Acceptability of Mebendazole Chewable Tablet in Children Aged 2 to 4 Years in Peru
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design, Objective, and Setting
2.2. Participants and Sample Size
- Receiving or received other anthelmintic treatment in the last 6 months;
- Receiving metronidazole for any other diagnosed illness in the last 15 days;
- Known allergies to the compounds in 500-mg mebendazole;
- Presence or history of important systemic or chronic diseases, diagnosed by a doctor;
- Fever at the time of observation;
- Acute respiratory disease;
- Acute or chronic diarrheal disease;
- Children, parents, or people who live within the child’s household currently diagnosed with COVID-19;
- Congenital malformation of the mouth or palate that hinders oral administration of medication;
- Participating in another clinical or similar study concurrently.
2.3. Data Collection
- Results of intake (the required dose was fully, partly, or not taken);
- Patient reaction during the administration using a 3-point facial hedonic scale (positive, neutral, or negative reaction);
- Time needed to:
- o Prepare the dose (from opening the packaging to having a required dose of medication ready to use, including all handling and modifications),
- o Administer the required dose of medication (from a required dose of medication ready to use to the end of the intake);
- Dividing the intake of a prescribed dose of the medication which cannot be taken as a whole (e.g., several sips of an oral preparations);
- Altering the use, such as
- o Modifying the dosage form (e.g., crushing, dissolving a tablet),
- o Using another route/mode of administration;
- Using food/drink
- o The prescribed dose of the medication had to be mixed with unintended drink or food (e.g., gelatin, yogurt),
- o The child had to take drink or food just before or after the dose administration (e.g., eat a cookie to mask the drug taste, take a spoon of honey for easier swallowing);
- Using a device not provided with the medication (e.g., disposable spoon or cup);
- Promising a reward;
- Using restraint (i.e., the child was forced to take it).
2.4. Data Analysis
2.4.1. Data Table
2.4.2. Acceptability Reference Framework
2.4.3. Acceptability Scoring
3. Results
3.1. Subjects
3.2. Overall Acceptability of 500-mg Mebendazole Chewable Tablet
3.3. Acceptability of 500-mg Mebendazole Chewable Tablet According to Age
3.4. Influence of Sex and Population Type on Acceptability
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hawdon, J.M. Controlling Soil-Transmitted Helminths: Time to Think Inside the Box? J. Parasitol. 2014, 100, 166–188. [Google Scholar] [CrossRef]
- Ojha, S.C.; Jaide, C.; Jinawath, N.; Rotjanapan, P.; Baral, P. Geohelminths: Public Health Significance. J. Infect. Dev. Ctries. 2014, 8, 005–016. [Google Scholar] [CrossRef] [Green Version]
- Gabrie, J.A.; Rueda, M.M.; Canales, M.; Gyorkos, T.W.; Sanchez, A.L. School Hygiene and Deworming Are Key Protective Factors for Reduced Transmission of Soil-Transmitted Helminths among Schoolchildren in Honduras. Parasites Vectors 2014, 7, 1–15. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. In Soil-Transmitted Helminth Infections; World Health Organization: Geneva, Switzerland; Available online: https://www.who.int/news-room/fact-sheets/detail/soil-transmitted-helminth-infections (accessed on 19 August 2021).
