Polyelectrolyte Matrices in the Modulation of Intermolecular Electrostatic Interactions for Amorphous Solid Dispersions: A Comprehensive Review
Abstract
:1. Introduction
2. General Principles of Amorphous Solid Dispersions
3. Pharmaceutical Polyelectrolytes
4. Polyelectrolytes in Hot-Melt Extrusion
5. Modified Polyelectrolyte Matrices
5.1. Combination of Polyelectrolytes with Polymers
5.2. Interpolyelectrolyte Complexes
5.3. Addition of Small Molecules
6. Physical Stability due to Intermolecular Interactions
The Antiplasticization Effect of Polyelectrolytes
7. The Impact of Polyelectrolytes on the Dissolution Rate and Supersaturation
8. Polyelectrolytes in Controlled-Drug Release/Colonic Targeting
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lipinski, C.A. Drug-like properties and the causes of poor solubility and poor permeability. J. Pharmacol. Toxicol. Methods 2000, 44, 235–249. [Google Scholar] [CrossRef]
- Kawabata, Y.; Wada, K.; Nakatani, M.; Yamada, S.; Onoue, S. Formulation design for poorly water-soluble drugs based on biopharmaceutics classification system: Basic approaches and practical applications. Int. J. Pharm. 2011, 420, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kansara, H.; Panola, R.; Chauhan, C.S.; Mishra, A. Bioavailability Enhancement Techniques for BCS Class II Drugs: A Review. Int. J. Drug Dev. Res. 2015, 7, 250–261. [Google Scholar]
- He, Y.; Ho, C. Amorphous Solid Dispersions: Utilization and Challenges in Drug Discovery and Development. J. Pharm. Sci. 2015, 104, 3237–3258. [Google Scholar] [CrossRef]
- Lee, T.; Boersen, N.; Hui, H.W.; Chow, S.F.; Wan, K.Y.; Chow, A. Delivery of Poorly Soluble Compounds by Amorphous Solid Dispersions. Curr. Pharm. Des. 2014, 20, 303–324. [Google Scholar] [CrossRef]
- Karagianni, A.; Malamatari, M.; Kachrimanis, K. Pharmaceutical cocrystals: New solid phase modification approaches for the formulation of APIs. Pharmaceutics 2018, 10, 18. [Google Scholar] [CrossRef] [Green Version]
- Mishra, D.K.; Dhote, V.; Bhargava, A.; Jain, D.K.; Mishra, P.K. Amorphous solid dispersion technique for improved drug delivery: Basics to clinical applications. Drug Deliv. Transl. Res. 2015, 5, 552–565. [Google Scholar] [CrossRef]
- Laitinen, R.; Löbmann, K.; Strachan, C.J.; Grohganz, H.; Rades, T. Emerging trends in the stabilization of amorphous drugs. Int. J. Pharm. 2013, 453, 65–79. [Google Scholar] [CrossRef]
- Vo, C.L.N.; Park, C.; Lee, B.J. Current trends and future perspectives of solid dispersions containing poorly water-soluble drugs. Eur. J. Pharm. Biopharm. 2013, 85, 799–813. [Google Scholar] [CrossRef]
- Wu, J.X.; Yang, M.; Van Den Berg, F.; Pajander, J.; Rades, T.; Rantanen, J. Influence of solvent evaporation rate and formulation factors on solid dispersion physical stability. Eur. J. Pharm. Sci. 2011, 44, 610–620. [Google Scholar] [CrossRef]
- Pandi, P.; Bulusu, R.; Kommineni, N.; Khan, W.; Singh, M. Amorphous solid dispersions: An update for preparation, characterization, mechanism on bioavailability, stability, regulatory considerations and marketed products. Int. J. Pharm. 2020, 586, 119560. [Google Scholar] [CrossRef]
- Zhang, J.; Han, R.; Chen, W.; Zhang, W.; Li, Y.; Ji, Y.; Chen, L.; Pan, H.; Yang, X.; Pan, W.; et al. Analysis of the literature and patents on solid dispersions from 1980 to 2015. Molecules 2018, 23, 1697. [Google Scholar] [CrossRef] [Green Version]
- Wilson, V.R.; Lou, X.; Osterling, D.J.; Stolarik, D.A.F.; Jenkins, G.J.; Nichols, B.L.B.; Dong, Y.; Edgar, K.J.; Zhang, G.G.Z.; Taylor, L.S. Amorphous solid dispersions of enzalutamide and novel polysaccharide derivatives: Investigation of relationships between polymer structure and performance. Sci. Rep. 2020, 10, 18535. [Google Scholar] [CrossRef] [PubMed]
- Frank, D.S.; Matzger, A.J. Probing the Interplay between Amorphous Solid Dispersion Stability and Polymer Functionality. Mol. Pharm. 2018, 15, 2714–2720. [Google Scholar] [CrossRef] [PubMed]
- Amponsah-Efah, K.K.; Mistry, P.; Eisenhart, R.; Suryanarayanan, R. The Influence of the Strength of Drug-Polymer Interactions on the Dissolution of Amorphous Solid Dispersions. Mol. Pharm. 2021, 18, 174–186. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Pang, H.; Guo, Z.; Lin, L.; Dong, Y.; Li, G.; Lu, M.; Wu, C. Interactions between drugs and polymers influencing hot melt extrusion. J. Pharm. Pharmacol. 2014, 66, 148–166. [Google Scholar] [CrossRef] [PubMed]
- Kojima, T.; Higashi, K.; Suzuki, T.; Tomono, K.; Moribe, K.; Yamamoto, K. Stabilization of a supersaturated solution of mefenamic acid from a solid dispersion with EUDRAGIT® EPO. Pharm. Res. 2012, 29, 2777–2791. [Google Scholar] [CrossRef]
- Beak, I.H.; Kim, M.S. Improved supersaturation and oral absorption of dutasteride by amorphous solid dispersions. Chem. Pharm. Bull. 2012, 60, 1468–1473. [Google Scholar] [CrossRef] [Green Version]
- Hess, M.; Jones, R.G.; Kahovec, J.; Kitayama, T.; Kratochvíl, P.; Kubisa, P.; Mormann, W.; Stepto, R.F.T.; Tabak, D.; Vohlídal, J.; et al. Terminology of polymers containing ionizable or ionic groups and of polymers containing ions (IUPAC Recommendations 2006). Pure Appl. Chem. 2006, 78, 2067–2074. [Google Scholar] [CrossRef]
- Lankalapalli, S.; Kolapalli, V.R.M. Polyelectrolyte complexes: A review of their applicability in drug delivery technology. Indian J. Pharm. Sci. 2009, 71, 481–487. [Google Scholar] [CrossRef] [Green Version]
- Chiou, W.L.; Riegelman, S. Pharmaceutical applications of solid dispersion systems. J. Pharm. Sci. 1971, 60, 1281–1302. [Google Scholar] [CrossRef]
