Inulin-Based Polymeric Micelles Functionalized with Ocular Permeation Enhancers: Improvement of Dexamethasone Permeation/Penetration through Bovine Corneas
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of the Inulin (INU) Derivatives
2.2.1. INU-EDA
2.2.2. INU-EDA-RA
2.2.3. INU-EDA-RA-PEG
2.2.4. INU-EDA-TAU-RA
2.2.5. INU-EDA-RA-CAR
2.2.6. INU-EDA-RA-CRE
2.3. Evaluation of Critical Aggregation Concentration (CAC)
2.4. Preparation of Empty, Fluorescent-Labelled and DEX-Loaded Polymeric Micelles
2.5. Dynamic Light Scattering (DLS) and Z-Potential Analysis
2.6. Mucoadhesion Studies
2.7. Drug Loading Percentage (DL%) Determination
2.8. In Vitro Drug Release Studies
2.9. Stability Studies of DEX-Loaded Micelles
2.10. In Vitro Biocompatibility Assay
2.11. Ex Vivo Evaluations
2.11.1. Tissue Preparation
2.11.2. Set-Up of the Experimental Model
2.11.3. Ex Vivo Transcorneal Permeation
2.11.4. Drug Retention in Corneal Tissue
2.12. Equations
2.13. Data Analysis
3. Results and Discussion
3.1. Preparation and Characterization of Micelles
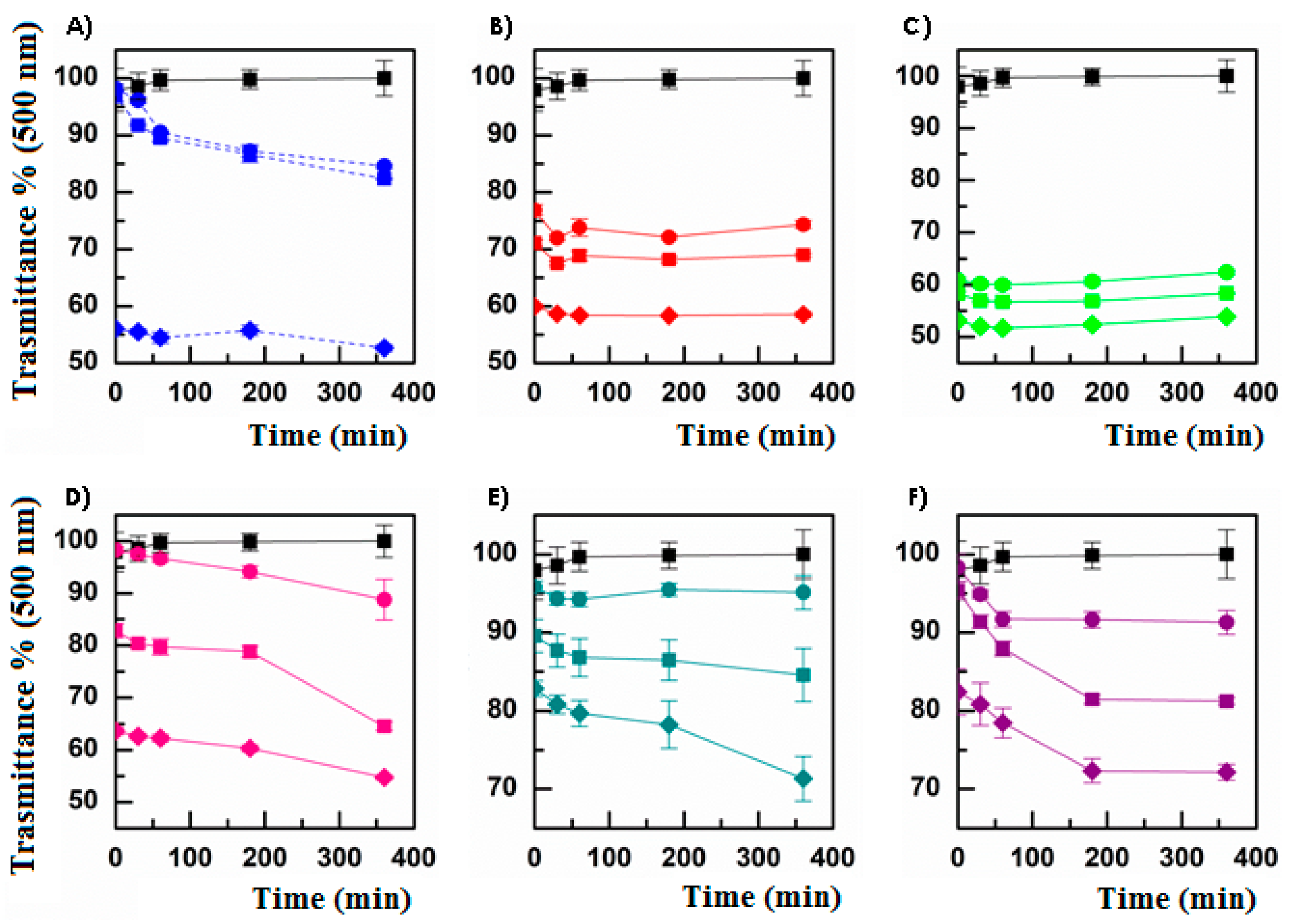
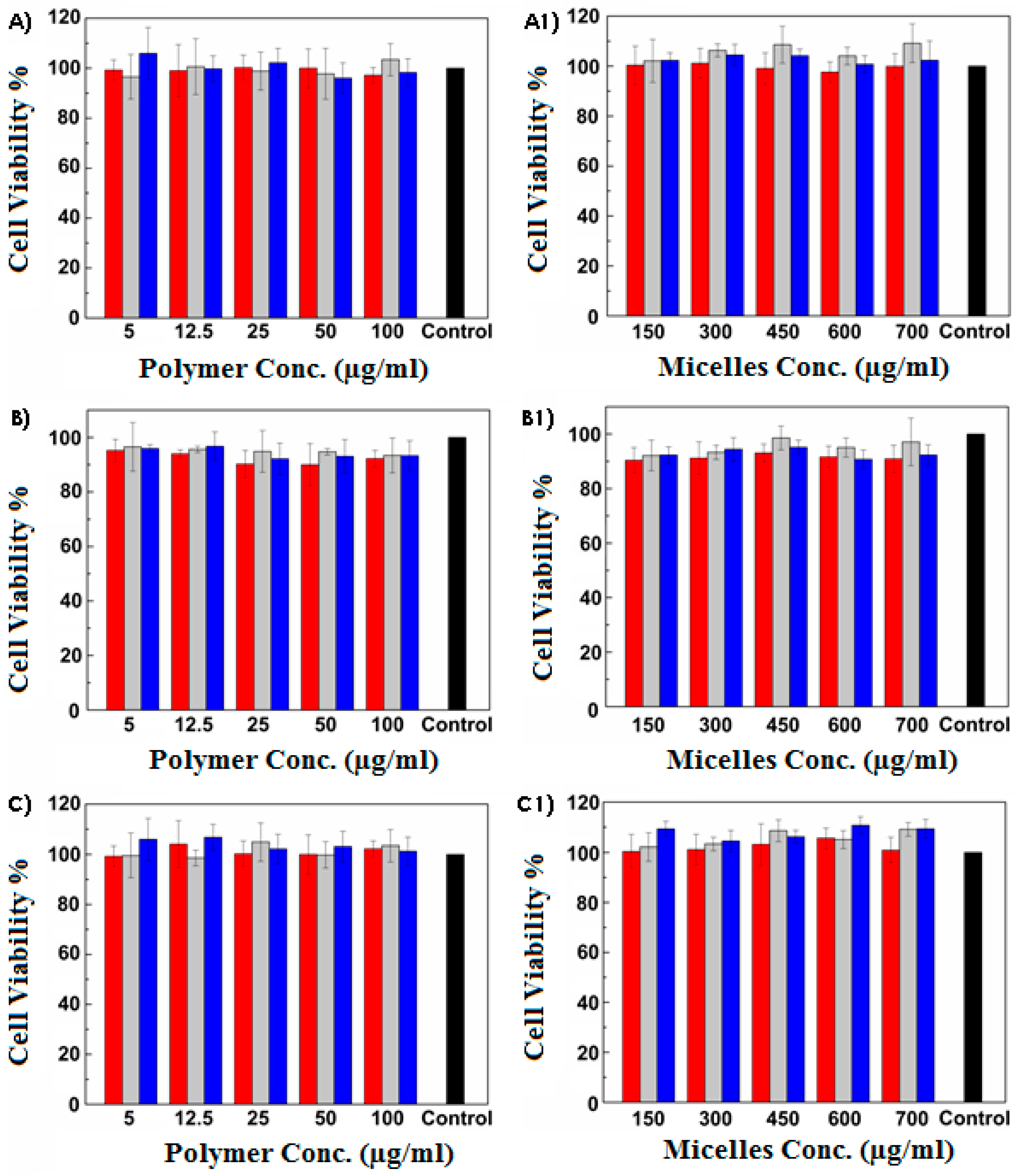
3.2. Mucoadhesiveness and Cytocompatibility of Micelles
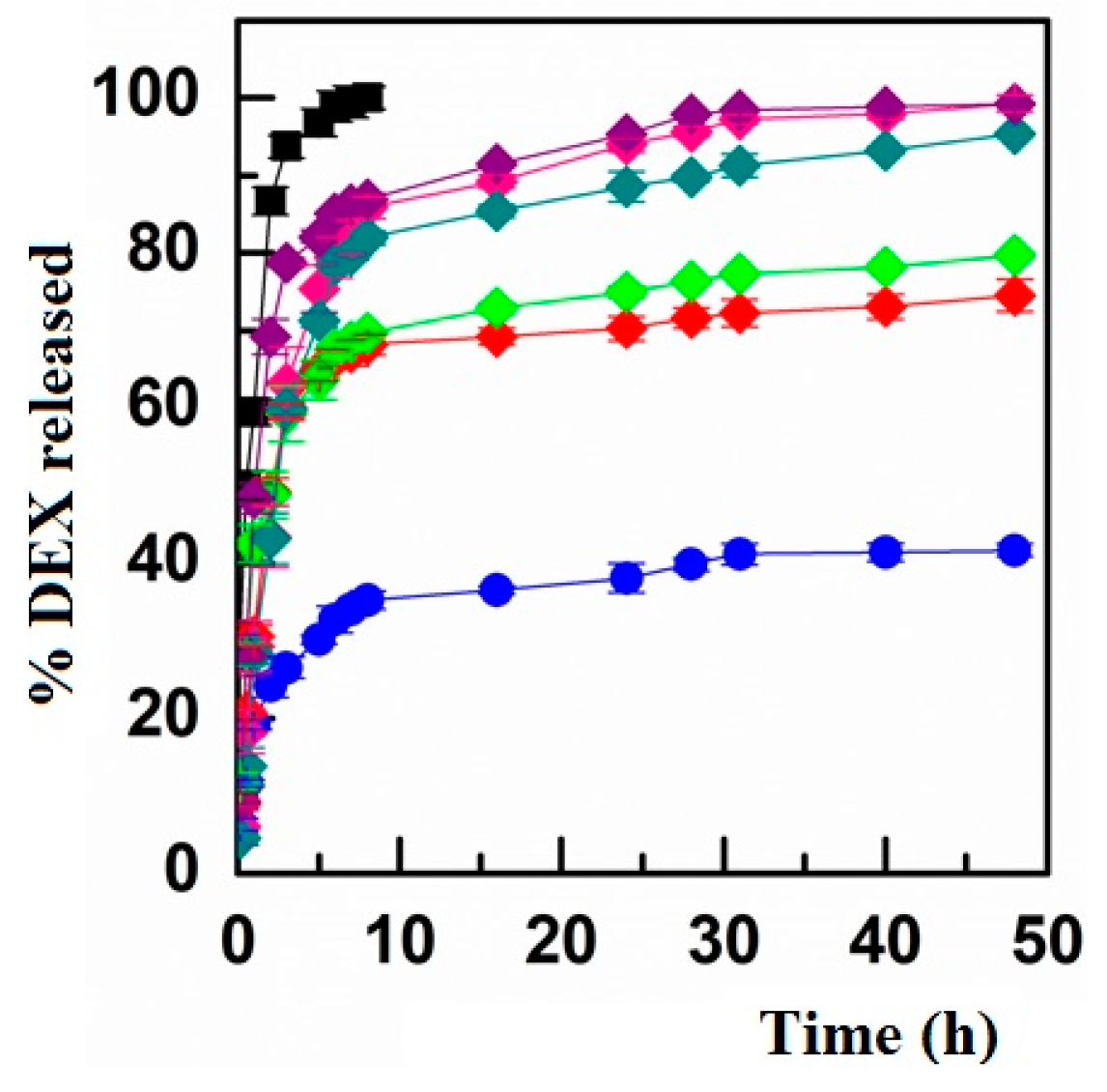
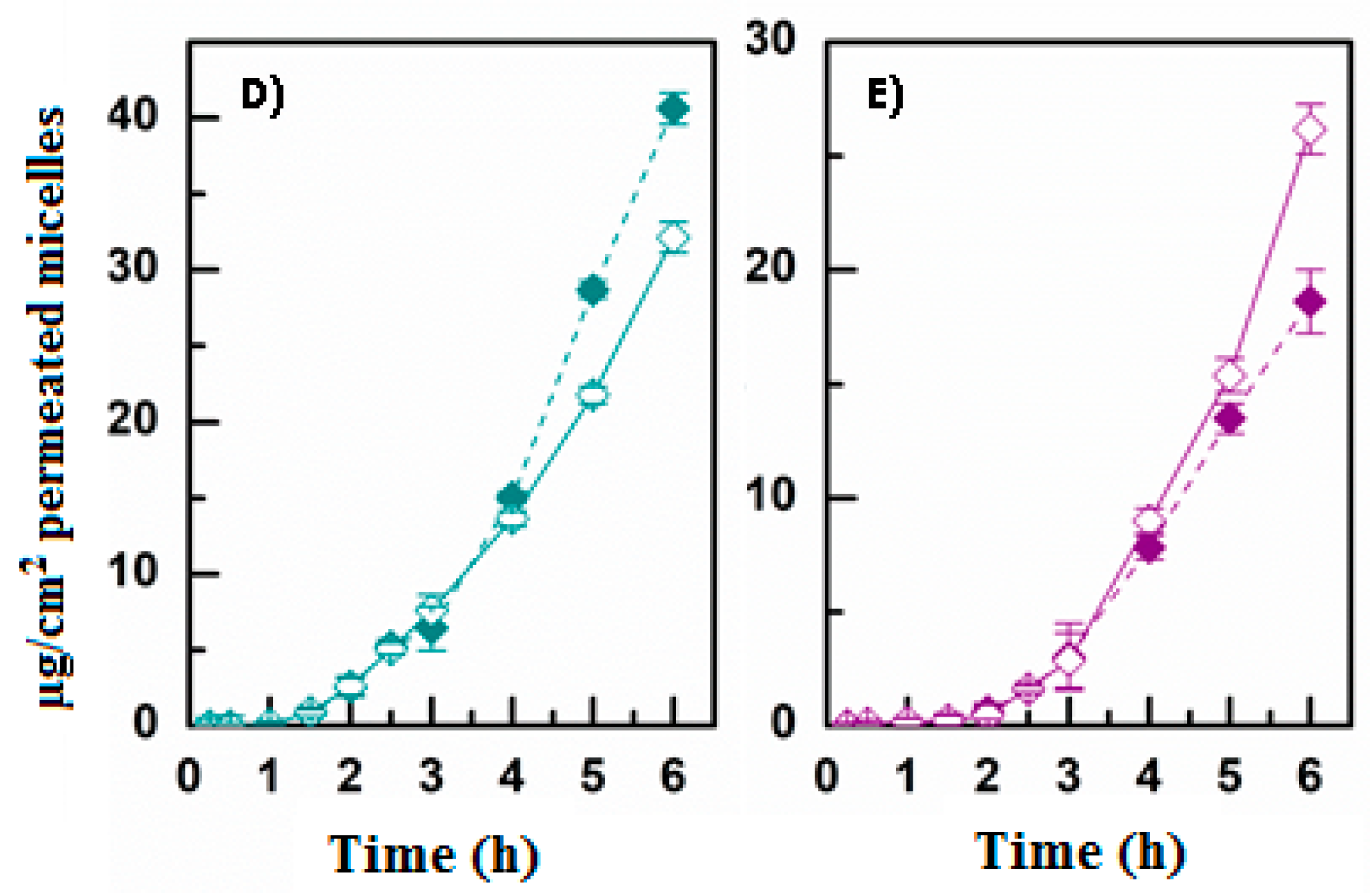
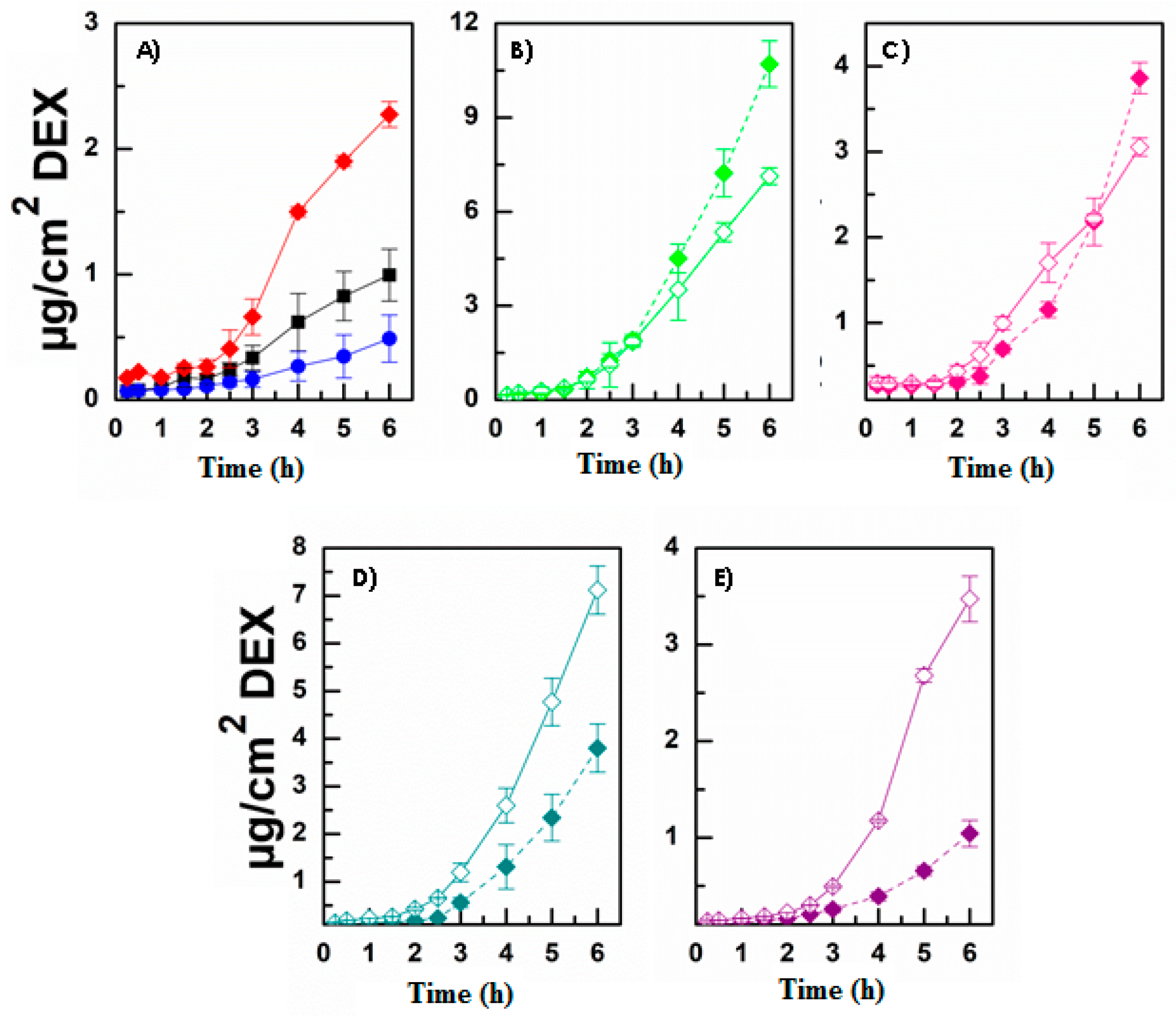
3.3. Evaluation of DEX Release and Permeation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Aungst, B.J. Absorption enhancers: Applications and advances. AAPS J. 2012, 14, 10–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maher, S.; Casettari, L.; Illum, L. Transmucosal absorption enhancers in the drug delivery field. Pharmaceutics 2019, 11, 339. [Google Scholar] [CrossRef] [Green Version]
- Rautio, J.; Kumpulainen, H.; Heimbach, T.; Oliyai, R.; Oh, D.; Järvinen, T.; Savolainen, J. Prodrugs: Design and clinical applications. Nat. Rev. Drug Discov. 2008, 7, 255–270. [Google Scholar] [CrossRef]