- World Health Organization. Mebendazole 500 mg Chewable Tablets-WHO-PQ Recommended Summary of Product Characteristics; WHO: Geneva, Switzerland, 2019. [Google Scholar]
- Friedman, A.J.; Ali, S.M.; Albonico, M. Safety of a New Chewable Formulation of Mebendazole for Preventive Chemotherapy Interventions to Treat Young Children in Countries with Moderate-to-High Prevalence of Soil Transmitted Helminth Infections. J. Trop. Med. 2012, 2012, 590463. [Google Scholar] [CrossRef]
- Silber, S.A.; Diro, E.; Workneh, N.; Mekonnen, Z.; Levecke, B.; Steinmann, P.; Umulisa, I.; Alemu, H.; Baeten, B.; Engelen, M. Efficacy and Safety of a Single-Dose Mebendazole 500 mg Chewable, Rapidly-Disintegrating Tablet for Ascaris Lumbricoides and Trichuris Trichiura Infection Treatment in Pediatric Patients: A Double-Blind, Randomized, Placebo-Controlled, Phase 3 Study. Am. J. Trop. Med. Hyg. 2017, 97, 1851. [Google Scholar] [CrossRef]
- Palmeirim, M.S.; Bosch, F.; Ame, S.M.; Ali, S.M.; Hattendorf, J.; Keiser, J. Efficacy, Safety and Acceptability of a New Chewable Formulation Versus the Solid Tablet of Mebendazole against Hookworm Infections in Children: An Open-Label, Randomized Controlled Trial. EClinicalMedicine 2020, 27, 100556. [Google Scholar] [CrossRef]
- European Medicine Agency. Guideline on Pharmaceutical Development of Medicines for Paediatric Use. EMA/CHMP/QWP/805880/2012 Rev. 2; EMA: London, UK, 2013. [Google Scholar]
- World Health Organization. Toolkit for Research and Development of Paediatric Antiretroviral Drugs and Formulations. Module 5: Acceptability; WHO: Geneva, Switzerland, 2018. [Google Scholar]
- Ruiz, F.; Vallet, T.; Pense-Lheritier, A.M.; Aoussat, A. Standardized Method to Assess Medicines′ Acceptability: Focus on Paediatric Population. J. Pharm. Pharmacol. 2017, 69, 406–416. [Google Scholar] [CrossRef]
- Vallet, T.; Ruiz, F.; Lavarde, M.; Pense-Lheritier, A.M.; Aoussat, A. Standardized Evaluation of Medicine Acceptability in Paediatric Population: Reliability of a Model. J. Pharm. Pharmacol. 2018, 70, 42–50. [Google Scholar] [CrossRef] [Green Version]
