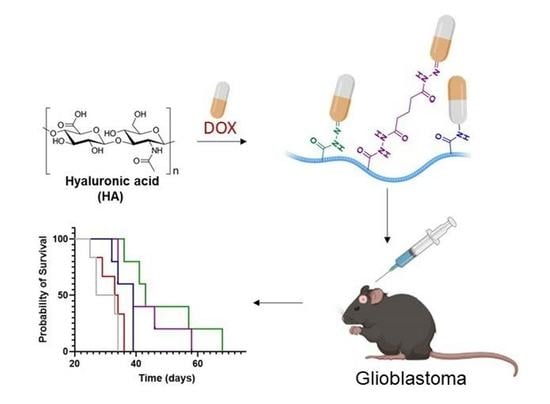
Design of Bio-Responsive Hyaluronic Acid–Doxorubicin Conjugates for the Local Treatment of Glioblastoma
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Synthesis and Characterization of HA-DOX Conjugates
2.2.1. Synthesis of HA-NH-DOX
2.2.2. General Protocol for the Synthesis of HA Hydrazides
2.2.3. General Protocol for the Synthesis of HA-Hydrazone-DOX
2.3. Characterization of HA-DOX Conjugates
2.3.1. Nuclear Magnetic Resonance (NMR)
2.3.2. Drug Loading
2.3.3. Free Drug Content
2.3.4. Dynamic Light Scattering (DLS)
2.3.5. Zeta Potential Analysis
2.4. Cell Lines
2.5. In Vitro Cell Viability
2.5.1. In Vitro Cell Viability on 2D Models
2.5.2. In Vitro Cell Viability on 3D Models
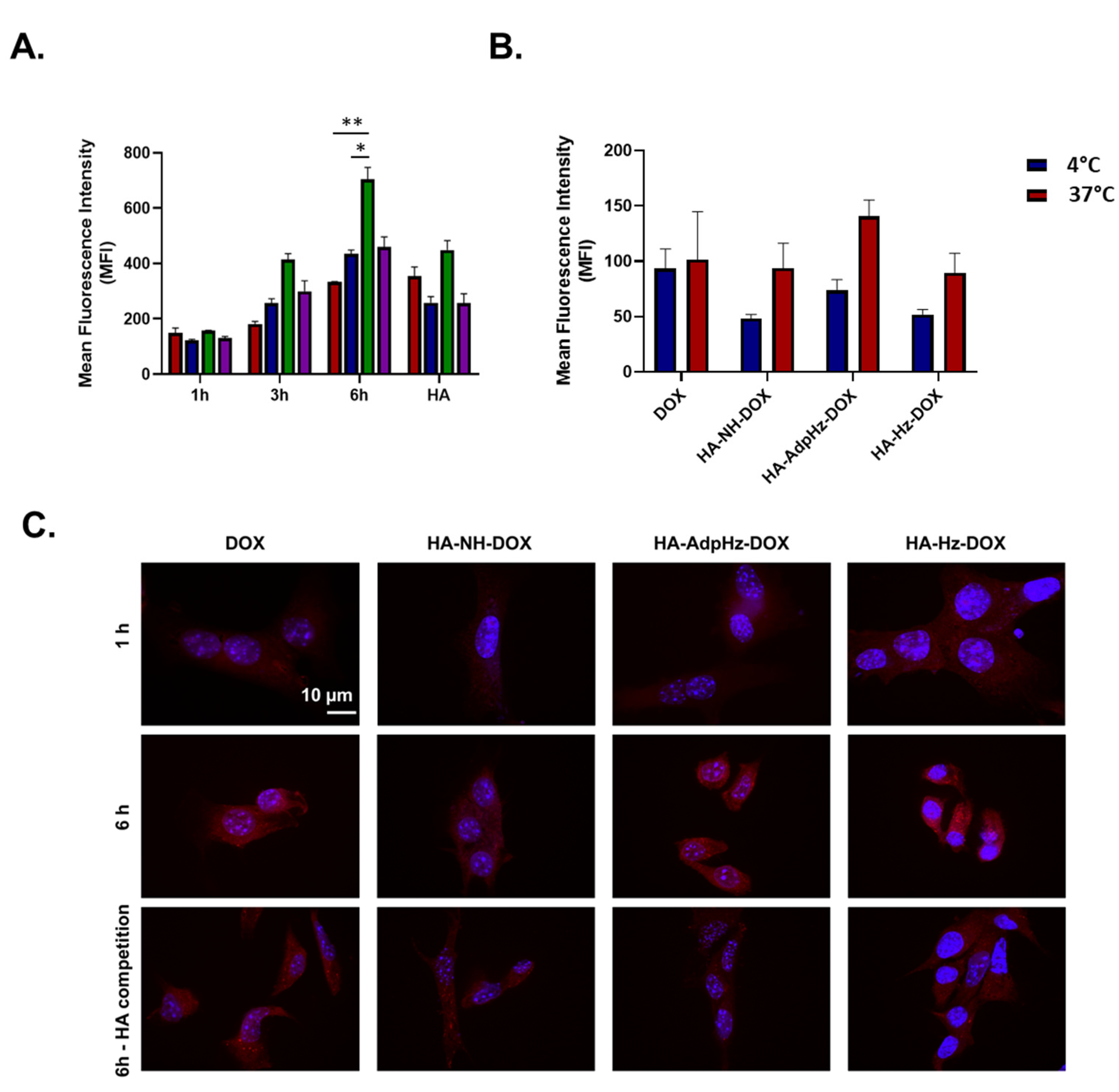
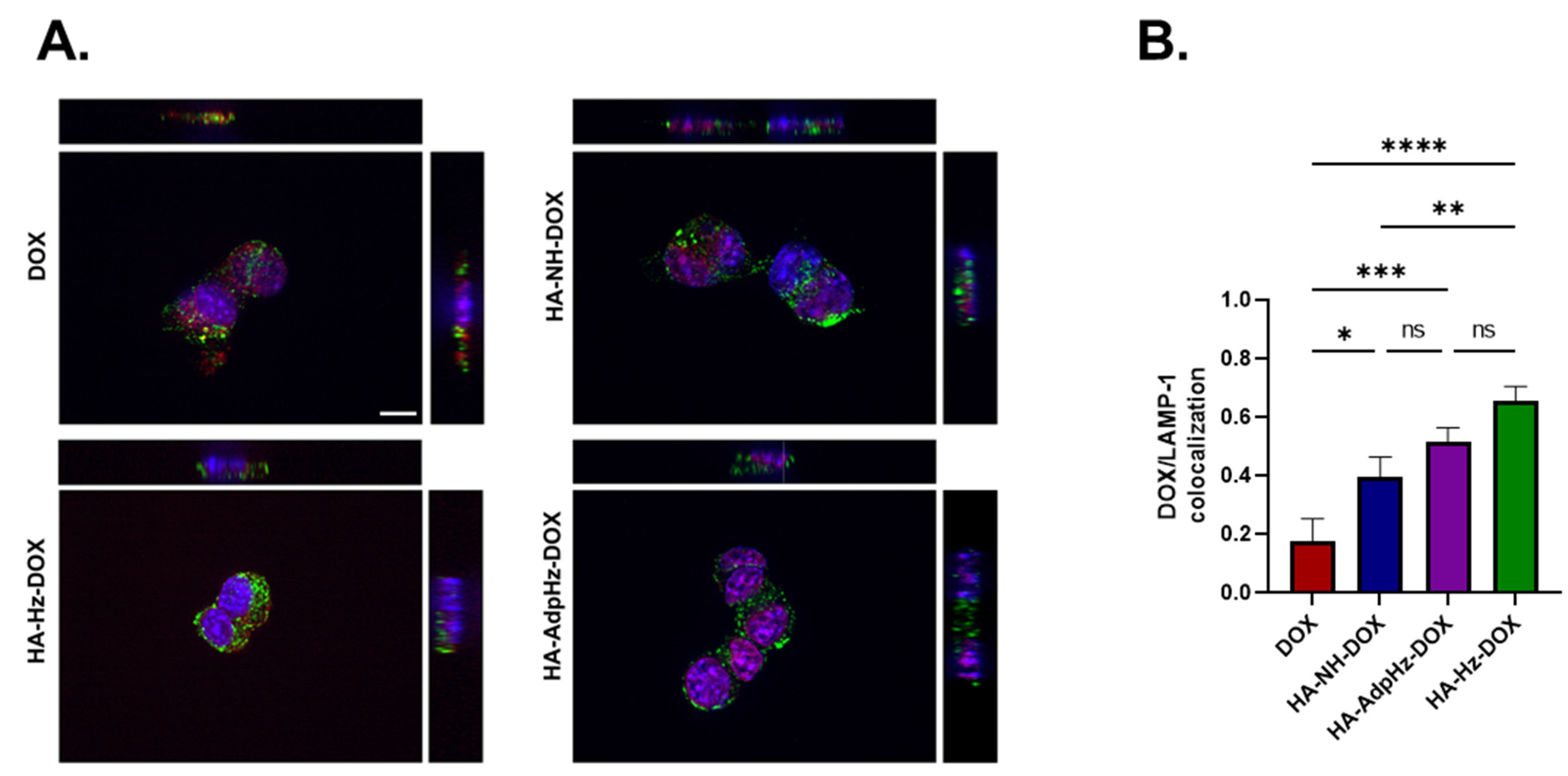
2.6. Cell Uptake and Trafficking
2.6.1. Flow Cytometry
2.6.2. Confocal Microscopy
2.7. In Vivo Study
Anticancer Activity on GBM Tumor Model
2.8. Statistical Analysis
3. Results and Discussion
3.1. Synthesis and Characterization of HA-DOX Conjugates
3.2. HA-DOX Conjugates Show Superior Cytotoxicity to Free Drug in Both 2D and 3D Models
3.3. HA-DOX Conjugates Facilitate DOX Internalization and Promote Chemotherapeutic Efficacy
3.4. Anticancer Activity on GBM Tumor Model
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ostrom, Q.T.; Gittleman, H.; Truitt, G.; Boscia, A.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS statistical report: Primary brain and other central nervous system tumors diagnosed in the United States in 2011–2015. Neuro-Oncology 2018, 20, iv1–iv86. [Google Scholar] [CrossRef] [Green Version]
- Tan, A.C.; Ashley, D.M.; López, G.Y.; Malinzak, M.; Friedman, H.S.; Khasraw, M. Management of glioblastoma: State of the art and future directions. CA A Cancer J. Clin. 2020, 70, 299–312. [Google Scholar] [CrossRef]
- Mutter, N.; Stupp, R. Temozolomide: A milestone in neuro-oncology and beyond? Expert Rev. Anticancer Ther. 2006, 6, 1187–1204. [Google Scholar] [CrossRef]
- Stupp, R.; Mason, W.P.; Van Den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef]
