Quercetin Liposomal Nanoformulation for Ischemia and Reperfusion Injury Treatment
Abstract
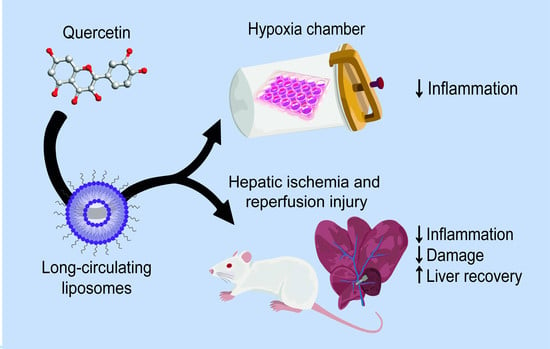
:1. Introduction
2. Materials and Methods
2.1. Preparation of Quercetin Liposomes
2.2. Characterization of Nanoformulations
2.3. Stability Studies
2.4. Release Studies
2.5. Hypoxia Chamber as In Vitro Model of Ischemia and Reperfusion
2.6. Cellular Viability Assay
2.7. RNA Extraction
2.8. RNA Purification
2.9. cDNA Synthesis
2.10. RT-qPCR
2.11. ROS Production
2.12. Animal Model and Surgical Procedure
2.13. mRNA Expression Analysis from Liver
2.14. Biochemical, Histological, and Histochemical Analysis
2.15. Statistical Analysis
3. Results and Discussion
3.1. Liposomal Formulation Characterization
3.2. In Vitro Studies
3.3. In Vivo Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Amaral, B.; Vicente, M.; Pereira, C.S.M.; Araújo, T.; Ribeiro, A.; Pereira, R.; Perdigoto, R.; Marcelino, P. Approach to the liver transplant early postoperative period: An institutional standpoint. Rev. Bras. Ter. Intensiva 2019, 31, 561–570. [Google Scholar] [CrossRef] [PubMed]
- Marcelino, P.; Marinho, H.S.; Campos, M.C.; Neves, A.R.; Real, C.; Fontes, F.S.; Carvalho, A.; Feio, G.; Martins, M.B.F.; Corvo, M.L. Therapeutic activity of superoxide dismutase-containing enzymosomes on rat liver ischaemia-reperfusion injury followed by magnetic resonance microscopy. Eur. J. Pharm. Sci. 2017, 109, 464–471. [Google Scholar] [CrossRef] [PubMed]
- Cannistrà, M.; Ruggiero, M.; Zullo, A.; Gallelli, G.; Serafini, S.; Maria, M.; Naso, A.; Grande, R.; Serra, R.; Nardo, B. Hepatic ischemia reperfusion injury: A systematic review of literature and the role of current drugs and biomarkers. Int. J. Surg. 2016, 33 (Suppl. 1), S57–S70. [Google Scholar] [CrossRef] [PubMed]
- Ferreira-Silva, M.; Faria-Silva, C.; Baptista, P.V.; Fernandes, E.; Fernandes, A.R.; Corvo, M.L. Drug delivery nanosystems targeted to hepatic ischemia and reperfusion injury. Drug Deliv. Transl. Res. 2021, 11, 397–410. [Google Scholar] [CrossRef]
- Couvreur, P.; Vauthier, C. Nanotechnology: Intelligent design to treat complex disease. Pharm. Res. 2006, 23, 1417–1450. [Google Scholar] [CrossRef] [PubMed]
- Moghimi, S.M.; Hunter, A.C.; Andresen, T.L. Factors controlling nanoparticle pharmacokinetics: An integrated analysis and perspective. Annu. Rev. Pharmacol. Toxicol. 2012, 52, 481–503. [Google Scholar] [CrossRef] [PubMed]
- Strehl, C.; van der Goes, M.C.; Bijlsma, J.W.J.; Jacobs, J.W.G.; Buttgereit, F. Glucocorticoid-targeted therapies for the treatment of rheumatoid arthritis. Expert Opin. Investig. Drugs 2017, 26, 187–195. [Google Scholar] [CrossRef]
- Anselmo, A.C.; Mitragotri, S. Nanoparticles in the clinic: An update. Bioeng. Transl. Med. 2019, 4, e10143. [Google Scholar] [CrossRef] [Green Version]
- Pattni, B.S.; Chupin, V.V.; Torchilin, V.P. New Developments in Liposomal Drug Delivery. Chem. Rev. 2015, 115, 10938–10966. [Google Scholar] [CrossRef]
- Sawant, R.R.; Torchilin, V.P. Challenges in development of targeted liposomal therapeutics. AAPS J. 2012, 14, 303–315. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Yao, J.; Han, C.; Yang, J.; Chaudhry, M.T.; Wang, S.; Liu, H.; Yin, Y. Quercetin, Inflammation and Immunity. Nutrients 2016, 8, 167. [Google Scholar] [CrossRef] [PubMed]