- Ma, X.; Williams, R.O. Characterization of amorphous solid dispersions: An update. J. Drug Deliv. Sci. Technol. 2019, 50, 113–124. [Google Scholar] [CrossRef]
- Beneš, M.; Pekárek, T.; Beránek, J.; Havlíček, J.; Krejčík, L.; Šimek, M.; Tkadlecová, M.; Doležal, P. Methods for the preparation of amorphous solid dispersions—A comparative study. J. Drug Deliv. Sci. Technol. 2017, 38, 125–134. [Google Scholar] [CrossRef]
- Jaiswar, D.R.; Jha, D.; Amin, P.D. Preparation and characterizations of stable amorphous solid solution of azithromycin by hot melt extrusion. J. Pharm. Investig. 2016, 46, 655–668. [Google Scholar] [CrossRef]
- Van Drooge, D.J.; Hinrichs, W.L.J.; Visser, M.R.; Frijlink, H.W. Characterization of the molecular distribution of drugs in glassy solid dispersions at the nano-meter scale, using differential scanning calorimetry and gravimetric water vapour sorption techniques. Int. J. Pharm. 2006, 310, 220–229. [Google Scholar] [CrossRef]
- Janssens, S.; Van den Mooter, G. Review: Physical chemistry of solid dispersions. J. Pharm. Pharmacol. 2009, 61, 1571–1586. [Google Scholar] [CrossRef] [PubMed]
- Jermain, S.V.; Brough, C.; Williams, R.O. Amorphous solid dispersions and nanocrystal technologies for poorly water-soluble drug delivery—An update. Int. J. Pharm. 2018, 535, 379–392. [Google Scholar] [CrossRef]
- Brough, C.; Williams, R.O. Amorphous solid dispersions and nano-crystal technologies for poorly water-soluble drug delivery. Int. J. Pharm. 2013, 453, 157–166. [Google Scholar] [CrossRef]
- Schittny, A.; Huwyler, J.; Puchkov, M. Mechanisms of increased bioavailability through amorphous solid dispersions: A review. Drug Deliv. 2020, 27, 110–127. [Google Scholar] [CrossRef]
- Chavan, R.B.; Lodagekar, A.; Yadav, B.; Shastri, N.R. Amorphous solid dispersion of nisoldipine by solvent evaporation technique: Preparation, characterization, in vitro, in vivo evaluation, and scale up feasibility study. Drug Deliv. Transl. Res. 2020, 10, 903–918. [Google Scholar] [CrossRef]
- Guan, J.; Jin, L.; Liu, Q.; Xu, H.; Wu, H.; Zhang, X.; Mao, S. Exploration of supersaturable lacidipine ternary amorphous solid dispersion for enhanced dissolution and in vivo absorption. Eur. J. Pharm. Sci. 2019, 139, 105043. [Google Scholar] [CrossRef]
- Shi, C.; Tong, Q.; Fang, J.; Wang, C.; Wu, J.; Wang, W. Preparation, characterization and in vivo studies of amorphous solid dispersion of berberine with hydrogenated phosphatidylcholine. Eur. J. Pharm. Sci. 2015, 74, 11–17. [Google Scholar] [CrossRef]
- Baghel, S.; Cathcart, H.; O’Reilly, N.J. Polymeric Amorphous Solid Dispersions: A Review of Amorphization, Crystallization, Stabilization, Solid-State Characterization, and Aqueous Solubilization of Biopharmaceutical Classification System Class II Drugs. J. Pharm. Sci. 2016, 105, 2527–2544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paudel, A.; Worku, Z.A.; Meeus, J.; Guns, S.; Van Den Mooter, G. ARTICLE IN PRESS Manufacturing of solid dispersions of poorly water soluble drugs by spray drying: Formulation and process considerations. Int. J. Pharm. 2012, 453, 253–284. [Google Scholar] [CrossRef]
- Filo Vasconcelos, T.; Sarmento, B.; Costa, P. Solid dispersions as strategy to improve oral bioavailability of poor water soluble drugs. Drug Discov. Today 2007, 12, 1068–1075. [Google Scholar] [CrossRef] [PubMed]
- Wyttenbach, N.; Kuentz, M. Glass-forming ability of compounds in marketed amorphous drug products. Eur. J. Pharm. Biopharm. 2017, 112, 204–208. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, D. Investigating the molecular structures of solid dispersions by the simulated annealing method. Chem. Phys. Lett. 2012, 554, 177–184. [Google Scholar] [CrossRef]
- Schönfeld, B.; Westedt, U.; Wagner, K.G. Vacuum drum drying—A novel solvent-evaporation based technology to manufacture amorphous solid dispersions in comparison to spray drying and hot melt extrusion. Int. J. Pharm. 2021, 596, 120233. [Google Scholar] [CrossRef]
- Guo, Z.; Boyce, C.; Rhodes, T.; Liu, L.; Salituro, G.M.; Lee, K.; Bak, A.; Leung, D.H. A novel method for preparing stabilized amorphous solid dispersion drug formulations using acoustic fusion. Int. J. Pharm. 2021, 592, 120026. [Google Scholar] [CrossRef]
- Kambayashi, A.; Kiyota, T.; Fujiwara, M.; Dressman, J.B. PBPK modeling coupled with biorelevant dissolution to forecast the oral performance of amorphous solid dispersion formulations. Eur. J. Pharm. Sci. 2019, 135, 83–90. [Google Scholar] [CrossRef]
- Everaerts, M.; Tigrine, A.; de la Rosa, V.R.; Hoogenboom, R.; Adriaensens, P.; Clasen, C.; Van den Mooter, G. Unravelling the Miscibility of Poly(2-oxazoline)s: A Novel Polymer Class for the Formulation of Amorphous Solid Dispersions. Molecules 2020, 25, 3587. [Google Scholar] [CrossRef]
- Ditzinger, F.; Wieland, R.; Statelova, M.; Vertzoni, M.; Holm, R.; Kuentz, M. In Vivo Performance of Innovative Polyelectrolyte Matrices for Hot Melt Extrusion of Amorphous Drug Systems. Mol. Pharm. 2020, 17, 3053–3061. [Google Scholar] [CrossRef] [PubMed]
- Brunsteiner, M.; Khinast, J.; Paudel, A. Relative contributions of solubility and mobility to the stability of amorphous solid dispersions of poorly soluble drugs: A molecular dynamics simulation study. Pharmaceutics 2018, 10, 101. [Google Scholar] [CrossRef] [Green Version]
- Barmpalexis, P.; Karagianni, A.; Katopodis, K.; Vardaka, E.; Kachrimanis, K. Molecular modelling and simulation of fusion-based amorphous drug dispersions in polymer/plasticizer blends. Eur. J. Pharm. Sci. 2019, 130, 260–268. [Google Scholar] [CrossRef]