- Ruiz, F.; Vallet, T.; Dufay Wojcicki, A.; Belissa, É.; Fontan, J.-E.; de Pontual, L.; Nathanson, S.; Chevallier, A.; Laribe-Caget, S.; Boudy, V. Dosage form Suitability in Vulnerable Populations: A Focus on Paracetamol Acceptability from Infants to Centenarians. PLoS ONE 2019, 14, e0221261. [Google Scholar] [CrossRef]
- Vallet, T.; Elhamdaoui, O.; Berraho, A.; Cherkaoui, L.O.; Kriouile, Y.; Mahraoui, C.; Mouane, N.; Pense-Lheritier, A.-M.; Ruiz, F.; Bensouda, Y. Medicines Acceptability in Hospitalized Children: An Ongoing Need for Age-Appropriate Formulations. Pharmaceutics 2020, 12, 766. [Google Scholar] [CrossRef]
- Emeryk, A.; Vallet, T.; Wawryk-Gawda, E.; Jędrzejewski, A.; Durmont, F.; Ruiz, F. Acceptability of a Sublingual Drug Formulation for Respiratory Tract Infections in Children Aged 3 to 5 Years. Pharmaceutics 2021, 13, 294. [Google Scholar] [CrossRef]
- Saito, J.; Miyamoto, S.; Yamada, M.; Yamatani, A.; Ruiz, F.; Vallet, T. Adherence and Acceptability of an Oral Antibiotic Used for the Prevention of Pediatric Urinary Tract Infection in Japan. Pharmaceutics 2021, 13, 345. [Google Scholar] [CrossRef] [PubMed]
- Vallet, T.; Bensouda, Y.; Saito, J.; Mathiesen, L.; Pokharkar, V.; Klingmann, V.; Peak, M.; Elhamdaoui, O.; Yamatani, A.; Ivanovic, I.; et al. Exploring Acceptability Drivers of Oral Antibiotics in Children: Findings from an International Observational Study. Pharmaceutics 2021, 13, 1721. [Google Scholar] [CrossRef]
- Pokharkar, V.; Sajith, M.; Vallet, T.; Akshantal, S.; Shah, R.; Ruiz, F.; Salunke, S. Acceptability of Different Oral Dosage Forms in Paediatric Patients in Hospital Setting. Arch. Dis. Child. 2021. [Google Scholar] [CrossRef]
- Le, S.; Josse, J.; Husson, F. FactoMineR: An R Package for Multivariate Analysis. J. Stat. Softw. 2008, 25, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Januskaite, P.; Xu, X.; Ranmal, S.R.; Gaisford, S.; Basit, A.W.; Tuleu, C.; Goyanes, A. I Spy with My Little Eye: A Paediatric Visual Preferences Survey of 3D Printed Tablets. Pharmaceutics 2020, 12, 1100. [Google Scholar] [CrossRef]
- Alyami, H.; Dahmash, E.; Alyami, F.; Dahmash, D.; Huynh, C.; Terry, D.; Mohammed, A.R. Dosage form Preference Consultation Study in Children and Young Adults: Paving the Way for Patient-Centred and Patient-Informed Dosage form Development. Eur. J. Hosp. Pharm. 2017, 24, 332–337. [Google Scholar] [CrossRef]
