The EyeFlowCell: Development of a 3D-Printed Dissolution Test Setup for Intravitreal Dosage Forms
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
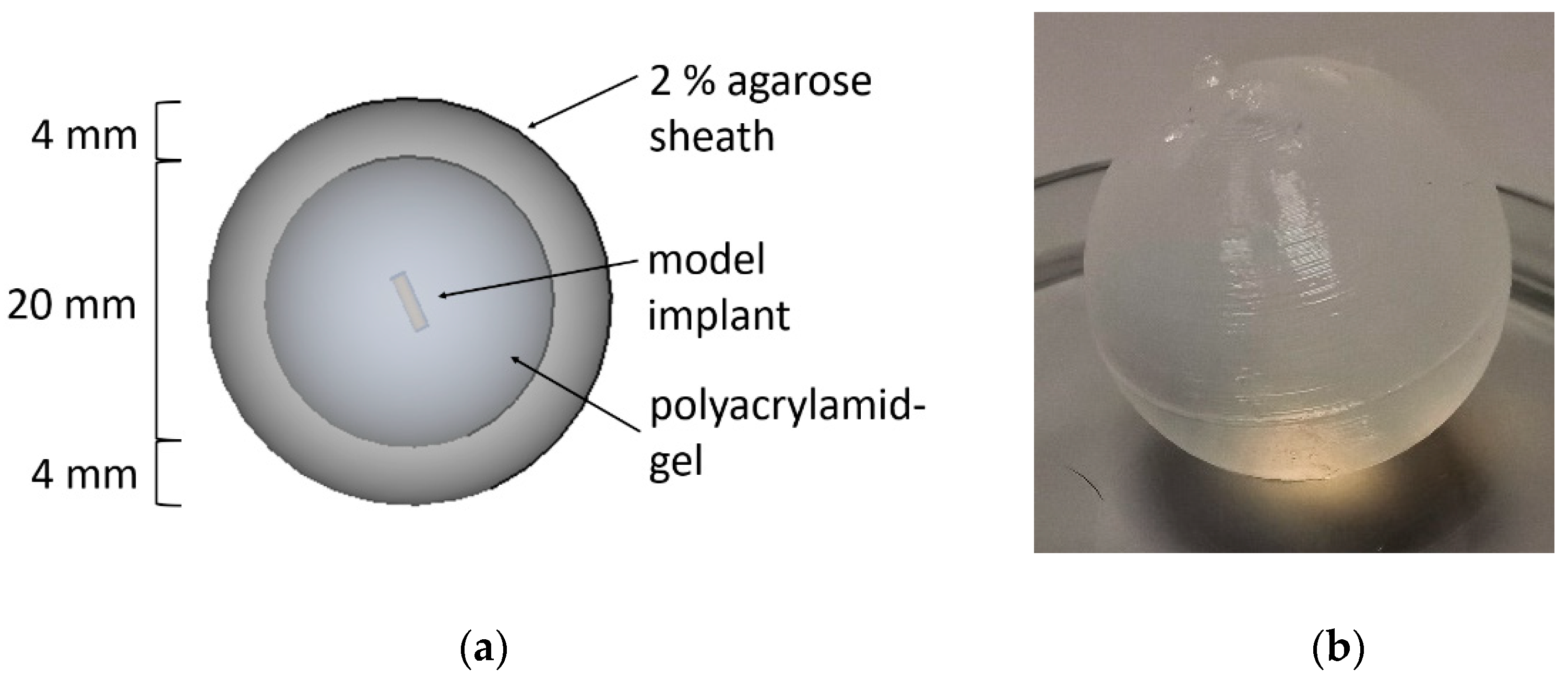
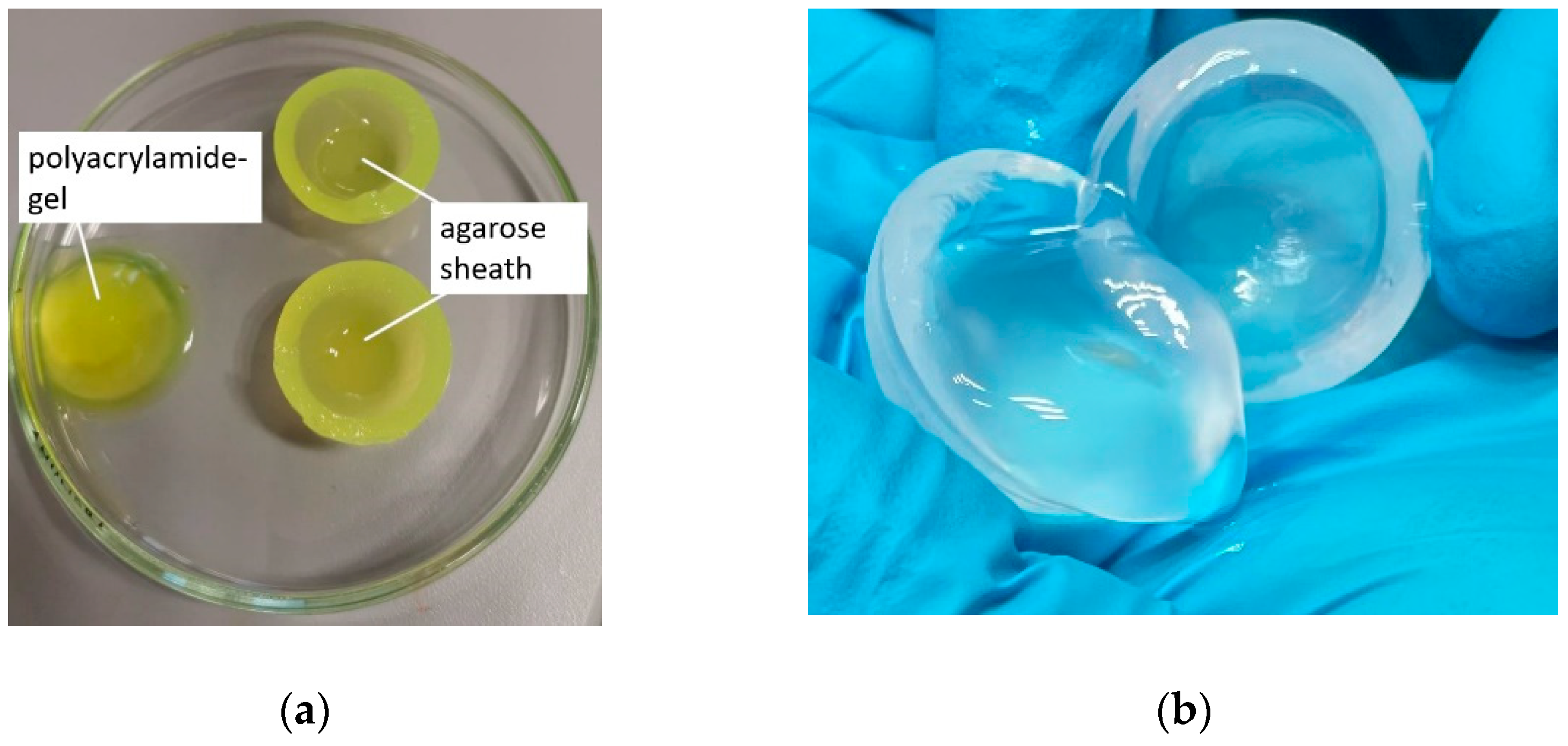
2.2.1. Preparation of the Model Implants
2.2.2. Fabrication of the EyeFlowCell
2.2.3. Fabrication of the Vitreous Substitute
2.2.4. In Vitro Drug Release Studies Using Compendial Methods
2.2.5. In Vitro Drug Release Studies in the EyeFlowCell
2.2.6. Quantification
3. Results
3.1. Preparation of the Vitreous Substitute
3.2. Fabrication of the EyeFlowCell
3.3. Drug Loaded Model Implants
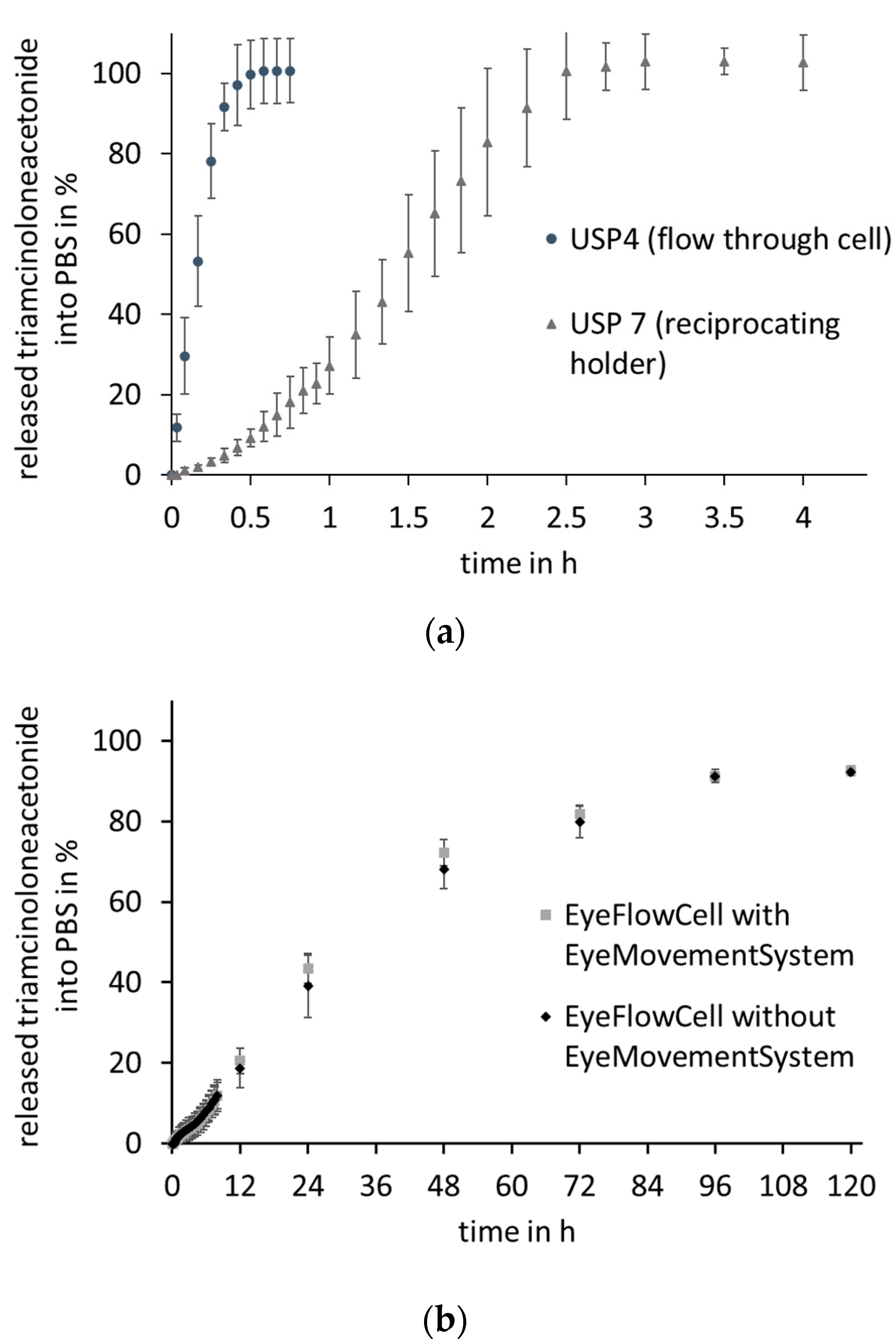
3.4. Dissolution Studies
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wong, W.L.; Su, X.; Li, X.; Cheung, C.M.G.; Klein, R.; Cheng, C.Y.; Wong, T.Y. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: A systematic review and meta-analysis. Lancet Glob. Health 2014, 2, e106–e116. [Google Scholar] [CrossRef] [Green Version]
- Voleti, V.B.; Hubschman, J.P. Age-related eye disease. Maturitas 2013, 75, 29–33. [Google Scholar] [CrossRef]
- Finger, R.P.; Fimmers, R.; Holz, F.G.; Scholl, H.P.N. Incidence of blindness and severe visual impairment in Germany: Projections for 2030. Investig. Ophthalmol. Vis. Sci. 2011, 52, 4381–4389. [Google Scholar] [CrossRef] [PubMed]
- Fogli, S.; Del Re, M.; Rofi, E.; Posarelli, C.; Figus, M.; Danesi, R. Clinical pharmacology of intravitreal anti-VEGF drugs. Eye 2018, 32, 1010–1020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fredenberg, S.; Wahlgren, M.; Reslow, M.; Axelsson, A. The mechanisms of drug release in poly(lactic-co-glycolic acid)-based drug delivery systems—A review. Int. J. Pharm. 2011, 415, 34–52. [Google Scholar] [CrossRef]
