Combining Therapeutic Drug Monitoring and Pharmacokinetic Modelling Deconvolutes Physiological and Environmental Sources of Variability in Clozapine Exposure
Abstract
:1. Introduction
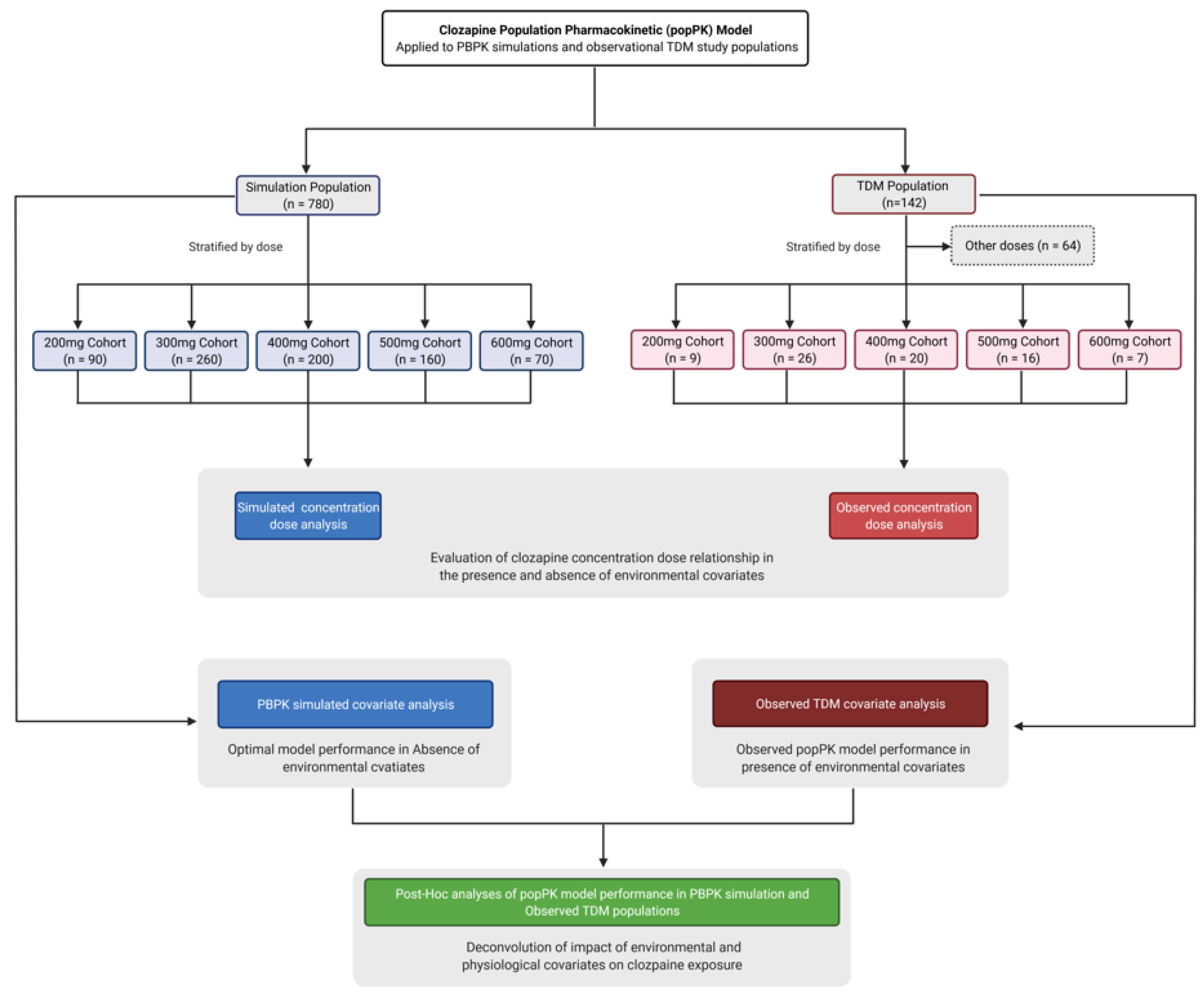
2. Materials and Methods
2.1. Physiologically Based Modelling and Simulation
2.2. Observed Clinical Data
2.3. Population Pharmacokinetic Model
2.4. Statistical Analysis
3. Results
3.1. TDM Population Demographics
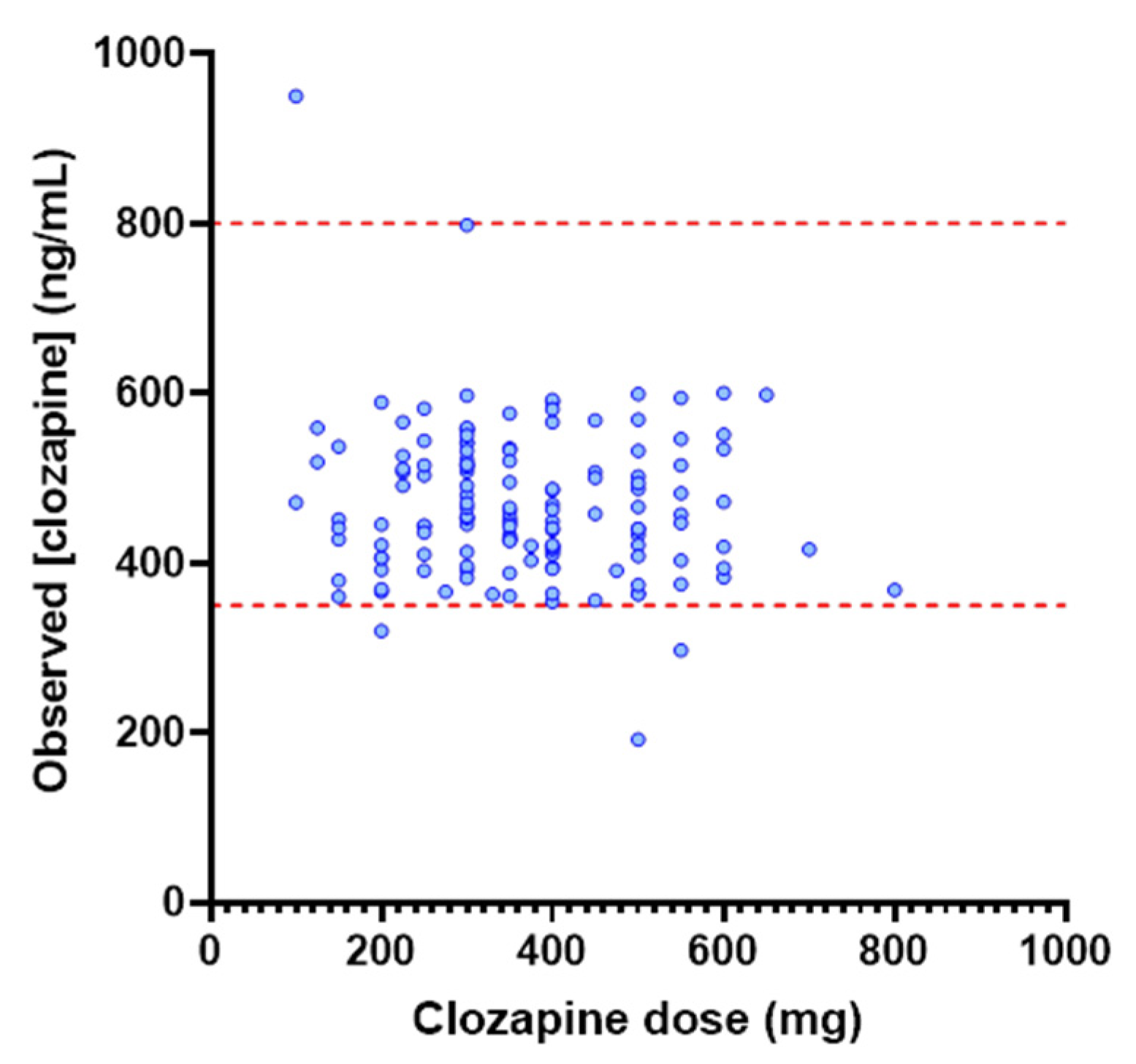
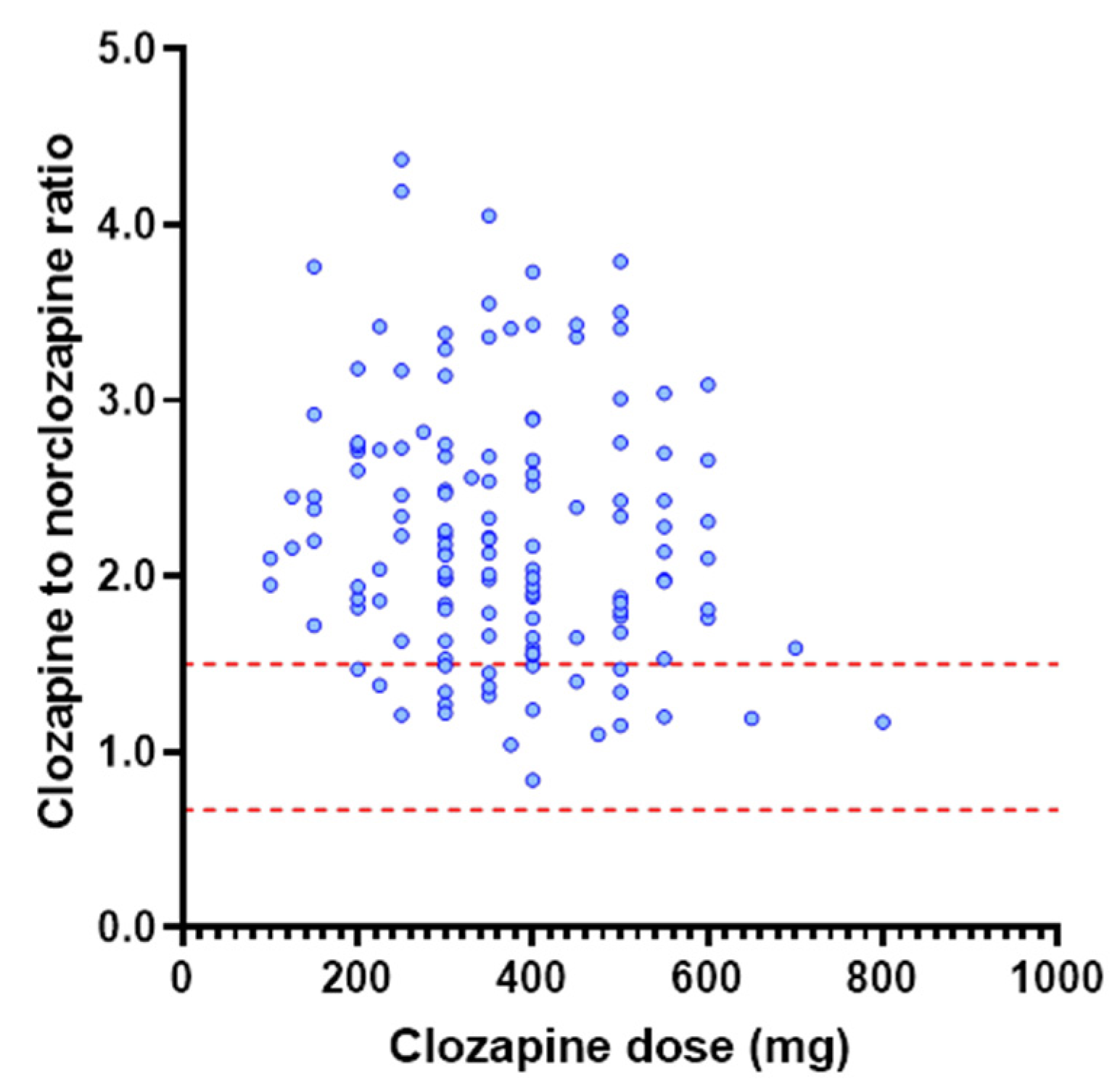
3.2. Investigation of Dose as a Determinant of Clozapine Exposure
3.3. Investigation of Physiological Covariates Influencing Clozapine Exposure
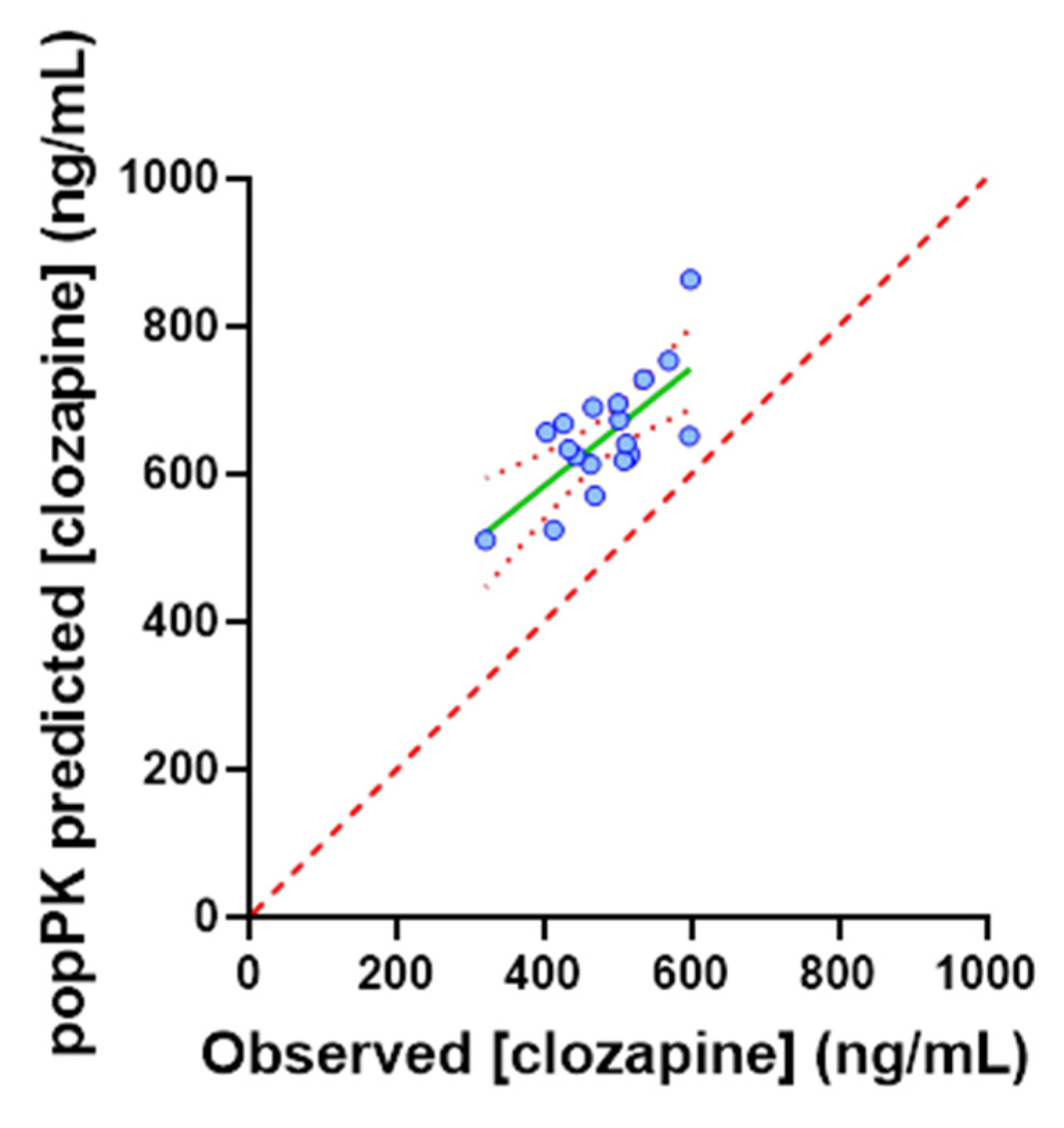
3.4. Application of the popPK Model to a TDM Population
3.5. Post Hoc Analyses
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Samara, M.T.; Dold, M.; Gianatsi, M.; Nikolakopoulou, A.; Helfer, B.; Salanti, G.; Leucht, S. Efficacy, Acceptability, and Tolerability of Antipsychotics in Treatment-Resistant Schizophrenia: A Network Meta-analysis. JAMA Psychiatry 2016, 73, 199–210. [Google Scholar] [CrossRef] [Green Version]
- Siskind, D.; McCartney, L.; Goldschlager, R.; Kisely, S. Clozapine v. first- and second-generation antipsychotics in treatment-refractory schizophrenia: Systematic review and meta-analysis. Br. J. Psychiatry 2016, 209, 385–392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forrester, T.; Siskind, D.; Winckel, K.; Wheeler, A.; Hollingworth, S. Increasing Clozapine Dispensing Trends in Queensland, Australia 2004–2013. Pharmacopsychiatry 2015, 48, 164–169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farooq, S.; Choudry, A.; Cohen, D.; Naeem, F.; Ayub, M. Barriers to using clozapine in treatment-resistant