- Bharti, K.; Mishra, B. Understanding the Drug-Polymer Interaction in Amorphous Solid Dispersion for BCS Class II Drug. In Proceedings of the International Conference on Drug Discovery (ICDD), Hyderabad, India, 29 February–2 March 2020. [Google Scholar]
- Olivera, M.E.; Manzo, R.H.; Alovero, F.; Jimenez-Kairuz, A.F.; Ramírez-Rigo, M.V. Polyelectrolyte-Drug Ionic Complexes as Nanostructured Drug Carriers to Design Solid and Liquid Oral Delivery Systems; Elsevier Inc.: Amsterdam, The Netherlands, 2017; ISBN 9780323477215. [Google Scholar]
- Rowe, R.C.; Sheskey, P.J.; Quinn, M.E. Handbook of Pharmaceutical Excipient, 6th ed.; American Pharmacists Association: Dublin, OH, USA, 2009; ISBN 978-0-85369-792-3. [Google Scholar]
- Am Ende, M.T.; Peppas, N.A. Transport of ionizable drugs and proteins in crosslinked poly(acrylic acid) and poly(acrylic acid-co-2-hydroxyethyl methacrylate) hydrogels. II. Diffusion and release studies. J. Control. Release 1997, 48, 47–56. [Google Scholar] [CrossRef]
- Jimenez-Kairuz, A.F.; Llabot, J.M.; Allemandi, D.A.; Manzo, R.H. Swellable drug-polyelectrolyte matrices (SDPM): Characterization and delivery properties. Int. J. Pharm. 2005, 288, 87–99. [Google Scholar] [CrossRef] [PubMed]
- Luo, R.; Venkatraman, S.S.; Neu, B. Layer-by-layer polyelectrolyte-polyester hybrid microcapsules for encapsulation and delivery of hydrophobic drugs. Biomacromolecules 2013, 14, 2262–2271. [Google Scholar] [CrossRef] [PubMed]
- Semdé, R.; Amighi, K.; Devleeschouwer, M.J.; Moës, A.J. Studies of pectin HM/Eudragit® RL/Eudragit® NE film-coating formulations intended for colonic drug delivery. Int. J. Pharm. 2000, 197, 181–192. [Google Scholar] [CrossRef]
- Zhang, B.; Pan, Y.; Chen, H.; Liu, T.; Tao, H.; Tian, Y. Stabilization of starch-based microgel-lysozyme complexes using a layer-by-layer assembly technique. Food Chem. 2017, 214, 213–217. [Google Scholar] [CrossRef]
- Tapia, C.; Escobar, Z.; Costa, E.; Sapag-Hagar, J.; Valenzuela, F.; Basualto, C.; Gai, M.N.; Yazdani-Pedram, M. Comparative studies on polyelectrolyte complexes and mixtures of chitosan-alginate and chitosan-carrageenan as prolonged diltiazem clorhydrate release systems. Eur. J. Pharm. Biopharm. 2004, 57, 65–75. [Google Scholar] [CrossRef]
- Ahmad, N.; Amin, M.C.I.M.; Mahali, S.M.; Ismail, I.; Chuang, V.T.G. Biocompatible and mucoadhesive bacterial cellulose-g-poly(acrylic acid) hydrogels for oral protein delivery. Mol. Pharm. 2014, 11, 4130–4142. [Google Scholar] [CrossRef] [PubMed]
- Andrianov, A.K.; Marin, A.; Roberts, B.E. Polyphosphazene polyelectrolytes: A link between the formation of noncovalent complexes with antigenic proteins and immunostimulating activity. Biomacromolecules 2005, 6, 1375–1379. [Google Scholar] [CrossRef] [PubMed]
- Mok, H.; Park, T.G. Functional polymers for targeted delivery of nucleic acid drugs. Macromol. Biosci. 2009, 9, 731–743. [Google Scholar] [CrossRef] [PubMed]
- Sonje, A.G.; Mahajan, H.S. Nasal inserts containing ondansetron hydrochloride based on Chitosan-gellan gum polyelectrolyte complex: In vitro-in vivo studies. Mater. Sci. Eng. C 2016, 64, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Daubiné, F.; Cortial, D.; Ladam, G.; Atmani, H.; Haïkel, Y.; Voegel, J.C.; Clézardin, P.; Benkirane-Jessel, N. Nanostructured polyelectrolyte multilayer drug delivery systems for bone metastasis prevention. Biomaterials 2009, 30, 6367. [Google Scholar] [CrossRef] [PubMed]
- Bigucci, F.; Abruzzo, A.; Vitali, B.; Saladini, B.; Cerchiara, T.; Gallucci, M.C.; Luppi, B. Vaginal inserts based on chitosan and carboxymethylcellulose complexes for local delivery of chlorhexidine: Preparation, characterization and antimicrobial activity. Int. J. Pharm. 2015, 478, 456–463. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, L.; Guo, B.; Ma, P.X. Cytocompatible injectable carboxymethyl chitosan/N-isopropylacrylamide hydrogels for localized drug delivery. Carbohydr. Polym. 2014, 103, 110–118. [Google Scholar] [CrossRef]
- Thakral, S.; Thakral, N.K.; Majumdar, D.K. Eudragit®: A technology evaluation. Expert Opin. Drug Deliv. 2013, 10, 131–149. [Google Scholar] [CrossRef] [PubMed]
- Gallardo, D.; Skalsky, B.; Kleinebudde, P. Controlled release solid dosage forms using combinations of (meth)acrylate copolymers. Pharm. Dev. Technol. 2008, 13, 413–423. [Google Scholar] [CrossRef]
- Tanno, F.; Nishiyama, Y.; Kokubo, H.; Obara, S. Evaluation of Hypromellose Acetate Succinate (HPMCAS) as a Carrier in Solid Dispersions. Drug Dev. Ind. Pharm. 2004, 30, 9–17. [Google Scholar] [CrossRef]
- Friesen, D.T.; Shanker, R.; Crew, M.; Smithey, D.T.; Curatolo, W.J.; Nightingale, J.A.S. Hydroxypropyl methylcellulose acetate succinate-based spray-dried dispersions: An overview. Mol. Pharm. 2008, 5, 1003–1019. [Google Scholar] [CrossRef] [Green Version]
- Weuts, I.; Kempen, D.; Verreck, G.; Peeters, J.; Brewster, M.; Blaton, N.; Van Den Mooter, G. Salt formation in solid dispersions consisting of polyacrylic acid as a carrier and three basic model compounds resulting in very high glass transition temperatures and constant dissolution properties upon storage. Eur. J. Pharm. Sci. 2005, 25, 387–393. [Google Scholar] [CrossRef]
- Wang, L.; De Cui, F.; Hayase, T.; Sunada, H. Preparation and evaluation of solid dispersion for nitrendipine-carbopol and nitrendipine-HPMCP systems using a twin screw extruder. Chem. Pharm. Bull. 2005, 53, 1240–1245. [Google Scholar] [CrossRef] [Green Version]
