho, S.M.; Mansur, A.A.P.; Mansur, H.S.; Guedes, M.I.M.C.; Lobato, Z.I.P.; Leite, M.F. In vitro and in vivo assessment of nanotoxicity of CdS quantum dot / aminopolysaccharide bionanoconjugates. Mater. Sci. Eng. C 2017, 71, 412–424. [Google Scholar] [CrossRef]
- Binu, P.J.; Muthukumaran, S. Optical and structural properties of Mn/Cu doped ZnS nanostructures. Mater. Today Proc. 2016, 43, 3762–3765. [Google Scholar] [CrossRef]
- Bwatanglang, I.B.; Mohammad, F.; Yusof, N.A.; Abdullah, J.; Hussein, M.Z.; Alitheen, N.B.; Abu, N. Folic acid targeted Mn:ZnS quantum dots for theranostic applications of cancer cell imaging and therapy. Int. J. Nanomed. 2016, 11, 413–428. [Google Scholar]
- Rasuli, H.; Rasuli, R.; Alizadeh, M.; BoonTong, G. Microwave-assisted exfoliation and tearing of graphene oxide in the presence of TiO2 nanoparticles. Results Phys. 2020, 18, 103200. [Google Scholar] [CrossRef]
- Alex, R.; Muñoz, M.; Rotstein, B.H.; Suuronen, E.J.; Alarcon, E.I. A low cost and open access system for rapid synthesis of large volumes of gold and silver nanoparticles. Sci. Rep. 2021, 11, 5420. [Google Scholar]
- Nguyen, T.P.; Leb, A.D.; Vu, T.B.; Lam, Q.V. Investigations on photoluminescence enhancement of Poly(vinyl alcohol)-encapsulated Mn-doped ZnS quantum dots. J. Lumin. 2017, 192, 166–172. [Google Scholar] [CrossRef]
- Samanta, D.; Basnet, P.; Chanu, T.I.; Chatterjee, S. Biomolecule assisted morphology-controllable synthesis of Zinc Sulphide nanomaterials for efficient photocatalytic activity under solar irradiation. J. Alloys Compd. 2020, 844, 155810. [Google Scholar] [CrossRef]
- Yang, H.; Changchun, H.; Hengyu, L.; Kunfeng, Z.; Runguang, S. Influence of bovine hemoglobin on the disruption of fluorescence resonance energy transfer between zinc sulfide quantum dots and fluoescent silica nanoparticles. J. Mol. Liq. 2021, 332, 115851. [Google Scholar] [CrossRef]
- Hir, Z.A.M.; Abdullah, A.H.; Zainal, Z.; Lim, H.N. Photoactive hybrid film photocatalyst of polyethersulfone-ZnO for the degradation of methyl orange dye: Kinetic study and operational parameters. Catalysts 2017, 7, 313. [Google Scholar]
- Babu, P.; Reddy, M.R.V.; Kondaiah, S.; Reddy, K.T.R.; Chinho, P. Chemical bath deposition of Mn-doped ZnS thin films using greener complexing agents: Effect of Mn-doping on the optical properties. Optics 2017, 130, 608–618. [Google Scholar]