schizophrenia: Systematic review. BJPsych Bull. 2018, 43, 8–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farooq, S.; Taylor, M. Clozapine: Dangerous orphan or neglected friend? Br. J. Psychiatry 2011, 198, 247–249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Howes, O.D.; Vergunst, F.; Gee, S.; McGuire, P.; Kapur, S.; Taylor, D. Adherence to treatment guidelines in clinical practice: Study of antipsychotic treatment prior to clozapine initiation. Br. J. Psychiatry 2012, 201, 481–485. [Google Scholar] [CrossRef] [Green Version]
- Mueller-Schoell, A.; Groenland, S.L.; Scherf-Clavel, O.; van Dyk, M.; Huisinga, W.; Michelet, R.; Jaehde, U.; Steeghs, N.; Huitema, A.D.; Kloft, C. Therapeutic drug monitoring of oral targeted antineoplastic drugs. Eur. J. Clin. Pharmacol. 2020, 77, 441–464. [Google Scholar] [CrossRef]
- Olsson, E.; Edman, G.; Bertilsson, L.; Hukic, D.S.; Lavebratt, C.; Eriksson, S.V.; Ösby, U. Genetic and Clinical Factors Affecting Plasma Clozapine Concentration. Prim. Care Companion CNS Disord. 2015, 17. [Google Scholar] [CrossRef] [Green Version]
- Olesen, O.V. Therapeutic Drug Monitoring of Clozapine Treatment. Clin. Pharmacokinet. 1998, 34, 497–502. [Google Scholar] [CrossRef]
- Stark, A.; Scott, J. A review of the use of clozapine levels to guide treatment and determine cause of death. Aust. N. Z. J. Psychiatry 2012, 46, 816–825. [Google Scholar] [CrossRef]
- Khan, A.Y.; Preskorn, S.H. Examining Concentration-Dependent Toxicity of Clozapine: Role of Therapeutic Drug Monitoring. J. Psychiatr. Pract. 2005, 11, 289–301. [Google Scholar] [CrossRef] [PubMed]
