From Microenvironment Remediation to Novel Anti-Cancer Strategy: The Emergence of Zero Valent Iron Nanoparticles
Abstract
:1. Introduction
2. Synthesis and Characterization of ZVI NPs
2.1. Synthetic Approaches of ZVI NPs
2.1.1. The Top-Down Synthesis
2.1.2. The Bottom-Up Synthesis
2.2. The Coating and the Storage of ZVI NPs
3. Mechanisms of the Endogenous Anti-Cancer Efficacy
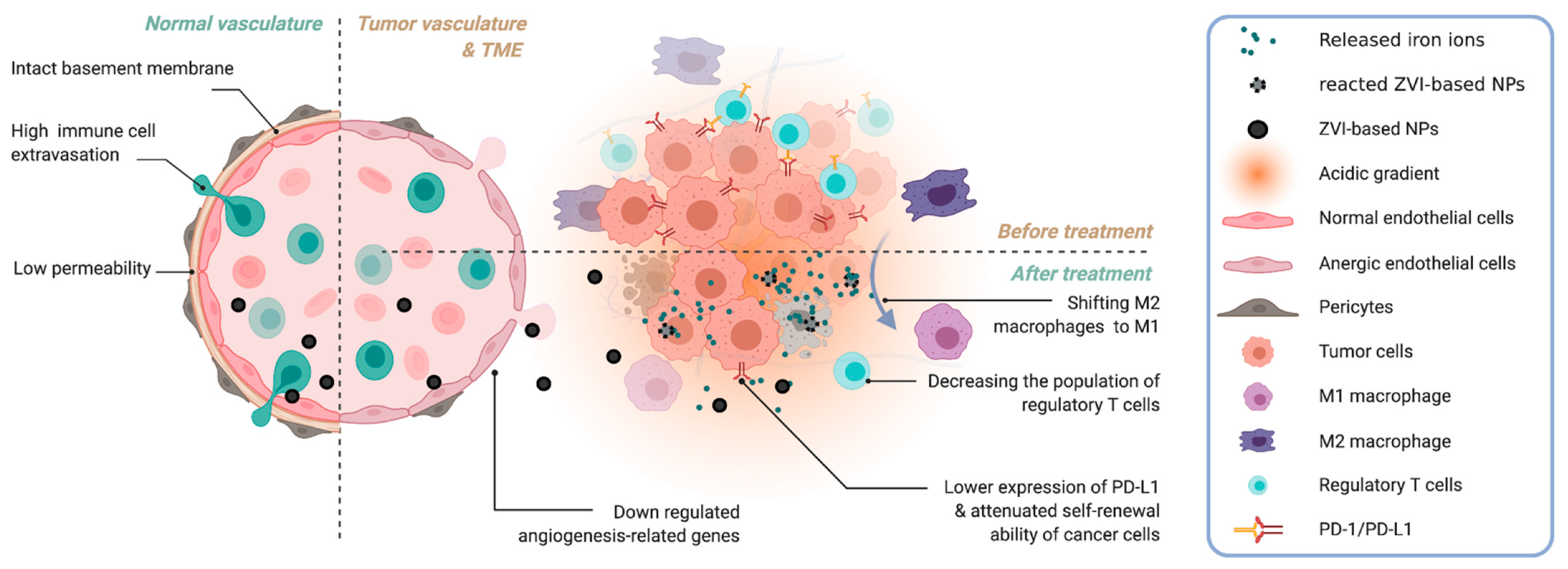
3.1. Tumors and Their Microenvironment
3.2. The Therapeutic Implication of ZVI
3.3. The Underlying Mechanisms
3.3.1. Lysosomes and Acidic TME
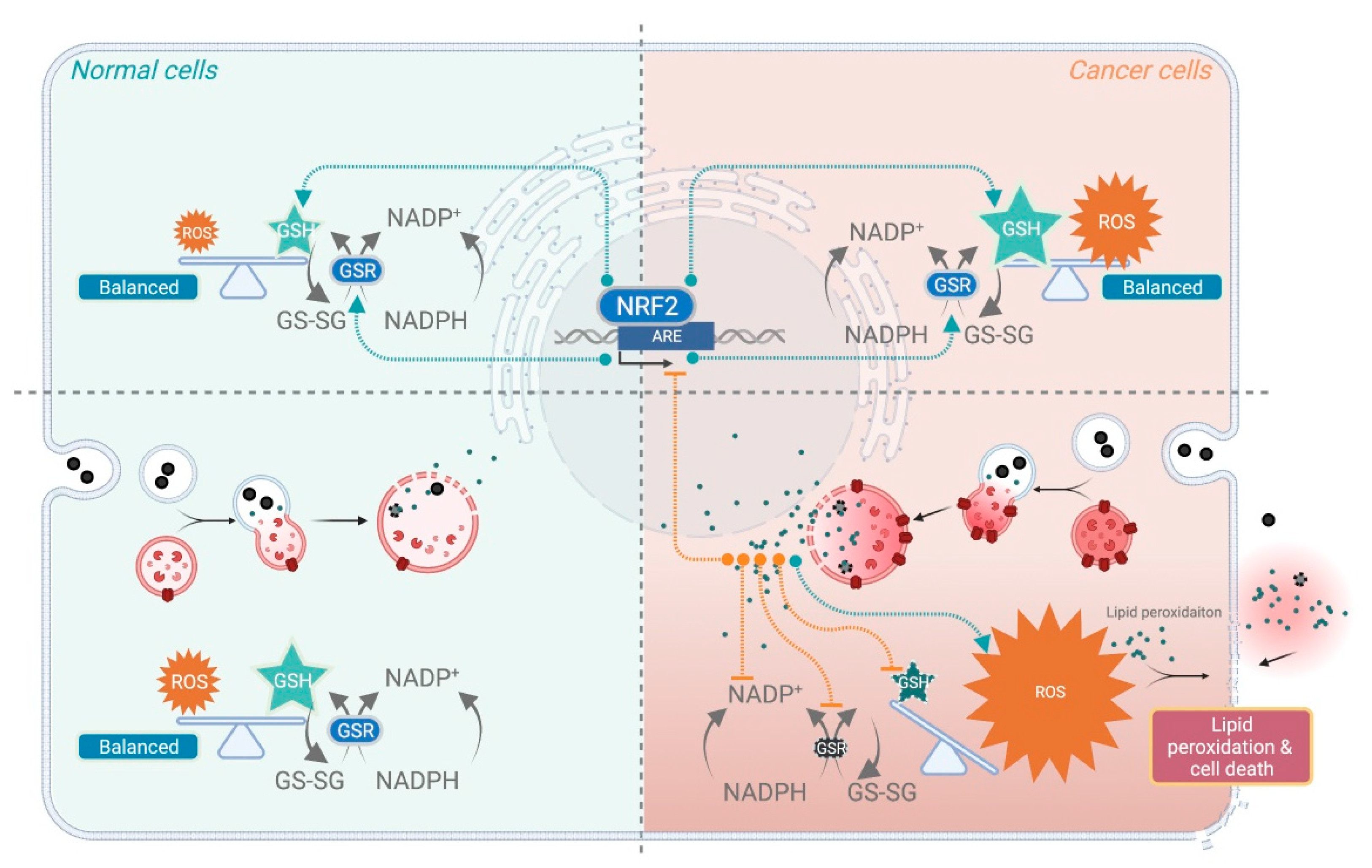
3.3.2. ROS and Lipid Peroxidation
3.3.3. ZVI-Based NPs Induced Programmed Cell Death
3.3.4. Tumor Micro-Environment
3.4. The Opportunity of Precision Medicine in ZVI-Based Nanotherapy
4. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Greenblatt, K.; Khaddour, K. Trastuzumab; StatPearls: Treasure Island, FL, USA, 2021. [Google Scholar]
- Holloway, R.W.; Marignani, P.A. Targeting mTOR and Glycolysis in HER2-Positive Breast Cancer. Cancers 2021, 13, 2922. [Google Scholar] [CrossRef]
- Brieger, K.; Schiavone, S.; Miller, F.J., Jr.; Krause, K.H. Reactive oxygen species: From health to disease. Swiss Med. Wkly. 2012, 142, w13659. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Berndt, C.; Jones, D.P. Oxidative Stress. Annu. Rev. Biochem. 2017, 86, 715–748. [Google Scholar] [CrossRef]
- Chen, K.; Lu, P.; Beeraka, N.M.; Sukocheva, O.A.; Madhunapantula, S.V.; Liu, J.; Sinelnikov, M.Y.; Nikolenko, V.N.; Bulygin, K.V.; Mikhaleva, L.M.; et al. Mitochondrial mutations and mitoepigenetics: Focus on regulation of oxidative stress-induced responses in breast cancers. Semin. Cancer Biol. 2020; online ahead of print. [Google Scholar] [CrossRef]
- Santos, F.S.d.; Lago, F.R.; Yokoyama, L.; Fonseca, F.V. Synthesis and characterization of zero-valent iron nanoparticles supported on SBA-15. J. Mater. Res. Technol. 2017, 6, 178–183. [Google Scholar] [CrossRef]
- Storz, P. KRas, ROS and the initiation of pancreatic cancer. Small GTPases 2017, 8, 38–42. [Google Scholar] [CrossRef]
- Trachootham, D.; Alexandre, J.; Huang, P. Targeting cancer cells by ROS-mediated mechanisms: A radical therapeutic approach? Nat. Rev. Drug Discov. 2009, 8, 579–591. [Google Scholar] [CrossRef]
- Raza, M.H.; Siraj, S.; Arshad, A.; Waheed, U.; Aldakheel, F.; Alduraywish, S.; Arshad, M. ROS-modulated therapeutic approaches in cancer treatment. J. Cancer Res. Clin. Oncol. 2017, 143, 1789–1809. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, H.; Takada, K. Reactive oxygen species in cancer: Current findings and future directions. Cancer Sci. 2021, 112, 3945–3952. [Google Scholar] [CrossRef] [PubMed]
- Conklin, K.A. Chemotherapy-associated oxidative stress: Impact on chemotherapeutic effectiveness. Integr. Cancer Ther. 2004, 3, 294–300. [Google Scholar] [CrossRef]
- van der Meel, R.; Sulheim, E.; Shi, Y.; Kiessling, F.; Mulder, W.J.M.; Lammers, T. Smart cancer nanomedicine. Nat. Nanotechnol. 2019, 14, 1007–1017. [Google Scholar] [CrossRef]