- Arshad, H.; Chaudhry, M.; Mehmood, S.; Farooq, A.; Wang, M.; Bhatti, A.S. The electrochemical reaction controlled optical response of cholestrol oxidase (COx) conjugated CdSe/ZnS quantum dots. Sci. Rep. 2020, 10, 1–10. [Google Scholar] [CrossRef]
- Zaini, M.S.; Liew, J.Y.C.; Alang Ahmad, S.A.; Mohmad, A.R.; Ahmad Kamarudin, M. Photoluminescence investigation of carrier localization in colloidal PbS and PbS/MnS quantum dots. ACS Omega 2020, 5, 30956–30962. [Google Scholar] [CrossRef] [PubMed]
- Sakthivel, P.; Rasu, K.K.; Venkatesan, G.K.D.P.; Viloria, A. Influence of Ag+ and Mn2+ ions on structural, optical and photoluminescence features of ZnS quantum dots. Spectrochim. Acta Mol. Biomol. Spectrosc. 2020, 241, 118666. [Google Scholar] [CrossRef]
- Ummartyotin, S.; Bunnak, N.; Juntaro, J.; Sain, M.; Manuspiya, H. Synthesis and Luminescence Properties of ZnS and Metal (Mn, Cu)-Doped-ZnS Ceramic Powder. Solid State Sci. 2012, 14, 299–304. [Google Scholar] [CrossRef]
- Lahariya, V.; Ramrakhiani, M. Luminescence study on Mn, Ni, Co-doped zinc sulfide nanocrystals. Luminescence 2020, 35, 924–933. [Google Scholar] [CrossRef]
- Ren, T.B.; Xu, W.; Zhang, W.; Zhang, X.X.; Wang, Z.Y.; Xiang, Z.; Yuan, L.; Zhang, X.B. A general method to increase stokes shift by introducing alternating vibronic structures. J. Am. Chem. Soc. 2018, 140, 7716–7722. [Google Scholar] [CrossRef]
- Premkumar, S.; Nataraj, D.; Bharathi, G.; Ramya, S.; Thangadurai, T.D. Highly Responsive Ultraviolet Sensor Based on ZnS Quantum Dot Solid with Enhanced Photocurrent. Sci. Rep. 2019, 9, 1–14. [Google Scholar] [CrossRef]
- Barman, B.; Chandra, K. Low temperature chemical synthesis of ZnS, Mn doped ZnS nanosized particles: Their structural, morphological and photophysical properties. Solid State Sci. 2020, 109, 106404. [Google Scholar] [CrossRef]
- Ranjbar-navazi, Z.; Omidi, Y.; Eskandani, M. Trends in Analytical Chemistry Cadmium-Free Quantum Dot-Based Theranostics. Trends Anal. Chem. 2019, 118, 386–400. [Google Scholar] [CrossRef]
- Madera-Santana, T.J.; Herrera-Méndez, C.H.; Núez, J.R.R. An Overview of the Chemical Modi Fi Cations of Chitosan and Their Advantages. Green Mater. 2018, 6, 31–42. [Google Scholar]
- Torkaman, S.; Hossein, R.; Alireza, A.; Seyed, H.; Najafi, M. Modification of chitosan using amino acids for wound healing purposes: A review. Carbohydr. Polym. 2021, 258, 117675. [Google Scholar] [CrossRef]