- Shergalis, A.; Bankhead, A.; Luesakul, U.; Muangsin, N.; Neamati, N. Current challenges and opportunities in treating glioblastoma. Pharmacol. Rev. 2018, 70, 412–445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bastiancich, C.; Malfanti, A.; Préat, V.; Rahman, R. Rationally designed drug delivery systems for the local treatment of resected glioblastoma. Adv. Drug Deliv. Rev. 2021, 177, 113951. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; van Straten, D.; Broekman, M.L.; Préat, V.; Schiffelers, R.M. Nanocarrier-based drug combination therapy for glioblastoma. Theranostics 2020, 10, 1355. [Google Scholar] [CrossRef] [PubMed]
- Chua, C.Y.X.; Ho, J.; Demaria, S.; Ferrari, M.; Grattoni, A. Emerging technologies for local cancer treatment. Adv. Ther. 2020, 3, 2000027. [Google Scholar] [CrossRef] [PubMed]
- Miglierini, P.; Bouchekoua, M.; Rousseau, B.; Hieu, P.D.; Malhaire, J.-P.; Pradier, O. Impact of the per-operatory application of GLIADEL wafers (BCNU, carmustine) in combination with temozolomide and radiotherapy in patients with glioblastoma multiforme: Efficacy and toxicity. Clin. Neurol. Neurosurg. 2012, 114, 1222–1225. [Google Scholar] [CrossRef] [PubMed]
- Bastiancich, C.; Danhier, P.; Préat, V.; Danhier, F. Anticancer drug-loaded hydrogels as drug delivery systems for the local treatment of glioblastoma. J. Control Release 2016, 243, 29–42. [Google Scholar] [CrossRef] [PubMed]
- McCrorie, P.; Vasey, C.E.; Smith, S.J.; Marlow, M.; Alexander, C.; Rahman, R. Biomedical engineering approaches to enhance therapeutic delivery for malignant glioma. J. Control Release 2020, 328, 917–931. [Google Scholar] [CrossRef]
- Erthal, L.C.; Gobbo, O.L.; Ruiz-Hernandez, E. Biocompatible copolymer formulations to treat glioblastoma multiforme. Acta Biomater. 2021, 121, 89–102. [Google Scholar] [CrossRef]
- Nance, E.; Zhang, C.; Shih, T.-Y.; Xu, Q.; Schuster, B.S.; Hanes, J. Brain-penetrating nanoparticles improve paclitaxel efficacy in malignant glioma following local administration. ACS Nano 2014, 8, 10655–10664. [Google Scholar] [CrossRef]
- Nance, E.A.; Woodworth, G.F.; Sailor, K.A.; Shih, T.-Y.; Xu, Q.; Swaminathan, G.; Xiang, D.; Eberhart, C.; Hanes, J. A dense poly (ethylene glycol) coating improves penetration of large polymeric nanoparticles within brain tissue. Sci. Transl. Med. 2012, 4, ra119–ra149. [Google Scholar] [CrossRef] [Green Version]
- Zhao, M.; Bozzato, E.; Joudiou, N.; Ghiassinejad, S.; Danhier, F.; Gallez, B.; Préat, V. Codelivery of paclitaxel and temozolomide through a photopolymerizable hydrogel prevents glioblastoma recurrence after surgical resection. J. Control Release 2019, 309, 72–81. [Google Scholar] [CrossRef]