- Awwad, S.; Henein, C.; Ibeanu, N.; Khaw, P.T.; Brocchini, S. Preclinical challenges for developing long acting intravitreal medicines. Eur. J. Pharm. Biopharm. 2020, 153, 130–149. [Google Scholar] [CrossRef]
- del Amo, E.M.; Urtti, A. Rabbit as an animal model for intravitreal pharmacokinetics: Clinical predictability and quality of the published data. Exp. Eye Res. 2015, 137, 111–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rowe-Rendleman, C.L.; Durazo, S.A.; Kompella, U.B.; Rittenhouse, K.D.; Di Polo, A.; Weiner, A.L.; Grossniklaus, H.E.; Naash, M.I.; Lewin, A.S.; Horsager, A.; et al. Drug and gene delivery to the back of the eye: From bench to bedside. Investig. Ophthalmol. Vis. Sci. 2014, 55, 2714–2730. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, L.E.; Orilla, W.; Hughes, P.M.; Tsai, S.; Burke, J.A.; Wilson, C.G. Effects of vitreous liquefaction on the intravitreal distribution of sodium fluorescein, fluorescein dextran, and fluorescent microparticles. Investig. Ophthalmol. Vis. Sci. 2011, 52, 1111–1118. [Google Scholar] [CrossRef]
- Stein, S.; Hadlich, S.; Langner, S.; Biesenack, A.; Zehm, N.; Kruschke, S.; Oelze, M.; Grimm, M.; Mahnhardt, S.; Weitschies, W.; et al. 7.1 T MRI and T2 mapping of the human and porcine vitreous body post mortem. Eur. J. Pharm. Biopharm. 2018, 131, 82–91. [Google Scholar] [CrossRef]
- Henein, C.; Awwad, S.; Ibeanu, N.; Vlatakis, S.; Brocchini, S.; Tee Khaw, P.; Bouremel, Y. Hydrodynamics of Intravitreal Injections into Liquid Vitreous Substitutes. Pharmaceutics 2019, 11, 371. [Google Scholar] [CrossRef] [Green Version]
