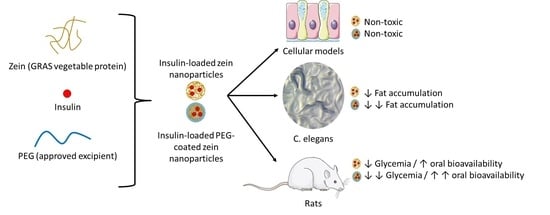
Zein-Based Nanoparticles as Oral Carriers for Insulin Delivery
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Nanoparticles
2.2.1. Preparation of Bare Nanoparticles Loaded with Insulin (I-NP)
2.2.2. Preparation of Insulin-Loaded PEG-Coated Nanoparticles (I-NP-PEG)
2.2.3. Preparation of Nanoparticles Fluorescently Labeled with Lumogen® F Red 305
2.3. Characterization of the Physico-Chemical Properties of the Resulting Nanoparticles
2.3.1. Mean Size, PDI (Polydispersity Index), ζ-Potential, and Total Process Yield
2.3.2. Morphology Evaluation
2.3.3. Assessment of the Surface Hydrophobicity of the Nanoparticles
2.3.4. Nanoparticles Surface Evaluation by Fourier Transform Infrared Resonance (FTIR)
2.4. Insulin Analysis
2.4.1. Insulin Payload and Encapsulation Efficiency
2.4.2. In Vitro Release Studies
2.5. Cellular Studies
2.5.1. Caco-2 Cell Culture
2.5.2. HT29-MTX Cell Culture
2.5.3. Cytotoxicity Evaluation
2.6. In Vivo Evaluation of Insulin-Loaded Nanoparticles in an Animal Model of Caenorhabditis elegans
2.6.1. Strain and Culture Conditions
2.6.2. Nanoparticles Intake
2.6.3. In Vivo Efficacy of the Nanoparticles in C. elegans
2.7. In Vivo Evaluation of Insulin-Loaded Nanoparticles in Diabetic Rats
2.7.1. Strain and Housing Conditions
2.7.2. Induction of Diabetes
2.7.3. Efficacy Evaluation
2.7.4. Pharmacokinetic and Pharmacodynamic Analysis
2.8. Statistical Analysis
3. Results
3.1. Characterization of Nanoparticles
3.2. In Vitro Release Behavior of Insulin-Loaded Nanoparticles
3.3. Cytotoxicity Evaluation of Insulin-Loaded Nanoparticles
3.4. Effect of Insulin-Loaded Nanoparticles in C. elegans
3.5. Evaluation of the Hypoglycemic Activity in Diabetic Rats
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mahmood, A.; Bernkop-Schnürch, A. SEDDS: A game changing approach for the oral administration of hydrophilic macromolecular drugs. Adv. Drug Deliv. Rev. 2019, 142, 91–101. [Google Scholar] [CrossRef]
- Sim, T.; Lim, C.; Hoang, N.H.; Joo, H.; Lee, J.W.; Kim, D.; won Lee, E.S.; Youn, Y.S.; Kim, J.O.; Oh, K.T. Nanomedicines for oral administration based on diverse nanoplatform. J. Pharm. Investig. 2016, 46, 351–362. [Google Scholar] [CrossRef]
- Brown, T.D.; Whitehead, K.A.; Mitragotri, S. Materials for oral delivery of proteins and peptides. Nat. Rev. Mater. 2020, 5, 127–148. [Google Scholar] [CrossRef]
- Ahmad, A.; Othman, I.; Zaini, A.; Chowdhury, E.H. Oral Nano-Insulin Therapy: Current Progress on Nanoparticle-Based Devices for Intestinal Epithelium-Targeted Insulin Delivery. J. Nanomed. Nanotechnol. 2012, s4, 007. [Google Scholar] [CrossRef]
- Frizzell, H.; Woodrow, K.A. Biomaterial Approaches for Understanding and Overcoming Immunological Barriers to Effective Oral Vaccinations. Adv. Funct. Mater. 2020, 30, 1907170. [Google Scholar] [CrossRef]
- Langguth, P.; Bohner, V.; Heizmann, J.; Merkle, H.P.; Wolffram, S.; Amidon, G.L.; Yamashita, S. The challenge of proteolysis enzymes in intestinal peptide delivery. J. Control. Release 1997, 46, 39–57. [Google Scholar] [CrossRef]