- Gaertner, I.; Gaertner, H.J.; Vonthein, R.; Dietz, K. Therapeutic Drug Monitoring of Clozapine in Relapse Prevention: A Five-Year Prospective Study. J. Clin. Psychopharmacol. 2001, 21, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Couchman, L.; Morgan, P.E.; Spencer, E.P.; Flanagan, R.J. Plasma Clozapine, Norclozapine, and the Clozapine:Norclozapine Ratio in Relation to Prescribed Dose and Other Factors: Data From a Therapeutic Drug Monitoring Service, 1993–2007. Ther. Drug Monit. 2010, 32, 438–447. [Google Scholar] [CrossRef] [PubMed]
- Green, K. Clozapine Plasma Level Monitoring Guide; Kingsley Green Pharmacy and Medicines Optimisation Team, Ed.; NHS Foundation Trust: Worthing, UK, 2019. [Google Scholar]
- Schoretsanitis, G.; Kane, J.M.; Ruan, C.-J.; Spina, E.; Hiemke, C.; De Leon, J. A comprehensive review of the clinical utility of and a combined analysis of the clozapine/norclozapine ratio in therapeutic drug monitoring for adult patients. Expert Rev. Clin. Pharmacol. 2019, 12, 603–621. [Google Scholar] [CrossRef] [PubMed]
- Polasek, T.M.; Tucker, G.T.; Sorich, M.J.; Wiese, M.D.; Mohan, T.; Rostami-Hodjegan, A.; Korprasertthaworn, P.; Perera, V.; Rowland, A. Prediction of olanzapine exposure in individual patients using PBPK modelling and simulation. Br. J. Clin. Pharmacol. 2018, 84, 462–476. [Google Scholar] [CrossRef] [Green Version]
- Van Dyk, M.; Rowland, A. PBPK modeling as an approach to evaluate the effect of covariates and drug-drug interactions on variability in EGFR kinase inhibitor exposure. Transl. Cancer Res. 2017, 6, S1600–S1615. [Google Scholar] [CrossRef]
- Rowland, A.; Van Dyk, M.; Hopkins, A.M.; Mounzer, R.; Polasek, T.M.; Rostami-Hodjegan, A.; Sorich, M.J. Physiologically Based Pharmacokinetic Modeling to Identify Physiological and Molecular Characteristics Driving Variability in Drug Exposure. Clin. Pharmacol. Ther. 2018, 104, 1219–1228. [Google Scholar] [CrossRef]
