Patterned Drug-Eluting Coatings for Tracheal Stents Based on PLA, PLGA, and PCL for the Granulation Formation Reduction: In Vivo Studies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fabrication of Sealed Free-Standing Polymer Films with Microchambers Array Containing Methylprednisolone Sodium Succinate and Fluorescein Isothiocyanate-Dextran
2.3. Functionalization of Tracheal Stent Surface by Microchamber Array Film
2.4. Characterization Technique
2.5. In Vivo Study
3. Results and Discussion
3.1. Functionalization of Tracheal Stent Surface by PLA, PLGA, and PCL Coatings Based on Microchamber Arrays
3.2. Comparison of PLA, PLGA, and PCL Coatings Mechanical Properties
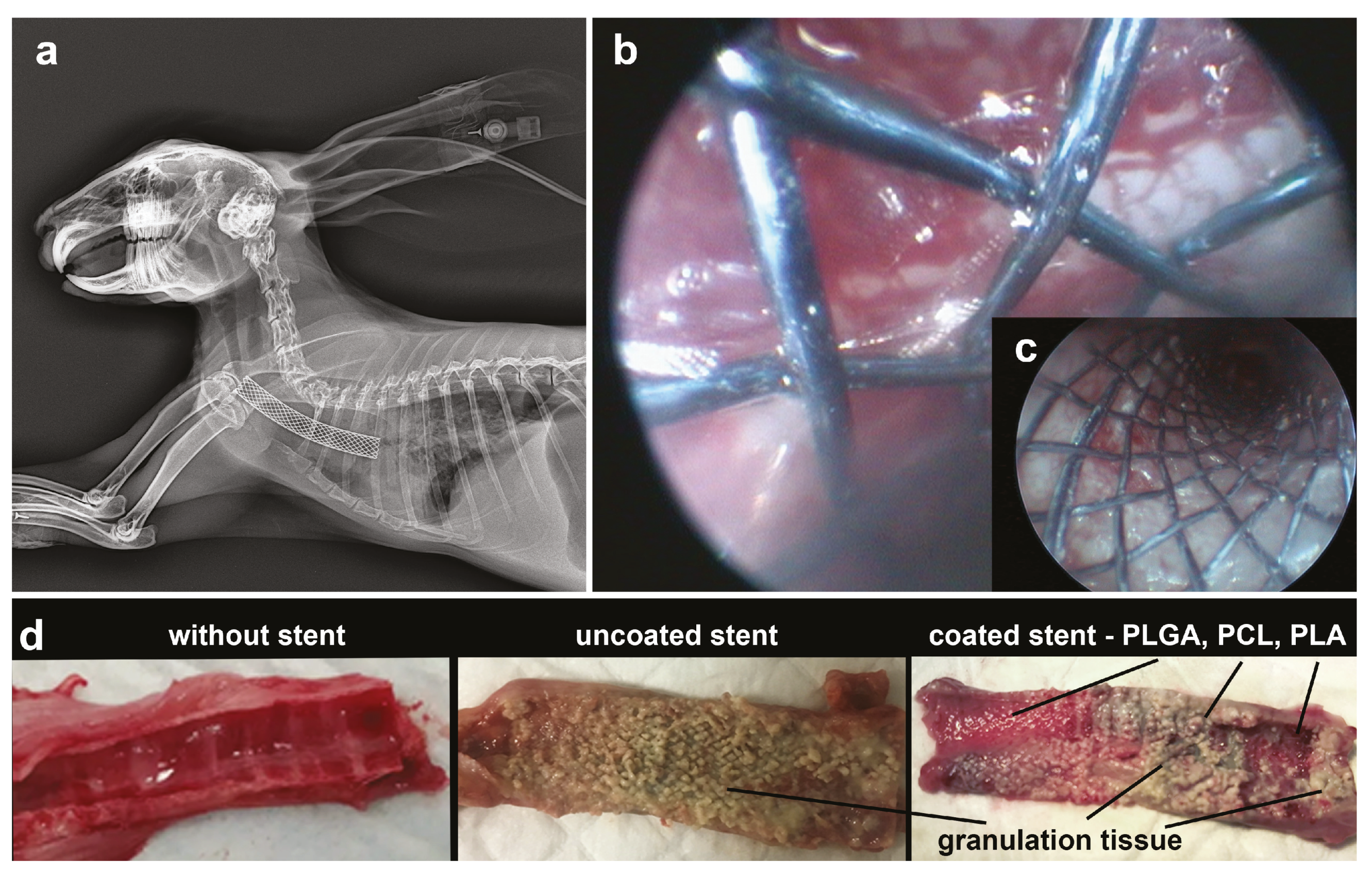
3.3. In Vivo Studies of the Use of PLA, PLGA, and PCL Coatings to Reduce Granulation Tissue
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huang, W.; Shan, Q.; Wu, Z.; Li, H.; Zhou, M.; Ding, X.; Wang, Z. Retrievable covered metallic segmented Y airway stent for gastrorespiratory fistula of carina or main bronchi. J. Thorac. Cardiovasc. Surg. 2021, 161, 1664–1671. [Google Scholar] [CrossRef]
- Freitag, L.; Gördes, M.; Zarogoulidis, P.; Darwiche, K.; Franzen, D.; Funke, F.; Hohenforst-Schmidt, W.; Dutau, H. Towards Individualized Tracheobronchial Stents: Technical, Practical and Legal Considerations. Respiration 2017, 94, 442–456. [Google Scholar] [CrossRef] [PubMed]
- Folch, E.; Keyes, C. Airway stents. Ann. Cardiothorac. Surg. 2018, 7, 273–283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, L.; Kong, J.; Georg, C. Recent advances in airway stenting. Shanghai Chest 2020, 4, 6. [Google Scholar] [CrossRef]
- Kang, Y. A Review of Self-Expanding Esophageal Stents for the Palliation Therapy of Inoperable Esophageal Malignancies. BioMed Res. Int. 2019, 2019, 9265017. [Google Scholar] [CrossRef] [Green Version]
- Schuurmans, M.M.; Palheiros Marques, M.; Freitag, L.; Zinkernagel, A.S.; Achermann, Y. Biofilm Formation in a Permanent Tracheal Stent Implanted for Twenty-Five Years. Respiration 2015, 90, 327–328. [Google Scholar] [CrossRef] [Green Version]
- Bi, Y.; Zhu, X.; Yu, Z.; Yi, M.; Han, X.; Ren, J. Clinical outcomes of self-expandable metallic stents for malignant obstructive atelectasis. Sci. Rep. 2020, 10, 3600. [Google Scholar] [CrossRef]
- Roscoe, A.; Kanellakos, G.W.; McRae, K.; Slinger, P. Pressures Exerted by Endobronchial Devices. Anesth. Analg. 2007, 104, 655–658. [Google Scholar] [CrossRef]
- Freitag, L.; Darwiche, K. Endoscopic Treatment of Tracheal Stenosis. Thorac. Surg. Clin. 2014, 24, 27–40. [Google Scholar] [CrossRef]