- Graham-Gurysh, E.; Moore, K.M.; Satterlee, A.B.; Sheets, K.T.; Lin, F.-C.; Bachelder, E.M.; Miller, C.R.; Hingtgen, S.D.; Ainslie, K.M. Sustained delivery of doxorubicin via acetalated dextran scaffold prevents glioblastoma recurrence after surgical resection. Mol. Pharm. 2018, 15, 1309–1318. [Google Scholar] [CrossRef] [PubMed]
- McCrorie, P.; Mistry, J.; Taresco, V.; Lovato, T.; Fay, M.; Ward, I.; Ritchie, A.A.; Clarke, P.A.; Smith, S.J.; Marlow, M. Etoposide and olaparib polymer-coated nanoparticles within a bioadhesive sprayable hydrogel for post-surgical localised delivery to brain tumours. Eur. J. Pharm. Biopharm. 2020, 157, 108–120. [Google Scholar] [CrossRef]
- Teleanu, D.M.; Chircov, C.; Grumezescu, A.M.; Teleanu, R.I. Neurotoxicity of nanomaterials: An up-to-date overview. Nanomaterials 2019, 9, 96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Passi, A.; Vigetti, D. Hyaluronan as tunable drug delivery system. Adv. Drug Deliv. Rev. 2019, 146, 83–96. [Google Scholar] [CrossRef] [PubMed]
- Dalla Pozza, E.; Lerda, C.; Costanzo, C.; Donadelli, M.; Dando, I.; Zoratti, E.; Scupoli, M.T.; Beghelli, S.; Scarpa, A.; Fattal, E. Targeting gemcitabine containing liposomes to CD44 expressing pancreatic adenocarcinoma cells causes an increase in the antitumoral activity. Biochim. Biophys. Acta Biomembr. 2013, 1828, 1396–1404. [Google Scholar] [CrossRef] [Green Version]
- Song, S.; Qi, H.; Xu, J.; Guo, P.; Chen, F.; Li, F.; Yang, X.; Sheng, N.; Wu, Y.; Pan, W. Hyaluronan-based nanocarriers with CD44-overexpressed cancer cell targeting. Pharm. Res. 2014, 31, 2988–3005. [Google Scholar] [CrossRef]
- Wu, G.; Song, X.; Liu, J.; Li, S.; Gao, W.; Qiu, M.; Yang, C.; Ma, Y.; Chen, Y. Expression of CD44 and the survival in glioma: A meta-analysis. Biosci. Rep. 2020, 40, BSR20200520. [Google Scholar] [CrossRef] [Green Version]
- Yin, T.; Wang, J.; Yin, L.; Shen, L.; Zhou, J.; Huo, M. Redox-sensitive hyaluronic acid–paclitaxel conjugate micelles with high physical drug loading for efficient tumor therapy. Polym. Chem. 2015, 6, 8047–8059. [Google Scholar] [CrossRef]
- Lee, H.; Lee, K.; Park, T.G. Hyaluronic acid-paclitaxel conjugate micelles: Synthesis, characterization, and antitumor activity. Bioconjug. Chem. 2008, 19, 1319–1325. [Google Scholar] [CrossRef] [PubMed]
