Co-Injection of Sulfotyrosine Facilitates Retinal Uptake of Hyaluronic Acid Nanospheres Following Intravitreal Injection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Fabrication of Fluorescent Hyaluronic Acid Nanospheres
2.3. High Performance Liquid Chromatography (HPLC) Measurements
2.4. Preparation of Sulfotyrosine Solution
2.5. Lipopolysaccharide (LPS)
2.5.1. Intravitreal and Subretinal Injection
2.5.2. Electroretinography, Fundus Imaging, and Optical Coherence Tomography (OCT)
2.5.3. Fluorescence Imaging and Counting HA-NS in the Retina
2.5.4. H&E Staining and Photoreceptor Nuclei Counts
2.5.5. Immunofluorescence Labeling
2.6. Electron Microscopy (EM)
Cytokine Protein Assay
3. Statistical Analysis
4. Results
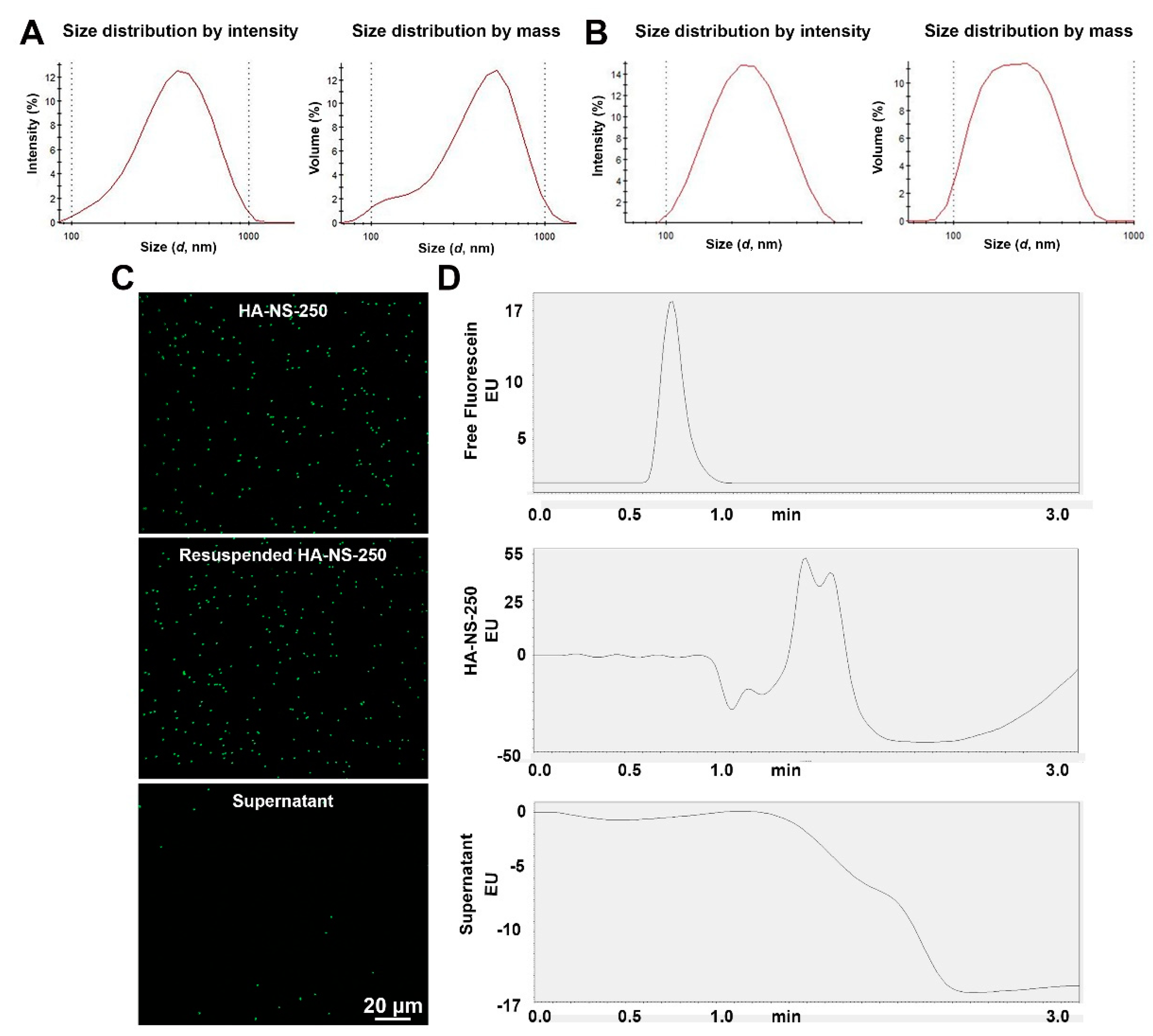
4.1. Characterization of Hyaluronic Acid Nanospheres
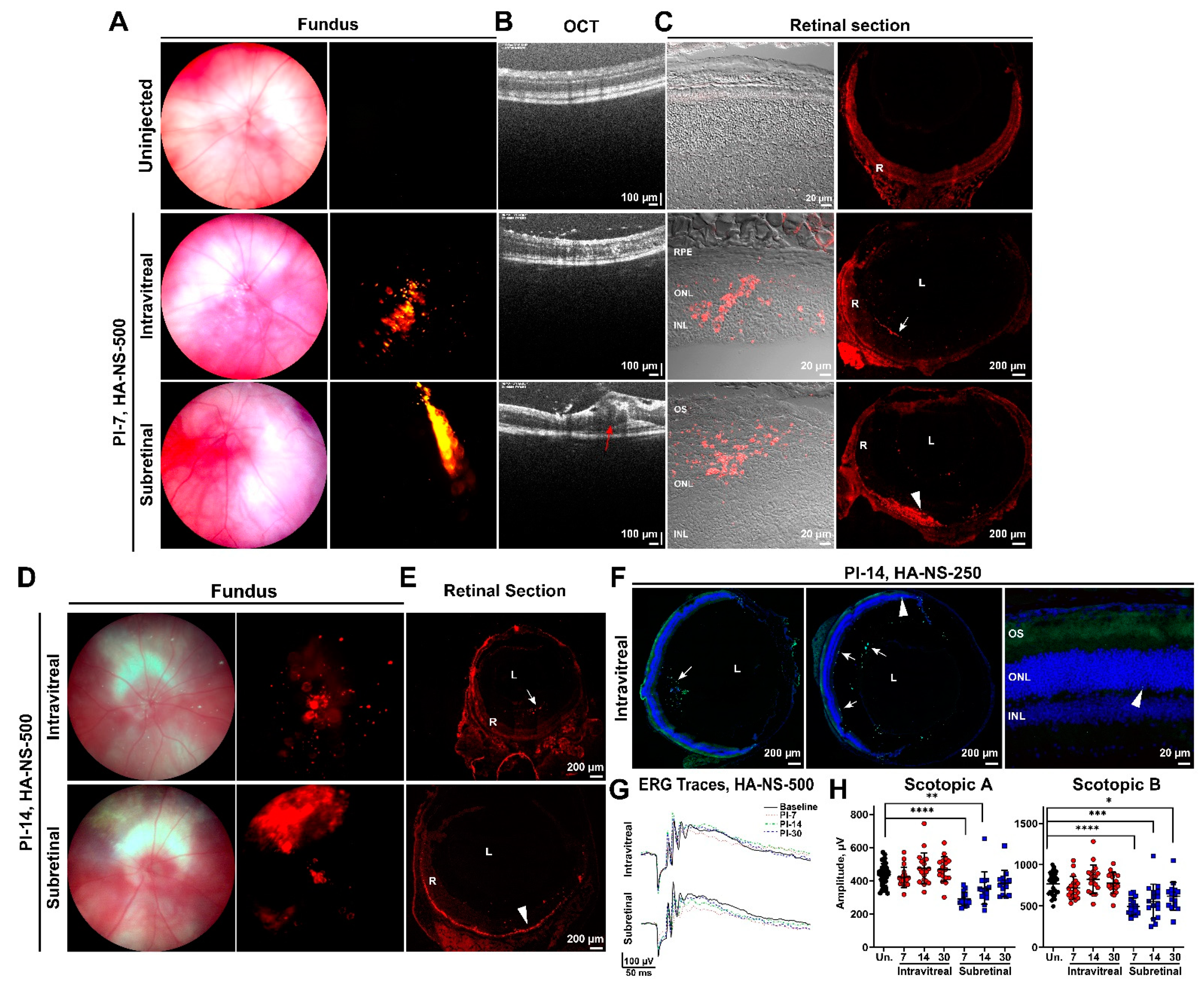
4.2. HA-NS Exhibit Persistence in the Vitreous but Low Retinal Penetration after Intravitreal Injection
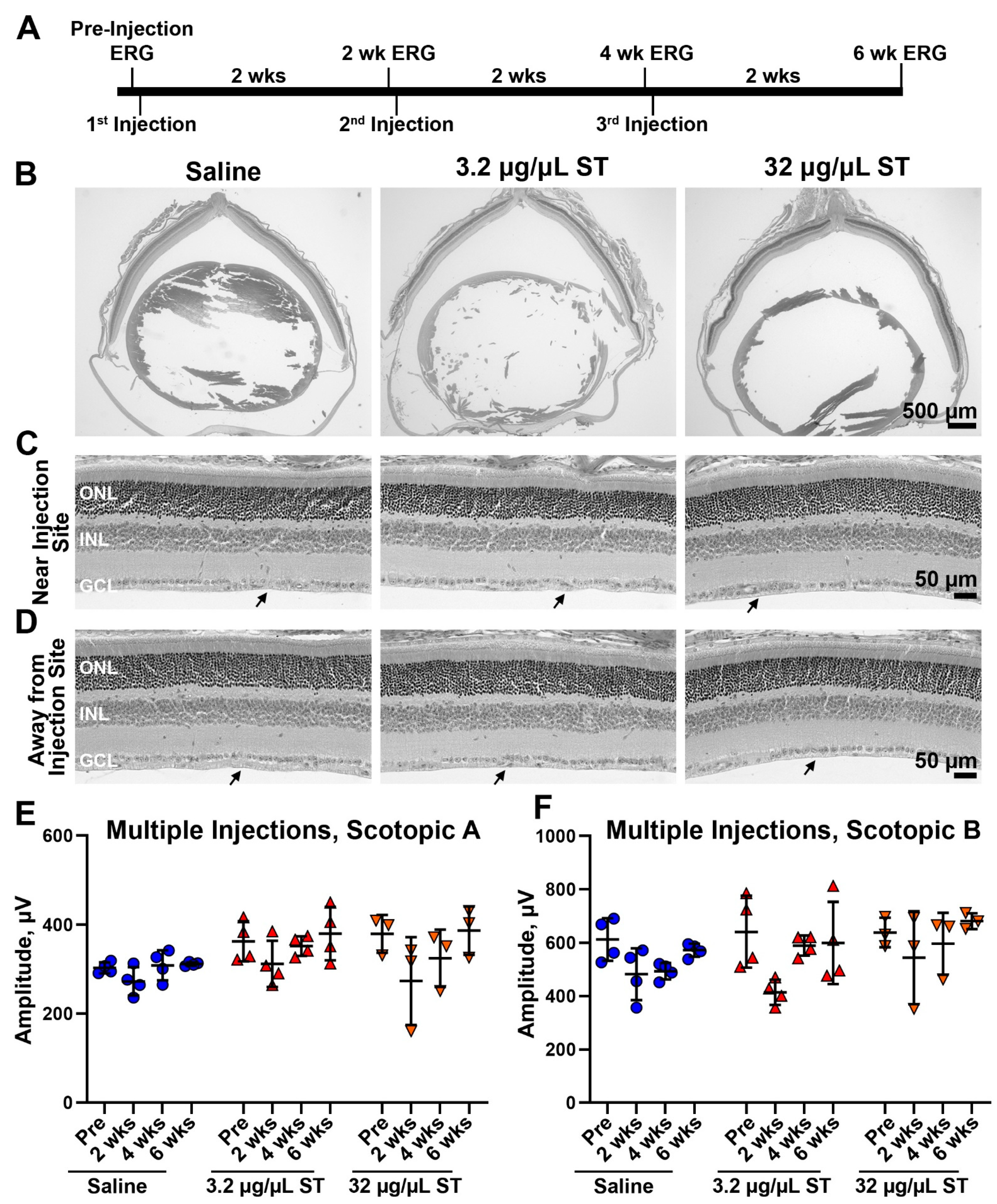
4.3. Sulfotyrosine Is Well-Tolerated in the Retina after Intravitreal Injection
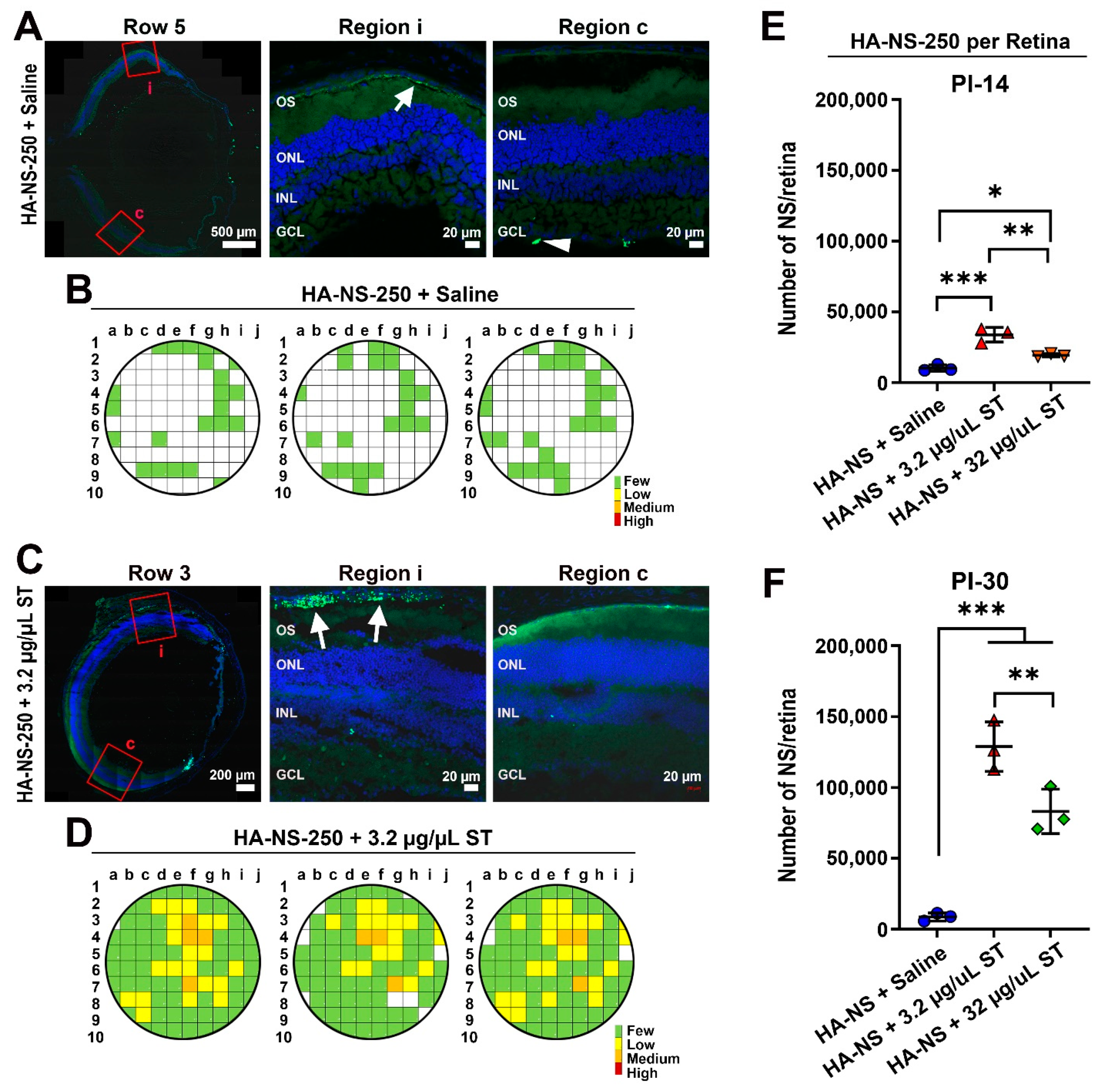
4.4. Co-Injection of Sulfotyrosine Facilitates NS Diffusion to the Outer Retina
5. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gregory-Evans, K.; Bhattacharya, S.S. Genetic blindness: Current concepts in the pathogenesis of human outer retinal dystrophies. Trends Genet. 1998, 14, 103–108. [Google Scholar] [CrossRef]
- Crane, R.; Conley, S.M.; Al-Ubaidi, M.R.; Naash, M.I. Gene Therapy to the Retina and the Cochlea. Front. Neurosci. 2021, 15, 652215. [Google Scholar] [CrossRef]
- Bainbridge, J.W.; Smith, A.J.; Barker, S.S.; Robbie, S.; Henderson, R.; Balaggan, K.; Viswanathan, A.; Holder, G.E.; Stockman, A.; Tyler, N.; et al. Effect of gene therapy on visual function in Leber’s congenital amaurosis. N. Engl. J. Med. 2008, 358, 2231–2239. [Google Scholar] [CrossRef] [PubMed]