- Bansal, S.; Dhingra, S.; Ghai, B.; Gupta, A.K. Metallic Stents for Proximal Tracheal Stenosis: Is It Worth the Risk? Case Rep. Otolaryngol. 2012, 2012, 1–3. [Google Scholar] [CrossRef] [Green Version]
- Shadmehr, M.B.; Abbasidezfouli, A.; Farzanegan, R.; Pejhan, S.; Daneshvar Kakhaki, A.; Sheikhy, K.; Saghebi, S.R.; Sadeghbeigee, F.; Gharedaghi, A.; Jahanshahi, N.; et al. The Role of Systemic Steroids in Postintubation Tracheal Stenosis: A Randomized Clinical Trial. Ann. Thorac. Surg. 2017, 103, 246–253. [Google Scholar] [CrossRef] [Green Version]
- Bruscoli, S.; Febo, M.; Riccardi, C.; Migliorati, G. Glucocorticoid Therapy in Inflammatory Bowel Disease: Mechanisms and Clinical Practice. Front. Immunol. 2021, 12, 2163. [Google Scholar] [CrossRef]
- Duvvuri, M.; Motz, K.; Murphy, M.; Feeley, M.; Ding, D.; Lee, A.; Elisseeff, J.H.; Hillel, A.T. Engineering an immunomodulatory drug-eluting stent to treat laryngotracheal stenosis. Biomater. Sci. 2019, 7, 1863–1874. [Google Scholar] [CrossRef] [PubMed]
- Arellano-Orden, E.; Serrano, C.; Montes-Worboys, A.; Sánchez-López, V.; Laborda, A.; Lostalé, F.; Lahuerta, C.; Rodríguez-Panadero, F.; de Gregorio, M.Á. Stent-induced tracheal stenosis can be predicted by IL-8 expression in rabbits. Eur. J. Clin. Investig. 2017, 47, 84–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beshchasna, N.; Saqib, M.; Kraskiewicz, H.; Wasyluk, Ł.; Kuzmin, O.; Duta, O.C.; Ficai, D.; Ghizdavet, Z.; Marin, A.; Ficai, A.; et al. Recent Advances in Manufacturing Innovative Stents. Pharmaceutics 2020, 12, 349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rykowska, I.; Nowak, I.; Nowak, R. Drug-Eluting Stents and Balloons—Materials, Structure Designs, and Coating Techniques: A Review. Molecules 2020, 25, 4624. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Yang, K.; Cheng, R.; Xiang, Y.; Yuan, T.; Cheng, Y.; Sarmento, B.; Cui, W. The current status of biodegradable stent to treat benign luminal disease. Mater. Today 2017, 20, 516–529. [Google Scholar] [CrossRef]
- Xu, J.; Ong, H.X.; Traini, D.; Byrom, M.; Williamson, J.; Young, P.M. The utility of 3D-printed airway stents to improve treatment strategies for central airway obstructions. Drug Dev. Ind. Pharm. 2019, 45, 1–10. [Google Scholar] [CrossRef]
- Feuerbach, T.; Kock, S.; Thommes, M. Slicing parameter optimization for 3D printing of biodegradable drug-eluting tracheal stents. Pharm. Dev. Technol. 2020, 25, 650–658. [Google Scholar] [CrossRef] [PubMed]