- Monschke, M.; Kayser, K.; Wagner, K.G. Processing of polyvinyl acetate phthalate in hot-melt extrusion-preparation of amorphous solid dispersions. Pharmaceutics 2020, 12, 337. [Google Scholar] [CrossRef]
- Kindermann, C.; Matthée, K.; Strohmeyer, J.; Sievert, F.; Breitkreutz, J. Tailor-made release triggering from hot-melt extruded complexes of basic polyelectrolyte and poorly water-soluble drugs. Eur. J. Pharm. Biopharm. 2011, 79, 372–381. [Google Scholar] [CrossRef] [PubMed]
- Sathigari, S.K.; Radhakrishnan, V.K.; Davis, V.A.; Parsons, D.L.; Babu, R.J. Amorphous-state characterization of efavirenz-polymer hot-melt extrusion systems for dissolution enhancement. J. Pharm. Sci. 2012, 101, 3456–3464. [Google Scholar] [CrossRef]
- Zheng, X.; Yang, R.; Tang, X.; Zheng, L. Part I: Characterization of solid dispersions of nimodipine prepared by hot-melt extrusion. Drug Dev. Ind. Pharm. 2007, 33, 791–802. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Jacobs, E.; Jones, D.S.; McCoy, C.P.; Wu, H.; Andrews, G.P. The design and development of high drug loading amorphous solid dispersion for hot-melt extrusion platform. Int. J. Pharm. 2020, 586, 119545. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.L.; Lin, S.Y.; Chen, T.F.; Cheng, W.T. Eudragit E accelerated the diketopiperazine formation of enalapril maleate determined by thermal FTIR microspectroscopic technique. Pharm. Res. 2004, 21, 2127–2132. [Google Scholar] [CrossRef]
- Maniruzzaman, M.; Morgan, D.J.; Mendham, A.P.; Pang, J.; Snowden, M.J.; Douroumis, D. Drug-polymer intermolecular interactions in hot-melt extruded solid dispersions. Int. J. Pharm. 2013, 443, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Sawant, K.P.; Fule, R.; Maniruzzaman, M.; Amin, P.D. Extended release delivery system of metoprolol succinate using hot-melt extrusion: Effect of release modifier on methacrylic acid copolymer. Drug Deliv. Transl. Res. 2018, 8, 1679–1693. [Google Scholar] [CrossRef]
- Monschke, M.; Wagner, K.G. Amorphous solid dispersions of weak bases with pH-dependent soluble polymers to overcome limited bioavailability due to gastric pH variability—An in-vitro approach. Int. J. Pharm. 2019, 564, 162–170. [Google Scholar] [CrossRef]
- Sarode, A.L.; Sandhu, H.; Shah, N.; Malick, W.; Zia, H. Hot melt extrusion (HME) for amorphous solid dispersions: Predictive tools for processing and impact of drug-polymer interactions on supersaturation. Eur. J. Pharm. Sci. 2013, 48, 371–384. [Google Scholar] [CrossRef]
- Miller, D.A.; DiNunzio, J.C.; Yang, W.; McGinity, J.W.; Williams, R.O. Enhanced in vivo absorption of itraconazole via stabilization of supersaturation following acidic-to-neutral pH transition. Drug Dev. Ind. Pharm. 2008, 34, 890–902. [Google Scholar] [CrossRef] [PubMed]
- Monschke, M.; Kayser, K.; Wagner, K.G. Influence of Particle Size and Drug Load on Amorphous Solid Dispersions Containing pH-Dependent Soluble Polymers and the Weak Base Ketoconazole. AAPS PharmSciTech 2021, 22, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Sarabu, S.; Kallakunta, V.R.; Bandari, S.; Batra, A.; Bi, V.; Durig, T.; Zhang, F.; Repka, M.A. Hypromellose acetate succinate based amorphous solid dispersions via hot melt extrusion: Effect of drug physicochemical properties. Carbohydr. Polym. 2020, 233, 115828. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zhao, Y.; Zhao, Y.; Ding, Z.; Fan, Z.; Zhang, H.; Liu, M.; Wang, Z.; Han, J. Effect of HPMCAS on recrystallization inhibition of nimodipine solid dispersions prepared by hot-melt extrusion and dissolution enhancement of nimodipine tablets. Colloids Surf. B Biointerfaces 2018, 172, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Solanki, N.G.; Lam, K.; Tahsin, M.; Gumaste, S.G.; Shah, A.V.; Serajuddin, A.T.M. Effects of Surfactants on Itraconazole-HPMCAS Solid Dispersion Prepared by Hot-Melt Extrusion I: Miscibility and Drug Release. J. Pharm. Sci. 2019, 108, 1453–1465. [Google Scholar] [CrossRef] [PubMed]
- Wegiel, L.A.; Zhao, Y.; Mauer, L.J.; Edgar, K.J.; Taylor, L.S. Curcumin amorphous solid dispersions: The influence of intra and intermolecular bonding on physical stability. Pharm. Dev. Technol. 2014, 19, 976–986. [Google Scholar] [CrossRef]
- Wegiel, L.A.; Mauer, L.J.; Edgar, K.J.; Taylor, L.S. Mid-infrared spectroscopy as a polymer selection tool for formulating amorphous solid dispersions. J. Pharm. Pharmacol. 2014, 66, 244–255. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Yang, X.; Chen, X.; Nie, H.; Byrn, S.; Lubach, J.W. Investigation of Drug-Excipient Interactions in Lapatinib Amorphous Solid Dispersions Using Solid-State NMR Spectroscopy. Mol. Pharm. 2015, 12, 857–866. [Google Scholar] [CrossRef]
- Mistry, P.; Mohapatra, S.; Gopinath, T.; Vogt, F.G.; Suryanarayanan, R. Role of the Strength of Drug-Polymer Interactions on the Molecular Mobility and Crystallization Inhibition in Ketoconazole Solid Dispersions. Mol. Pharm. 2015, 12, 3339–3350. [Google Scholar] [CrossRef]
- Sarode, A.L.; Sandhu, H.; Shah, N.; Malick, W.; Zia, H. Hot melt extrusion for amorphous solid dispersions: Temperature and moisture activated drug-polymer interactions for enhanced stability. Mol. Pharm. 2013, 10, 3665–3675. [Google Scholar] [CrossRef] [PubMed]
- Lubach, J.W.; Hau, J. Solid-State NMR Investigation of Drug-Excipient Interactions and Phase Behavior in Indomethacin-Eudragit E Amorphous Solid Dispersions. Pharm. Res. 2018, 35, 65. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhang, X.; Suwardie, H.; Wang, P.; Gogos, C.G. Miscibility studies of indomethacin and Eudragit® E PO by thermal, rheological, and spectroscopic analysis. J. Pharm. Sci. 2012, 101, 2204–2212. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Taylor, L.S. Tailoring supersaturation from amorphous solid dispersions. J. Control. Release 2018, 279, 114–125. [Google Scholar] [CrossRef]