- Maguire, A.M.; Simonelli, F.; Pierce, E.A.; Pugh, E.N., Jr.; Mingozzi, F.; Bennicelli, J.; Banfi, S.; Marshall, K.A.; Testa, F.; Surace, E.M.; et al. Safety and efficacy of gene transfer for Leber’s congenital amaurosis. N. Engl. J. Med. 2008, 358, 2240–2248. [Google Scholar] [CrossRef] [Green Version]
- Cideciyan, A.V.; Aleman, T.S.; Boye, S.L.; Schwartz, S.B.; Kaushal, S.; Roman, A.J.; Pang, J.-J.; Sumaroka, A.; Windsor, E.A.M.; Wilson, J.; et al. Human gene therapy for RPE65 isomerase deficiency activates the retinoid cycle of vision but with slow rod kinetics. Proc. Natl. Acad. Sci. USA 2008, 105, 15112–15117. [Google Scholar] [CrossRef] [Green Version]
- Zulliger, R.; Conley, S.M.; Naash, M.I. Non-viral therapeutic approaches to ocular diseases: An overview and future directions. J. Control. Release 2015, 219, 471–487. [Google Scholar] [CrossRef] [Green Version]
- Bisht, R.; Mandal, A.; Jaiswal, J.K.; Rupenthal, I.D. Nanocarrier mediated retinal drug delivery: Overcoming ocular barriers to treat posterior eye diseases. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2018, 10, e1473. [Google Scholar] [CrossRef]
- da Silva, A.L.; Martini, S.V.; Abreu, S.C.; Samary, C.d.S.; Diaz, B.L.; Fernezlian, S.; de Sá, V.K.; Capelozzi, V.L.; Boylan, N.J.; Goya, R.G.; et al. DNA nanoparticle-mediated thymulin gene therapy prevents airway remodeling in experimental allergic asthma. J. Control. Release 2014, 180, 125–133. [Google Scholar] [CrossRef] [Green Version]
- Han, Z.; Conley, S.M.; Makkia, R.S.; Cooper, M.J.; Naash, M.I. DNA nanoparticle-mediated ABCA4 delivery rescues Stargardt dystrophy in mice. J. Clin. Investig. 2012, 122, 3221–3226. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.