- Manthrammel, M.A.; Mohd, A.; Alfaify, S. A systematic investigation on physical properties of spray pyrolysis–fabricated CdS thin films for opto-nonlinear applications: An effect of Na doping. J. Mater. Res. 2020, 35, 410–421. [Google Scholar] [CrossRef]
- Hir, Z.A.M.; Abdullah, A.H.; Zainal, Z.; Lim, H.N. Visible light-active hybrid film photocatalyst of polyethersulfone–reduced TiO2: Photocatalytic response and radical trapping investigation. J. Mater Sci. 2018, 53, 13264–13279. [Google Scholar]
- Vinh, N.D.; Tan, P.M.; Do, P.V.; Bharti, S.; Hoa, V.X.; Hien, N.T.; Luyen, N.T.; Ca, N.X. Effect of dopant concentration and the role of ZnS shell on optical properties of Sm3+ doped CdS quantum dots. RSC Adv. 2021, 11, 7961–7971. [Google Scholar] [CrossRef]
- Ismail, A.F.; Khulbe, K.C.; Matsuura, T. RO membrane characterization. In Reverse Osmosys; Ismail, A.F., Khulbe, K.C., Matsuura, T., Eds.; Elsevier Inc.: New York, NY, USA, 2019; pp. 57–90. [Google Scholar]
- Othman, N.; Siti, N.; Ain, J.; Mas, J.M.; Luqman, C.A.; Rusli, D.; Nor, S.S. L-Ascorbic acid and thymoquinone dual-loaded palmitoyl-chitosan nanoparticles: Improved preparation method, encapsulation and release efficiency. Processes 2020, 8, 1040. [Google Scholar] [CrossRef]
- Nair, R.S.; Morris, A.; Billa, N.; Leong, C. An evaluation of curcumin-encapsulated chitosan nanoparticles for transdermal delivery. AAPS PharmSciTech 2019, 20, 69. [Google Scholar] [CrossRef]
- Maluin, F.N.; Hussein, M.Z.; Yusof, N.A.; Fakurazi, S.; Abu Seman, I.; Hilmi, N.H.Z.; Daim, L.D.J. Enhanced fungicidal efficacy on Ganoderma Boninense by simultaneous co-delivery of hexaconazole and dazomet from their chitosan nanoparticles. RSC Adv. 2019, 9, 27083–27095. [Google Scholar] [CrossRef] [Green Version]
- Ramalingam, P.; Ko, Y.T. Improved oral delivery of resveratrol from N-trimethyl chitosan-g-palmitic acid surface-modified solid lipid nanoparticles. Colloids Surf. B 2016, 139, 52–61. [Google Scholar] [CrossRef]
- Thotakura, N.; Dadarwal, M.; Kumar, R.; Singh, B.; Sharma, G.; Kumar, P.; Katare, O.P.; Raza, K. “International Journal of Biological Macromolecules Chitosan-Palmitic Acid Based Polymeric Micelles as Promising Carrier for Circumventing Pharmacokinetic and Drug Delivery Concerns of Tamoxifen. Int. J. Biol. Macromol. 2017, 102, 1220–1225. [Google Scholar] [CrossRef]
- Shnoudeh, A.J.; Hamad, I.; Abdo, R.W. Synthesis, Characterization, and Applications of Metal Nanoparticles. In Biomaterials and Bionanotechnology; Tekade, R.K., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; Chapter 15; pp. 527–612. [Google Scholar]