- Higashi, K.; Yamamoto, K.; Pandey, M.K.; Mroue, K.H.; Moribe, K.; Yamamoto, K.; Ramamoorthy, A. Insights into atomic-level interaction between mefenamic acid and Eudragit EPO in a supersaturated solution by high-resolution magic-angle spinning NMR spectroscopy. Mol. Pharm. 2014, 11, 351–357. [Google Scholar] [CrossRef]
- Cho, Y.; Ha, E.S.; Baek, I.H.; Kim, M.S.; Cho, C.W.; Hwang, S.J. Enhanced supersaturation and oral absorption of sirolimus using an amorphous solid dispersion based on Eudragit® E. Molecules 2015, 20, 9496–9509. [Google Scholar] [CrossRef] [Green Version]
- Elkhabaz, A.; Sarkar, S.; Dinh, J.K.; Simpson, G.J.; Taylor, L.S. Variation in Supersaturation and Phase Behavior of Ezetimibe Amorphous Solid Dispersions upon Dissolution in Different Biorelevant Media. Mol. Pharm. 2018, 15, 193–206. [Google Scholar] [CrossRef]
- Xie, T.; Taylor, L.S. Improved Release of Celecoxib from High Drug Loading Amorphous Solid Dispersions Formulated with Polyacrylic Acid and Cellulose Derivatives. Mol. Pharm. 2016, 13, 873–884. [Google Scholar] [CrossRef]
- Balogh, A.; Farkas, B.; Domokos, A.; Farkas, A.; Démuth, B.; Borbás, E.; Nagy, B.; Marosi, G.; Nagy, Z.K. Controlled-release solid dispersions of Eudragit® FS 100 and poorly soluble spironolactone prepared by electrospinning and melt extrusion. Eur. Polym. J. 2017, 95, 406–417. [Google Scholar] [CrossRef]
- Guo, S.; Wang, G.; Wu, T.; Bai, F.; Xu, J.; Zhang, X. Solid dispersion of berberine hydrochloride and Eudragit® S100: Formulation, physicochemical characterization and cytotoxicity evaluation. J. Drug Deliv. Sci. Technol. 2017, 40, 21–27. [Google Scholar] [CrossRef]
- Bhairav, B.A.; Kokane, P.A.; Saudagar, R.B. Hot Melt Extrusion Technique—A Review. Res. J. Sci. Technol. 2016, 8, 155. [Google Scholar] [CrossRef]
- Censi, R.; Gigliobianco, M.R.; Casadidio, C.; Di Martino, P. Hot melt extrusion: Highlighting physicochemical factors to be investigated while designing and optimizing a hot melt extrusion process. Pharmaceutics 2018, 10, 89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Den Mooter, G. The use of amorphous solid dispersions: A formulation strategy to overcome poor solubility and dissolution rate. Drug Discov. Today Technol. 2012, 9, e79–e85. [Google Scholar] [CrossRef]
- Ditzinger, F.; Dejoie, C.; Jung, D.S.; Kuentz, M. Polyelectrolytes in hot melt extrusion: A combined solvent-based and interacting additive technique for solid dispersions. Pharmaceutics 2019, 11, 174. [Google Scholar] [CrossRef] [Green Version]
- Ditzinger, F.; Scherer, U.; Schönenberger, M.; Holm, R.; Kuentz, M. Modified Polymer Matrix in Pharmaceutical Hot Melt Extrusion by Molecular Interactions with a Carboxylic Coformer. Mol. Pharm. 2019, 16, 141–150. [Google Scholar] [CrossRef]
- Sarode, A.L.; Obara, S.; Tanno, F.K.; Sandhu, H.; Iyer, R.; Shah, N. Stability assessment of hypromellose acetate succinate (HPMCAS) NF for application in hot melt extrusion (HME). Carbohydr. Polym. 2014, 101, 146–153. [Google Scholar] [CrossRef]
- Kallakunta, V.R.; Sarabu, S.; Bandari, S.; Batra, A.; Bi, V.; Durig, T.; Repka, M.A. Stable amorphous solid dispersions of fenofibrate using hot melt extrusion technology: Effect of formulation and process parameters for a low glass transition temperature drug. J. Drug Deliv. Sci. Technol. 2020, 58, 101395. [Google Scholar] [CrossRef]
- Solanki, N.G.; Gumaste, S.G.; Shah, A.V.; Serajuddin, A.T.M. Effects of Surfactants on Itraconazole-Hydroxypropyl Methylcellulose Acetate Succinate Solid Dispersion Prepared by Hot Melt Extrusion. II: Rheological Analysis and Extrudability Testing. J. Pharm. Sci. 2019, 108, 3063–3073. [Google Scholar] [CrossRef]
- Ohyagi, N.; Ueda, K.; Higashi, K.; Yamamoto, K.; Kawakami, K.; Moribe, K. Synergetic Role of Hypromellose and Methacrylic Acid Copolymer in the Dissolution Improvement of Amorphous Solid Dispersions. J. Pharm. Sci. 2017, 106, 1042–1050. [Google Scholar] [CrossRef]
- Alshahrani, S.M.; Lu, W.; Park, J.B.; Morott, J.T.; Alsulays, B.B.; Majumdar, S.; Langley, N.; Kolter, K.; Gryczke, A.; Repka, M.A. Stability-enhanced Hot-melt Extruded Amorphous Solid Dispersions via Combinations of Soluplus® and HPMCAS-HF. AAPS PharmSciTech 2015, 16, 824–834. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Cao, F.; Zhang, C.; Ping, Q. Use of polymer combinations in the preparation of solid dispersions of a thermally unstable drug by hot-melt extrusion. Acta Pharm. Sin. B 2013, 3, 263–272. [Google Scholar] [CrossRef] [Green Version]
- Miller, D.A.; DiNunzio, J.C.; Yang, W.; McGinity, J.W.; Williams, R.O. Targeted intestinal delivery of supersaturated itraconazole for improved oral absorption. Pharm. Res. 2008, 25, 1450–1459. [Google Scholar] [CrossRef] [PubMed]
- Nollenberger, K.; Gryczke, A.; Meier, C.; Dressman, J.; Schmidt, M.U.; Brühne, S. Pair distribution function X-ray analysis explains dissolution characteristics of felodipine melt extrusion products. J. Pharm. Sci. 2009, 98, 1476–1486. [Google Scholar] [CrossRef]
- Moustafine, R.I.; Kabanova, T.V.; Kemenova, V.A.; Van Den Mooter, G. Characteristics of interpolyelectrolyte complexes of Eudragit E100 with Eudragit L100. J. Control. Release 2005, 103, 191–198. [Google Scholar] [CrossRef]
- Mustafin, R.I.; Bilan, A.B.; Bukhovets, A.V.; Kemenova, V.A. Comparative study of structural and compositional changes of polycomplex matrices based on eudragit® EPO and eudragit® L100. Pharm. Chem. J. 2011, 45, 114–117. [Google Scholar] [CrossRef]