- Chao, Y.K.; Liu, K.S.; Wang, Y.C.; Huang, Y.L.; Liu, S.J. Biodegradable Cisplatin-Eluting Tracheal Stent for Malignant Airway Obstruction. Chest 2013, 144, 193–199. [Google Scholar] [CrossRef]
- Stramiello, J.A.; Mohammadzadeh, A.; Ryan, J.; Brigger, M.T. The role of bioresorbable intraluminal airway stents in pediatric tracheobronchial obstruction: A systematic review. Int. J. Pediatr. Otorhinolaryngol. 2020, 139, 110405. [Google Scholar] [CrossRef]
- Wang, S.; Chen, Z.; Lin, Q.; Lin, Q.; Geng, Y.; Wang, S. In vitro pharmacokinetics of sirolimus-coated stent for tracheal stenosis. Trop. J. Pharm. Res. 2017, 16, 2033. [Google Scholar] [CrossRef] [Green Version]
- Serrano, C.; Lostalé, F.; Rodríguez-Panadero, F.; de Blas, I.; Laborda, A.; de Gregorio, M.A. Tracheal Self-Expandable Metallic Stents: A Comparative Study of Three Different Stents in a Rabbit Model. Arch. Bronconeumol. (Engl. Ed.) 2016, 52, 123–130. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.; Zhang, J.; Wang, J.; Pei, Y.H.; Qiu, X.J.; Wang, Y.L. Paclitaxel Drug-eluting Tracheal Stent Could Reduce Granulation Tissue Formation in a Canine Model. Chin. Med. J. 2016, 129, 2708–2713. [Google Scholar] [CrossRef]
- Kong, Y.; Zhang, J.; Wang, T.; Qiu, X.; Wang, Y. Preparation and characterization of paclitaxel-loaded poly lactic acid-co-glycolic acid coating tracheal stent. Chin. Med J. 2014, 127, 2236–2240. [Google Scholar]
- Heo, D.N.; Lee, J.B.; Bae, M.S.; Hwang, Y.S.; Kwon, K.H.; Kwon, I.K. Development of Nanofiber Coated Indomethacin—Eluting Stent for Tracheal Regeneration. J. Nanosci. Nanotechnol. 2011, 11, 5711–5716. [Google Scholar] [CrossRef] [PubMed]
- Kuznetsov, K.; Stepanova, A.; Kvon, R.; Douglas, T.; Kuznetsov, N.; Chernonosova, V.; Zaporozhchenko, I.; Kharkova, M.; Romanova, I.; Karpenko, A.; et al. Electrospun Produced 3D Matrices for Covering of Vascular Stents: Paclitaxel Release Depending on Fiber Structure and Composition of the External Environment. Materials 2018, 11, 2176. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Jiao, D.; Zhang, W.; Ren, K.; Qiu, L.; Tian, C.; Li, Y.; Li, J.; Zhou, X.; Zhao, Y.; et al. Antibacterial and antihyperplasia polylactic acid/silver nanoparticles nanofiber membrane-coated airway stent for tracheal stenosis. Colloids Surf. B Biointerfaces 2021, 206, 111949. [Google Scholar] [CrossRef] [PubMed]
- Antipina, M.N.; Kiryukhin, M.V.; Skirtach, A.G.; Sukhorukov, G.B. Micropackaging via layer-by-layer assembly: Microcapsules and microchamber arrays. Int. Mater. Rev. 2014, 59, 224–244. [Google Scholar] [CrossRef]