- Pan, D.C.; Krishnan, V.; Salinas, A.K.; Kim, J.; Sun, T.; Ravid, S.; Peng, K.; Wu, D.; Nurunnabi, M.; Nelson, J.A. Hyaluronic a cid–doxorubicin nanoparticles for targeted treatment of colorectal cancer. Bioeng. Transl. Med. 2021, 6, e10166. [Google Scholar] [CrossRef] [PubMed]
- Oommen, O.P.; Garousi, J.; Sloff, M.; Varghese, O.P. Tailored doxorubicin—H yaluronan conjugate as a potent anticancer glyco-D rug: An alternative to prodrug approach. Macromol. Biosci. 2014, 14, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.; Xie, Y.; Bagby, T.R.; Cohen, M.S.; Forrest, M.L. Intralymphatic chemotherapy using a hyaluronan–cisplatin conjugate. J. Surg. Res. 2008, 147, 247–252. [Google Scholar] [CrossRef] [Green Version]
- Vogus, D.R.; Evans, M.A.; Pusuluri, A.; Barajas, A.; Zhang, M.; Krishnan, V.; Nowak, M.; Menegatti, S.; Helgeson, M.E.; Squires, T.M. A hyaluronic acid conjugate engineered to synergistically and sequentially deliver gemcitabine and doxorubicin to treat triple negative breast cancer. J. Control Release 2017, 267, 191–202. [Google Scholar] [CrossRef]
- Camacho, K.M.; Kumar, S.; Menegatti, S.; Vogus, D.R.; Anselmo, A.C.; Mitragotri, S. Synergistic antitumor activity of camptothecin–doxorubicin combinations and their conjugates with hyaluronic acid. J. Control Release 2015, 210, 198–207. [Google Scholar] [CrossRef] [Green Version]
- Cohen, S.M.; Mukerji, R.; Cai, S.; Damjanov, I.; Forrest, M.L.; Cohen, M.S. Subcutaneous delivery of nanoconjugated doxorubicin and cisplatin for locally advanced breast cancer demonstrates improved efficacy and decreased toxicity at lower doses than standard systemic combination therapy in vivo. Am. J. Surg. 2011, 202, 646–653. [Google Scholar] [CrossRef] [Green Version]
- Dubey, R.D.; Klippstein, R.; Wang, J.T.-W.; Hodgins, N.; Mei, K.-C.; Sosabowski, J.; Hider, R.C.; Abbate, V.; Gupta, P.N.; Al-Jamal, K.T. Novel hyaluronic acid conjugates for dual nuclear imaging and therapy in CD44-expressing tumors in mice in vivo. Nanotheranostics 2017, 1, 59. [Google Scholar] [CrossRef] [Green Version]
- Van Heeswijk, W.; Stoffer, T.; Eenink, M.; Potman, W.; Van der Vijgh, W.; vd Poort, J.; Pinedo, H.; Lelieveld, P.; Feijen, J. Synthesis, characterization and antitumor activity of macromolecular prodrugs of adriamycin. In Recent Advances in Drug Delivery Systems; Springer: Berlin/Heidelberg, Germany, 1984; pp. 77–100. [Google Scholar]