- Moustafine, R.I.; Sitenkov, A.Y.; Bukhovets, A.V.; Nasibullin, S.F.; Appeltans, B.; Kabanova, T.V.; Khutoryanskiy, V.V.; Van den Mooter, G. Indomethacin-containing interpolyelectrolyte complexes based on Eudragit® E PO/S 100 copolymers as a novel drug delivery system. Int. J. Pharm. 2017, 524, 121–133. [Google Scholar] [CrossRef]
- Lounis, F.M.; Chamieh, J.; Gonzalez, P.; Cottet, H.; Leclercq, L. Prediction of Polyelectrolyte Complex Stoichiometry for Highly Hydrophilic Polyelectrolytes. Macromolecules 2016, 49, 3881–3888. [Google Scholar] [CrossRef]
- Dakhara, S.L.; Anajwala, C.C. Polyelectrolyte complex: A pharmaceutical review. Syst. Rev. Pharm. 2010, 1, 121–127. [Google Scholar] [CrossRef] [Green Version]
- Tsuchida, E. Formation of Polyelectrolyte Complexes and their Structures. J. Macromol. Sci. Part A 1994, 31, 1–15. [Google Scholar] [CrossRef]
- Zhang, Y.; Yildirim, E.; Antila, H.S.; Valenzuela, L.D.; Sammalkorpi, M.; Lutkenhaus, J.L. The influence of ionic strength and mixing ratio on the colloidal stability of PDAC/PSS polyelectrolyte complexes. Soft Matter 2015, 11, 7392–7401. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Wang, C.; Zhu, G.; Zacharia, N.S. Self-Healing of Bulk Polyelectrolyte Complex Material as a Function of pH and Salt. ACS Appl. Mater. Interfaces 2016, 8, 26258–26265. [Google Scholar] [CrossRef] [PubMed]
- Siyawamwaya, M.; Choonara, Y.E.; Bijukumar, D.; Kumar, P.; Du Toit, L.C.; Pillay, V. A Review: Overview of Novel Polyelectrolyte Complexes as Prospective Drug Bioavailability Enhancers. Int. J. Polym. Mater. Polym. Biomater. 2015, 64, 955–968. [Google Scholar] [CrossRef]
- Hamman, J.H. Chitosan based polyelectrolyte complexes as potential carrier materials in drug delivery systems. Mar. Drugs 2010, 8, 1305–1322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montaña, J.A.; Perez, L.D.; Baena, Y. A pH-responsive drug delivery matrix from an interpolyelectrolyte complex: Preparation and pharmacotechnical properties. Braz. J. Pharm. Sci. 2018, 54. [Google Scholar] [CrossRef]
- Moustafine, R.I.; Zaharov, I.M.; Kemenova, V.A. Physicochemical characterization and drug release properties of Eudragit® E PO/Eudragit® L 100-55 interpolyelectrolyte complexes. Eur. J. Pharm. Biopharm. 2006, 63, 26–36. [Google Scholar] [CrossRef]
- Jeganathan, B.; Prakya, V. Interpolyelectrolyte Complexes of Eudragit® EPO with Hypromellose Acetate Succinate and Eudragit® EPO with Hypromellose Phthalate as Potential Carriers for Oral Controlled Drug Delivery. AAPS PharmSciTech 2015, 16, 878–888. [Google Scholar] [CrossRef] [Green Version]
- Ngwuluka, N.C.; Choonara, Y.E.; Kumar, P.; Modi, G.; Du Toit, L.C.; Pillay, V. A hybrid methacrylate-sodium carboxymethylcellulose interpolyelectrolyte complex: Rheometry and in silico disposition for controlled drug release. Materials 2013, 6, 4284–4308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mustafin, R.I.; Bukhovets, A.V.; Sitenkov, A.Y.; Garipova, V.R.; Kemenova, V.A.; Rombaut, P.; Van Den Mooter, G. Synthesis and characterization of a new carrier based on Eudragit® EPO/S100 interpolyelectrolyte complex for controlled colon-specific drug delivery. Pharm. Chem. J. 2011, 45, 568–574. [Google Scholar] [CrossRef]
- Mustafin, R.I.; Kabanova, T.V.; Zhdanova, E.R.; Bukhovets, A.V.; Garipova, V.R.; Nasibullin, S.F.; Kemenova, V.A. Diffusion-transport properties of a polycomplex matrix system based on eudragit® EPO and Carbomer 940. Pharm. Chem. J. 2010, 44, 147–150. [Google Scholar] [CrossRef]
- Higashi, K.; Seo, A.; Egami, K.; Otsuka, N.; Limwikrant, W.; Yamamoto, K.; Moribe, K. Mechanistic insight into the dramatic improvement of probucol dissolution in neutral solutions by solid dispersion in Eudragit e PO with saccharin. J. Pharm. Pharmacol. 2016, 68, 655–664. [Google Scholar] [CrossRef] [PubMed]
- Ueda, K.; Kanaya, H.; Higashi, K.; Yamamoto, K.; Moribe, K. Molecular-level elucidation of saccharin-assisted rapid dissolution and high supersaturation level of drug from Eudragit® E solid dispersion. Int. J. Pharm. 2018, 538, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Hu, Y.; Liu, L.; Su, L.; Li, N.; Yu, J.; Tang, B.; Yang, Z. Physical Stability of Amorphous Solid Dispersions: A Physicochemical Perspective with Thermodynamic, Kinetic and Environmental Aspects. Pharm. Res. 2018, 35, 125. [Google Scholar] [CrossRef]
- Wang, B.; Wang, D.; Zhao, S.; Huang, X.; Zhang, J.; Lv, Y.; Liu, X.; Lv, G.; Ma, X. Evaluate the ability of PVP to inhibit crystallization of amorphous solid dispersions by density functional theory and experimental verify. Eur. J. Pharm. Sci. 2017, 96, 45–52. [Google Scholar] [CrossRef]
- Yoo, S.U.; Krill, S.L.; Wang, Z.; Telang, C. Miscibility/stability considerations in binary solid dispersion systems composed of functional excipients towards the design of multi-component amorphous systems. J. Pharm. Sci. 2009, 98, 4711–4723. [Google Scholar] [CrossRef]
- Kindermann, C.; Matthée, K.; Sievert, F.; Breitkreutz, J. Electrolyte-Stimulated biphasic dissolution profile and stability enhancement for tablets containing drug-Polyelectrolyte complexes. Pharm. Res. 2012, 29, 2710–2721. [Google Scholar] [CrossRef] [PubMed]
- Bevernage, J.; Brouwers, J.; Annaert, P.; Augustijns, P. Drug precipitation-permeation interplay: Supersaturation in an absorptive environment. Eur. J. Pharm. Biopharm. 2012, 82, 424–428. [Google Scholar] [CrossRef]
