Report on Webinar Series Cell and Gene Therapy: From Concept to Clinical Use
Abstract
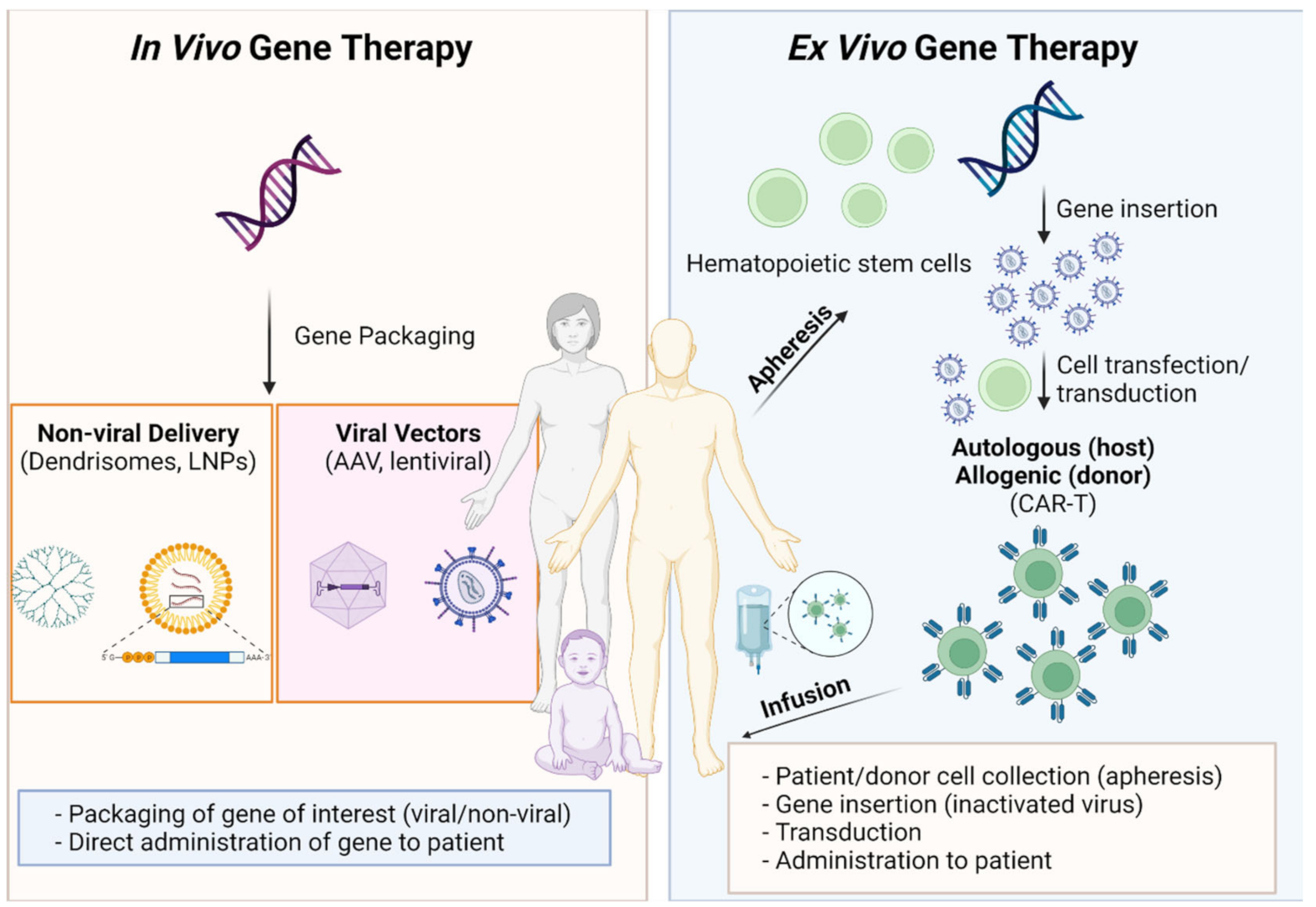
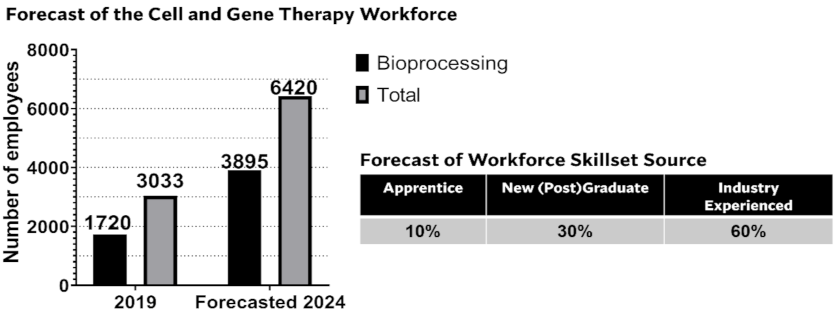
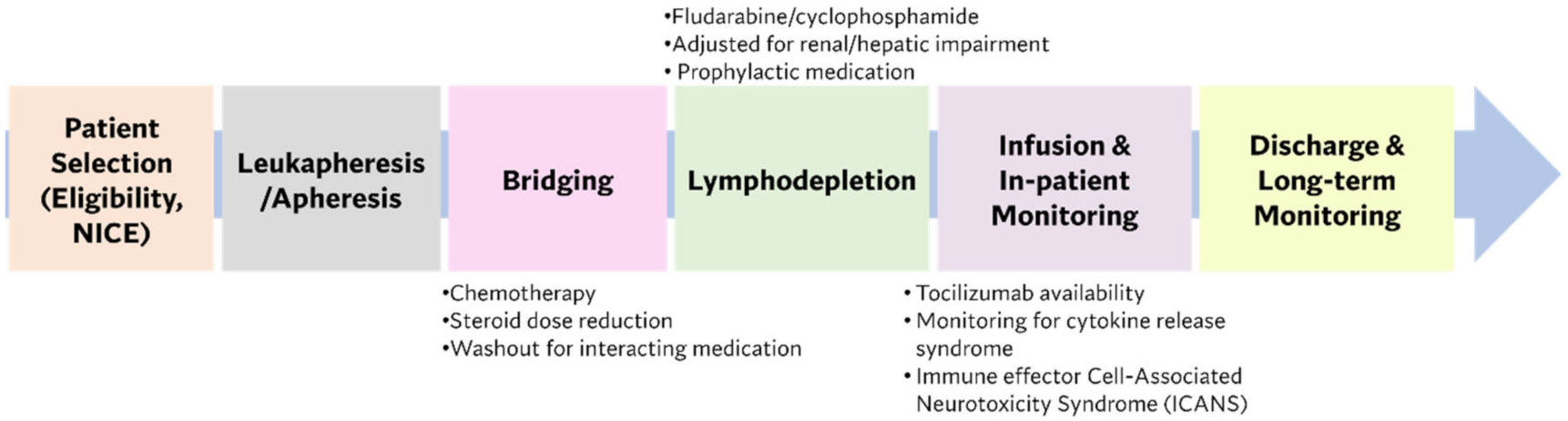
:1. Proceedings
2. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Advanced Therapy Medicinal Products Market Size, Share & Trends Analysis Report by Therapy Type (CAR-T, Gene, Cell, Stem Cell Therapy), By Region (North America, Europe, APAC, ROW), and Segment Forecasts, 2021–2028; Electronic; Grand View Research: San Francisco, CA, USA, 2021; 225p, Available online: https://www.marketresearch.com/Grand-View-Research-v4060/Advanced-Therapy-Medicinal-Products-Size-14624207/ (accessed on 10 September 2021).
- U.S. Food & Drug Administration. Approved Cellular and Gene Therapy Products. Available online: https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-products/approved-cellular-and-gene-therapy-products (accessed on 15 October 2021).
- Cell and Gene Therapy Catapult. The Cell and Gene Therapy Catapult UK Clinical Trials Database. 2020. Available online: https://ct.catapult.org.uk/clinical-trials-database (accessed on 15 October 2021).
- GlobeNewswire. Global Cell and Gene Therapy Clinical Trials Markets 2021–2028—Growing Interest in CGT/Favorable Regulatory Environment/Increasing R&D Funding. 2021. Available online: https://www.globenewswire.com/news-release/2021/09/29/2305079/28124/en/Global-Cell-and-Gene-Therapy-Clinical-Trials-Markets-2021-2028-Growing-Interest-in-CGT-Favorable-Regulatory-Environment-Increasing-R-D-Funding.html (accessed on 15 October 2021).