- Sorich, M.; Mulflib, F.; van Dyk, M.; Hopkins, A.; Polasek, T.; Marshall, J. Use of predictive analytics to identify physiological and molecular characteristics driving variability in axitinib exposure: A fresh approach to precision dosing in oncology. JCO Precis Oncol. 2018. under review. [Google Scholar]
- Mennickent, S.; Sobarzo, A.; Vega, M.; De Diego, M.; Godoy, G.; Rioseco, P.; Saavedra, L. Determination of clozapine in serum of patients with schizophrenia as a measurement of medication compliance. Int. J. Psychiatry Clin. Pract. 2010, 14, 41–46. [Google Scholar] [CrossRef]
- Bersani, F.S.; Capra, E.; Minichino, A.; Pannese, R.; Girardi, N.; Marini, I.; Chiaie, R.D.; Biondi, M. Factors affecting interindividual differences in clozapine response: A review and case report. Hum. Psychopharmacol. Clin. Exp. 2011, 26, 177–187. [Google Scholar] [CrossRef]
- Rostami-Hodjegan, A.; Amin, A.M.; Spencer, E.P.; Lennard, M.S.; Tucker, G.T.; Flanagan, R.J. Influence of dose, cigarette smoking, age, sex, and metabolic activity on plasma clozapine concentrations: A predictive model and nomograms to aid clozapine dose adjustment and to assess compliance in individual patients. J. Clin. Psychopharmacol. 2004, 24, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Darwich, A.; Ogungbenro, K.; Vinks, A.; Powell, J.R.; Reny, J.-L.; Marsousi, N.; Daali, Y.; Fairman, D.; Cook, J.; Lesko, L.J.; et al. Why Has Model-Informed Precision Dosing Not Yet Become Common Clinical Reality? Lessons From the Past and a Roadmap for the Future. Clin. Pharmacol. Ther. 2017, 101, 646–656. [Google Scholar] [CrossRef] [PubMed]