- Taylor, L.S.; Zhang, G.G.Z. Physical chemistry of supersaturated solutions and implications for oral absorption. Adv. Drug Deliv. Rev. 2016, 101, 122–142. [Google Scholar] [CrossRef]
- Miller, J.M.; Beig, A.; Carr, R.A.; Spence, J.K.; Dahan, A. A win-win solution in oral delivery of lipophilic drugs: Supersaturation via amorphous solid dispersions increases apparent solubility without sacrifice of intestinal membrane permeability. Mol. Pharm. 2012, 9, 2009–2016. [Google Scholar] [CrossRef]
- Sun, D.D.; Lee, P.I. Evolution of supersaturation of amorphous pharmaceuticals: The effect of rate of supersaturation generation. Mol. Pharm. 2013, 10, 4330–4346. [Google Scholar] [CrossRef] [PubMed]
- Curatolo, W.; Nightingale, J.A.; Herbig, S.M. Utility of Hydroxypropylmethylcellulose Acetate Succinate (HPMCAS) for Initiation and Maintenance of Drug Supersaturation in the GI Milieu. Pharm. Res. 2009, 26, 1419–1431. [Google Scholar] [CrossRef]
- Aceves, J.M.; Cruz, R.; Hernandez, E. Preparation and characterization of Furosemide-Eudragit controlled release systems. Int. J. Pharm. 2000, 195, 45–53. [Google Scholar] [CrossRef]
- Tran, T.T.D.; Tran, P.H.L. Insoluble polymers in solid dispersions for improving bioavailability of poorly water-soluble drugs. Polymers 2020, 12, 1679. [Google Scholar] [CrossRef]
- Huang, S.; Williams, R.; Mao, C.; Iii, R.O.W.; Yang, C.-Y. Solubility Advantage (and Disadvantage) of Pharmaceutical Amorphous Solid Dispersions. Artic. J. Pharm. Sci. 2016, 105, 3549–3561. [Google Scholar] [CrossRef]
- Maincent, J.; Williams, R.O. Sustained-release amorphous solid dispersions. Drug Deliv. Transl. Res. 2018, 8, 1714–1725. [Google Scholar] [CrossRef]
- Albarahmieh, E.; Qi, S.; Craig, D.Q.M. Hot melt extruded transdermal films based on amorphous solid dispersions in Eudragit RS PO: The inclusion of hydrophilic additives to develop moisture-activated release systems. Int. J. Pharm. 2016, 514, 270–281. [Google Scholar] [CrossRef]
- Van den Mooter, G. Colon drug delivery. Expert Opin. Drug Deliv. 2006, 3, 111–125. [Google Scholar] [CrossRef] [PubMed]
- Mustafin, R.I.; Kabanova, T.V.; Zhdanova, E.R.; Bukhovets, A.V.; Garipova, V.R.; Nasibullin, S.F.; Kemenova, V.A. Synthesis and physicochemical evaluation of a new carrier based on an interpolyelectrolyte complex formed by Eudragit® EPO and Carbomer 940. Pharm. Chem. J. 2010, 44, 271–273. [Google Scholar] [CrossRef]
- Mustafin, R.I.; Kabanova, T.V.; Semina, I.I.; Bukhovets, A.V.; Garipova, V.R.; Shilovskaya, E.V.; Nasibullin, S.F.; Sitenkov, A.Y.; Kazakova, R.R.; Kemenova, V.A. Biopharmaceutical assessment of a polycomplex matrix system based on carbomer 940 and Eudragit® EPO for colon-specific drug delivery. Pharm. Chem. J. 2011, 45, 491–494. [Google Scholar] [CrossRef]


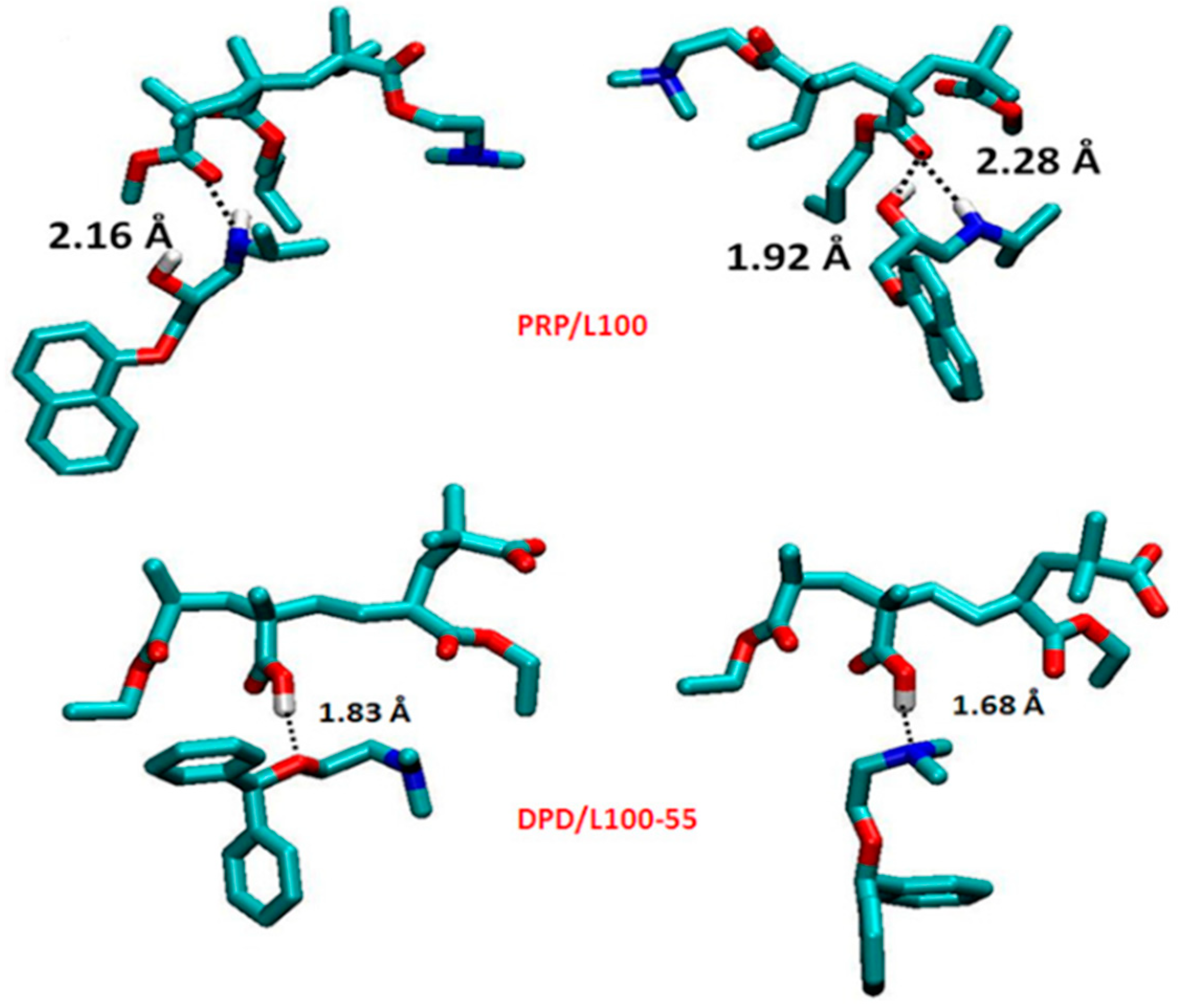




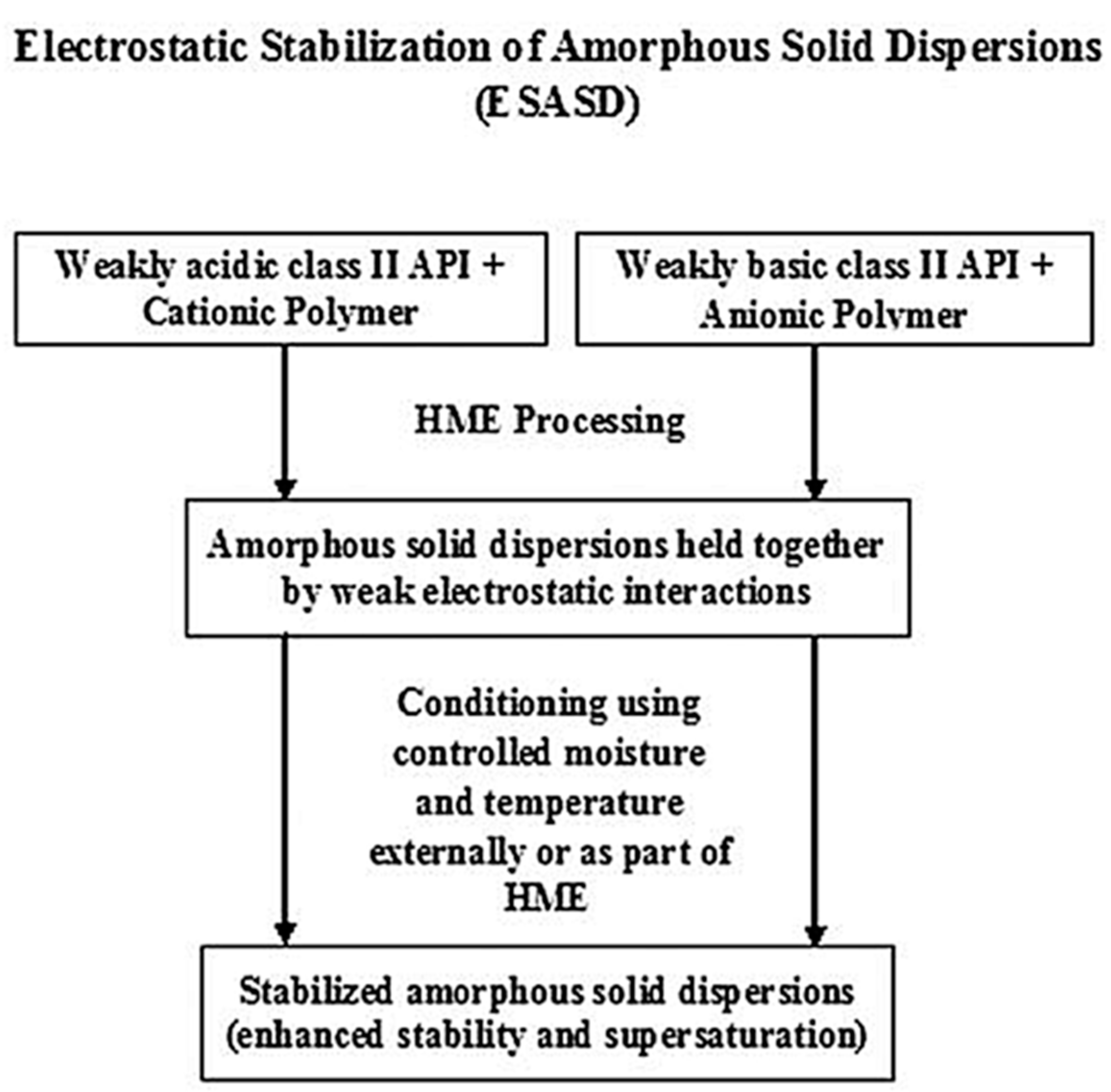
| API | Polyelectrolyte Matrix | Preparation Method | ASDs | Ref. |
|---|---|---|---|---|
| Naproxen, Furosemide | Eudragit EPO | Hot-melt extrusion | Existence of ionic interactions in the melt | [68] |
| Efavirenz | Eudragit EPO | Hot-melt extrusion | Plasticization effect of efavirenz/Facilitation of extrusion | [69] |
| Nimodipine | Eudragit EPO, PVP/VA | Hot-melt extrusion | Higher miscibility compared to nonionic polymers, due to intermolecular interaction | [70] |
| Indomethacin, Ibuprofen, Naproxen | Eudragit EPO | Hot-melt extrusion | High drug-loading amorphous extrudates/Strong intermolecular interactions | [71] |
| Enalapril maleate | Eudragit EPO | Hot-melt extrusion | Formation of carcinogen enalapril diketopiperazine at high temperatures | [72] |
| Propranolol HCL, Diphenhydramine HCL | Eudragit L 100, Eudragit L 100/55 | Hot-melt extrusion | Intermolecular interactions between the amide groups of the drugs and carboxyl group of polyelectrolytes | [73] |
| Metoprolol succinate | Eudragit S 100, Eudragit L 100 | Hot-melt extrusion | Advanced dissolution performance with addition of Eudragit L100-55 to the extrudates | [74] |
| Nevirapine | HPMCAS, HPMCP (HP-55, HP-50), Eudragit L 100-55 | Hot-melt extrusion | Stable final formulations without crystalline fragments with improved in vivo absorption | [75] |
| Indomethacin, Itraconazole, Grizeofulvin | Eudragit EPO Eudragit L 100-55, Eudragit L 100, HPMC AS-LF and HPMC AS-MF | Hot-melt extrusion | improved supersaturation and dissolution levels due to drug–polymer ionic interactions | [76] |
| Itraconazole | Eudragit L 100-55, HPCP (HP-55 and HP-55S grades) | Hot-melt extrusion | Maintenance of supersaturation of the amorphous extrudates | [77] |
| Ketoconazole | HPMCAS LG and Eudragit L100-55 | Hot-melt extrusion | ASDs with Eudragit L100-55 in a drug load of 10% the optimal formulation | [78] |
| Nifedipine, Efavirenz | HPMCAS grades | Hot-melt extrusion | Strong intermolecular interactions in the melt | [79] |
| Nimodipine | HPMCAS-HF | Hot-melt extrusion | High physical stability due to intermolecular interactions | [80] |
| Itraconazole | HPMCAS | Hot-melt extrusion | Improvement of extrusion with the addition of poloxamer 188, poloxamer 407 and d-alpha tocopheryl polyethylene glycol 1000 succinate as plasticizers | [81] |
| Nitrendipine | HPMCP, Carbopol | Hot-melt extrusion | Deceleration of dissolution due to electrostatic interactions between nitrendipine and HPMCP/Carbopol more suitable | [66] |
| Indomethacin | PVP-VA | Hot-melt extrusion | Amorphous extrudates with addition of PEG 3000 as plasticizer | [67] |
| Curcumin | Eudragit EPO | Solvent evaporation, cryo-milling | Enhanced physical stability due to electrostatic drug–polymer interactions | [82,83] |