- Das, P.; Das, M.; Chinnadayyala, S.R.; Singha, I.M.; Goswami, P. Recent advances on developing 3rd generation enzyme electrode for biosensor applications. Biosens. Bioelectron. 2016, 79, 386–397. [Google Scholar] [CrossRef]
- Kathe, N.; Brian, H.; Harsh, C. “Physicochemical Characterization Techniques for Solid Lipid Nanoparticles: Principles and Limitations. Drug Dev. Ind. Pharm. 2014, 46, 1565–1575. [Google Scholar] [CrossRef]
- Samimi, S.; Maghsoudnia, N.; Eftekhari, R.B.; Dorkoosh, F. Lipid-Based Nanoparticles for Drug Delivery Systems. In Characterization and Biology of Nanomaterials for Drug Delivery; Mohapatra, S.S., Ranjan, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; Chapter 3; pp. 47–76. [Google Scholar]
- Bhattacharjee, S. DLS and zeta potential–What they are and what they are not? J. Controll. Release 2016, 235, 337–351. [Google Scholar] [CrossRef]
- Jasmani, L.; Sharmiza, A. Preparation and characterization of nanocrystalline cellulose from acacia mangium and its rein-forcement potential. Carbohydr. Polym. 2017, 161, 166–171. [Google Scholar] [CrossRef]
- Alam, S.; Khan, Z.I.; Mustafa, G.; Kumar, M.; Islam, F.; Bhatnagar, A.; Ahmad, F.J. Development and evaluation of thymo-quinone- encapsulated chitosan nanoparticles for nose-to- brain targeting: A pharmacoscinti-graphic study. Int. J. Nanomed. 2012, 7, 5705–5718. [Google Scholar] [CrossRef] [Green Version]
- Du, X.-J.; Wang, J.-L.; Iqbal, S.; Li, H.-J.; Cao, Z.-T.; Wang, Y.-C.; Du, J.-Z.; Wang, J. The Effect of Surface Charge on Oral Absorption of Polymeric Nanoparticles. Biomater. Sci. 2018, 6, 642–650. [Google Scholar] [CrossRef]
- Basu, T.; Pal, B.; Singh, S. Hollow chitosan nanocomposite as drug carrier system for controlled delivery of ramipril. Chem. Phys. Lett. 2018, 706, 465–471. [Google Scholar] [CrossRef]
- Janeth, C.; Rivas, M.; Tarhini, M.; Badri, W.; Miladi, K.; Greige-gerges, H.; Agha, Q.; Arturo, S.; Rodríguez, G.; Álvarez, R.; et al. Nanoprecipitation process: From encapsulation to drug delivery. Int. J. Pharm. 2017, 532, 66–81. [Google Scholar]
- Markam, R.; Bajpai, A.K. Functionalization of ginger derived nanoparticles with chitosan to design drug delivery system for controlled release of 5-amino salicylic acid (5-ASA) in treatment of inflammatory bowel diseases: An in vitro study. React. Funct. Polym. 2020, 149, 104520. [Google Scholar] [CrossRef]