- Anselmo, A.C.; Mitragotri, S. Nanoparticles in the clinic: An update post COVID-19 vaccines. Bioeng. Transl. Med. 2021, 6, e10246. [Google Scholar] [CrossRef] [PubMed]
- Takano, S.; Islam, W.; Nakazawa, K.; Maeda, H.; Sakurai, K.; Fujii, S. Phosphorylcholine-Grafted Molecular Bottlebrush-Doxorubicin Conjugates: High Structural Stability, Long Circulation in Blood, and Efficient Anticancer Activity. Biomacromolecules 2021, 22, 1186–1196. [Google Scholar] [CrossRef]
- Lin, W.; Ma, G.; Yuan, Z.; Qian, H.; Xu, L.; Sidransky, E.; Chen, S. Development of Zwitterionic Polypeptide Nanoformulation with High Doxorubicin Loading Content for Targeted Drug Delivery. Langmuir 2019, 35, 1273–1283. [Google Scholar] [CrossRef] [PubMed]
- Hare, J.I.; Lammers, T.; Ashford, M.B.; Puri, S.; Storm, G.; Barry, S.T. Challenges and strategies in anti-cancer nanomedicine development: An industry perspective. Adv. Drug Deliv. Rev. 2017, 108, 25–38. [Google Scholar] [CrossRef] [Green Version]
- Wicki, A.; Witzigmann, D.; Balasubramanian, V.; Huwyler, J. Nanomedicine in cancer therapy: Challenges, opportunities, and clinical applications. J. Control. Release 2015, 200, 138–157. [Google Scholar] [CrossRef]
- Villa, I.; Villa, C.; Crapanzano, R.; Secchi, V.; Tawfilas, M.; Trombetta, E.; Porretti, L.; Brambilla, A.; Campione, M.; Torrente, Y.; et al. Functionalized Scintillating Nanotubes for Simultaneous Radio- and Photodynamic Therapy of Cancer. ACS Appl. Mater. Interfaces 2021, 13, 12997–13008. [Google Scholar] [CrossRef] [PubMed]
- Jasim, K.A.; Gesquiere, A.J. Ultrastable and Biofunctionalizable Conjugated Polymer Nanoparticles with Encapsulated Iron for Ferroptosis Assisted Chemodynamic Therapy. Mol. Pharm. 2019, 16, 4852–4866. [Google Scholar] [CrossRef] [PubMed]
- Steinborn, B.; Lachelt, U. Metal-organic Nanopharmaceuticals. Pharm. Nanotechnol. 2020, 8, 163–190. [Google Scholar] [CrossRef]
- Son, S.; Kim, J.H.; Wang, X.; Zhang, C.; Yoon, S.A.; Shin, J.; Sharma, A.; Lee, M.H.; Cheng, L.; Wu, J.; et al. Multifunctional sonosensitizers in sonodynamic cancer therapy. Chem. Soc. Rev. 2020, 49, 3244–3261. [Google Scholar] [CrossRef] [PubMed]
- Hanley, C.; Layne, J.; Punnoose, A.; Reddy, K.M.; Coombs, I.; Coombs, A.; Feris, K.; Wingett, D. Preferential killing of cancer cells and activated human T cells using ZnO nanoparticles. Nanotechnology 2008, 19, 295103. [Google Scholar] [CrossRef] [Green Version]
- Mauro, N.; Utzeri, M.A.; Varvara, P.; Cavallaro, G. Functionalization of Metal and Carbon Nanoparticles with Potential in Cancer Theranostics. Molecules 2021, 26, 3085. [Google Scholar] [CrossRef]
- Monga, Y.; Kumar, P.; Sharma, R.K.; Filip, J.; Varma, R.S.; Zboril, R.; Gawande, M.B. Sustainable Synthesis of Nanoscale Zerovalent Iron Particles for Environmental Remediation. ChemSusChem 2020, 13, 3288–3305. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Wang, X.; Khan, A.; Wang, P.; Liu, Y.; Alsaedi, A.; Hayat, T.; Wang, X. Environmental Remediation and Application of Nanoscale Zero-Valent Iron and Its Composites for the Removal of Heavy Metal Ions: A Review. Environ. Sci. Technol. 2016, 50, 7290–7304. [Google Scholar] [CrossRef]