- Gote, V.; Sikder, S.; Sicotte, J.; Pal, D. Ocular drug delivery: Present innovations and future challenges. J. Pharmacol. Exp. Ther. 2019, 370, 602–624. [Google Scholar] [CrossRef]
- Achouri, D.; Alhanout, K.; Piccerelle, P.; Andrieu, V. Recent advances in ocular drug delivery. Drug Dev. Ind. Pharm. 2013, 39, 1599–1617. [Google Scholar] [CrossRef]
- Joana, F.F.; Francisco, V.; Amelia, M.S.; Eliana, B.S. Ocular Drug Delivery-New Strategies for Targeting Anterior and Posterior Segments of the Eye. Curr. Pharm. Des. 2016, 22, 1135–1146. [Google Scholar]
- Gote, V.; Ansong, M.; Pal, D. Prodrugs and nanomicelles to overcome ocular barriers for drug penetration. Expert Opin. Drug Metab. Toxicol. 2020, 16, 885–906. [Google Scholar] [CrossRef] [PubMed]
- Mandal, A.; Gote, V.; Pal, D.; Ogundele, A.; Mitra, A.K. Ocular Pharmacokinetics of a Topical Ophthalmic Nanomicellar Solution of Cyclosporine (Cequa®) for Dry Eye Disease. Pharm. Res. 2019, 36, 36. [Google Scholar] [CrossRef]
- Pepić, I.; Lovrić, J.; Filipović-Grčić, J. How do polymeric micelles cross epithelial barriers? Eur. J. Pharm. Sci. 2013, 50, 42–55. [Google Scholar] [CrossRef] [PubMed]
- de Cogan, F.; Hill, L.J.; Lynch, A.; Morgan-Warren, P.J.; Lechner, J.; Berwick, M.R.; Peacock, A.F.A.; Chen, M.; Scott, R.A.H.; Xu, H.; et al. Topical delivery of anti-VEGF drugs to the ocular posterior segment using cell-penetrating peptides. Investig. Ophthalmol. Vis. Sci. 2017, 58, 2578–2590. [Google Scholar] [CrossRef] [Green Version]
- Zhou, T.; Zhu, L.; Xia, H.; He, J.; Liu, S.; He, S.; Wang, L.; Zhang, J. Micelle carriers based on macrogol 15 hydroxystearate for ocular delivery of terbinafine hydrochloride: In vitro characterization and in vivo permeation. Eur. J. Pharm. Sci. 2017, 109, 288–296. [Google Scholar] [CrossRef]