- Li, L.D.; Crouzier, T.; Sarkar, A.; Dunphy, L.; Han, J.; Ribbeck, K. Spatial configuration and composition of charge modulates transport into a mucin hydrogel barrier. Biophys. J. 2013, 105, 1357–1365. [Google Scholar] [CrossRef] [PubMed]
- Johansson, M.E.V.; Ambort, D.; Pelaseyed, T.; Schütte, A.; Gustafsson, J.K.; Ermund, A.; Subramani, D.B.; Holmén-Larsson, J.M.; Thomsson, K.A.; Bergström, J.H.; et al. Composition and functional role of the mucus layers in the intestine. Cell. Mol. Life Sci. 2011, 68, 3635–3641. [Google Scholar] [CrossRef]
- Hwang, S.R.; Byun, Y. Advances in oral macromolecular drug delivery. Expert Opin. Drug Deliv. 2014, 11, 1955–1967. [Google Scholar] [CrossRef]
- Lee, B.; Moon, K.M.; Kim, C.Y. Tight junction in the intestinal epithelium: Its association with diseases and regulation by phytochemicals. J. Immunol. Res. 2018, 2018, 2645465. [Google Scholar] [CrossRef]
- Xu, Q.; Hong, H.; Wu, J.; Yan, X. Bioavailability of bioactive peptides derived from food proteins across the intestinal epithelial membrane: A review. Trends Food Sci. Technol. 2019, 86, 399–411. [Google Scholar] [CrossRef]
- Li, R.; Laurent, F.; Taverner, A.; Mackay, J.; De Bank, P.A.; Mrsny, R.J. Intestinal transcytosis of a protein cargo and nanoparticles mediated by a non-toxic form of pseudomonas aeruginosa exotoxin A. Pharmaceutics 2021, 13, 1171. [Google Scholar] [CrossRef] [PubMed]
- Shakweh, M.; Ponchel, G.; Fattal, E. Particle uptake by Peyer’s patches: A pathway for drug and vaccine delivery. Expert Opin. Drug Deliv. 2004, 1, 141–163. [Google Scholar] [CrossRef]
- Vakilian, M.; Tahamtani, Y.; Ghaedi, K. A review on insulin trafficking and exocytosis. Gene 2019, 706, 52–61. [Google Scholar] [CrossRef] [PubMed]
- McGinn, B.J.; Morrison, J.D. Investigations into the absorption of insulin and insulin derivatives from the small intestine of the anaesthetised rat. J. Control. Release 2016, 232, 120–130. [Google Scholar] [CrossRef][Green Version]
- Ben Lulu, S.; Coran, A.G.; Mogilner, J.G.; Shaoul, R.; Shamir, R.; Shehadeh, N.; Sukhotnik, I. Oral insulin stimulates intestinal epithelial cell turnover in correlation with insulin-receptor expression along the villus-crypt axis in a rat model of short bowel syndrome. Pediatr. Surg. Int. 2010, 26, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Hall, C.; Yu, H.; Choi, E. Insulin receptor endocytosis in the pathophysiology of insulin resistance. Exp. Mol. Med. 2020, 52, 911–920. [Google Scholar] [CrossRef]
- Marschütz, M.K.; Bernkop-Schnürch, A. Oral peptide drug delivery: Polymer-inhibitor conjugates protecting insulin from enzymatic degradation in vitro. Biomaterials 2000, 21, 1499–1507. [Google Scholar] [CrossRef]
- Ibie, C.O.; Knott, R.M.; Thompson, C.J. Complexation of novel thiomers and insulin to protect against in vitro enzymatic degradation–towards oral insulin delivery. Drug Dev. Ind. Pharm. 2019, 45, 67–75. [Google Scholar] [CrossRef]