- Pigeau, G.M.; Csaszar, E.; Dulgar-Tulloch, A. Commercial Scale Manufacturing of Allogeneic Cell Therapy. Front. Med. 2018, 5, 233. [Google Scholar] [CrossRef]
- Mendell, J.R.; Al-Zaidy, S.A.; Rodino-Klapac, L.R.; Goodspeed, K.; Gray, S.J.; Kay, C.N.; Boye, S.L.; Boye, S.E.; George, L.A.; Salabarria, S.; et al. Current Clinical Applications of In vivo Gene Therapy with AAVs. Mol. Ther. 2021, 29, 464–488. [Google Scholar] [CrossRef]
- Lo Presti, V.; Buitenwerf, F.; van Til, N.P.; Nierkens, S. Gene Augmentation and Editing to Improve TCR Engineered T Cell Therapy against Solid Tumors. Vaccines 2020, 8, 733. [Google Scholar] [CrossRef] [PubMed]
- Abramson, J.S.; Palomba, M.L.; Gordon, L.I.; Lunning, M.A.; Wang, M.; Arnason, J.; Mehta, A.; Purev, E.; Maloney, D.G.; Andreadis, C.; et al. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): A multicentre seamless design study. Lancet 2020, 396, 839–852. [Google Scholar] [CrossRef]
- Chen, Y.H.; Pallant, C.; Sampson, C.J.; Boiti, A.; Johnson, S.; Brazauskas, P.; Hardwicke, P.; Marongiu, M.; Marinova, V.M.; Carmo, M.; et al. Rapid Lentiviral Vector Producer Cell Line Generation Using a Single DNA Construct. Mol. Ther. Methods Clin. Dev. 2020, 19, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Moreira, A.S.; Cavaco, D.G.; Faria, T.Q.; Alves, P.M.; Carrondo, M.J.T.; Peixoto, C. Advances in Lentivirus Purification. Biotechnol. J. 2021, 16, 2000019. [Google Scholar] [CrossRef] [PubMed]
- Ausubel, L.J.; Hall, C.; Sharma, A.; Shakeley, R.; Lopez, P.; Quezada, V.; Couture, S.; Laderman, K.; McMahon, R.; Huang, P.; et al. Production of CGMP-Grade Lentiviral Vectors. BioProcess Int. 2012, 10, 32–43. [Google Scholar] [PubMed]
- Krishna, D.; Rittié, L.; Tran, H.; Zheng, X.; Chen-Rogers, C.; McGillivray, A.; Clay, T.; Ketkar, A.; Tarnowski, J. Short Time to Market and Forward Planning Will Enable Cell Therapies to Deliver R&D Pipeline Value. Hum. Gene Ther. 2021, 32, 433–445. [Google Scholar] [CrossRef]
- Grabarek, A.D.; Senel, E.; Menzen, T.; Hoogendoorn, K.H.; Pike-Overzet, K.; Hawe, A.; Jiskoot, W. Particulate impurities in cell-based medicinal products traced by flow imaging microscopy combined with deep learning for image analysis. Cytotherapy 2021, 23, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Castella, M.; Caballero-Baños, M.; Ortiz-Maldonado, V.; González-Navarro, E.A.; Suñé, G.; Antoñana-Vidósola, A.; Boronat, A.; Marzal, B.; Millán, L.; Martín-Antonio, B.; et al. Point-Of-Care CAR T-Cell Production (ARI-0001) Using a Closed Semi-automatic Bioreactor: Experience from an Academic Phase I Clinical Trial. Front. Immunol. 2020, 11, 482. [Google Scholar] [CrossRef]
- Desai, A.S.; Hunter, M.R.; Kapustin, A.N. Using macropinocytosis for intracellular delivery of therapeutic nucleic acids to tumour cells. Philos. Trans. R. Soc. B Biol. Sci. 2019, 374, 20180156. [Google Scholar] [CrossRef] [Green Version]
- Sayers, E.J.; Peel, S.E.; Schantz, A.; England, R.M.; Beano, M.; Bates, S.M.; Desai, A.S.; Puri, S.; Ashford, M.B.; Jones, A.T. Endocytic Profiling of Cancer Cell Models Reveals Critical Factors Influencing LNP-Mediated mRNA Delivery and Protein Expression. Mol. Ther. J. Am. Soc. Gene Ther. 2019, 27, 1950–1962. [Google Scholar] [CrossRef]