- Seidlitz, A.; Weitschies, W. In-vitro dissolution methods for controlled release parenterals and their applicability to drug-eluting stent testing. J. Pharm. Pharmacol. 2012, 64, 969–985. [Google Scholar] [CrossRef]
- Fialho, S.L.; Behar-Cohen, F.; Silva-Cunha, A. Dexamethasone-loaded poly(ε-caprolactone) intravitreal implants: A pilot study. Eur. J. Pharm. Biopharm. 2008, 68, 637–646. [Google Scholar] [CrossRef]
- Matter, B.; Ghaffari, A.; Bourne, D.; Wang, Y.; Choi, S.; Kompella, U.B. Dexamethasone Degradation in Aqueous Medium and Implications for Correction of In Vitro Release from Sustained Release Delivery Systems. AAPS PharmSciTech 2019, 20. [Google Scholar] [CrossRef]
- Awwad, S.; Lockwood, A.; Brocchini, S.; Khaw, P.T. The PK-Eye: A Novel in Vitro Ocular Flow Model for Use in Preclinical Drug Development. J. Pharm. Sci. 2015, 104, 3330–3342. [Google Scholar] [CrossRef] [Green Version]
- Loch, C.; Nagel, S.; Guthoff, R.; Seidlitz, A.; Weitschies, W. The vitreous model—A new in vitro test method simulating the vitreous body. Biomed. Tech. 2012, 57, 281–284. [Google Scholar] [CrossRef]
- Stein, S.; Auel, T.; Kempin, W.; Bogdahn, M.; Weitschies, W.; Seidlitz, A. Influence of the test method on in vitro drug release from intravitreal model implants containing dexamethasone or fluorescein sodium in poly (D,L-lactide-co-glycolide) or polycaprolactone. Eur. J. Pharm. Biopharm. 2018, 127, 270–278. [Google Scholar] [CrossRef]
- Spaide, R.F.; Klancnik, J.M.; Cooney, M.J. Retinal vascular layers imaged by fluorescein angiography and optical coherence tomography angiography. JAMA Ophthalmol. 2015, 133, 45–50. [Google Scholar] [CrossRef]