-Q.; Quiambao, A.B.; Fitzgerald, J.B.; Cooper, M.J.; Conley, S.M.; Naash, M.I. Ocular Delivery of Compacted DNA-Nanoparticles Does Not Elicit Toxicity in the Mouse Retina. PLoS ONE 2009, 4, e7410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fink, T.L.; Klepcyk, P.J.; Oette, S.M.; Gedeon, C.R.; Hyatt, S.L.; Kowalczyk, T.H.; Moen, R.C.; Cooper, M.J. Plasmid size up to 20 kbp does not limit effective in vivo lung gene transfer using compacted DNA nanoparticles. Gene Ther. 2006, 13, 1048–1051. [Google Scholar] [CrossRef]
- Han, Z.; Banworth, M.J.; Makkia, R.; Conley, S.M.; Al-Ubaidi, M.R.; Cooper, M.J.; Naash, M.I. Genomic DNA nanoparticles rescue rhodopsin-associated retinitis pigmentosa phenotype. FASEB J. 2015, 29, 2535–2544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koirala, A.; Makkia, R.S.; Conley, S.M.; Cooper, M.J.; Naash, M.I. S/MAR-containing DNA nanoparticles promote persistent RPE gene expression and improvement in RPE65-associated LCA. Hum. Mol. Genet. 2013, 22, 1632–1642. [Google Scholar] [CrossRef] [Green Version]
- MacLaren, R.E.; Groppe, M.; Barnard, A.R.; Cottriall, C.L.; Tolmachova, T.; Seymour, L.; Clark, K.R.; During, M.J.; Cremers, F.P.M.; Seabra, M.C.; et al. Retinal gene therapy in patients with choroideremia: Initial findings from a phase 1/2 clinical trial. Lancet 2014, 383, 1129–1137. [Google Scholar] [CrossRef] [Green Version]
- Boye, S.E.; Boye, S.L.; Lewin, A.S.; Hauswirth, W.W. A comprehensive review of retinal gene therapy. Mol. Ther. 2013, 21, 509–519. [Google Scholar] [CrossRef] [Green Version]
- Gauthier, R.; Joly, S.; Pernet, V.; Lachapelle, P.; Polo, A.D. Brain-derived neurotrophic factor gene delivery to muller glia preserves structure and function of light-damaged photoreceptors. Investig. Ophthalmol. Vis. Sci. 2005, 46, 3383–3392. [Google Scholar] [CrossRef] [Green Version]
- Busskamp, V.; Picaud, S.; Sahel, J.-A.; Roska, B. Optogenetic therapy for retinitis pigmentosa. Gene Ther. 2011, 19, 169–175. [Google Scholar] [CrossRef] [Green Version]