- Moiseev, R.V.; Morrison, P.W.J.; Steele, F.; Khutoryanskiy, V.V. Penetration enhancers in ocular drug delivery. Pharmaceutics 2019, 11, 321. [Google Scholar] [CrossRef] [Green Version]
- Di Prima, G.; Saladino, S.; Bongiovì, F.; Adamo, G.; Ghersi, G.; Pitarresi, G.; Giammona, G. Novel inulin-based mucoadhesive micelles loaded with corticosteroids as potential transcorneal permeation enhancers. Eur. J. Pharm. Biopharm. 2017, 117, 385–399. [Google Scholar] [CrossRef]
- Di Prima, G.; Bongiovì, F.; Palumbo, F.S.; Pitarresi, G.; Licciardi, M.; Giammona, G. Mucoadhesive PEGylated inulin-based self-assembling nanoparticles: In vitro and ex vivo transcorneal permeation enhancement of corticosteroids. J. Drug Deliv. Sci. Technol. 2019, 49, 195–208. [Google Scholar] [CrossRef]
- Vaishya, R.D.; Gokulgandhi, M.; Patel, S.; Minocha, M.; Mitra, A.K. Novel dexamethasone-loaded nanomicelles for the intermediate and posterior segment uveitis. AAPS PharmSciTech 2014, 15, 1238–1251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xua, X.; Sun, L.; Zhou, L.; Cheng, Y.; Cao, F. Functional chitosan oligosaccharide nanomicelles for topical ocular drug delivery of dexamethasone. Carbohydr. Polym. 2020, 227, 115356. [Google Scholar] [CrossRef]
- Alami-Milani, M.; Zakeri-Milani, P.; Valizadeh, H.; Fathi, M.; Salatin, S.; Salehi, R.; Jelvehgari, M. PLA-PCL-PEG-PCL-PLA based micelles for improving the ocular permeability of dexamethasone: Development, characterization, and in vitro evaluation. Pharm. Dev. Technol. 2020, 25, 704–719. [Google Scholar] [CrossRef]
- Chaudhuri, A.; Haldar, S.; Chattopadhyay, A. Organization and dynamics in micellar structural transition monitored by pyrene fluorescence. Biochem. Biophys. Res. Commun. 2009, 390, 728–732. [Google Scholar] [CrossRef]