- Torchilin, V.P. Recent advances with liposomes as pharmaceutical carriers. Nat. Rev. Drug Discov. 2005, 4, 145–160. [Google Scholar] [CrossRef]
- Wong, C.Y.; Al-Salami, H.; Dass, C.R. Recent advancements in oral administration of insulin-loaded liposomal drug delivery systems for diabetes mellitus. Int. J. Pharm. 2018, 549, 201–217. [Google Scholar] [CrossRef]
- Li, P.; Tan, A.; Prestidge, C.A.; Nielsen, H.M.; Müllertz, A. Self-nanoemulsifying drug delivery systems for oral insulin delivery: In vitro and in vivo evaluations of enteric coating and drug loading. Int. J. Pharm. 2014, 477, 390–398. [Google Scholar] [CrossRef]
- Irache, J.M.; Esparza, I.; Gamazo, C.; Agüeros, M.; Espuelas, S. Nanomedicine: Novel approaches in human and veterinary therapeutics. Vet. Parasitol. 2011, 180, 47–71. [Google Scholar] [CrossRef] [PubMed]
- Fonte, P.; Araújo, F.; Silva, C.; Pereira, C.; Reis, S.; Santos, H.A.; Sarmento, B. Polymer-based nanoparticles for oral insulin delivery: Revisited approaches. Biotechnol. Adv. 2014, 33, 1342–1354. [Google Scholar] [CrossRef] [PubMed]
- Hirlekar, R.S.; Patil, E.J.; Bhairy, S.R. Oral insulin delivery: Novel strategies. Asian J. Pharm. 2017, 11, S434–S443. [Google Scholar]
- Paques, J.P.; Van Der Linden, E.; Van Rijn, C.J.M.; Sagis, L.M.C. Preparation methods of alginate nanoparticles. Adv. Colloid Interface Sci. 2014, 209, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Wang, Q. Zein-based micro- and nano-particles for drug and nutrient delivery: A review. J. Appl. Polym. Sci. 2014, 131. [Google Scholar] [CrossRef]
- Penalva, R.; Esparza, I.; Larraneta, E.; González-Navarro, C.J.; Gamazo, C.; Irache, J.M. Zein-Based Nanoparticles Improve the Oral Bioavailability of Resveratrol and Its Anti-inflammatory Effects in a Mouse Model of Endotoxic Shock. J. Agric. Food Chem. 2015, 63, 5603–5611. [Google Scholar] [CrossRef]
- Inchaurraga, L.; Martínez-López, A.L.; Martin-Arbella, N.; Irache, J.M. Zein-based nanoparticles for the oral delivery of insulin. Drug Deliv. Transl. Res. 2020, 10, 1601–1611. [Google Scholar] [CrossRef] [PubMed]
- Peñalva, R.; Esparza, I.; González-Navarro, C.J.; Quincoces, G.; Peñuelas, I.; Irache, J.M. Zein nanoparticles for oral folic acid delivery. J. Drug Deliv. Sci. Technol. 2015, 30, 450–457. [Google Scholar] [CrossRef]
- Reboredo, C.; González-Navarro, C.J.; Martínez-Oharriz, C.; Martínez-López, A.L.; Irache, J.M. Preparation and evaluation of PEG-coated zein nanoparticles for oral drug delivery purposes. Int. J. Pharm. 2021, 597, 120287. [Google Scholar] [CrossRef] [PubMed]
- Arbós, P.; Arangoa, M.A.; Campanero, M.A.; Irache, J.M. Quantification of the bioadhesive properties of protein-coated PVM/MA nanoparticles. Int. J. Pharm. 2002, 242, 129–136. [Google Scholar] [CrossRef]
- Doktorovova, S.; Shegokar, R.; Martins-Lopes, P.; Silva, A.M.; Lopes, C.M.; Müller, R.H.; Souto, E.B. Modified Rose Bengal assay for surface hydrophobicity evaluation of cationic solid lipid nanoparticles (cSLN). Eur. J. Pharm. Sci. 2012, 45, 606–612. [Google Scholar] [CrossRef]