- Bourges, J.-L.; Gautier, S.E.; Delie, F.; Bejjani, R.A.; Jeanny, J.-C.; Gurny, R.; Benezra, D.; Behar-Cohen, F.F. Ocular Drug Delivery Targeting the Retina and Retinal Pigment Epithelium Using Polylactide Nanoparticles. Investig. Opthalmol. Vis. Sci. 2003, 44, 3562–3569. [Google Scholar] [CrossRef] [PubMed]
- Cabrera, F.J.; Wang, D.C.; Reddy, K.; Acharya, G.; Shin, C.S. Challenges and opportunities for drug delivery to the posterior of the eye. Drug Discov. Today 2019, 24, 1679–1684. [Google Scholar] [CrossRef] [PubMed]
- Peynshaert, K.; Devoldere, J.; Forster, V.; Picaud, S.; Vanhove, C.; De Smedt, S.C.; Remaut, K. Toward smart design of retinal drug carriers: A novel bovine retinal explant model to study the barrier role of the vitreoretinal interface. Drug Deliv. 2017, 24, 1384–1394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peynshaert, K.; Devoldere, J.; Minnaert, A.-K.; De Smedt, S.C.; Remaut, K. Morphology and Composition of the Inner Limiting Membrane: Species-Specific Variations and Relevance toward Drug Delivery Research. Curr. Eye Res. 2019, 44, 465–475. [Google Scholar] [CrossRef]
- Acharya, G.; Shin, C.S.; McDermott, M.; Mishra, H.; Park, H.; Kwon, I.C.; Park, K. The hydrogel template method for fabrication of homogeneous nano/microparticles. J. Control. Release 2010, 141, 314–319. [Google Scholar] [CrossRef]
- Yuan, X.; Marcano, D.C.; Shin, C.; Hua, X.; Isenhart, L.C.; Pflugfelder, S.C.; Acharya, G. Ocular Drug Delivery Nanowafer with Enhanced Therapeutic Efficacy. ACS Nano 2015, 9, 1749–1758. [Google Scholar] [CrossRef] [Green Version]
- Cai, X.; Conley, S.M.; Nash, Z.; Fliesler, S.J.; Cooper, M.J.; Naash, M.I. Gene delivery to mitotic and postmitotic photoreceptors Via compacted DNA nanoparticles results in improved phenotype in a mouse model of retinitis pigmentosa. FASEB J. 2010, 24, 1178–1191. [Google Scholar] [CrossRef] [Green Version]