- Darwich, A.S.; Ogungbenro, K.; Hatley, O.J.; Rostami-Hodjegan, A. Role of pharmacokinetic modeling and simulation in precision dosing of anticancer drugs. Transl. Cancer Res. 2017, 6, S1512–S1529. [Google Scholar] [CrossRef] [Green Version]
- Rowland, A.; Sorich, M.J. Preface to Precision Dosing of Targeted Anti-Cancer Drugs. Transl. Cancer Res. 2017, 6, S1498–S1499. [Google Scholar] [CrossRef]
- Jamei, M.; Marciniak, S.; Feng, K.; Barnett, A.; Tucker, G.; Rostami-Hodjegan, A. The Simcyp® Population-based ADME Simulator. Expert Opin. Drug Metab. Toxicol. 2009, 5, 211–223. [Google Scholar] [CrossRef] [PubMed]
- Yeo, K.R.; Jamei, M.; Yang, J.; Tucker, G.T.; Rostami-Hodjegan, A. Physiologically based mechanistic modelling to predict complex drug–drug interactions involving simultaneous competitive and time-dependent enzyme inhibition by parent compound and its metabolite in both liver and gut—The effect of diltiazem on the time-course of exposure to triazolam. Eur. J. Pharm. Sci. 2010, 39, 298–309. [Google Scholar] [CrossRef]
- Howgate, E.M.; Rowland Yeo, K.; Proctor, N.J.; Tucker, G.T.; Rostami-Hodjegan, A. Prediction of in vivo drug clearance from in vitro data. I: Impact of inter-individual variability. Xenobiotica 2006, 36, 473–497. [Google Scholar] [CrossRef]
- Polasek, T.M.; Polak, S.; Doogue, M.P.; Rostami-Hodjegan, A.; Miners, J.O. Assessment of inter-individual variability in predicted phenytoin clearance. Eur. J. Clin. Pharmacol. 2009, 65, 1203–1210. [Google Scholar] [CrossRef]
- Wattanachai, N.; Polasek, T.M.; Heath, T.M.; Uchaipichat, V.; Tassaneeyakul, W.; Tassaneeyakul, W.; Miners, J.O. In vitro–in vivo extrapolation of CYP2C8-catalyzed paclitaxel 6α-hydroxylation: Effects of albumin on in vitro kinetic parameters and assessment of interindividual variability in predicted clearance. Eur. J. Clin. Pharmacol. 2011, 67, 815–824. [Google Scholar] [CrossRef]