- Antipina, M.N.; Kiryukhin, M.V.; Sukhorukov, G.B. Stimuli-Responsive Polymer Composite Multilayer Microcapsules and Microchamber Arrays. In Multilayer Thin Films; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2012; pp. 851–890. [Google Scholar] [CrossRef]
- Kiryukhin, M.V.; Gorelik, S.R.; Man, S.M.; Subramanian, G.S.; Antipina, M.N.; Low, H.Y.; Sukhorukov, G.B. Individually Addressable Patterned Multilayer Microchambers for Site-Specific Release-On-Demand. Macromol. Rapid Commun. 2013, 34, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Kiryukhin, M.V.; Man, S.M.; Tonoyan, A.; Low, H.Y.; Sukhorukov, G.B. Adhesion of Polyelectrolyte Multilayers: Sealing and Transfer of Microchamber Arrays. Langmuir 2012, 28, 5678–5686. [Google Scholar] [CrossRef]
- Gai, M.; Frueh, J.; Tao, T.; Petrov, A.V.; Petrov, V.V.; Shesterikov, E.V.; Tverdokhlebov, S.I.; Sukhorukov, G.B. Polylactic acid nano- and microchamber arrays for encapsulation of small hydrophilic molecules featuring drug release via high intensity focused ultrasound. Nanoscale 2017, 9, 7063–7070. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sindeeva, O.A.; Gusliakova, O.I.; Inozemtseva, O.A.; Abdurashitov, A.S.; Brodovskaya, E.P.; Gai, M.; Tuchin, V.V.; Gorin, D.A.; Sukhorukov, G.B. Effect of a Controlled Release of Epinephrine Hydrochloride from PLGA Microchamber Array: In Vivo Studies. ACS Appl. Mater. Interfaces 2018, 10, 37855–37864. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Sun, R.; DeSouza-Edwards, A.O.; Frueh, J.; Sukhorukov, G.B. Microchamber arrays made of biodegradable polymers for enzymatic release of small hydrophilic cargos. Soft Matter 2020, 16, 2266–2275. [Google Scholar] [CrossRef] [PubMed]
- Kopach, O.; Zheng, K.; Sindeeva, O.A.; Gai, M.; Sukhorukov, G.B.; Rusakov, D.A. Polymer microchamber arrays for geometry-controlled drug release: A functional study in human cells of neuronal phenotype. Biomater. Sci. 2019, 7, 2358–2371. [Google Scholar] [CrossRef] [Green Version]
- Sindeeva, O.A.; Kopach, O.; Kurochkin, M.A.; Sapelkin, A.; Gould, D.J.; Rusakov, D.A.; Sukhorukov, G.B. Polylactic Acid-Based Patterned Matrixes for Site-Specific Delivery of Neuropeptides On-Demand: Functional NGF Effects on Human Neuronal Cells. Front. Bioeng. Biotechnol. 2020, 8, 497. [Google Scholar] [CrossRef]
- Sindeeva, O.A.; Prikhozhdenko, E.S.; Bratashov, D.N.; Vostrikova, A.M.; Atkin, V.S.; Ermakov, A.V.; Khlebtsov, B.N.; Sapelkin, A.V.; Goryacheva, I.Y.; Sukhorukov, G.B. Carbon dot aggregates as an alternative to gold nanoparticles for the laser-induced opening of microchamber arrays. Soft Matter 2018, 14, 9012–9019. [Google Scholar] [CrossRef]