- Kelley, R.A.; Conley, S.M.; Makkia, R.; Watson, J.N.; Han, Z.; Cooper, M.J.; Naash, M.I. DNA nanoparticles are safe and nontoxic in non-human primate eyes. Int. J. Nanomed. 2018, 13, 1361–1379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farjo, R.; Skaggs, J.S.; Nagel, B.A.; Quiambao, A.B.; Nash, Z.A.; Fliesler, S.J.; Naash, M.I. Retention of function without normal disc morphogenesis occurs in cone but not rod photoreceptors. J. Cell Biol. 2006, 173, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Kelley, R.A.; Al-Ubaidi, M.R.; Sinha, T.; Genc, A.M.; Makia, M.S.; Ikelle, L.; Naash, M.I. Ablation of the riboflavin-binding protein retbindin reduces flavin levels and leads to progressive and dose-dependent degeneration of rods and cones. J. Biol. Chem. 2017, 292, 21023–21034. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eblimit, A.; Nguyen, T.-M.T.; Chen, Y.; Esteve-Rudd, J.; Zhong, H.; Letteboer, S.; Van Reeuwijk, J.; Simons, D.; Ding, Q.; Wu, K.M.; et al. Spata7 is a retinal ciliopathy gene critical for correct RPGRIP1 localization and protein trafficking in the retina. Hum. Mol. Genet. 2014, 24, 1584–1601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.-S.; Wang, C.-C.; Chen, B.-H.; Hou, Y.-H.; Hung, K.-S.; Mao, Y.-C. Tyrosine Sulfation as a Protein Post-Translational Modification. Molecules 2015, 20, 2138–2164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanan, Y.; Hoffhines, A.; Rauhauser, A.; Murray, A.; Al-Ubaidi, M.R. Protein tyrosine-O-sulfation in the retina. Exp. Eye Res. 2009, 89, 559–567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bertram, K.M.; Bula, D.V.; Pulido, J.S.; Shippy, S.A.; Gautam, S.; Lu, M.-J.; Hatfield, R.M.; Kim, J.-H.; Quirk, M.T.; Arroyo, J.G. Amino-acid levels in subretinal and vitreous fluid of patients with retinal detachment. Eye 2007, 22, 582–589. [Google Scholar] [CrossRef]