- Chang, W.H.; Lin, S.K.; Lane, H.Y.; Hu, W.H.; Jann, M.W.; Lin, H.N. Clozapine dosages and plasma drug concentrations. J. Formos. Med Assoc. 1997, 96, 599–605. [Google Scholar]
- Kuzin, M.; Haen, E.; Hiemke, C.; Bochon, B.; Bochon, K.; Gründer, G.; Paulzen, M.; Schoretsanitis, G. Body mass index as a determinant of clozapine plasma concentrations: A pharmacokinetic-based hypothesis. J. Psychopharmacol. 2021, 35, 273–278. [Google Scholar] [CrossRef]
- Cheymol, G. Effects of Obesity on Pharmacokinetics. Clin. Pharmacokinet. 2000, 39, 215–231. [Google Scholar] [CrossRef] [PubMed]
- Morrish, G.A.; Pai, M.P.; Green, B. The effects of obesity on drug pharmacokinetics in humans. Expert Opin. Drug Metab. Toxicol. 2011, 7, 697–706. [Google Scholar] [CrossRef] [PubMed]
- Dayabandara, M.; Hanwella, R.; Ratnatunage, S.; Seneviratne, S.; Suraweera, C.; de Silva, V.A. Antipsychotic-associated weight gain: Management strategies and impact on treatment adherence. Neuropsychiatr. Dis. Treat. 2017, 13, 2231–2241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Theisen, F.M.; Cichon, S.; Linden, A.; Martin, M.; Remschmidt, H.; Hebebrand, J. Clozapine and Weight Gain. Am. J. Psychiatry 2001, 158, 816. [Google Scholar] [CrossRef]
- Maixner, S.M.; Mellow, A.M.; Tandon, R. The efficacy, safety, and tolerability of antipsychotics in the elderly. J. Clin. Psychiatry 1999, 60. [Google Scholar]
- Albitar, O.; Harun, S.N.; Zainal, H.; Ibrahim, B.; Ghadzi, S.M.S. Population Pharmacokinetics of Clozapine: A Systematic Review. BioMed Res. Int. 2020, 2020, 9872936. [Google Scholar] [CrossRef]
- Winklbaur, B.; Ebner, N.; Sachs, G.; Thau, K.; Fischer, G. Substance abuse in patients with schizophrenia. Dialog-Clin. Neurosci. 2006, 8, 37–43. [Google Scholar]