- Prokopowicz, M.; Adrian, S.; Adrianna, S.; Rafał, S.; Gavin, W. Biphasic composite of calcium phosphate-based mesoporous silica as a novel bone drug delivery system. Drug Deliv. Transl. Res. 2020, 10, 455–470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asghar, K.; Qasim, M.; Dharmapurib, G.; Das, D. Investigation on a smart nanocarrier with a mesoporous magnetic core and thermo-responsive shell for co-delivery of doxorubicin and curcumin: A new approach towards combination therapy of cancer. RSC Adv. 2017, 7, 28802–28818. [Google Scholar] [CrossRef] [Green Version]
- Lisik, A.; Musiał, W. Conductomeric evaluation of the release kinetics of active substances from pharmaceutical preparations containing iron ions. Materials 2019, 12, 730. [Google Scholar] [CrossRef] [Green Version]
- Ikrima, K.; Ahmad, M.; Minhas, M.U.; Barkat, K. Synthesis and evaluation of chondroitin sulfate based hydrogels of loxoprofen with adjustable properties as controlled release carriers. Carbohydr. Polym. 2018, 181, 1169–1179. [Google Scholar]


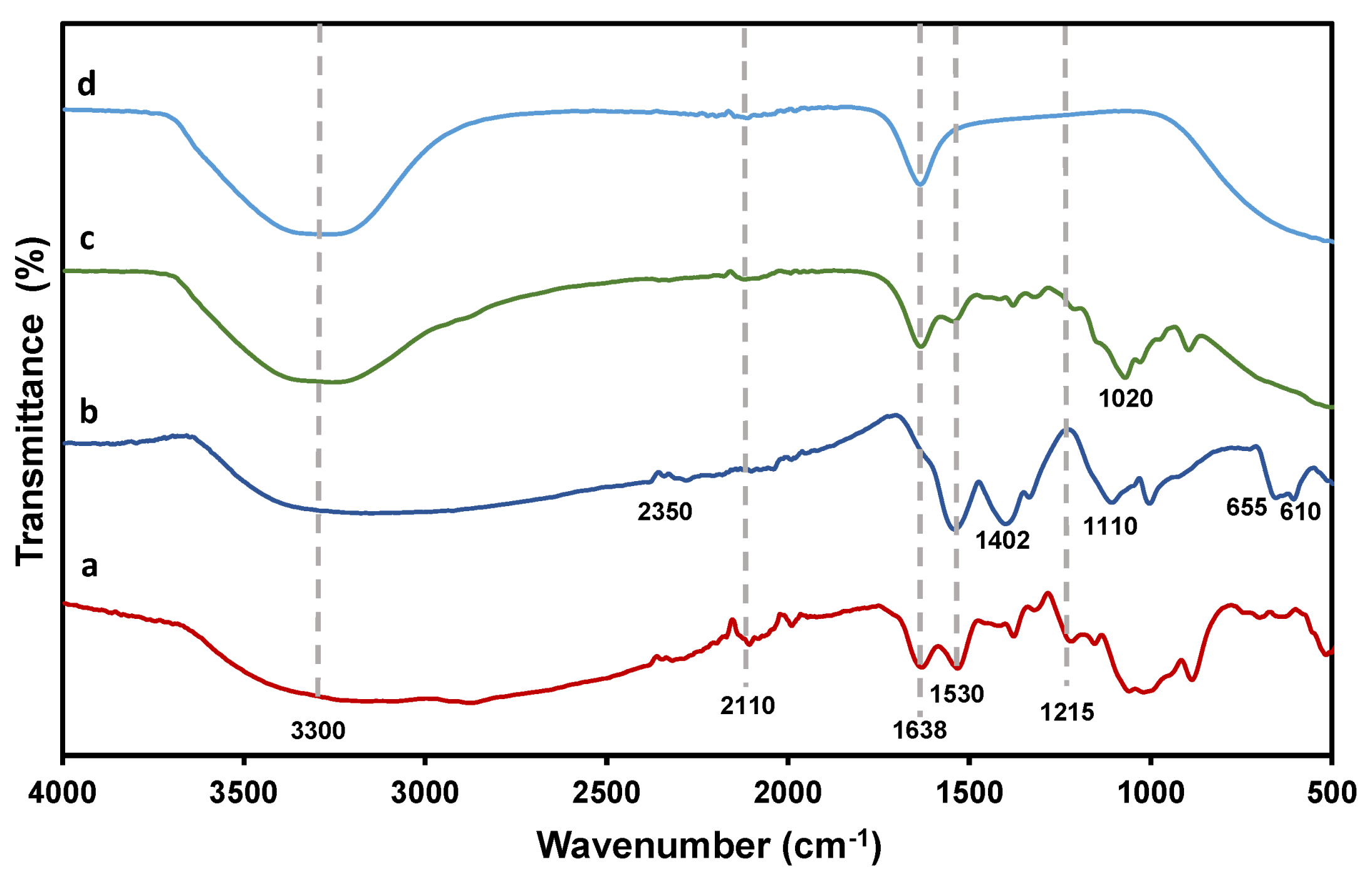

| Wavenumber (nm−1) | Functional Group |
|---|---|
| 3300 | C–H and NH2 stretching in CS and MMC |
| 2110 | C–O stretching in CS |
| 1638 | NH2 bending in CS and MMC |
| 1530 and 1402 | C–O stretching of carboxylic group |
| 1110, 655 and 610 | Zn–S vibration |
| Sample | hkl | 2θ (deg.) | β (rad.) | D (nm) | dhkl (nm) | a (nm) | ||
|---|---|---|---|---|---|---|---|---|
| ZnS | [111] | 28.69 | 0.0805 | 1.775 | 3.172 | 1.951 | 0.311 | 0.733 |
| Mn:ZnS | [111] | 28.70 | 0.0951 | 1.503 | 4.421 | 2.300 | 0.311 | 0.734 |
| CS-Mn:ZnS | [111] | 28.22 | 0.1117 | 1.279 | 6.111 | 2.709 | 0.316 | 0.740 |
| MMC@CS-Mn:ZnS | [111] | 28.75 | 0.0749 | 1.909 | 2.741 | 1.814 | 0.310 | 0.733 |
| Sample | Particle Sizes (nm) | PDI | Zeta Potential (mV) |
|---|---|---|---|
| Mn:ZnS | 46 ± 0.56 | 0.564 | −22.80 ± 0.33 |
| CS NPs | 158 ± 0.21 | 0.289 | +32.60 ± 0.29 |
| CS-Mn:ZnS | 161 ± 0.67 | 0.320 | +32.70 ± 0.46 |
| MMC@CS-Mn:ZnS | 175 ± 0.33 | 0.448 | +33.20 ± 0.38 |
| Nanocarriers | Drug Loading Capacity (%) | Encapsulation Efficiency (%) | Reaction Yield (%) |
|---|---|---|---|
| MMC@CS-Mn:ZnS a | 35.55 ± 1.25 | 50.13 ± 0.54 | 50.75 |
| MMC@CS-Mn:ZnS b | 42.36 ± 1.80 | 60.01 ± 0.28 | 54.17 |
| MMC@CS-Mn:ZnS c | 44.52 ± 1.05 | 60.31 ± 0.49 | 53.30 |
| MMC@CS-Mn:ZnS d | 37.82 ± 1.45 | 55.28 ± 0.31 | 52.29 |