- Gai, M.; Li, W.; Frueh, J.; Sukhorukov, G.B. Polylactic acid sealed polyelectrolyte complex microcontainers for controlled encapsulation and NIR-Laser based release of cargo. Colloids Surf. B Biointerfaces 2019, 173, 521–528. [Google Scholar] [CrossRef]
- Gai, M.; Kurochkin, M.A.; Li, D.; Khlebtsov, B.N.; Dong, L.; Tarakina, N.; Poston, R.; Gould, D.J.; Frueh, J.; Sukhorukov, G.B. In-situ NIR-laser mediated bioactive substance delivery to single cell for EGFP expression based on biocompatible microchamber-arrays. J. Control. Release 2018, 276, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Kurochkin, M.A.; Sindeeva, O.A.; Brodovskaya, E.P.; Gai, M.; Frueh, J.; Su, L.; Sapelkin, A.; Tuchin, V.V.; Sukhorukov, G.B. Laser-triggered drug release from polymeric 3-D micro-structured films via optical fibers. Mater. Sci. Eng. C 2020, 110, 110664. [Google Scholar] [CrossRef]
- Ermakov, A.V.; Kudryavtseva, V.L.; Demina, P.A.; Verkhovskii, R.A.; Zhang, J.; Lengert, E.V.; Sapelkin, A.V.; Goryacheva, I.Y.; Sukhorukov, G.B. Site-specific release of reactive oxygen species from ordered arrays of microchambers based on polylactic acid and carbon nanodots. J. Mater. Chem. B 2020, 8, 7977–7986. [Google Scholar] [CrossRef]
- Zykova, Y.; Kudryavtseva, V.; Gai, M.; Kozelskaya, A.; Frueh, J.; Sukhorukov, G.; Tverdokhlebov, S. Free-standing microchamber arrays as a biodegradable drug depot system for implant coatings. Eur. Polym. J. 2019, 114, 72–80. [Google Scholar] [CrossRef]
- Antipina, M.N.; Kiryukhin, M.V.; Chong, K.; Low, H.Y.; Sukhorukov, G.B. Patterned microcontainers as novel functional elements for µTAS and LOC. Lab Chip 2009, 9, 1472. [Google Scholar] [CrossRef]
- Gai, M.; Frueh, J.; Kudryavtseva, V.L.; Mao, R.; Kiryukhin, M.V.; Sukhorukov, G.B. Patterned Microstructure Fabrication: Polyelectrolyte Complexes vs Polyelectrolyte Multilayers. Sci. Rep. 2016, 6, 37000. [Google Scholar] [CrossRef] [Green Version]
- Marmur, A. Wetting on Hydrophobic Rough Surfaces: To Be Heterogeneous or Not To Be? Langmuir 2003, 19, 8343–8348. [Google Scholar] [CrossRef]
- Shimada, K.; Mitamura, K.; Higashi, T. Gas chromatography and high-performance liquid chromatography of natural steroids. J. Chromatogr. A 2001, 935, 141–172. [Google Scholar] [CrossRef]
- Delaere, P.R.; Liu, Z.Y.; Hermans, R.; Sciot, R.; Feenstra, L. Experimental tracheal allograft revascularization and transplantation. J. Thorac. Cardiovasc. Surg. 1995, 110, 728–737. [Google Scholar] [CrossRef] [Green Version]