- Çevik, Ö.; Çadırcı, S.; Şener, T.E.; Tinay, I.; Akbal, C.; Tavukçu, H.H.; Çetinel, S.; Kıran, D.; Şener, G. Quercetin treatment against ischemia/reperfusion injury in rat corpus cavernosum tissue: A role on apoptosis and oxidative stress. Free Radic. Res. 2013, 47, 683–691. [Google Scholar] [CrossRef] [PubMed]
- Proença, C.; Ribeiro, D.; Soares, T.; Tomé, S.M.; Silva, A.M.S.; Lima, J.L.F.C.; Fernandes, E.; Freitas, M. Chlorinated Flavonoids Modulate the Inflammatory Process in Human Blood. Inflammation 2017, 40, 1155–1165. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Hu, M.-J.; Wang, Y.-Q.; Cui, Y.-L. Antioxidant Activities of Quercetin and Its Complexes for Medicinal Application. Molecules 2019, 24, 1123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.; Yin, O.Q.P.; Zuo, Z.; Chow, M.S.S. Pharmacokinetics and modeling of quercetin and metabolites. Pharm. Res. 2005, 22, 892–901. [Google Scholar] [CrossRef]
- Corvo, M.L.; Marinho, H.S.; Marcelino, P.; Lopes, R.M.; Vale, C.A.; Marques, C.R.; Martins, L.C.D.; Laverman, P.; Storm, G.; Martins, M.B.A.F. Superoxide dismutase enzymosomes: Carrier capacity optimization, in vivo behaviour and therapeutic activity. Pharm. Res. 2015, 32, 91–102. [Google Scholar] [CrossRef] [Green Version]
- Rouser, G.; Fleischer, S.; Yamamoto, A. Two dimensional thin layer chromatographic separation of polar lipids and determination of phospholipids by phosphorus analysis of spots. Lipids 1970, 5, 494–496. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Caddeo, C.; Nacher, A.; Vassallo, A.; Armentano, M.F.; Pons, R.; Fernàndez-Busquets, X.; Carbone, C.; Valenti, D.; Fadda, A.M.; Manconi, M. Effect of quercetin and resveratrol co-incorporated in liposomes against inflammatory/oxidative response associated with skin cancer. Int. J. Pharm. 2016, 513, 153–163. [Google Scholar] [CrossRef]
- Gang, W.; Jie, W.J.; Ping, Z.L.; Ming, D.S.; Ying, L.J.; Lei, W.; Fang, Y. Liposomal quercetin: Evaluating drug delivery in vitro and biodistribution in vivo. Expert Opin. Drug Deliv. 2012, 9, 599–613. [Google Scholar] [CrossRef]
- Priprem, A.; Watanatorn, J.; Sutthiparinyanont, S.; Phachonpai, W.; Muchimapura, S. Anxiety and cognitive effects of quercetin liposomes in rats. Nanomedicine 2008, 4, 70–78. [Google Scholar] [CrossRef]
- Hioki, A.; Wakasugi, A.; Kawano, K.; Hattori, Y.; Maitani, Y. Development of an in vitro drug release assay of PEGylated liposome using bovine serum albumin and high temperature. Biol. Pharm. Bull. 2010, 33, 1466–1470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Durymanov, M.; Kamaletdinova, T.; Lehmann, S.E.; Reineke, J. Exploiting passive nanomedicine accumulation at sites of enhanced vascular permeability for non-cancerous applications. J. Control. Release 2017, 261, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Ju, C.; Colgan, S.P.; Eltzschig, H.K. Hypoxia-inducible factors as molecular targets for liver diseases. J. Mol. Med. 2016, 94, 613–627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilson, G.K.; Tennant, D.A.; McKeating, J.A. Hypoxia inducible factors in liver disease and hepatocellular carcinoma: Current understanding and future directions. J. Hepatol. 2014, 61, 1397–1406. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.W.; Ko, J.; Ju, C.; Eltzschig, H.K. Hypoxia signaling in human diseases and therapeutic targets. Exp. Mol. Med. 2019, 51, 68. [Google Scholar] [CrossRef]