- Arroyo-Crespo, J.J.; Armiñán, A.; Charbonnier, D.; Balzano-Nogueira, L.; Huertas-López, F.; Martí, C.; Tarazona, S.; Forteza, J.; Conesa, A.; Vicent, M.J. Tumor microenvironment-targeted poly-L-glutamic acid-based combination conjugate for enhanced triple negative breast cancer treatment. Biomaterials 2018, 186, 8–21. [Google Scholar] [CrossRef]
- Bastiancich, C.; Bozzato, E.; Luyten, U.; Danhier, F.; Bastiat, G.; Préat, V. Drug combination using an injectable nanomedicine hydrogel for glioblastoma treatment. Int. J. Pharm. 2019, 559, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Bae, Y.; Jang, W.-D.; Nishiyama, N.; Fukushima, S.; Kataoka, K. Multifunctional polymeric micelles with folate-mediated cancer cell targeting and pH-triggered drug releasing properties for active intracellular drug delivery. Mol. BioSyst. 2005, 1, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Balasso, A.; Salmaso, S.; Pontisso, P.; Rosato, A.; Quarta, S.; Malfanti, A.; Mastrotto, F.; Caliceti, P. Re-programming pullulan for targeting and controlled release of doxorubicin to the hepatocellular carcinoma cells. Eur. J. Pharm. Sci. 2017, 103, 104–115. [Google Scholar] [CrossRef]
- Perides, G.; Zhuge, Y.; Lin, T.; Stins, M.F.; Bronson, R.T.; Wu, J.K. The fibrinolytic system facilitates tumor cell migration across the blood-brain barrier in experimental melanoma brain metastasis. BMC Cancer 2006, 6, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pibuel, M.A.; Poodts, D.; Díaz, M.; Hajos, S.E.; Lompardía, S.L. The scrambled story between hyaluronan and glioblastoma. J. Biol. Chem. 2021, 296, 100549. [Google Scholar] [CrossRef] [PubMed]
- Tavianatou, A.G.; Caon, I.; Franchi, M.; Piperigkou, Z.; Galesso, D.; Karamanos, N.K. Hyaluronan: Molecular size—dependent signaling and biological functions in inflammation and cancer. FEBS J. 2019, 286, 2883–2908. [Google Scholar] [CrossRef]
- Chen, J.W.E.; Pedron, S.; Harley, B.A. The Combined Influence of Hydrogel Stiffness and Matrix—Bound Hyaluronic Acid Content on Glioblastoma Invasion. Macromol. Biosci. 2017, 17, 1700018. [Google Scholar] [CrossRef]
- Paolillo, M.; Comincini, S.; Schinelli, S. In Vitro Glioblastoma Models: A Journey into the Third Dimension. Cancers 2021, 13, 2449. [Google Scholar] [CrossRef]