- Colt, H.G.; Harrell, J.; Neuman, T.R.; Robbins, T. External Fixation of Subglottic Tracheal Stents. Chest 1994, 105, 1653–1657. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daumerie, G.; Su, S.; Ochroch, E.A. Anesthesia for the Patient with Tracheal Stenosis. Anesthesiol. Clin. 2010, 28, 157–174. [Google Scholar] [CrossRef] [PubMed]
- Palinko, D.; Matievics, V.; Szegesdi, I.; Sztano, B.; Rovo, L. Minimally invasive endoscopic treatment for pediatric combined high grade stenosis as a laryngeal manifestation of epidermolysis bullosa. Int. J. Pediatr. Otorhinolaryngol. 2017, 92, 126–129. [Google Scholar] [CrossRef] [PubMed]
- Gai, M.; Kudryavtseva, V.L.; Sukhorukov, G.B.; Frueh, J. Micro-Patterned Polystyrene Sheets as Templates for Interlinked 3D Polyelectrolyte Multilayer Microstructures. BioNanoScience 2018, 8, 654–660. [Google Scholar] [CrossRef]
- Wenzel, R.N. Resistance of solid surfaces to wetting by water. Ind. Eng. Chem. 1936, 28, 988–994. [Google Scholar] [CrossRef]
- Gai, M.; Frueh, J.; Kudryavtseva, V.L.; Yashchenok, A.M.; Sukhorukov, G.B. Polylactic Acid Sealed Polyelectrolyte Multilayer Microchambers for Entrapment of Salts and Small Hydrophilic Molecules Precipitates. ACS Appl. Mater. Interfaces 2017, 9, 16536–16545. [Google Scholar] [CrossRef]
- Nair, N.R.; Sekhar, V.C.; Nampoothiri, K.M. Augmentation of a Microbial Consortium for Enhanced Polylactide (PLA) Degradation. Indian J. Microbiol. 2016, 56, 59–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sabino, V.G.; Ginani, F.; Silva, T.N.; Cabral, A.A.; Mota-Filho, H.G.; Freire, M.C.L.C.; Souza Furtado, P.; Assumpção, P.W.M.C.; Cabral, L.M.; Moura, C.E.; et al. Laser therapy increases the proliferation of preosteoblastic MC3T3-E1 cells cultured on poly(lactic acid) films. J. Tissue Eng. Regen. Med. 2020, 14, 1792–1803. [Google Scholar] [CrossRef] [PubMed]
- Foldberg, S.; Petersen, M.; Fojan, P.; Gurevich, L.; Fink, T.; Pennisi, C.P.; Zachar, V. Patterned poly(lactic acid) films support growth and spontaneous multilineage gene expression of adipose-derived stem cells. Colloids Surf. B Biointerfaces 2012, 93, 92–99. [Google Scholar] [CrossRef]
- Maeda, K.; Ono, S.; Tazuke, Y.; Baba, K. Long-term outcomes of congenital tracheal stenosis treated by metallic airway stenting. J. Pediatr. Surg. 2013, 48, 293–296. [Google Scholar] [CrossRef]