- Michele, T.M.; Knorr, B.; Vadas, E.B.; Reiss, T.F. Safety of Chewable Tablets for Children. J. Asthma 2002, 39, 391–403. [Google Scholar] [CrossRef] [PubMed]
- Lopez, F.L.; Ernest, T.B.; Tuleu, C.; Gul, M.O. Formulation Approaches to Pediatric Oral Drug Delivery: Benefits and Limitations of Current Platforms. Expert Opin. Drug Deliv. 2015, 12, 1727–1740. [Google Scholar] [CrossRef]
- Bhusnure, O.; Shaikh, F.; Sugave, B.; Kavale, B.; Sayyed, R.; Hucche, B. Formulation Strategies for Taste-Masking of Chewable Tablets. Am. J. Pharm. Res. 2015, 5, 3836–3849. [Google Scholar]
- Gala, U.; Chauhan, H. Taste Masking Techniques in the Pharmaceutical Industry. Am. Pharm. Rev. 2014, 17. [Google Scholar]
- Mennella, J.A.; Reed, D.R.; Mathew, P.S.; Roberts, K.M.; Mansfield, C.J. “A Spoonful of Sugar Helps the Medicine Go Down”: Bitter Masking by Sucrose Among Children and Adults. Chem. Senses 2015, 40, 17–25. [Google Scholar] [CrossRef]
- Schiffman, S.S.; Gatlin, L.A.; Sattely-Miller, E.A.; Graham, B.G.; Heiman, S.A.; Stagner, W.C.; Erickson, R.P. The Effect of Sweeteners on Bitter Taste in Young and Elderly Subjects. Brain Res. Bull. 1994, 35, 189–204. [Google Scholar] [CrossRef]
- World Health Organization. Guideline: Preventive Chemotherapy to Control Soil-Transmitted Helminth Infections in at-Risk Population Groups; WHO: Geneva, Switzerland, 2017. [Google Scholar]
- Venables, R.; Batchelor, H.; Hodson, J.; Stirling, H.; Marriott, J. Determination of Formulation Factors that Affect Oral Medicines Acceptability in a Domiciliary Paediatric Population. Int. J. Pharm. 2015, 480, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Klingmann, V.; Spomer, N.; Lerch, C.; Stoltenberg, I.; Fromke, C.; Bosse, H.M.; Breitkreutz, J.; Meissner, T. Favorable Acceptance of Mini-Tablets Compared with Syrup: A Randomized Controlled Trial in Infants and Preschool Children. J. Pediatr. 2013, 163, 1728–1732. [Google Scholar] [CrossRef]