- Martínez-López, A.L.; González-Navarro, C.J.; Vizmanos, J.L.; Irache, J.M. Zein-based nanocarriers for the oral delivery of insulin. In vivo evaluation in Caenorhabditis elegans. Drug Deliv. Transl. Res. 2021, 11, 647–658. [Google Scholar] [CrossRef] [PubMed]
- Lechanteur, A.; Almeida, A.; Sarmento, B. Elucidation of the impact of cell culture conditions of Caco-2 cell monolayer on barrier integrity and intestinal permeability. Eur. J. Pharm. Biopharm. 2017, 119, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Hilgendorf, C.; Spahn-Langguth, H.; Regårdh, C.G.; Lipka, E.; Amidon, G.L.; Langguth, P. Caco-2 versus Caco-2/HT29-MTX co-cultured cell lines: Permeabilities via diffusion, inside- and outside-directed carrier-mediated transport. J. Pharm. Sci. 2000, 89, 63–75. [Google Scholar] [CrossRef]
- Lazzaro, B.P.; Schneider, D.S. The genetics of immunity. Genetics 2014, 197, 467–470. [Google Scholar] [CrossRef]
- Lucio, D.; Martínez-Ohárriz, M.C.; Jaras, G.; Aranaz, P.; González-Navarro, C.J.; Radulescu, A.; Irache, J.M. Optimization and evaluation of zein nanoparticles to improve the oral delivery of glibenclamide. In vivo study using C. elegans. Eur. J. Pharm. Biopharm. 2017, 121, 104–112. [Google Scholar] [CrossRef]
- Zhang, Y.; Huo, M.; Zhou, J.; Xie, S. PKSolver: An add-in program for pharmacokinetic and pharmacodynamic data analysis in Microsoft Excel. Comput. Methods Programs Biomed. 2010, 99, 306–314. [Google Scholar] [CrossRef]
- Homayun, B.; Lin, X.; Choi, H.J. Challenges and recent progress in oral drug delivery systems for biopharmaceuticals. Pharmaceutics 2019, 11, 129. [Google Scholar] [CrossRef]
- Wu, S.; Bin, W.; Tu, B.; Li, X.; Wang, W.; Liao, S.; Sun, C. A Delivery System for Oral Administration of Proteins/Peptides Through Bile Acid Transport Channels. J. Pharm. Sci. 2019, 108, 2143–2152. [Google Scholar] [CrossRef]
- Jain, A.; Singh, S.K.; Arya, S.K.; Kundu, S.C.; Kapoor, S. Protein Nanoparticles: Promising Platforms for Drug Delivery Applications. ACS Biomater. Sci. Eng. 2018, 4, 3939–3961. [Google Scholar] [CrossRef]
- Pascoli, M.; de Lima, R.; Fraceto, L.F. Zein nanoparticles and strategies to improve colloidal stability: A mini-review. Front. Chem. 2018, 6, 6. [Google Scholar] [CrossRef]
- Reddy, N.; Rapisarda, M. Properties and applications of nanoparticles from plant proteins. Materials 2021, 14, 3607. [Google Scholar] [CrossRef] [PubMed]
- Calvez, J.; Benoit, S.; Fleury, L.; Khodorova, N.; Piedcoq, J.; Tomé, D.; Airinei, G.; Ben-Amouzig, R.; Gaudichon, C. True Ileal Protein Digestibility of Zein and Whey Protein Isolate in Healthy Humans (OR27-06-19). Curr. Dev. Nutr. 2019, 3. [Google Scholar] [CrossRef]
- Ye, D.; Bramini, M.; Hristov, D.R.; Wan, S.; Salvati, A.; Åberg, C.; Dawson, K.A. Low uptake of silica nanoparticles in Caco-2 intestinal epithelial barriers. Beilstein J. Nanotechnol. 2017, 8, 1396–1406. [Google Scholar] [CrossRef] [PubMed]