- German Society of Ophthalmology (DOG); German Retina Society (RG); Professional Association of Ophthalmologists in Germany (BVA). Statement of the German Ophthalmological Society, the German Retina Society, and the Professional Association of Ophthalmologists in Germany on treatment of diabetic macular edema. Ophthalmologe 2021, 118, 40–67. [Google Scholar] [CrossRef]
- Mondelo-García, C.; Bandín-Vilar, E.; García-Quintanilla, L.; Castro-Balado, A.; del Amo, E.M.; Gil-Martínez, M.; Blanco-Teijeiro, M.J.; González-Barcia, M.; Zarra-Ferro, I.; Fernández-Ferreiro, A.; et al. Current Situation and Challenges in Vitreous Substitutes. Macromol. Biosci. 2021, 2100066. [Google Scholar] [CrossRef]
- Yu, Z.; Ma, S.; Wu, M.; Cui, H.; Wu, R.; Chen, S.; Xu, C.; Lu, X.; Feng, S. Self-assembling hydrogel loaded with 5-FU PLGA microspheres as a novel vitreous substitute for proliferative vitreoretinopathy. J. Biomed. Mater. Res. Part A 2020, 108, 2435–2446. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Goyanes, A.; Gaisford, S.; Basit, A.W. Stereolithographic (SLA) 3D printing of oral modified-release dosage forms. Int. J. Pharm. 2016, 503, 207–212. [Google Scholar] [CrossRef]
- Sil, B.C.; Alvarez, M.P.; Zhang, Y.; Kung, C.P.; Hossain, M.; Iliopoulos, F.; Luo, L.; Crowther, J.M.; Moore, D.J.; Hadgraft, J.; et al. 3D-printed Franz type diffusion cells. Int. J. Cosmet. Sci. 2018, 40, 604–609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Käsdorf, B.T.; Arends, F.; Lieleg, O. Diffusion Regulation in the Vitreous Humor. Biophys. J. 2015, 109, 2171–2181. [Google Scholar] [CrossRef] [Green Version]