- Xin, J.; Deng, C.; Aras, O.; Zhou, M.; Wu, C.; An, F. Chemodynamic nanomaterials for cancer theranostics. J. Nanobiotechnol. 2021, 19, 192. [Google Scholar] [CrossRef] [PubMed]
- Du, W.; Liu, T.; Xue, F.; Cai, X.; Chen, Q.; Zheng, Y.; Chen, H. Fe3O4 Mesocrystals with Distinctive Magnetothermal and Nanoenzyme Activity Enabling Self-Reinforcing Synergistic Cancer Therapy. ACS Appl. Mater. Interfaces 2020, 12, 19285–19294. [Google Scholar] [CrossRef]
- Li, S.; Shang, L.; Xu, B.; Wang, S.; Gu, K.; Wu, Q.; Sun, Y.; Zhang, Q.; Yang, H.; Zhang, F.; et al. A Nanozyme with Photo-Enhanced Dual Enzyme-Like Activities for Deep Pancreatic Cancer Therapy. Angew. Chem. Int. Ed. Engl. 2019, 58, 12624–12631. [Google Scholar] [CrossRef]
- Tate, J.A.; Petryk, A.A.; Giustini, A.J.; Hoopes, P.J. In vivo biodistribution of iron oxide nanoparticles: An overview. Proc. SPIE Int. Soc. Opt. Eng. 2011, 7901, 790117. [Google Scholar] [CrossRef] [Green Version]
- Arami, H.; Khandhar, A.; Liggitt, D.; Krishnan, K.M. In vivo delivery, pharmacokinetics, biodistribution and toxicity of iron oxide nanoparticles. Chem. Soc. Rev. 2015, 44, 8576–8607. [Google Scholar] [CrossRef]
- Mulens-Arias, V.; Rojas, J.M.; Barber, D.F. The Use of Iron Oxide Nanoparticles to Reprogram Macrophage Responses and the Immunological Tumor Microenvironment. Front. Immunol. 2021, 12, 693709. [Google Scholar] [CrossRef]
- Adusei-Gyamfi, J.; Acha, V. Carriers for nano zerovalent iron (nZVI): Synthesis, application and efficiency. RSC Adv. 2016, 6, 91025–91044. [Google Scholar] [CrossRef]
- Pasinszki, T.; Krebsz, M. Synthesis and Application of Zero-Valent Iron Nanoparticles in Water Treatment, Environmental Remediation, Catalysis, and Their Biological Effects. Nanomaterials 2020, 10, 917. [Google Scholar] [CrossRef]
- Ling, L.; Pan, B.C.; Zhang, W.X. Removal of selenium from water with nanoscale zero-valent iron: Mechanisms of intraparticle reduction of Se(IV). Water Res. 2015, 71, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.R.; Liu, J.; Pan, B.C.; Zhang, W.X. Formation of lepidocrocite (gamma-FeOOH) from oxidation of nanoscale zero-valent iron (nZVI) in oxygenated water. RSC Adv. 2014, 4, 57377–57382. [Google Scholar] [CrossRef]
- Li, S.L.; Yan, W.L.; Zhang, W.X. Solvent-free production of nanoscale zero-valent iron (nZVI) with precision milling. Green Chem. 2009, 11, 1618–1626. [Google Scholar] [CrossRef]
- Kober, R.; Hollert, H.; Hornbruch, G.; Jekel, M.; Kamptner, A.; Klaas, N.; Maes, H.; Mangold, K.M.; Martac, E.; Matheis, A.; et al. Nanoscale zero-valent iron flakes for groundwater treatment. Environ. Earth Sci. 2014, 72, 3339–3352. [Google Scholar] [CrossRef]
- Gao, J.; Wang, W.; Rondinone, A.J.; He, F.; Liang, L.Y. Degradation of Trichloroethene with a Novel Ball Milled Fe-C Nanocomposite. J. Hazard. Mater. 2015, 300, 443–450. [Google Scholar] [CrossRef] [Green Version]