- Reale, O.; Huguet, A.; Fessard, V. Co-culture model of Caco-2/HT29-MTX cells: A promising tool for investigation of phycotoxins toxicity on the intestinal barrier. Chemosphere 2021, 273, 128497. [Google Scholar] [CrossRef] [PubMed]
- Pierce, S.B.; Costa, M.; Wisotzkey, R.; Devadhar, S.; Homburger, S.A.; Buchman, A.R.; Ferguson, K.C.; Heller, J.; Platt, D.M.; Pasquinelli, A.A.; et al. Regulation of DAF-2 receptor signaling by human insulin and ins-1, a member of the unusually large and diverse C. elegans insulin gene family. Genes Dev. 2001, 15, 672–686. [Google Scholar] [CrossRef]
- Martínez-López, A.L.; González-Navarro, C.J.; Aranaz, P.; Vizmanos, J.L.; Irache, J.M. In vivo testing of mucus-permeating nanoparticles for oral insulin delivery using Caenorhabditis elegans as a model under hyperglycemic conditions. Acta Pharm. Sin. B 2021, 11, 989–1002. [Google Scholar] [CrossRef]
- Cohen, J.D.; Sundaram, M.V.C. Elegans apical extracellular matrices shape epithelia. J. Dev. Biol. 2020, 8, 23. [Google Scholar] [CrossRef]
- Sonaje, K.; Lin, Y.H.; Juang, J.H.; Wey, S.P.; Chen, C.T.; Sung, H.W. In vivo evaluation of safety and efficacy of self-assembled nanoparticles for oral insulin delivery. Biomaterials 2009, 30, 2329–2339. [Google Scholar] [CrossRef]
- Jin, Y.; Song, Y.; Zhu, X.; Zhou, D.; Chen, C.; Zhang, Z.; Huang, Y. Goblet cell-targeting nanoparticles for oral insulin delivery and the influence of mucus on insulin transport. Biomaterials 2012, 33, 1573–1582. [Google Scholar] [CrossRef] [PubMed]
- Sarmento, B.; Ribeiro, A.; Veiga, F.; Sampaio, P.; Neufeld, R.; Ferreira, D. Alginate/chitosan nanoparticles are effective for oral insulin delivery. Pharm. Res. 2007, 24, 2198–2206. [Google Scholar] [CrossRef]
- Mumuni, M.A.; Kenechukwu, F.C.; Ofokansi, K.C.; Attama, A.A.; Díaz, D.D. Insulin-loaded mucoadhesive nanoparticles based on mucin-chitosan complexes for oral delivery and diabetes treatment. Carbohydr. Polym. 2020, 229, 115506. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Guo, F.; Deng, T.; Zhu, S.; Liu, W.; Zhong, H.; Yu, H.; Luo, R.; Deng, Z. Eudragit S100-Coated Chitosan Nanoparticles Co-loading Tat for Enhanced Oral Colon Absorption of Insulin. AAPS PharmSciTech 2017, 18, 1277–1287. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Zhang, M.; Gao, Y.; Zhu, S.; Chen, S.; Liu, W.; Zhong, H.; Liu, J. Modified nanoparticles with cell-penetrating peptide and amphipathic chitosan derivative for enhanced oral colon absorption of insulin: Preparation and evaluation. Drug Deliv. 2016, 23, 2003–2014. [Google Scholar] [CrossRef]
- Xu, B.; Jiang, G.; Yu, W.; Liu, D.; Liu, Y.; Kong, X.; Yao, J. Preparation of poly(lactic-co-glycolic acid) and chitosan composite nanocarriers via electrostatic self assembly for oral delivery of insulin. Mater. Sci. Eng. C 2017, 78, 420–428. [Google Scholar] [CrossRef]
- Bravo-Alfaro, D.A.; Muñoz-Correa, M.O.F.; Santos-Luna, D.; Toro-Vazquez, J.F.; Cano-Sarmiento, C.; García-Varela, R.; García, H.S. Encapsulation of an insulin-modified phosphatidylcholine complex in a self-nanoemulsifying drug delivery system (SNEDDS) for oral insulin delivery. J. Drug Deliv. Sci. Technol. 2020, 57, 101622. [Google Scholar] [CrossRef]