- Beyer, U.; Roth, T.; Schumacher, P.; Maier, G.; Unold, A.; Frahm, A.W.; Fiebig, H.H.; Unger, C.; Kratz, F. Synthesis and in vitro efficacy of transferrin conjugates of the anticancer drug chlorambucil. J. Med. Chem. 1998, 41, 2701–2708. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.Y.; Saravanakumar, G.; Park, J.H.; Park, K. Hyaluronic acid-based nanocarriers for intracellular targeting: Interfacial interactions with proteins in cancer. Colloids Surf. B Biointerfaces 2012, 99, 82–94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Finbloom, J.A.; Aanei, I.L.; Bernard, J.M.; Klass, S.H.; Elledge, S.K.; Han, K.; Ozawa, T.; Nicolaides, T.P.; Berger, M.S.; Francis, M.B. Evaluation of three morphologically distinct virus-like particles as nanocarriers for convection-enhanced drug delivery to glioblastoma. Nanomaterials 2018, 8, 1007. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Conjugate | DOX Loading (%w/w) | Size (nm) | Zeta Potential (mV) | Free Dox (%w/w of Total DOX) |
|---|---|---|---|---|
| HA-NH-DOX | 6.12 | 6.9 | −21.6 | 1.32 |
| HA-AdpHz-DOX | 5.72 | 25.2 | −22.0 | <LOD |
| HA-Hz-DOX | 6.71 | 15.9 | −21.4 | <LOD |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malfanti, A.; Catania, G.; Degros, Q.; Wang, M.; Bausart, M.; Préat, V. Design of Bio-Responsive Hyaluronic Acid–Doxorubicin Conjugates for the Local Treatment of Glioblastoma. Pharmaceutics 2022, 14, 124. https://doi.org/10.3390/pharmaceutics14010124
Malfanti A, Catania G, Degros Q, Wang M, Bausart M, Préat V. Design of Bio-Responsive Hyaluronic Acid–Doxorubicin Conjugates for the Local Treatment of Glioblastoma. Pharmaceutics. 2022; 14(1):124. https://doi.org/10.3390/pharmaceutics14010124
Chicago/Turabian StyleMalfanti, Alessio, Giuseppina Catania, Quentin Degros, Mingchao Wang, Mathilde Bausart, and Véronique Préat. 2022. "Design of Bio-Responsive Hyaluronic Acid–Doxorubicin Conjugates for the Local Treatment of Glioblastoma" Pharmaceutics 14, no. 1: 124. https://doi.org/10.3390/pharmaceutics14010124
APA StyleMalfanti, A., Catania, G., Degros, Q., Wang, M., Bausart, M., & Préat, V. (2022). Design of Bio-Responsive Hyaluronic Acid–Doxorubicin Conjugates for the Local Treatment of Glioblastoma. Pharmaceutics, 14(1), 124. https://doi.org/10.3390/pharmaceutics14010124