- Polasek, T.M.; Rayner, C.R.; Peck, R.W.; Rowland, A.; Kimko, H.; Rostami-Hodjegan, A. Toward Dynamic Prescribing Information: Codevelopment of Companion Model-Informed Precision Dosing Tools in Drug Development. Clin. Pharmacol. Drug Dev. 2018, 8, 418–425. [Google Scholar] [CrossRef]
- Rodrigues, A.; Rowland, A. From Endogenous Compounds as Biomarkers to Plasma-Derived Nanovesicles as Liquid Biopsy; Has the Golden Age of Translational PK-ADME-DDI Science Finally Arrived? Clin. Pharmacol. Ther. 2019, 105, 1407–1420. [Google Scholar] [CrossRef]
- Kluwe, F.; Michelet, R.; Mueller-Schoell, A.; Maier, C.; Klopp-Schulze, L.; Van Dyk, M.; Mikus, G.; Huisinga, W.; Kloft, C. Perspectives on Model-Informed Precision Dosing in the Digital Health Era: Challenges, Opportunities, and Recommendations. Clin. Pharmacol. Ther. 2021, 109, 29–36. [Google Scholar] [CrossRef] [PubMed]




| Full | 200 mg | 300 mg | 400 mg | 500 mg | 600 mg | Sig. | |
|---|---|---|---|---|---|---|---|
| Subjects (n) | 142 | 9 | 26 | 20 | 16 | 7 | |
| Dose (mg) | 366 (100–800) | 200 | 300 | 400 | 500 | 600 | |
| Sex (% female) | 27 | 44 | 27 | 10 | 56 | 0 | |
| Age (years) | 42 (21–69) | (46(21–63) | 38 (21–59) | 41 (27–60) | 38 (28–63) | 40 (28–63) | 0.29 |
| Weight (kg) | 95 (37–176) | 94 (65–109) | 88 (59–162) | 99 (60–146) | 91 (68–120) | 102 (82–119) | 0.46 |
| BMI (kg/m2) | 31(18–48) | 32 (23–37) | 29 (18–48) | 30 (18–42) | 31 (19–45) | 32 (25–37) | 0.65 |
| Smoker (%) | 65 | 44 | 62 | 90 | 75 | 86 |
| Population | 200 mg | 300 mg | 400 mg | 500 mg | 600 mg | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | 95% CI | Mean | 95% CI | Mean | 95% CI | Mean | 95% CI | Mean | 95% CI | |