- Martens, T.F.; Remaut, K.; Deschout, H.; Engbersen, J.F.; Hennink, W.E.; van Steenbergen, M.J.; Demeester, J.; De Smedt, S.C.; Braeckmans, K. Coating nanocarriers with hyaluronic acid facilitates intravitreal drug delivery for retinal gene therapy. J. Control. Release 2015, 202, 83–92. [Google Scholar] [CrossRef]
- De La Fuente, M.; Seijo, B.; Alonso, M.J. Novel Hyaluronic Acid-Chitosan Nanoparticles for Ocular Gene Therapy. Investig. Opthalmol. Vis. Sci. 2008, 49, 2016–2024. [Google Scholar] [CrossRef] [Green Version]
- Apaolaza, P.S.; Delgado, D.; del Pozo-Rodriguez, A.; Rodríguez-Gascón, A.; Solinís, M. A novel gene therapy vector based on hyaluronic acid and solid lipid nanoparticles for ocular diseases. Int. J. Pharm. 2014, 465, 413–426. [Google Scholar] [CrossRef]
- Devoldere, J.; Wels, M.; Peynshaert, K.; Dewitte, H.; De Smedt, S.C.; Remaut, K. The obstacle course to the inner retina: Hyaluronic acid-coated lipoplexes cross the vitreous but fail to overcome the inner limiting membrane. Eur. J. Pharm. Biopharm. 2019, 141, 161–171. [Google Scholar] [CrossRef]
- Ruponen, M.; Rönkkö, S.; Honkakoski, P.; Pelkonen, J.; Tammi, M.; Urtti, A. Extracellular Glycosaminoglycans Modify Cellular Trafficking of Lipoplexes and Polyplexes. J. Biol. Chem. 2001, 276, 33875–33880. [Google Scholar] [CrossRef] [Green Version]
- Lesley, J.; Hascall, V.C.; Markwald, I.R.; Ghatak, I. Hyaluronan binding by cell surface CD44. J. Biol. Chem. 2000, 275, 26967–26975. [Google Scholar] [CrossRef]
- Gan, L.; Wang, J.; Zhao, Y.; Chen, D.; Zhu, C.; Liu, J.; Gan, Y. Hyaluronan-modified core–shell liponanoparticles targeting CD44-positive retinal pigment epithelium cells via intravitreal injection. Biomaterials 2013, 34, 5978–5987. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Tian, Y.; Liu, Y.; Li, D.; Zhang, H.; Yang, Y.; Qi, J.; Wang, H.; Gan, L. Hyaluronic acid-modified cationic niosomes for ocular gene delivery: Improving transfection efficiency in retinal pigment epithelium. J. Pharm. Pharmacol. 2018, 70, 1139–1151. [Google Scholar] [CrossRef] [PubMed]