- Van Riet-Nales, D.A.; de Neef, B.J.; Schobben, A.F.; Ferreira, J.A.; Egberts, T.C.; Rademaker, C.M. Acceptability of Different Oral Formulations in Infants and Preschool Children. Arch. Dis. Child. 2013, 98, 725–731. [Google Scholar] [CrossRef] [Green Version]
- European Medicine Agency. Reflection Paper: Formulations of Choice for the Paediatric Population; EMA: London, UK, 2006. [Google Scholar]
- Zahn, J.; Hoerning, A.; Trollmann, R.; Rascher, W.; Neubert, A. Manipulation of Medicinal Products for Oral Administration to Paediatric Patients at a German University Hospital: An Observational Study. Pharmaceutics 2020, 12, 583. [Google Scholar] [CrossRef]
- Bjerknes, K.; Bøyum, S.; Kristensen, S.; Brustugun, J.; Wang, S. Manipulating Tablets and Capsules Given to Hospitalised Children in Norway Is Common Practice. Acta Paediatr. 2017, 106, 503–508. [Google Scholar] [CrossRef]
- Richey, R.H.; Shah, U.U.; Peak, M.; Craig, J.V.; Ford, J.L.; Barker, C.E.; Nunn, A.J.; Turner, M.A. Manipulation of Drugs to Achieve the Required Dose Is Intrinsic to Paediatric Practice But Is Not Supported by Guidelines or Evidence. BMC Pediatr. 2013, 13, 81. [Google Scholar] [CrossRef] [Green Version]
- Magalhães, J.; Rodrigues, A.T.; Roque, F.; Figueiras, A.; Falcão, A.; Herdeiro, M.T. Use of off-Label and Unlicenced Drugs in Hospitalised Paediatric Patients: A Systematic Review. Eur. J. Clin. Pharmacol. 2015, 71, 1–13. [Google Scholar] [CrossRef]
- World Health Organization. Report of the WHO Informal Consultation on the Use of Praziquantel during Pregnancy/Lactation and Albendazole/Mebendazole in Children under 24 Months: Geneva, 8–9 April 2002; WHO: Geneva, Switzerland, 2003. [Google Scholar]
- Moser, W.; Schindler, C.; Keiser, J. Efficacy of Recommended Drugs against Soil Transmitted Helminths: Systematic Review and Network Meta-Analysis. BMJ 2017, 358, j4307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
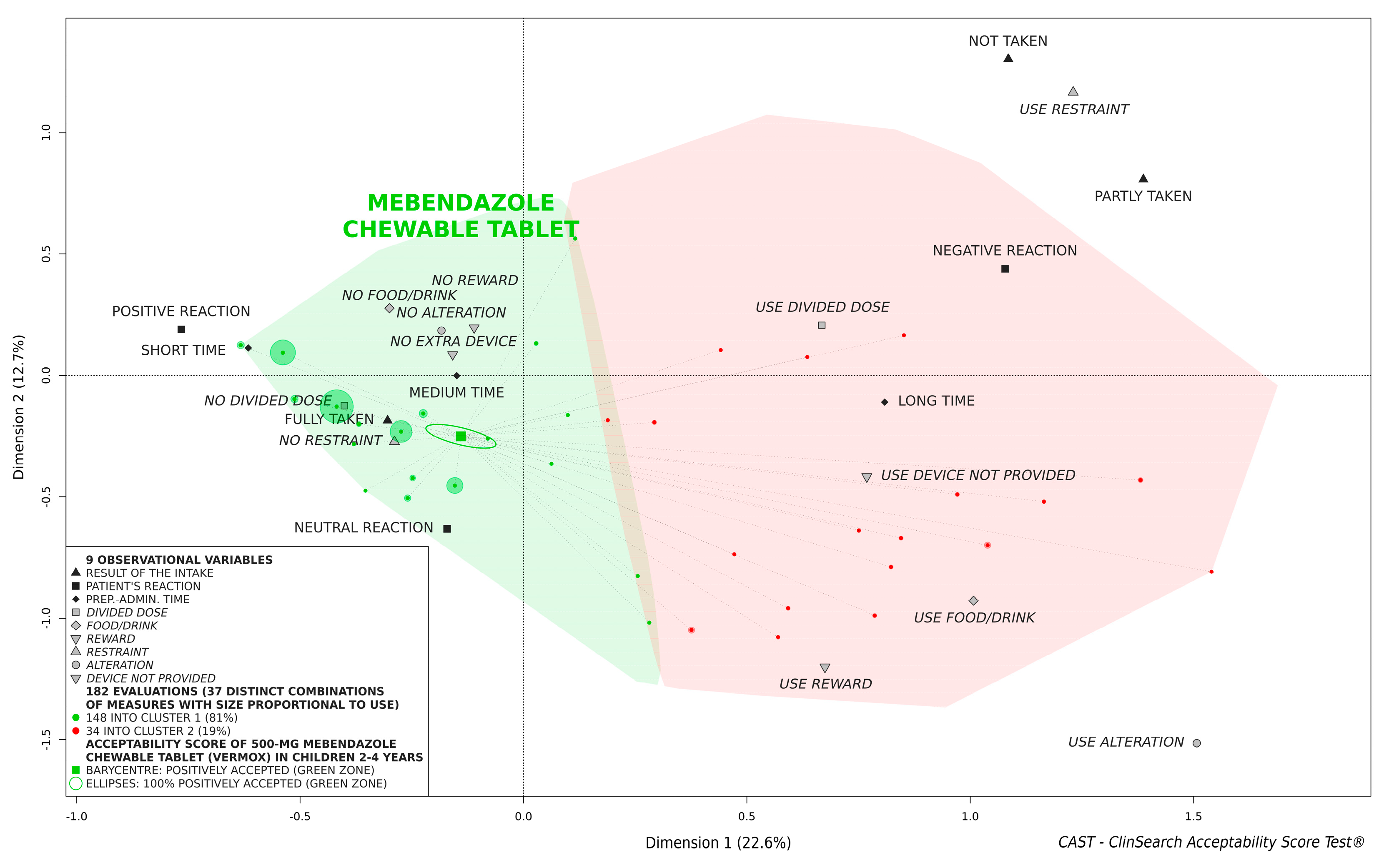




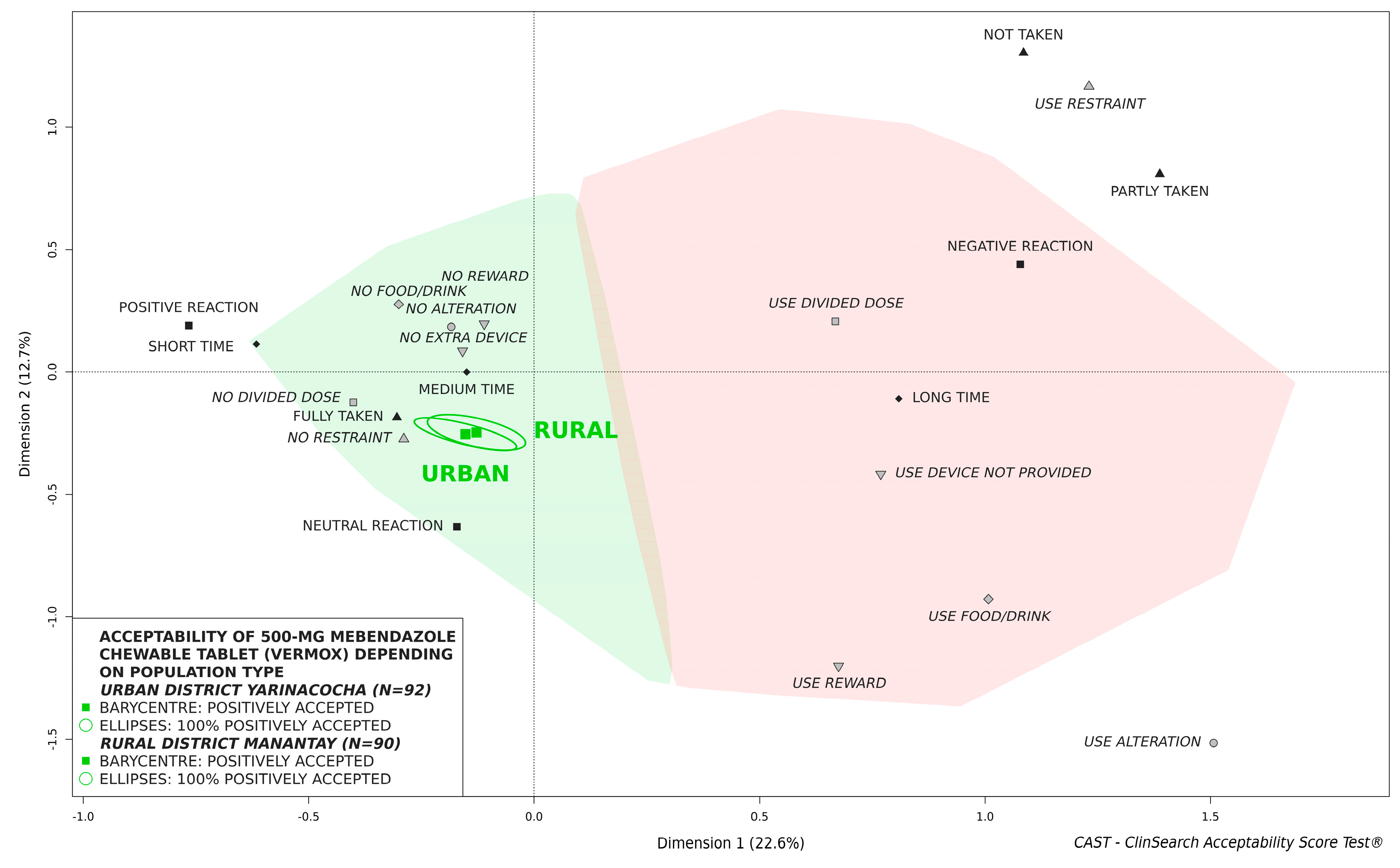
| Characteristics | Patient Age | Statistical Test | ||
|---|---|---|---|---|
| 2 Years (n = 61) | 3 Years (n = 60) | 4 Years (n = 61) | ||
| Sex | χ2 b: p = 0.98 | |||
| Female | 31 (51) a | 30 (50) | 30 (49) | |
| Male | 30 (49) | 30 (50) | 31 (51) | |
| District | χ2: p = 0.99 | |||