| Clozapine tough concentration (ng/mL) | 413 | 355–471 | 504 | 470–537 | 449 | 418–481 | 443 | 392–494 | 479 | 401–557 |
| Clozapine to norclozapine ratio | 2.34 | 1.90–2.79 | 2.08 | 1.83–2.33 | 2.12 | 1.77–2.46 | 2.22 | 1.77–2.67 | 2.34 | 1.89–2.79 |
| % Therapeutic | 89 | 100 | 100 | 94 | 100 | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wills, K.H.; Behan, S.J.; Nance, M.J.; Dawson, J.L.; Polasek, T.M.; Hopkins, A.M.; van Dyk, M.; Rowland, A. Combining Therapeutic Drug Monitoring and Pharmacokinetic Modelling Deconvolutes Physiological and Environmental Sources of Variability in Clozapine Exposure. Pharmaceutics 2022, 14, 47. https://doi.org/10.3390/pharmaceutics14010047
Wills KH, Behan SJ, Nance MJ, Dawson JL, Polasek TM, Hopkins AM, van Dyk M, Rowland A. Combining Therapeutic Drug Monitoring and Pharmacokinetic Modelling Deconvolutes Physiological and Environmental Sources of Variability in Clozapine Exposure. Pharmaceutics. 2022; 14(1):47. https://doi.org/10.3390/pharmaceutics14010047
Chicago/Turabian StyleWills, Kenneth H., Stephen J. Behan, Michael J. Nance, Jessica L. Dawson, Thomas M. Polasek, Ashley M. Hopkins, Madelé van Dyk, and Andrew Rowland. 2022. "Combining Therapeutic Drug Monitoring and Pharmacokinetic Modelling Deconvolutes Physiological and Environmental Sources of Variability in Clozapine Exposure" Pharmaceutics 14, no. 1: 47. https://doi.org/10.3390/pharmaceutics14010047
APA StyleWills, K. H., Behan, S. J., Nance, M. J., Dawson, J. L., Polasek, T. M., Hopkins, A. M., van Dyk, M., & Rowland, A. (2022). Combining Therapeutic Drug Monitoring and Pharmacokinetic Modelling Deconvolutes Physiological and Environmental Sources of Variability in Clozapine Exposure. Pharmaceutics, 14(1), 47. https://doi.org/10.3390/pharmaceutics14010047