- Sarthy, V.; Hoshi, H.; Mills, S.; Dudley, V.J. Characterization of green fluorescent protein-expressing retinal cells in CD 44-transgenic mice. Neuroscience 2007, 144, 1087–1093. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaitin, M.H.; Brun-Zinkernagel, A.-M. Immunolocalization of CD44 in the Dystrophic Rat Retina. Exp. Eye Res. 1998, 67, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Chaitin, M.H.; Wortham, H.S.; Zinkernagel, A.-M.B. Immunocytochemical Localization of CD44 in the Mouse Retina. Exp. Eye Res. 1994, 58, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Collis, L.; Hall, C.; Lange, L.; Ziebell, M.; Prestwich, R.; Turley, E.A. Rapid hyaluronan uptake is associated with enhanced motility: Implications for an intracellular mode of action. FEBS Lett. 1998, 440, 444–449. [Google Scholar] [CrossRef] [Green Version]
- Tammi, R.; Rilla, K.; Pienimäki, J.-P.; MacCallum, D.K.; Hogg, M.; Luukkonen, M.; Hascall, V.C.; Tammi, M. Hyaluronan Enters Keratinocytes by a Novel Endocytic Route for Catabolism. J. Biol. Chem. 2001, 276, 35111–35122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ito, T.; Iida-Tanaka, N.; Niidome, T.; Kawano, T.; Kubo, K.; Yoshikawa, K.; Sato, T.; Yang, Z.; Koyama, Y. Hyaluronic acid and its derivative as a multi-functional gene expression enhancer: Protection from non-specific interactions, adhesion to targeted cells, and transcriptional activation. J. Control. Release 2006, 112, 382–388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, K.; Yang, J.-A.; Lee, M.-Y.; Lee, H.; Hahn, S.K. Reducible hyaluronic acid-siRNA conjugate for target specific gene silencing. Bioconjug. Chem. 2013, 24, 1201–1209. [Google Scholar] [CrossRef] [PubMed]