- Sebastiani, F.; Yanez Arteta, M.; Lerche, M.; Porcar, L.; Lang, C.; Bragg, R.A.; Elmore, C.S.; Krishnamurthy, V.R.; Russell, R.A.; Darwish, T.; et al. Apolipoprotein E Binding Drives Structural and Compositional Rearrangement of mRNA-Containing Lipid Nanoparticles. ACS Nano 2021, 15, 6709–6722. [Google Scholar] [CrossRef] [PubMed]
- Ross-Thriepland, D.; Bornot, A.; Butler, L.; Desai, A.; Jaiswal, H.; Peel, S.; Hunter, M.R.; Odunze, U.; Isherwood, B.; Gianni, D. Arrayed CRISPR Screening Identifies Novel Targets That Enhance the Productive Delivery of mRNA by MC3-Based Lipid Nanoparticles. SLAS Discov. Adv. Sci. Drug Discov. 2020, 25, 605–617. [Google Scholar] [CrossRef] [PubMed]
- Sakpakdeejaroen, I.; Somani, S.; Laskar, P.; Mullin, M.; Dufès, C. Regression of Melanoma Following Intravenous Injection of Plumbagin Entrapped in Transferrin-Conjugated, Lipid–Polymer Hybrid Nanoparticles. Int. J. Nanomed. 2021, 16, 2615. [Google Scholar] [CrossRef]
- Somani, S.; Laskar, P.; Altwaijry, N.; Kewcharoenvong, P.; Irving, C.; Robb, G.; Pickard, B.S.; Dufès, C. PEGylation of polypropylenimine dendrimers: Effects on cytotoxicity, DNA condensation, gene delivery and expression in cancer cells. Sci. Rep. 2018, 8, 9410. [Google Scholar] [CrossRef] [Green Version]
- Laskar, P.; Somani, S.; Campbell, S.J.; Mullin, M.; Keating, P.; Tate, R.J.; Irving, C.; Leung, H.Y.; Dufès, C. Camptothecin-based dendrimersomes for gene delivery and redox-responsive drug delivery to cancer cells. Nanoscale 2019, 11, 20058–20071. [Google Scholar] [CrossRef] [PubMed]
- Laskar, P.; Somani, S.; Mullin, M.; Tate, R.J.; Warzecha, M.; Bowering, D.; Keating, P.; Irving, C.; Leung, H.Y.; Dufès, C. Octadecyl chain-bearing PEGylated poly(propyleneimine)-based dendrimersomes: Physicochemical studies, redox-responsiveness, DNA condensation, cytotoxicity and gene delivery to cancer cells. Biomater. Sci. 2021, 9, 1431–1448. [Google Scholar] [CrossRef]
- Altwaijry, N.; Somani, S.; Dufès, C. Targeted nonviral gene therapy in prostate cancer. Int. J. Nanomed. 2018, 13, 5753–5767. [Google Scholar] [CrossRef] [Green Version]
- Koppu, S.; Oh, Y.J.; Edrada-Ebel, R.; Blatchford, D.R.; Tetley, L.; Tate, R.J.; Dufès, C. Tumor regression after systemic administration of a novel tumor-targeted gene delivery system carrying a therapeutic plasmid DNA. J. Control. Release 2010, 143, 215–221. [Google Scholar] [CrossRef] [Green Version]
- Lim, L.Y.; Koh, P.Y.; Somani, S.; Al Robaian, M.; Karim, R.; Yean, Y.L.; Mitchell, J.; Tate, R.J.; Edrada-Ebel, R.; Blatchford, D.R.; et al. Tumor regression following intravenous administration of lactoferrin- and lactoferricin-bearing dendriplexes. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 1445–1454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Catapult. UK Cell and Gene Therapy Skills Demand Report 2019. 2019. Available online: https://ct.catapult.org.uk/resources/publications/manufacturing-surveys/all (accessed on 15 October 2021).