- Yazdi, J.R.; Tafaghodi, M.; Sadri, K.; Mashreghi, M.; Nikpoor, A.R.; Nikoofal-Sahlabadi, S.; Chamani, J.; Vakili, R.; Moosavian, S.A.; Jaafari, M.R. Folate targeted PEGylated liposomes for the oral delivery of insulin: In vitro and in vivo studies. Colloids Surf. B Biointerfaces 2020, 194, 111203. [Google Scholar] [CrossRef]
- El Leithy, E.S.; Abdel-Bar, H.M.; Ali, R.A.M. Folate-chitosan nanoparticles triggered insulin cellular uptake and improved in vivo hypoglycemic activity. Int. J. Pharm. 2019, 571, 118708. [Google Scholar] [CrossRef]
- Bao, X.; Qian, K.; Yao, P. Insulin- And cholic acid-loaded zein/casein-dextran nanoparticles enhance the oral absorption and hypoglycemic effect of insulin. J. Mater. Chem. B 2021, 9, 6234–6245. [Google Scholar] [CrossRef] [PubMed]









| Formulation | Size (nm) | PDI | Zeta Potential (mV) | Insulin Payload (µg/mg) | E. E. (%) |
|---|---|---|---|---|---|
| NP | 239 ± 19 | 0.17 ± 0.07 | −56.7 ± 3.4 | - | - |
| NP-PEG | 222 ± 19 | 0.14 ± 0.07 | −51.2 ± 1.8 | - | - |
| I-NP | 277 ± 14 | 0.17 ± 0.06 | −39.4 ± 0.2 | 76.1 ± 2 | 77.3 ± 3 |
| I-NP-PEG | 263 ± 19 | 0.15 ± 0.05 | −38.9 ± 2.3 | 81.1 ± 6 | 84.8 ± 3 |
| Treatment | Dose (IU/kg) | AAC (μg/hmL) | Tmax (h) | Cmin (% of Initial Values) | PA (%) |
|---|---|---|---|---|---|
| Ins sc | 5 | 1789.8 ± 155.5 | 3.1 ± 0.9 | 22.4 ± 8.7 | 100 |
| I-NP | 50 | 845.9 ± 357.6 | 5.1 ± 0.9 | 56.7 ± 15.6 | 4.7 ± 1.9 |
| I-NP-PEG | 50 | 1267.3 ± 297.0 | 5.6 ± 0.7 | 31.9 ± 20.1 | 14.9 ± 1.6 *** |
| Treatment | Dose (IU/kg) | Cmax (ng/mL) | Tmax (h) | AUC (ng/hmL) | Fr% |
|---|---|---|---|---|---|
| Ins sc | 5 | 5.87 ± 2.07 | 1.37 ± 0.48 | 15.86 ± 4.13 | 100 |
| I-NP | 50 | 2.10 ± 0.93 ** | 6.00 ± 0.00 | 6.65 ± 2.74 * | 4.2 |
| I-NP-PEG | 50 | 4.02 ± 1.67 | 5.49 ± 0.90 | 16.18 ± 9.76 | 10.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reboredo, C.; González-Navarro, C.J.; Martínez-López, A.L.; Martínez-Ohárriz, C.; Sarmento, B.; Irache, J.M. Zein-Based Nanoparticles as Oral Carriers for Insulin Delivery. Pharmaceutics 2022, 14, 39. https://doi.org/10.3390/pharmaceutics14010039
Reboredo C, González-Navarro CJ, Martínez-López AL, Martínez-Ohárriz C, Sarmento B, Irache JM. Zein-Based Nanoparticles as Oral Carriers for Insulin Delivery. Pharmaceutics. 2022; 14(1):39. https://doi.org/10.3390/pharmaceutics14010039
Chicago/Turabian StyleReboredo, Cristian, Carlos J. González-Navarro, Ana Luisa Martínez-López, Cristina Martínez-Ohárriz, Bruno Sarmento, and Juan M. Irache. 2022. "Zein-Based Nanoparticles as Oral Carriers for Insulin Delivery" Pharmaceutics 14, no. 1: 39. https://doi.org/10.3390/pharmaceutics14010039
APA StyleReboredo, C., González-Navarro, C. J., Martínez-López, A. L., Martínez-Ohárriz, C., Sarmento, B., & Irache, J. M. (2022). Zein-Based Nanoparticles as Oral Carriers for Insulin Delivery. Pharmaceutics, 14(1), 39. https://doi.org/10.3390/pharmaceutics14010039