- Cursio, R.; Miele, C.; Filippa, N.; Van Obberghen, E.; Gugenheim, J. Liver HIF-1 Alpha Induction Precedes Apoptosis Following Normothermic Ischemia-Reperfusion in Rats. Transplant. Proc. 2008, 40, 2042–2045. [Google Scholar] [CrossRef] [PubMed]
- Bartneck, M.; Peters, F.M.; Warzecha, K.T.; Bienert, M.; van Bloois, L.; Trautwein, C.; Lammers, T.; Tacke, F. Liposomal encapsulation of dexamethasone modulates cytotoxicity, inflammatory cytokine response, and migratory properties of primary human macrophages. Nanomed. Nanotechnol. Biol. Med. 2014, 10, 1209–1220. [Google Scholar] [CrossRef]
- Gao, B.; Jeong, W.-I.; Tian, Z. Liver: An organ with predominant innate immunity. Hepatology 2007, 47, 729–736. [Google Scholar] [CrossRef] [PubMed]
- Racanelli, V.; Rehermann, B. The liver as an immunological organ. Hepatology 2006, 43, S54–S62. [Google Scholar] [CrossRef]
- Toyoda, Y.; Tamai, M.; Kashikura, K.; Kobayashi, S.; Fujiyama, Y.; Soga, T.; Tagawa, Y. Acetaminophen-Induced Hepatotoxicity in a Liver Tissue Model Consisting of Primary Hepatocytes Assembling around an Endothelial Cell Network. Drug Metab. Dispos. 2012, 40, 169–177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salerno, S.; Campana, C.; Morelli, S.; Drioli, E.; De Bartolo, L. Human hepatocytes and endothelial cells in organotypic membrane systems. Biomaterials 2011, 32, 8848–8859. [Google Scholar] [CrossRef] [PubMed]
- Nelson, L.J.; Navarro, M.; Treskes, P.; Samuel, K.; Tura-Ceide, O.; Morley, S.D.; Hayes, P.C.; Plevris, J.N. Acetaminophen cytotoxicity is ameliorated in a human liver organotypic co-culture model. Sci. Rep. 2015, 5, 17455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piantadosi, C.A.; Suliman, H.B. Transcriptional control of mitochondrial biogenesis and its interface with inflammatory processes. Biochim. Biophys. Acta 2012, 1820, 532–541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, M.; Swarts, S.G.; Yin, L.; Liu, C.; Tian, Y.; Cao, Y.; Swarts, M.; Yang, S.; Zhang, S.B.; Zhang, K.; et al. Antioxidant properties of quercetin. Adv. Exp. Med. Biol. 2011, 701, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Lin, B.; Tan, X.; Liang, J.; Wu, S.; Liu, J.; Zhang, Q.; Zhu, R. A reduction in reactive oxygen species contributes to dihydromyricetin-induced apoptosis in human hepatocellular carcinoma cells. Sci. Rep. 2014, 4, 7041. [Google Scholar] [CrossRef] [Green Version]
- Martín, M.A.; Ramos, S.; Mateos, R.; Granado Serrano, A.B.; Izquierdo-Pulido, M.; Bravo, L.; Goya, L. Protection of human HepG2 cells against oxidative stress by cocoa phenolic extract. J. Agric. Food Chem. 2008, 56, 7765–7772. [Google Scholar] [CrossRef] [PubMed]
- Martín, M.A.; Ramos, S.; Mateos, R.; Izquierdo-Pulido, M.; Bravo, L.; Goya, L. Protection of human HepG2 cells against oxidative stress by the flavonoid epicatechin. Phyther. Res. 2010, 24, 503–509. [Google Scholar] [CrossRef]
- Martins, M.B.A.F.; Corvo, M.L.; Marcelino, P.; Marinho, H.S.; Feio, G.; Carvalho, A. New long circulating magnetoliposomes as contrast agents for detection of ischemia-reperfusion injuries by MRI. Nanomedicine 2014, 10, 207–214. [Google Scholar] [CrossRef]