- Stewart, M.W. Corticosteroid Use for Diabetic Macular Edema: Old Fad or New Trend? Curr. Diab. Rep. 2012, 12, 364–375. [Google Scholar] [CrossRef]
- Duh, E.J.; Sun, J.K.; Stitt, A.W. Diabetic retinopathy: Current understanding, mechanisms, and treatment strategies. JCI Insight 2017, 2, e93751. [Google Scholar] [CrossRef] [PubMed]
- Wilson, C.A.; Berkowitz, B.A.; Sato, Y.; Ando, N.; Handa, J.T.; Juan, E. Treatment with Intravitreal Steroid Reduces Blood-Retinal Barrier Breakdown due to Retinal Photocoagulation. Arch. Ophthalmol. 1992, 110, 1155–1159. [Google Scholar] [CrossRef]
- Ali, Y.; Lehmussaari, K. Industrial perspective in ocular drug delivery. Adv. Drug Deliv. Rev. 2006, 58, 1258–1268. [Google Scholar] [CrossRef]
- Licciardi, M.; Scialabba, C.; Sardo, C.; Cavallaro, G.; Giammona, G. Amphiphilic inulin graft co-polymers as self-assembling micelles for doxorubicin delivery. J. Mater. Chem. B 2014, 2, 4262–4271. [Google Scholar] [CrossRef] [PubMed]
- Di Prima, G. Ocular Drug Delivery Systems per la Veicolazione di Molecole Bioattive al Segmento Posteriore dell’Occhio. Ph.D. Thesis, University of Palermo, Palermo, Italy, 2018. [Google Scholar]
- Bhatta, R.S.; Chandasana, H.; Chhonker, Y.S.; Rathi, C.; Kumar, D.; Mitra, K.; Shukla, P.K. Mucoadhesive nanoparticles for prolonged ocular delivery of natamycin: In vitro and pharmacokinetics studies. Int. J. Pharm. 2012, 432, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Licciardi, M.; Pitarresi, G.; Cavallaro, G.; Giammona, G. Nanoaggregates based on new poly-hydroxyethyl-aspartamide copolymers for oral insulin absorption. Mol. Pharm. 2013, 10, 1644–1654. [Google Scholar] [CrossRef]
- Friedrich, R.B.; Ravanello, A.; Cichota, L.C.; Rolim, C.M.B.; Beck, R.C.R. Validation of a simple and rapid UV spectrophotometric method for dexamethasone assay in tablets. Quim. Nova 2009, 32, 1052–1054. [Google Scholar] [CrossRef]