| Rural (Manantay) | 30 (49) | 30 (50) | 30 (49) | |
| Urban (Yarinacocha) | 31 (51) | 30 (50) | 31 (51) | |
| Place of administration | χ2: p = 0.4 | |||
| Home | 38 (62) | 41 (68) | 45 (74) | |
| Local facilities | 23 (38) | 19 (32) | 16 (26) | |
| Time of administration | F c: p = 0.015 | |||
| Morning (breakfast) | 0 (0) | 1 (2) | 1 (2) | |
| Mid-morning | 43 (70) | 45 (75) | 35 (57) | |
| Noon (lunch) | 4 (7) | 0 (0) | 0 (0) | |
| Mid-afternoon | 14 (23) | 14 (23) | 25 (41) | |
| Treatment exposure | χ2: p < 0.001 | |||
| Previous exposure | 4 (7) | 28 (47) | 25 (41) | |
| First exposure | 57 (93) | 32 (53) | 36 (59) | |
| Observer-Reported Outcomes | 500-mg Mebendazole Chewable Tablet (n = 182) | Hard Tablet Regardless of Treatment (n = 71) | Statistical Test |
|---|---|---|---|
| Result intake | |||
| Fully taken | 170 (93) a | 49 (69) | F b: p < 0.001 |
| Partly taken | 11 (6) | 22 (31) | |
| Not taken | 1 (1) | 0 (0) | |
| Patient reaction | |||
| Positive | 65 (36) | 13 (18) | χ2 c: p < 0.001 |
| Neutral | 91 (50) | 22 (31) | |
| Negative | 26 (14) | 36 (51) | |
| Preparation and administration time | |||
| Short | 22 (12) | 9 (13) | χ2: p < 0.001 |
| Medium | 131 (72) | 24 (34) | |
| Long | 29 (16) | 38 (54) | |
| Divided dose | |||
| No divided dose | 163 (90) | 30 (42) | χ2: p < 0.001 |
| Use divided dose | 19 (10) | 41 (58) | |
| Food/drinkd | |||
| No food/drink | 95 (52) | 18 (25) | χ2: p < 0.001 |
| Use food/drink | 87 (48) | 53 (75) | |
| Alteratione | |||
| No alteration | 152 (84) | 15 (21) | χ2: p < 0.001 |
| Use alteration | 30 (16) | 56 (79) | |
| Extra devicef | |||
| No extra device | 152 (84) | 55 (77) | χ2: p = 0.35 |
| Use extra device | 30 (16) | 16 (23) | |
| Reward | |||
| No reward | 174 (96) | 36 (51) | χ2: p < 0.001 |
| Use reward | 8 (4) | 35 (49) | |
| Restraint | |||
| No restraint | 182 (100) | 59 (83) | χ2: p < 0.001 |
| Use restraint | 0 (0) | 12 (17) |
| Observer-Reported Outcomes | Patient Age | Statistical Test | ||
|---|---|---|---|---|
| 2 Years (n = 61) | 3 Years (n = 60) | 4 Years (n = 61) | ||
| Result intake | ||||
| Fully taken | 55 (90) a | 55 (92) | 60 (98) | F b: p = 0.21 |