- Ribas, D.; Peskova, K.; Jubany, I.; Parma, P.; Cernik, M.; Benito, J.A.; Marti, V. High reactive nano zero-valent iron produced via wet milling through abrasion by alumina. Chem. Eng. J. 2019, 366, 235–245. [Google Scholar] [CrossRef]
- Okazoe, S.; Yasaka, Y.; Kudo, M.; Maeno, H.; Murakami, Y.; Kimura, Y. Synthesis of zero-valent iron nanoparticles via laser ablation in a formate ionic liquid under atmospheric conditions. Chem. Commun. 2018, 54, 7834–7837. [Google Scholar] [CrossRef]
- Kuhn, L.T.; Bojesen, A.; Timmermann, L.; Nielsen, M.M.; Morup, S. Structural and magnetic properties of core-shell iron-iron oxide nanoparticles. J. Phys. Condens. Mat. 2002, 14, 13551–13567. [Google Scholar] [CrossRef]
- Wu, Y.N.; Chen, D.H.; Shi, X.Y.; Lian, C.C.; Wang, T.Y.; Yeh, C.S.; Ratinac, K.R.; Thordarson, P.; Braet, F.; Shieh, D.B. Cancer-cell-specific cytotoxicity of non-oxidized iron elements in iron core-gold shell NPs. Nanomedicine 2011, 7, 420–427. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Zhou, W.L.; Kumbhar, A.; Wiemann, J.; Fang, J.Y.; Carpenter, E.E.; O’Connor, C.J. Gold-coated iron (Fe@Au) nanoparticles: Synthesis, characterization, and magnetic field-induced self-assembly. J. Solid State Chem. 2001, 159, 26–31. [Google Scholar] [CrossRef] [Green Version]
- Barreto-Rodrigues, M.; Silveira, J.; Zazo, J.A.; Rodriguez, J.J. Synthesis, characterization and application of nanoscale zero-valent iron in the degradation of the azo dye Disperse Red 1. J. Environ. Chem. Eng. 2017, 5, 628–634. [Google Scholar] [CrossRef]
- Han, Y.L.; Yang, M.D.Y.; Zhang, W.X.; Yan, W.L. Optimizing synthesis conditions of nanoscale zero-valent iron (nZVI) through aqueous reactivity assessment. Front. Environ. Sci. Eng. 2015, 9, 813–822. [Google Scholar] [CrossRef]
- Jamei, M.R.; Khosravi, M.R.; Anvaripour, B. A novel ultrasound assisted method in synthesis of NZVI particles. Ultrason. Sonochem. 2014, 21, 226–233. [Google Scholar] [CrossRef]
- Kamali, M.; Costa, M.E.V.; Otero-Irurueta, G.; Capela, I. Ultrasonic irradiation as a green production route for coupling crystallinity and high specific surface area in iron nanomaterials. J. Clean Prod. 2019, 211, 185–197. [Google Scholar] [CrossRef]
- Jamei, M.R.; Khosravi, M.R.; Anvaripour, B. Investigation of ultrasonic effect on synthesis of nano zero valent iron particles and comparison with conventional method. Asia Pac. J. Chem. Eng. 2013, 8, 767–774. [Google Scholar] [CrossRef]
- Liu, T.; Wang, Z.-L.; Yan, X.; Zhang, B. Removal of mercury (II) and chromium (VI) from wastewater using a new and effective composite: Pumice-supported nanoscale zero-valent iron. Chem. Eng. J. 2014, 245, 34–40. [Google Scholar] [CrossRef]
- Hu, Y.-b.; Li, X.-y. Influence of a thin aluminum hydroxide coating layer on the suspension stability and reductive reactivity of nanoscale zero-valent iron. Appl. Catal. B Environ. 2018, 226, 554–564. [Google Scholar] [CrossRef]
- Dongsheng, Z.; Wenqiang, G.; Guozhang, C.; Shuai, L.; Weizhou, J.; Youzhi, L. Removal of heavy metal lead(II) using nanoscale zero-valent iron with different preservation methods. Adv. Powder Technol. 2019, 30, 581–589. [Google Scholar] [CrossRef]