- Pescina, S.; Govoni, P.; Potenza, A.; Padula, C.; Santi, P.; Nicoli, S. Development of a convenient ex vivo model for the study of the transcorneal permeation of drugs: Histological and permeability evaluation. J. Pharm. Sci. 2015, 104, 63–71. [Google Scholar] [CrossRef]
- Di Prima, G.; Conigliaro, A.; De Caro, V. Mucoadhesive Polymeric Films to Enhance Barbaloin Penetration Into Buccal Mucosa: A Novel Approach to Chemoprevention. AAPS PharmSciTech 2019, 20, 1–12. [Google Scholar] [CrossRef]
- Di Prima, G.; Campisi, G.; de Caro, V. Amorphous ropinirole-loaded mucoadhesive buccal film: A potential patient-friendly tool to improve drug pharmacokinetic profile and effectiveness. J. Pers. Med. 2020, 10, 242. [Google Scholar] [CrossRef]
- D’souza, A.A.; Shegokar, R. Polyethylene glycol (PEG): A versatile polymer for pharmaceutical applications. Expert Opin. Drug Deliv. 2016, 13, 1257–1275. [Google Scholar] [CrossRef]
- Pasut, G.; Veronese, F.M. State of the art in PEGylation: The great versatility achieved after forty years of research. J. Control. Release 2012, 161, 461–472. [Google Scholar] [CrossRef]
- Liu, S.; Jones, L.; Gu, F.X. Nanomaterials for Ocular Drug Delivery. Macromol. Biosci. 2012, 12, 608–620. [Google Scholar] [CrossRef]
- Cholkar, K.; Patel, S.P.; Vadlapudi, A.D.; Mitra, A.K. Novel strategies for anterior segment ocular drug delivery. J. Ocul. Pharmacol. Ther. 2013, 29, 106–123. [Google Scholar] [CrossRef] [Green Version]
- Khar, R.K.; Jain, G.K.; Warsi, M.H.; Mallick, N.; Akhter, S.; Pathan, S.A.; Ahmad, F.J. Nano-vectors for the ocular delivery of nucleic acid-based therapeutics. Indian J. Pharm. Sci. 2010, 72, 675–688. [Google Scholar] [CrossRef] [Green Version]