| Partly taken | 5 (8) | 5 (8) | 1 (2) | |
| Not taken | 1 (2) | 0 (0) | 0 (0) | |
| Patient reaction | ||||
| Positive | 7 (11) | 27 (45) | 31 (51) | χ2 c: p < 0.001 |
| Neutral | 36 (59) | 26 (43) | 29 (48) | |
| Negative | 18 (30) | 7 (12) | 1 (2) | |
| Preparation and administration time | ||||
| Short | 3 (5) | 5 (8) | 14 (23) | χ2: p < 0.001 |
| Medium | 36 (59) | 48 (80) | 47 (77) | |
| Long | 22 (36) | 7 (12) | 0 (0) | |
| Divided dose | ||||
| No divided dose | 50 (82) | 53 (88) | 60 (98) | χ2: p = 0.012 |
| Use divided dose | 11 (18) | 7 (12) | 1 (2) | |
| Food/drinkd | ||||
| No food/drink | 35 (57) | 21 (35) | 39 (64) | χ2: p = 0.004 |
| Use food/drink | 26 (43) | 39 (65) | 22 (36) | |
| Alteratione | ||||
| No alteration | 43 (70) | 51 (85) | 58 (95) | χ2: p = 0.001 |
| Use alteration | 18 (30) | 9 (15) | 3 (5) | |
| Extra devicef | ||||
| No extra device | 43 (70) | 51 (85) | 58 (95) | χ2: p = 0.001 |
| Use extra device | 18 (30) | 9 (15) | 3 (5) | |
| Reward | ||||
| No reward | 57 (93) | 57 (95) | 60 (98) | F: p = 0.44 |
| Use reward | 4 (7) | 3 (5) | 1 (2) | |
| Restraint | ||||
| No restraint | 61 (100) | 60 (100) | 61 (100) | |
| Use restraint | 0 (0) | 0 (0) | 0 (0) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perez, F.; Vallet, T.; Bravo, Z.; Callahan, K.; Ruiz, F. Acceptability of Mebendazole Chewable Tablet in Children Aged 2 to 4 Years in Peru. Pharmaceutics 2022, 14, 27. https://doi.org/10.3390/pharmaceutics14010027
Perez F, Vallet T, Bravo Z, Callahan K, Ruiz F. Acceptability of Mebendazole Chewable Tablet in Children Aged 2 to 4 Years in Peru. Pharmaceutics. 2022; 14(1):27. https://doi.org/10.3390/pharmaceutics14010027
Chicago/Turabian StylePerez, Fernando, Thibault Vallet, Zarela Bravo, Kristin Callahan, and Fabrice Ruiz. 2022. "Acceptability of Mebendazole Chewable Tablet in Children Aged 2 to 4 Years in Peru" Pharmaceutics 14, no. 1: 27. https://doi.org/10.3390/pharmaceutics14010027
APA StylePerez, F., Vallet, T., Bravo, Z., Callahan, K., & Ruiz, F. (2022). Acceptability of Mebendazole Chewable Tablet in Children Aged 2 to 4 Years in Peru. Pharmaceutics, 14(1), 27. https://doi.org/10.3390/pharmaceutics14010027