- Parimala, L.; Santhanalakshmi, J. Studies on the Iron Nanoparticles Catalyzed Reduction of Substituted Aromatic Ketones to Alcohols. J. Nanoparticles 2014, 2014, 156868. [Google Scholar] [CrossRef] [Green Version]
- Xie, Y.; Cwiertny, D.M. Use of dithionite to extend the reactive lifetime of nanoscale zero-valent iron treatment systems. Environ. Sci. Technol. 2010, 44, 8649–8655. [Google Scholar] [CrossRef] [PubMed]
- Hoag, G.E.; Collins, J.B.; Holcomb, J.L.; Hoag, J.R.; Nadagouda, M.N.; Varma, R.S. Degradation of bromothymol blue by ‘greener’ nano-scale zero-valent iron synthesized using tea polyphenols. J. Mater. Chem. 2009, 19, 8671–8677. [Google Scholar] [CrossRef]
- Machado, S.; Pacheco, J.G.; Nouws, H.P.A.; Albergaria, J.T.; Delerue-Matos, C. Characterization of green zero-valent iron nanoparticles produced with tree leaf extracts. Sci. Total. Environ. 2015, 533, 76–81. [Google Scholar] [CrossRef] [Green Version]
- Kupka, D.; Lovás, M.; Šepelák, V. Deferrization of Kaolinic Sand by Iron Oxidizing and Iron Reducing Bacteria. Adv. Mater. Res. 2007, 20–21, 130–133. [Google Scholar] [CrossRef]
- Durán, N.; Seabra, A.B. Metallic oxide nanoparticles: State of the art in biogenic syntheses and their mechanisms. Appl. Microbiol. Biotechnol. 2012, 95, 275–288. [Google Scholar] [CrossRef] [PubMed]
- Mehrotra, N.; Tripathi, R.M.; Zafar, F.; Singh, M.P. Catalytic Degradation of Dichlorvos Using Biosynthesized Zero Valent Iron Nanoparticles. IEEE Trans. NanoBiosci. 2017, 16, 280–286. [Google Scholar] [CrossRef]
- Lacroix, L.M.; Huls, N.F.; Ho, D.; Sun, X.; Cheng, K.; Sun, S. Stable single-crystalline body centered cubic Fe nanoparticles. Nano Lett. 2011, 11, 1641–1645. [Google Scholar] [CrossRef] [PubMed]
- Suslick, K.S.; Choe, S.-B.; Cichowlas, A.A.; Grinstaff, M.W. Sonochemical synthesis of amorphous iron. Nature 1991, 353, 414–416. [Google Scholar] [CrossRef]
- Hoch, L.B.; Mack, E.J.; Hydutsky, B.W.; Hershman, J.M.; Skluzacek, J.M.; Mallouk, T.E. Carbothermal synthesis of carbon-supported nanoscale zero-valent iron particles for the remediation of hexavalent chromium. Environ. Sci. Technol. 2008, 42, 2600–2605. [Google Scholar] [CrossRef]
- Glasgow, W.; Fellows, B.; Qi, B.; Darroudi, T.; Kitchens, C.; Ye, L.; Crawford, T.M.; Mefford, O.T. Continuous synthesis of iron oxide (Fe3O4) nanoparticles via thermal decomposition. Particuology 2016, 26, 47–53. [Google Scholar] [CrossRef] [Green Version]
- Xia, Q.; Jiang, Z.; Wang, J.; Yao, Z. A facile preparation of hierarchical dendritic zero-valent iron for Fenton-like degradation of phenol. Catal. Commun. 2017, 100, 57–61. [Google Scholar] [CrossRef]
- Chen, S.-S.; Hsu, H.-D.; Li, C.-W. A new method to produce nanoscale iron for nitrate removal. J. Nanoparticle Res. 2004, 6, 639–647. [Google Scholar] [CrossRef]