- Serio, P.; Fainardi, V.; Leone, R.; Baggi, R.; Grisotto, L.; Biggeri, A.; Mirabile, L. Tracheobronchial obstruction: Follow-up study of 100 children treated with airway stenting. Eur. J. Cardio-Thorac. Surg. 2014, 45, e100–e109. [Google Scholar] [CrossRef] [Green Version]
- Simoni, P.; Wiatrak, B.J. Microbiology of Stents in Laryngotracheal Reconstruction. Laryngoscope 2004, 114, 364–367. [Google Scholar] [CrossRef]
- Yuasa, R.; Hata, Y.; Otsuka, H.; Makino, T.; Koezuka, S.; Sato, F.; Tamaki, K.; Sasamoto, S.; Takagi, K.; Iyoda, A. Placement of self-expandable metallic stents for tracheal stenosis secondary to thyroid cancer. Mol. Clin. Oncol. 2014, 2, 1003–1008. [Google Scholar] [CrossRef]
- Vitsas, V.; Touman, A.; Agelakis, L.; Moraitou, E.; Vogiatzakis, E.; Koulouris, N.; Stratakos, G. Bacterial colonization/infection of the airway stents. In Interventional Pulmonology; European Respiratory Society: Sheffield, UK, 2017; p. PA3792. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sindeeva, O.A.; Prikhozhdenko, E.S.; Schurov, I.; Sedykh, N.; Goriainov, S.; Karamyan, A.; Mordovina, E.A.; Inozemtseva, O.A.; Kudryavtseva, V.; Shchesnyak, L.E.; et al. Patterned Drug-Eluting Coatings for Tracheal Stents Based on PLA, PLGA, and PCL for the Granulation Formation Reduction: In Vivo Studies. Pharmaceutics 2021, 13, 1437. https://doi.org/10.3390/pharmaceutics13091437
Sindeeva OA, Prikhozhdenko ES, Schurov I, Sedykh N, Goriainov S, Karamyan A, Mordovina EA, Inozemtseva OA, Kudryavtseva V, Shchesnyak LE, et al. Patterned Drug-Eluting Coatings for Tracheal Stents Based on PLA, PLGA, and PCL for the Granulation Formation Reduction: In Vivo Studies. Pharmaceutics. 2021; 13(9):1437. https://doi.org/10.3390/pharmaceutics13091437
Chicago/Turabian StyleSindeeva, Olga A., Ekaterina S. Prikhozhdenko, Igor Schurov, Nikolay Sedykh, Sergey Goriainov, Arfenya Karamyan, Ekaterina A. Mordovina, Olga A. Inozemtseva, Valeriya Kudryavtseva, Leonid E. Shchesnyak, and et al. 2021. "Patterned Drug-Eluting Coatings for Tracheal Stents Based on PLA, PLGA, and PCL for the Granulation Formation Reduction: In Vivo Studies" Pharmaceutics 13, no. 9: 1437. https://doi.org/10.3390/pharmaceutics13091437
APA StyleSindeeva, O. A., Prikhozhdenko, E. S., Schurov, I., Sedykh, N., Goriainov, S., Karamyan, A., Mordovina, E. A., Inozemtseva, O. A., Kudryavtseva, V., Shchesnyak, L. E., Abramovich, R. A., Mikhajlov, S., & Sukhorukov, G. B. (2021). Patterned Drug-Eluting Coatings for Tracheal Stents Based on PLA, PLGA, and PCL for the Granulation Formation Reduction: In Vivo Studies. Pharmaceutics, 13(9), 1437. https://doi.org/10.3390/pharmaceutics13091437