- McGill, M.R. The past and present of serum aminotransferases and the future of liver injury biomarkers. EXCLI J. 2016, 15, 817–828. [Google Scholar] [CrossRef]
- Giannini, E.G. Liver enzyme alteration: A guide for clinicians. Can. Med. Assoc. J. 2005, 172, 367–379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dufour, D.R.; Lott, J.A.; Nolte, F.S.; Gretch, D.R.; Koff, R.S.; Seeff, L.B. Diagnosis and monitoring of hepatic injury. II. Recommendations for use of laboratory tests in screening, diagnosis, and monitoring. Clin. Chem. 2000, 46, 2050–2068. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, A.K.; Singh, H.V.; Raizada, A.; Singh, S.K.; Pandey, A.; Singh, N.; Yadav, D.S.; Sharma, H. Inflammatory markers in patients with rheumatoid arthritis. Allergol. Immunopathol. 2015, 43, 81–87. [Google Scholar] [CrossRef]
- Wright, T.M. Cytokines in acute and chronic inflammation. Front. Biosci. 1997, 2, A171. [Google Scholar] [CrossRef] [Green Version]
- Turner, M.D.; Nedjai, B.; Hurst, T.; Pennington, D.J. Cytokines and chemokines: At the crossroads of cell signalling and inflammatory disease. Biochim. Biophys. Acta Mol. Cell Res. 2014, 1843, 2563–2582. [Google Scholar] [CrossRef] [Green Version]
- Ksontini, R. Revisiting the Role of Tumor Necrosis Factor α and the Response to Surgical Injury and Inflammation. Arch. Surg. 1998, 133, 558. [Google Scholar] [CrossRef] [PubMed]
- Atef, Y.; El-Fayoumi, H.M.; Abdel-Mottaleb, Y.; Mahmoud, M.F. Quercetin and tin protoporphyrin attenuate hepatic ischemia reperfusion injury: Role of HO-1. Naunyn. Schmiedebergs. Arch. Pharmacol. 2017, 390, 871–881. [Google Scholar] [CrossRef]
- Amira, M.; Abo, F.E.A. Protective Effects of Quercetin and Ursodeoxycholic Acid on Hepatic Ischemia-Reperfusion Injury in Rats. Clin. Pharmacol. Biopharm. 2015, 4, 128. [Google Scholar] [CrossRef] [Green Version]
- Uylaş, M.U.; Şahin, A.; Şahintürk, V.; Alataş, İ.Ö. Quercetin dose affects the fate of hepatic ischemia and reperfusion injury in rats: An experimental research. Int. J. Surg. 2018, 53, 117–121. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Y.; Liu, L.; Pan, Y.; Hu, Y.; Yang, P.; Liao, M. Protective and therapeutic effects of nanoliposomal quercetin on acute liver injury in rats. BMC Pharmacol. Toxicol. 2020, 21, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, J.-F.; Guo, C.-J.; Wei, J.-Y.; Yang, J.-J.; Jiang, Y.-G.; Li, Y.-F. Protection against hepatic ischemia-reperfusion injury in rats by oral pretreatment with quercetin. Biomed. Environ. Sci. 2003, 16, 1–8. [Google Scholar] [PubMed]
- Arash, K. Protective effect of quercetin against necrosis and apoptosis induced by experimental ischemia and reperfusion in rat liver. Afr. J. Pharm. Pharmacol. 2010, 4, 022–026. [Google Scholar]
- Lingappan, K. NF-κB in Oxidative Stress. Curr. Opin. Toxicol. 2018, 7, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Morgan, M.J.; Liu, Z. Crosstalk of reactive oxygen species and NF-κB signaling. Cell Res. 2011, 21, 103–115. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Yang, L.; Tao, K.; Vizcaychipi, M.P.; Lloyd, D.M.; Sun, X.; Irwin, M.G.; Ma, D.; Yu, W. Protective effects of hydrogen enriched saline on liver ischemia reperfusion injury by reducing oxidative stress and HMGB1 release. BMC Gastroenterol. 2014, 14, 12. [Google Scholar] [CrossRef] [Green Version]