- Wu, Y.N.; Shieh, D.B.; Yang, L.X.; Sheu, H.S.; Zheng, R.Z.; Thordarson, P.; Chen, D.H.; Braet, F. Characterization of Iron Core–Gold Shell Nanoparticles for Anti-Cancer Treatments: Chemical and Structural Transformations During Storage and Use. Materials 2018, 11, 2572. [Google Scholar] [CrossRef] [Green Version]
- Liang, H.; Guo, J.; Shi, Y.; Zhao, G.; Sun, S.; Sun, X. Porous yolk-shell Fe/Fe3O4 nanoparticles with controlled exposure of highly active Fe(0) for cancer therapy. Biomaterials 2021, 268, 120530. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.X.; Wu, Y.N.; Wang, P.W.; Huang, K.J.; Su, W.C.; Shieh, D.B. Silver-coated zero-valent iron nanoparticles enhance cancer therapy in mice through lysosome-dependent dual programed cell death pathways: Triggering simultaneous apoptosis and autophagy only in cancerous cells. J. Mater. Chem. B 2020, 8, 4122–4131. [Google Scholar] [CrossRef]
- Yang, L.X.; Wu, Y.N.; Wang, P.W.; Su, W.C.; Shieh, D.B. Iron Release Profile of Silica-Modified Zero-Valent Iron NPs and Their Implication in Cancer Therapy. Int. J. Mol. Sci. 2019, 20, 4336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shevtsov, M.A.; Parr, M.A.; Ryzhov, V.A.; Zemtsova, E.G.; Arbenin, A.Y.; Ponomareva, A.N.; Smirnov, V.M.; Multhoff, G. Zero-valent Fe confined mesoporous silica nanocarriers (Fe(0) @ MCM-41) for targeting experimental orthotopic glioma in rats. Sci. Rep. 2016, 6, 29247. [Google Scholar] [CrossRef]
- Wu, Y.N.; Yang, L.X.; Shi, X.Y.; Li, I.C.; Biazik, J.M.; Ratinac, K.R.; Chen, D.H.; Thordarson, P.; Shieh, D.B.; Braet, F. The selective growth inhibition of oral cancer by iron core-gold shell nanoparticles through mitochondria-mediated autophagy. Biomaterials 2011, 32, 4565–4573. [Google Scholar] [CrossRef]
- Hsieh, C.H.; Hsieh, H.C.; Shih, F.S.; Wang, P.W.; Yang, L.X.; Shieh, D.B.; Wang, Y.C. An innovative NRF2 nano-modulator induces lung cancer ferroptosis and elicits an immunostimulatory tumor microenvironment. Theranostics 2021, 11, 7072–7091. [Google Scholar] [CrossRef]
- Hashemi, Z.; Ebrahimzadeh, M.A.; Biparva, P.; Mortazavi-Derazkola, S.; Goli, H.R.; Sadeghian, F.; Kardan, M.; Rafiei, A. Biogenic Silver and Zero-Valent Iron Nanoparticles by Feijoa: Biosynthesis, Characterization, Cytotoxic, Antibacterial and Antioxidant Activities. Anticancer. Agents Med. Chem. 2020, 20, 1673–1687. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.J.; Wei, Y.H.; Chiu, Y.C.; Wu, S.R.; Shieh, D.B. Assessment of zero-valent iron-based nanotherapeutics for ferroptosis induction and resensitization strategy in cancer cells. Biomater. Sci. 2019, 7, 1311–1322. [Google Scholar] [CrossRef]
- FDA; Center for Drug Evaluation and Research. Inactive Ingredient Search for Approved Drug Products. Available online: https://www.accessdata.fda.gov/scripts/cder/iig/index.cfm?event=browseByLetter.page&Letter=C (accessed on 22 December 2021).