- Reimondez-Troitiño, S.; Csaba, N.; Alonso, M.J.; De La Fuente, M. Nanotherapies for the treatment of ocular diseases. Eur. J. Pharm. Biopharm. 2015, 95, 279–293. [Google Scholar] [CrossRef]
- Vadlapudi, A.D.; Mitra, A.K. Nanomicelles: An emerging platform for drug delivery to the eye. Ther. Deliv. 2013, 4, 1–3. [Google Scholar] [CrossRef] [Green Version]
- Marques, M.R.C.; Loebenberg, R.; Almukainzi, M. Simulated Biological Fluids with Possible Application in Dissolution Testing. Dissolution Technol. 2011, 18, 15–28. [Google Scholar] [CrossRef]
- Morgan, P.B.; Maldonado-Codina, C. Corneal staining: Do we really understand what we are seeing? Cont. Lens Anterior Eye 2009, 32, 48–54. [Google Scholar] [CrossRef]



| DD mol% EDA | DD mol% RA | DD mol% PEG | DD mol% CAR | DD mol% CRE | DD mol% TAU | |
|---|---|---|---|---|---|---|
| INU-EDA-RA | 11.30 ± 1.50 | 4.30 ± 0.30 | - | - | - | - |
| INU-EDA-RA-PEG | 11.30 ± 1.50 | 4.30 ± 0.30 | 1.50 ± 0.25 | - | - | - |
| INU-EDA-RA-CAR | 11.30 ± 1.50 | 4.30 ± 0.30 | - | 2.00 ± 0.13 | - | - |
| INU-EDA-RA-CRE | 11.30 ± 1.50 | 4.30 ± 0.30 | - | - | 1.90 ± 0.15 | - |
| INU-EDA-TAU-RA | 11.30 ± 1.50 | 4.30 ± 0.30 | - | - | - | 2.16 ± 0.10 |
| Bidistilled Water (mg/mL) | DPBS pH 7.4 (mg/mL) | HEPES pH 7.4 (mg/mL) | |
|---|---|---|---|
| INU-EDA-RA | 0.135 ± 0.003 | 0.136 ± 0.002 | 0.135 ± 0.003 |
| INU-EDA-RA-PEG | 0.209 ± 0.004 | 0.073 ± 0.001 | 0.185 ± 0.002 |
| INU-EDA-RA-CAR | 0.100 ± 0.002 | 0.110 ± 0.002 | 0.140 ± 0.003 |
| INU-EDA-RA-CRE | 0.245 ± 0.004 | 0.150 ± 0.003 | 0.125 ± 0.003 |
| INU-EDA-TAU-RA | 0.142 ± 0.003 | 0.125 ± 0.002 | 0.135 ± 0.002 |
| DL% (w/w) | Particle Size (nm) | PDI | Z-Potential (mV) | Medium | |
|---|---|---|---|---|---|
| INU-EDA-TAU-RA | − | 227.41 ± 12.33 | 0.159 | −7.72 ± 0.88 | Water |
| 225.62 ± 11.70 | 0.145 | −2.65 ± 0.13 | DPBS | ||
| 234.40 ± 15.62 | 0.149 | −2.96 ± 0.20 | HEPES | ||