- Le Goff, M.M.; Bishop, P.N. Adult vitreous structure and postnatal changes. Eye 2008, 22, 1214–1222. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.; Robinson, S.B.; Csaky, K.G. Investigating the movement of intravitreal human serum albumin nanoparticles in the vitreous and retina. Pharm. Res. 2009, 26, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Soheilian, M.; Eskandari, A.; Ramezani, A.; Rabbanikhah, Z.; Soheilian, R. A pilot study of intravitreal diclofenac versus intravitreal triamcinolone for uveitic cystoid macular edema. Ocul. Immunol. Inflamm. 2013, 21, 124–129. [Google Scholar] [CrossRef]
- del Amo, E.M.; Rimpelä, A.K.; Heikkinen, E.; Kari, O.K.; Ramsay, E.; Lajunen, T.; Schmitt, M.; Pelkonen, L.; Bhattacharya, M.; Richardson, D.; et al. Pharmacokinetic aspects of retinal drug delivery. Prog. Retin. Eye Res. 2017, 57, 134–185. [Google Scholar] [CrossRef]
- Rauck, B.M.; Friberg, T.R.; Medina Mendez, C.A.; Park, D.; Shah, V.; Bilonick, R.A.; Wang, Y. Biocompatible reverse thermal gel sustains the release of intravitreal bevacizumab in vivo. Investig. Ophthalmol. Vis. Sci. 2013, 55, 469–470. [Google Scholar] [CrossRef]
- Stein, S.; Bogdahn, M.; Rosenbaum, C.; Weitschies, W.; Seidlitz, A. Distribution of fluorescein sodium and triamcinolone acetonide in the simulated liquefied and vitrectomized Vitreous Model with simulated eye movements. Eur. J. Pharm. Sci. 2017, 109, 233–243. [Google Scholar] [CrossRef]






| Compound | Content (%) |
|---|---|
| phosphate buffered saline pH 7.4 | 92.21 |
| rotiphoresis gel 30 | 6.69 |
| ammonium peroxodisulfate | 1 |
| tetramethylethylendiamine | 0.1 |
| Compound | Batch 1 Content (%) | Batch 2 Content (%) |
|---|---|---|
| fluorescein sodium | 40 | - |
| triamcinolone acetonide | - | 20 |
| silicone dioxide | 0.5 | 0.5 |