- Anbouhi, T.S.; Esfidvajani, E.M.; Nemati, F.; Haghighat, S.; Sari, S.; Attar, F.; Pakaghideh, A.; Sohrabi, M.J.; Mousavi, S.E.; Falahati, M. Albumin binding, anticancer and antibacterial properties of synthesized zero valent iron nanoparticles. Int. J. Nanomed. 2019, 14, 243–256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, C.; Bu, W.; Ni, D.; Zhang, S.; Li, Q.; Yao, Z.; Zhang, J.; Yao, H.; Wang, Z.; Shi, J. Synthesis of Iron Nanometallic Glasses and Their Application in Cancer Therapy by a Localized Fenton Reaction. Angew. Chem. Int. Ed. Engl. 2016, 55, 2101–2106. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [Green Version]
- Toyokuni, S.; Ito, F.; Yamashita, K.; Okazaki, Y.; Akatsuka, S. Iron and thiol redox signaling in cancer: An exquisite balance to escape ferroptosis. Free Radic. Biol. Med. 2017, 108, 610–626. [Google Scholar] [CrossRef]
- Toyokuni, S.; Okamoto, K.; Yodoi, J.; Hiai, H. Persistent oxidative stress in cancer. FEBS Lett. 1995, 358, 1–3. [Google Scholar] [CrossRef] [Green Version]
- Harris, I.S.; Treloar, A.E.; Inoue, S.; Sasaki, M.; Gorrini, C.; Lee, K.C.; Yung, K.Y.; Brenner, D.; Knobbe-Thomsen, C.B.; Cox, M.A.; et al. Glutathione and thioredoxin antioxidant pathways synergize to drive cancer initiation and progression. Cancer Cell 2015, 27, 211–222. [Google Scholar] [CrossRef] [Green Version]
- Benhar, M.; Shytaj, I.L.; Stamler, J.S.; Savarino, A. Dual targeting of the thioredoxin and glutathione systems in cancer and HIV. J. Clin. Investig. 2016, 126, 1630–1639. [Google Scholar] [CrossRef] [Green Version]
- Surnar, B.; Sharma, K.; Jayakannan, M. Core-shell polymer nanoparticles for prevention of GSH drug detoxification and cisplatin delivery to breast cancer cells. Nanoscale 2015, 7, 17964–17979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tuy, K.; Rickenbacker, L.; Hjelmeland, A.B. Reactive oxygen species produced by altered tumor metabolism impacts cancer stem cell maintenance. Redox Biol. 2021, 44, 101953. [Google Scholar] [CrossRef] [PubMed]
- Sporn, M.B.; Liby, K.T. NRF2 and cancer: The good, the bad and the importance of context. Nat. Rev. Cancer 2012, 12, 564–571. [Google Scholar] [CrossRef]
- Wang, X.J.; Sun, Z.; Villeneuve, N.F.; Zhang, S.; Zhao, F.; Li, Y.; Chen, W.; Yi, X.; Zheng, W.; Wondrak, G.T.; et al. Nrf2 enhances resistance of cancer cells to chemotherapeutic drugs, the dark side of Nrf2. Carcinogenesis 2008, 29, 1235–1243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hughes, R.; Qian, B.Z.; Rowan, C.; Muthana, M.; Keklikoglou, I.; Olson, O.C.; Tazzyman, S.; Danson, S.; Addison, C.; Clemons, M.; et al. Perivascular M2 Macrophages Stimulate Tumor Relapse after Chemotherapy. Cancer Res. 2015, 75, 3479–3491. [Google Scholar] [CrossRef] [Green Version]
- Dong, D.; Zhang, G.; Yang, J.; Zhao, B.; Wang, S.; Wang, L.; Zhang, G.; Shang, P. The role of iron metabolism in cancer therapy focusing on tumor-associated macrophages. J. Cell Physiol. 2019, 234, 8028–8039. [Google Scholar] [CrossRef]
- Recalcati, S.; Locati, M.; Gammella, E.; Invernizzi, P.; Cairo, G. Iron levels in polarized macrophages: Regulation of immunity and autoimmunity. Autoimmun. Rev. 2012, 11, 883–889. [Google Scholar] [CrossRef]