- Wright, J.F. Quality Control Testing, Characterization and Critical Quality Attributes of Adeno-Associated Virus Vectors Used for Human Gene Therapy. Biotechnol. J. 2021, 16, 2000022. [Google Scholar] [CrossRef]
- Guideline on Quality, Non-Clinical and Clinical Requirements for Investigational Advanced Therapy Medicinal Products in Clinical Trials; Committee for Advanced Therapies (Ed.) European Medicines Agency: Amsterdam, The Netherlands, 2019; Available online: https://www.ema.europa.eu/en/guideline-quality-non-clinical-clinical-requirements-investigational-advanced-therapy-medicinal (accessed on 1 September 2021).
- Chemistry, Manufacturing, and Control (CMC) Information for Human Gene Therapy Investigational New Drug Applications (INDs): Guidance for Industry; U.S. Food & Drug Administration: Silver Spring, MD, USA, 2020. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/chemistry-manufacturing-and-control-cmc-information-human-gene-therapy-investigational-new-drug (accessed on 1 September 2021).
- Guideline on the Quality, Non-Clinical and Clinical Aspects of Gene Therapy Medicinal Products; Committee for Advanced Therapies (Ed.) European Medicines Agency: Amsterdam, The Netherlands, 2018; Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-quality-non-clinical-clinical-aspects-gene-therapy-medicinal-products_en.pdf (accessed on 1 September 2021).
- Sheth, V.S.; Gauthier, J. Taming the beast: CRS and ICANS after CAR T-cell therapy for ALL. Bone Marrow Transplant. 2021, 56, 552–566. [Google Scholar] [CrossRef] [PubMed]
- Siegler, E.L.; Kenderian, S.S. Neurotoxicity and Cytokine Release Syndrome After Chimeric Antigen Receptor T Cell Therapy: Insights Into Mechanisms and Novel Therapies. Front. Immunol. 2020, 11, 1973. [Google Scholar] [CrossRef]
- Lee, D.W.; Santomasso, B.D.; Locke, F.L.; Ghobadi, A.; Turtle, C.J.; Brudno, J.N.; Maus, M.V.; Park, J.H.; Mead, E.; Pavletic, S.; et al. ASTCT Consensus Grading for Cytokine Release Syndrome and Neurologic Toxicity Associated with Immune Effector Cells. Biol. Blood Marrow Transpl. 2019, 25, 625–638. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Category | Cost ($M) | Patient COGs Subcategory | % Contribution to Patient COGs |
|---|---|---|---|
| Patient COGs | 55 | Apheresis | 1 |
| Cell, PD & TT * | 18 | Processing apheresis material | 10 |
| Vector, PD & TT | 25 | GMP vector/batch | 26 |
| Plasmids/cell banks | 8 | GMP T cell process/batch | 42 |
| R&D consumables | 4 | Release and stability testing, per specifications (analytics) | 17 |
| Supply chain | 4 | Qualified Person, release DP | 3 |
| Shipping | 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
van der Walle, C.F.; Dufès, C.; Desai, A.S.; Kerby, J.; Broadhead, J.; Tam, A.; Rattray, Z. Report on Webinar Series Cell and Gene Therapy: From Concept to Clinical Use. Pharmaceutics 2022, 14, 168. https://doi.org/10.3390/pharmaceutics14010168
van der Walle CF, Dufès C, Desai AS, Kerby J, Broadhead J, Tam A, Rattray Z. Report on Webinar Series Cell and Gene Therapy: From Concept to Clinical Use. Pharmaceutics. 2022; 14(1):168. https://doi.org/10.3390/pharmaceutics14010168
Chicago/Turabian Stylevan der Walle, Christopher F., Christine Dufès, Arpan S. Desai, Julie Kerby, Joanne Broadhead, Alice Tam, and Zahra Rattray. 2022. "Report on Webinar Series Cell and Gene Therapy: From Concept to Clinical Use" Pharmaceutics 14, no. 1: 168. https://doi.org/10.3390/pharmaceutics14010168
APA Stylevan der Walle, C. F., Dufès, C., Desai, A. S., Kerby, J., Broadhead, J., Tam, A., & Rattray, Z. (2022). Report on Webinar Series Cell and Gene Therapy: From Concept to Clinical Use. Pharmaceutics, 14(1), 168. https://doi.org/10.3390/pharmaceutics14010168