| Model | Pseudo-First Order | Pseudo-Second Order | Hixson–Crowell | Korsmeyer–Peppas | Higuchi | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Release medium | K1 | R2 | K2 | R2 | KHC | R2 | n | KKP | R2 | KH | R2 |
| pH 6.5 | 0.0058 | 0.7003 | 0.0266 | 0.9631 | 0.0054 | 0.9567 | 0.63 | 0.0324 | 0.9680 | 1.8734 | 0.9849 |
| pH 6.8 | 0.0077 | 0.7808 | 0.0001 | 0.0064 | 0.0066 | 0.8007 | 0.78 | 0.0694 | 0.9821 | 4.0951 | 0.9604 |
| pH 7.2 | 0.0074 | 0.7621 | 0.0006 | 0.0636 | 0.0058 | 0.9006 | 0.74 | 0.0919 | 0.9727 | 3.1640 | 0.9783 |
| pH 7.5 | 0.0057 | 0.6094 | 0.0066 | 0.6431 | 0.0016 | 0.3702 | 0.52 | 0.1460 | 0.8790 | 0.7776 | 0.7989 |
| Release Exponent (n) | Drug Transport Mechanism | Rate as a Function of Time (t) Transformation |
|---|---|---|
| n ≤ 0.45 | Fickian diffusion | t−0.5 |
| 0.45 ≤ n ≤ 0.89 | Non-Fickian transport | tn−1 |
| n ≥ 0.89 | Case II transport | t |
| n ≥ 1 | Super case II transport | tn−1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manan, F.A.A.; Yusof, N.A.; Abdullah, J.; Mohammad, F.; Nurdin, A.; Yazan, L.S.; Khiste, S.K.; Al-Lohedan, H.A. Drug Release Profiles of Mitomycin C Encapsulated Quantum Dots–Chitosan Nanocarrier System for the Possible Treatment of Non-Muscle Invasive Bladder Cancer. Pharmaceutics 2021, 13, 1379. https://doi.org/10.3390/pharmaceutics13091379
Manan FAA, Yusof NA, Abdullah J, Mohammad F, Nurdin A, Yazan LS, Khiste SK, Al-Lohedan HA. Drug Release Profiles of Mitomycin C Encapsulated Quantum Dots–Chitosan Nanocarrier System for the Possible Treatment of Non-Muscle Invasive Bladder Cancer. Pharmaceutics. 2021; 13(9):1379. https://doi.org/10.3390/pharmaceutics13091379
Chicago/Turabian StyleManan, Fariza Aina Abd, Nor Azah Yusof, Jaafar Abdullah, Faruq Mohammad, Armania Nurdin, Latifah Saiful Yazan, Sachin K. Khiste, and Hamad A. Al-Lohedan. 2021. "Drug Release Profiles of Mitomycin C Encapsulated Quantum Dots–Chitosan Nanocarrier System for the Possible Treatment of Non-Muscle Invasive Bladder Cancer" Pharmaceutics 13, no. 9: 1379. https://doi.org/10.3390/pharmaceutics13091379
APA StyleManan, F. A. A., Yusof, N. A., Abdullah, J., Mohammad, F., Nurdin, A., Yazan, L. S., Khiste, S. K., & Al-Lohedan, H. A. (2021). Drug Release Profiles of Mitomycin C Encapsulated Quantum Dots–Chitosan Nanocarrier System for the Possible Treatment of Non-Muscle Invasive Bladder Cancer. Pharmaceutics, 13(9), 1379. https://doi.org/10.3390/pharmaceutics13091379