- Colletti, L.M.; Remick, D.G.; Burtch, G.D.; Kunkel, S.L.; Strieter, R.M.; Campbell, D.A. Role of tumor necrosis factor-alpha in the pathophysiologic alterations after hepatic ischemia/reperfusion injury in the rat. J. Clin. Investig. 1990, 85, 1936–1943. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, Y.; Gong, J.-P. Impact of Recombinant Globular Adiponectin on Early Warm Ischemia-Reperfusion Injury in Rat Bile Duct after Liver Transplantation. Sci. Rep. 2015, 4, 6426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hai, Y.; Zhang, Y.; Liang, Y.; Ma, X.; Qi, X.; Xiao, J.; Xue, W.; Luo, Y.; Yue, T. Advance on the absorption, metabolism, and efficacy exertion of quercetin and its important derivatives. Food Front. 2020, 1, 420–434. [Google Scholar] [CrossRef]














| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| HIF1A | 5′–TTG ATG GGA TAT GAG CCA GA–3′ | 5′–TGT CCT GTG ACT TGT CC– 3′ |
| IL10 | 5′–GGT TGC CAA GCC TTG TCT GA–3′ | 5′–CCC CCA GGG AGT TCA CAT G–3′ |
| 18S | 5′–GTA ACC CGT TGA ACC CCA TT–3′ | 5′–CCA TCC AAT CGG TAG CG–3′ |
| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| TNFA | 5′–ACC ATG AGC ACG GAA AGC AT–3′ | 5′–AAC TGA GAG GGA GCC CA–3′ |
| 18S | 5′–GTA GTG ACG AAA AAT AAC AAT–3′ | 5′–TTG CCC TCC AAT GGA TCC T–3′ |
| Nomenclature | Lipid Composition (Molar Ratio) | ([Quer]/[Lipid]) f (µg/µmol) | I.E. (%) | Mean Size (µm) (PdI) | ζ at pH 6.0 (mV) |
|---|---|---|---|---|---|
| SPC_Querc | SPC:Chol:DSPE-mPEG2000 (8:1.5:0.5) | 31 ± 3 | 96 ± 3 | 0.12 ± 0.01 (0.058 ± 0.010) | −2 ± 1 |
| Unloaded-Lip | SPC:Chol:DSPE-mPEG2000 (8:1.5:0.5) | n.a. | n.a. | 0.12 ± 0.01 (0.074 ± 0.014) | −2 ± 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferreira-Silva, M.; Faria-Silva, C.; Carvalheiro, M.C.; Simões, S.; Marinho, H.S.; Marcelino, P.; Campos, M.C.; Metselaar, J.M.; Fernandes, E.; Baptista, P.V.; et al. Quercetin Liposomal Nanoformulation for Ischemia and Reperfusion Injury Treatment. Pharmaceutics 2022, 14, 104. https://doi.org/10.3390/pharmaceutics14010104
Ferreira-Silva M, Faria-Silva C, Carvalheiro MC, Simões S, Marinho HS, Marcelino P, Campos MC, Metselaar JM, Fernandes E, Baptista PV, et al. Quercetin Liposomal Nanoformulation for Ischemia and Reperfusion Injury Treatment. Pharmaceutics. 2022; 14(1):104. https://doi.org/10.3390/pharmaceutics14010104
Chicago/Turabian StyleFerreira-Silva, Margarida, Catarina Faria-Silva, Manuela C. Carvalheiro, Sandra Simões, H. Susana Marinho, Paulo Marcelino, Maria Celeste Campos, Josbert M. Metselaar, Eduarda Fernandes, Pedro V. Baptista, and et al. 2022. "Quercetin Liposomal Nanoformulation for Ischemia and Reperfusion Injury Treatment" Pharmaceutics 14, no. 1: 104. https://doi.org/10.3390/pharmaceutics14010104
APA StyleFerreira-Silva, M., Faria-Silva, C., Carvalheiro, M. C., Simões, S., Marinho, H. S., Marcelino, P., Campos, M. C., Metselaar, J. M., Fernandes, E., Baptista, P. V., Fernandes, A. R., & Corvo, M. L. (2022). Quercetin Liposomal Nanoformulation for Ischemia and Reperfusion Injury Treatment. Pharmaceutics, 14(1), 104. https://doi.org/10.3390/pharmaceutics14010104