- Sherry, D.M.; Kanan, Y.; Hamilton, R.; Hoffhines, A.; Arbogast, K.L.; Fliesler, S.J.; Naash, M.I.; Moore, K.L.; Al-Ubaidi, M.R. Differential Developmental Deficits in Retinal Function in the Absence of either Protein Tyrosine Sulfotransferase-1 or -2. PLoS ONE 2012, 7, e39702. [Google Scholar] [CrossRef] [Green Version]
- Moore, K.L. The Biology and Enzymology of Protein Tyrosine O-Sulfation. J. Biol. Chem. 2003, 278, 24243–24246. [Google Scholar] [CrossRef] [Green Version]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eblimit, A.; Makia, M.S.; Strayve, D.; Crane, R.; Conley, S.M.; Sinha, T.; Acharya, G.; Al-Ubaidi, M.R.; Naash, M.I. Co-Injection of Sulfotyrosine Facilitates Retinal Uptake of Hyaluronic Acid Nanospheres Following Intravitreal Injection. Pharmaceutics 2021, 13, 1510. https://doi.org/10.3390/pharmaceutics13091510
Eblimit A, Makia MS, Strayve D, Crane R, Conley SM, Sinha T, Acharya G, Al-Ubaidi MR, Naash MI. Co-Injection of Sulfotyrosine Facilitates Retinal Uptake of Hyaluronic Acid Nanospheres Following Intravitreal Injection. Pharmaceutics. 2021; 13(9):1510. https://doi.org/10.3390/pharmaceutics13091510
Chicago/Turabian StyleEblimit, Aiden, Mustafa S. Makia, Daniel Strayve, Ryan Crane, Shannon M. Conley, Tirthankar Sinha, Ghanashyam Acharya, Muayyad R. Al-Ubaidi, and Muna I. Naash. 2021. "Co-Injection of Sulfotyrosine Facilitates Retinal Uptake of Hyaluronic Acid Nanospheres Following Intravitreal Injection" Pharmaceutics 13, no. 9: 1510. https://doi.org/10.3390/pharmaceutics13091510
APA StyleEblimit, A., Makia, M. S., Strayve, D., Crane, R., Conley, S. M., Sinha, T., Acharya, G., Al-Ubaidi, M. R., & Naash, M. I. (2021). Co-Injection of Sulfotyrosine Facilitates Retinal Uptake of Hyaluronic Acid Nanospheres Following Intravitreal Injection. Pharmaceutics, 13(9), 1510. https://doi.org/10.3390/pharmaceutics13091510