| INU-EDA-TAU-RA-Alexa Fluor488 | − | 222.78 ± 13.81 | 0.169 | −6.99 ± 0.40 | Water |
| 228.98 ± 15.00 | 0.160 | −2.06 ± 0.11 | DPBS | ||
| 229.99 ± 14.67 | 0.159 | −2.49 ± 0.16 | HEPES | ||
| INU-EDA-TAU-RA/DEX | 6.60 ± 0.56% | 226.00 ± 10.42 | 0.100 | −9.10 ± 0.74 | Water |
| 250.63 ± 18.17 | 0.102 | −3.22 ± 0.54 | DPBS | ||
| 235.00 ± 13.31 | 0.198 | −0.27 ± 0.01 | HEPES | ||
| INU-EDA-RA-CAR | − | 220.22 ± 12.03 | 0.109 | 18.72 ± 1.03 | Water |
| 223.94 ± 16.00 | 0.125 | −1.65 ± 0.12 | DPBS | ||
| 225.49 ± 15.47 | 0.139 | −1.96 ± 0.10 | HEPES | ||
| INU-EDA-RA-CAR-Alexa Fluor488 | − | 224.54 ± 11.19 | 0.115 | 16.99 ± 0.80 | Water |
| 228.89 ± 12.09 | 0.150 | −1.09 ± 0.10 | DPBS | ||
| 226.97 ± 13.40 | 0.147 | −1.40 ± 0.14 | HEPES | ||
| INU-EDA-RA-CAR/DEX | 4.10 ± 0.33% | 224.00 ± 11.87 | 0.131 | 20.33 ± 1.13 | Water |
| 230.60 ± 14.14 | 0.109 | −1.22 ± 0.21 | DPBS | ||
| 231.20 ± 13.99 | 0.118 | −2.27 ± 0.15 | HEPES | ||
| INU-EDA-RA-CRE | − | 227.41 ± 13.09 | 0.159 | 12.30 ± 0.94 | Water |
| 225.66 ± 12.00 | 0.196 | −1.65 ± 0.18 | DPBS | ||
| 234.43 ± 18.01 | 0.138 | −2.96 ± 0.22 | HEPES | ||
| INU-EDA-RA-CRE-Alexa Fluor488 | − | 229.01 ± 11.50 | 0.163 | 11.98 ± 0.99 | Water |
| 228.81 ± 12.09 | 0.158 | −1.49 ± 0.23 | DPBS | ||
| 232.32 ± 17.00 | 0.166 | −2.50 ± 0.30 | HEPES | ||
| INU-EDA-RA-CRE/DEX | 4.20 ± 0.20% | 226.00 ± 17.08 | 0.140 | 10.5 ± 0.79 | Water |
| 250.60 ± 19.22 | 0.191 | −3.63 ± 0.33 | DPBS | ||
| 235.01 ± 13.66 | 0.108 | −4.8 ± 0.35 | HEPES |
| Js (µg/cm2·h−1) | Kp (cm/h) | tlag (min) | De (µg/cm2) | Ac (cm) | |
|---|---|---|---|---|---|
| DEX solution | 0.218 ± 0.009 | 0.0044 ± 0.0004 | 90 | 1.736 ± 0.379 | 0.0347 ± 0.0012 |
| DEX suspension | 0.093 ± 0.005 | 0.0006 ± 0.0001 | 90 | 2.101 ± 0.835 | 0.0128 ± 0.0014 |
| INU-EDA-RA/DEX | 0.535 ± 0.009 | 0.0016 ± 0.0001 | 90 | 14.622 ± 0.616 | 0.0441 ± 0.0013 |