| Lapatinib | HPMCAS, HPMCP | Spray drying | Stabilization of the ASD due to ionic interactions between protonated lapatinib with the phthalate groups of the polymer | [84] |
| Ketoconazole | PAA | Solvent evaporation and melt-quenching | Intermolecular interactions led to inhibition of recrystallization | [85] |
| Indomethacin, Itraconazole | Eudragit EPO, HPMCAS-LF | Hot-melt extrusion | Electrostatic stabilization of ASDs | [86] |
| Indomethacin | Eudragit EPO | Spray drying | Strong ionic interactions in the matrix | [87,88] |
| Loperamide | PAA | Spray drying | Inhibition of recrystallization | [65] |
| Lopinavir | HPMCAS, HPMCP | Solvent evaporation | Variation in the extent of ASD concentration depend on drug-polymer interactions | [89] |
| Mefenamic acid | Eudragit EPO | Cryogenic grinding | Maintenance of supersaturation in solution due to electrostatic interactions | [17,90] |
| Dutasteride | Eudragit E | Spray drying | Extended supersaturation compared to ASDs with nonionic polymers | [18] |
| Sirolimus | Eudragit E | Spray drying | Enhancement in physical stability and dissolution profile with addition of TPGS | [91] |
| Ezetimibe monohydrate | PAA | Solvent evaporation | Fast crystallization in sodium acetate buffer | [92] |
| Celecoxib | PAA | Solvent evaporation | Rapid decrease of supersaturation of ASDs with high drug loading | [93] |
| Spironolactone | Eudragit FS100 | Electrospinning/Hot-melt extrusion | Stronger polymer-drug interactions in the electrospun fibers than extrudates | [94] |
| Berberine Hydrochloride | Eudragit S100 | Solvent evaporation | Enhancement in antitumor activity due to electrostatic interactions | [95] |
| Polyelectrolyte | Nonionic Polymer | API | Advantage of Synergistic Role | Ref. |
|---|---|---|---|---|
| Eudragit L100, Eudragit S100 | HPMC | Griseofulvin | Improvement of dissolution profile/Increase in supersaturation level of the API | [104] |
| HPMCAS-HF | Soluplus | Carbamazepine | Molecular stabilization of carbamazepine in the amorphous extrudates | [105] |
| Eudragit EPO | Soluplus | Carbamazepine | Extrusion of carbamazepine below its melting point/Enhanced physicochemical stability of the ASD | [106] |
| Eudragit L100/55 | Carbopol 974P | Itraconazole | Delayed precipitation and improvement of supersaturation | [107] |
| Eudragit E | Eudragit NE | Felodipine | Enhancement of dissolution rate/Prevention of recrystallization | [108] |
| Anionic Polyelectrolytes | Cationic Polyelectrolytes | IPEC Preparation Method | Intermolecular Interactions | Ref. |
|---|---|---|---|---|
| Eudragit L100 | Eudragit EPO | Solvent evaporation at pH 6.0, 6.5, 7.0 | Ionized functional groups of L100 with the protonated dimethylamino-groups of EPO | [109,110,119] |
| Eudragit L100/55 | Eudragit EPO | Solvent evaporation at pH 5.5 | Carboxylate groups of L100-55 with the protonated dimethylamino groups of EPO | [120] |
| HPMC-AS/HPMCP | Eudragit EPO | Dissolution method at pH 6.8 (in situ) | Carboxylic group of HPMCAS and HPMCP with the dimethylamino groups of EPO | [121] |
| NaCMC | Eudragit E100 | Solvent evaporation | Functional groups of NaCMC with dimethylamino groups of E100 after 1 h of the synthesis | [122] |
| Eudragit S 100 | Eudragit EPO | Solvent evaporation | S 100 carboxylic groups with the dimethylamino groups of EPO | [123] |
| Carbopol 940P | Eudragit EPO | Solvent evaporation | carboxyl groups in C940 with protonated dimethylamino groups in EPO | [124] |
| Polyelectrolyte | Molecular Additive | API | Preparation Method | Advantage of Synergistic Role | Ref. |
|---|---|---|---|---|---|
| Eudragit EPO | Saccharin | Probucol | Cryogenic grinding | Enhanced dissolution profile due to intermolecular interactions compared to binary mixtures without additive | [125] |
| Eudragit EPO | Saccharin | Phenytoin | Cryogenic grinding | Improved supersaturation level and dissolution profile of phenytoin due to ionic and hydrophobic interactions in the matrix | [126] |
| Eudragit E | Maleic acid | Fenofibrate | Hot-melt extrusion | Facilitation of extrusion/ Advanced physical stability of amorphous extrudates and drug release profile | [100] |
| NaCMC | Lysine/Meglumine | Fenofibrate | Solvent evaporation—Hot-melt extrusion | Fully amorphous NaCMC extrudates | [42,99] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsiaxerli, A.; Karagianni, A.; Ouranidis, A.; Kachrimanis, K. Polyelectrolyte Matrices in the Modulation of Intermolecular Electrostatic Interactions for Amorphous Solid Dispersions: A Comprehensive Review. Pharmaceutics 2021, 13, 1467. https://doi.org/10.3390/pharmaceutics13091467
Tsiaxerli A, Karagianni A, Ouranidis A, Kachrimanis K. Polyelectrolyte Matrices in the Modulation of Intermolecular Electrostatic Interactions for Amorphous Solid Dispersions: A Comprehensive Review. Pharmaceutics. 2021; 13(9):1467. https://doi.org/10.3390/pharmaceutics13091467
Chicago/Turabian StyleTsiaxerli, Anastasia, Anna Karagianni, Andreas Ouranidis, and Kyriakos Kachrimanis. 2021. "Polyelectrolyte Matrices in the Modulation of Intermolecular Electrostatic Interactions for Amorphous Solid Dispersions: A Comprehensive Review" Pharmaceutics 13, no. 9: 1467. https://doi.org/10.3390/pharmaceutics13091467
APA StyleTsiaxerli, A., Karagianni, A., Ouranidis, A., & Kachrimanis, K. (2021). Polyelectrolyte Matrices in the Modulation of Intermolecular Electrostatic Interactions for Amorphous Solid Dispersions: A Comprehensive Review. Pharmaceutics, 13(9), 1467. https://doi.org/10.3390/pharmaceutics13091467