| polyethylene glycol 6000 | 10 | 10 |
| hydroxypropyl methylcellulose | 49.5 | 69.5 |
| Movement Pattern (x-Axis) | Angle (°) | Angular Velocity (°/s) | |
|---|---|---|---|
| Mode 1 | Slow pursuit movement | 35 | 41 |
| Mode 2 | Fast pursuit movement | 33 | 83 |
| Mode 3 | Saccadic movement | 42 | 165 |
| Mode 4 | Pursuit movement with distinct amplitudes | 90 | 330 |
| Movement pattern y-axis | 20 | 60 | |
| 24 h day rhythm Minutes (mode) | 290 min (mode 1), 5 min (mode 3) 280 min (mode 4), 5 min (mode 3) 260 min (mode 2), 5 min (mode 3) 300 min (mode 1), 5 min (mode 3) 285 min (mode 4), 5 min (mode 3) | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Auel, T.; Großmann, L.; Schulig, L.; Weitschies, W.; Seidlitz, A. The EyeFlowCell: Development of a 3D-Printed Dissolution Test Setup for Intravitreal Dosage Forms. Pharmaceutics 2021, 13, 1394. https://doi.org/10.3390/pharmaceutics13091394
Auel T, Großmann L, Schulig L, Weitschies W, Seidlitz A. The EyeFlowCell: Development of a 3D-Printed Dissolution Test Setup for Intravitreal Dosage Forms. Pharmaceutics. 2021; 13(9):1394. https://doi.org/10.3390/pharmaceutics13091394
Chicago/Turabian StyleAuel, Tobias, Linus Großmann, Lukas Schulig, Werner Weitschies, and Anne Seidlitz. 2021. "The EyeFlowCell: Development of a 3D-Printed Dissolution Test Setup for Intravitreal Dosage Forms" Pharmaceutics 13, no. 9: 1394. https://doi.org/10.3390/pharmaceutics13091394
APA StyleAuel, T., Großmann, L., Schulig, L., Weitschies, W., & Seidlitz, A. (2021). The EyeFlowCell: Development of a 3D-Printed Dissolution Test Setup for Intravitreal Dosage Forms. Pharmaceutics, 13(9), 1394. https://doi.org/10.3390/pharmaceutics13091394