| INU-EDA-RA-PEG/DEX | 2.909 ± 0.010 | 0.0086 ± 0.0006 | 138 | 20.231 ± 0.048 | 0.0600 ± 0.0015 |
| INU-EDA-RA/DEX + PEG | 1.731 ± 0.011 | 0.0052 ± 0.0004 | 102 | 18.455 ± 0.053 | 0.0556 ± 0.0012 |
| INU-EDA-TAU-RA/DEX | 0.953 ± 0.008 | 0.0024 ± 0.0002 | 192 | 25.903 ± 0.120 | 0.0652 ± 0.0013 |
| INU-EDA-RA/DEX + TAU | 0.713 ± 0.008 | 0.0022 ± 0.0001 | 100 | 25.673 ± 0.136 | 0.0773 ± 0.0014 |
| INU-EDA-RA-CAR/DEX | 1.001 ± 0.010 | 0.0026 ± 0.0001 | 162 | 37.722 ± 0.245 | 0.0961 ± 0.0013 |
| INU-EDA-RA/DEX + CAR | 1.994 ± 0.012 | 0.0060 ± 0.0005 | 168 | 31.527 ± 0.233 | 0.0950 ± 0.0011 |
| INU-EDA-RA-CRE/DEX | 0.326 ± 0.003 | 0.0008 ± 0.0001 | 156 | 15.886 ± 0.197 | 0.0373 ± 0.0010 |
| INU-EDA-RA/DEX + CRE | 1.044 ± 0.009 | 0.0031 ± 0.0002 | 138 | 15.090 ± 0.188 | 0.0455 ± 0.0011 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Prima, G.; Licciardi, M.; Bongiovì, F.; Pitarresi, G.; Giammona, G. Inulin-Based Polymeric Micelles Functionalized with Ocular Permeation Enhancers: Improvement of Dexamethasone Permeation/Penetration through Bovine Corneas. Pharmaceutics 2021, 13, 1431. https://doi.org/10.3390/pharmaceutics13091431
Di Prima G, Licciardi M, Bongiovì F, Pitarresi G, Giammona G. Inulin-Based Polymeric Micelles Functionalized with Ocular Permeation Enhancers: Improvement of Dexamethasone Permeation/Penetration through Bovine Corneas. Pharmaceutics. 2021; 13(9):1431. https://doi.org/10.3390/pharmaceutics13091431
Chicago/Turabian StyleDi Prima, Giulia, Mariano Licciardi, Flavia Bongiovì, Giovanna Pitarresi, and Gaetano Giammona. 2021. "Inulin-Based Polymeric Micelles Functionalized with Ocular Permeation Enhancers: Improvement of Dexamethasone Permeation/Penetration through Bovine Corneas" Pharmaceutics 13, no. 9: 1431. https://doi.org/10.3390/pharmaceutics13091431