- Laskar, A.; Eilertsen, J.; Li, W.; Yuan, X.M. SPION primes THP1 derived M2 macrophages towards M1-like macrophages. Biochem. Biophys. Res. Commun. 2013, 441, 737–742. [Google Scholar] [CrossRef]
- Zanganeh, S.; Hutter, G.; Spitler, R.; Lenkov, O.; Mahmoudi, M.; Shaw, A.; Pajarinen, J.S.; Nejadnik, H.; Goodman, S.; Moseley, M.; et al. Iron oxide nanoparticles inhibit tumour growth by inducing pro-inflammatory macrophage polarization in tumour tissues. Nat. Nanotechnol. 2016, 11, 986–994. [Google Scholar] [CrossRef] [PubMed]
- Galdames, A.; Ruiz-Rubio, L.; Orueta, M.; Sanchez-Arzalluz, M.; Vilas-Vilela, J.L. Zero-Valent Iron Nanoparticles for Soil and Groundwater Remediation. Int. J. Environ. Res. Public Health 2020, 17, 5817. [Google Scholar] [CrossRef]
- Rizzollo, F.; More, S.; Vangheluwe, P.; Agostinis, P. The lysosome as a master regulator of iron metabolism. Trends Biochem. Sci. 2021; online ahead of print. [Google Scholar] [CrossRef]
- Gotink, K.J.; Broxterman, H.J.; Labots, M.; de Haas, R.R.; Dekker, H.; Honeywell, R.J.; Rudek, M.A.; Beerepoot, L.V.; Musters, R.J.; Jansen, G.; et al. Lysosomal sequestration of sunitinib: A novel mechanism of drug resistance. Clin. Cancer Res. 2011, 17, 7337–7346. [Google Scholar] [CrossRef] [Green Version]
- Zheng, T.; Jaattela, M.; Liu, B. pH gradient reversal fuels cancer progression. Int J. Biochem. Cell Biol. 2020, 125, 105796. [Google Scholar] [CrossRef]
- Piao, S.; Amaravadi, R.K. Targeting the lysosome in cancer. Ann. N. Y. Acad. Sci. 2016, 1371, 45–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stransky, L.; Cotter, K.; Forgac, M. The Function of V-ATPases in Cancer. Physiol. Rev. 2016, 96, 1071–1091. [Google Scholar] [CrossRef] [Green Version]
- Sabella, S.; Carney, R.P.; Brunetti, V.; Malvindi, M.A.; Al-Juffali, N.; Vecchio, G.; Janes, S.M.; Bakr, O.M.; Cingolani, R.; Stellacci, F.; et al. A general mechanism for intracellular toxicity of metal-containing nanoparticles. Nanoscale 2014, 6, 7052–7061. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biemond, P.; van Eijk, H.G.; Swaak, A.J.; Koster, J.F. Iron mobilization from ferritin by superoxide derived from stimulated polymorphonuclear leukocytes. Possible mechanism in inflammation diseases. J. Clin. Investig. 1984, 73, 1576–1579. [Google Scholar] [CrossRef]
- Liang, R.; Li, Y.; Huo, M.; Lin, H.; Chen, Y. Triggering Sequential Catalytic Fenton Reaction on 2D MXenes for Hyperthermia-Augmented Synergistic Nanocatalytic Cancer Therapy. ACS Appl. Mater. Interfaces 2019, 11, 42917–42931. [Google Scholar] [CrossRef] [PubMed]
- Van der Paal, J.; Neyts, E.C.; Verlackt, C.C.W.; Bogaerts, A. Effect of lipid peroxidation on membrane permeability of cancer and normal cells subjected to oxidative stress. Chem. Sci. 2016, 7, 489–498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clemente, S.M.; Martinez-Costa, O.H.; Monsalve, M.; Samhan-Arias, A.K. Targeting Lipid Peroxidation for Cancer Treatment. Molecules 2020, 25, 5144. [Google Scholar] [CrossRef]
- Minotti, G.; Aust, S.D. The role of iron in the initiation of lipid peroxidation. Chem Phys. Lipids 1987, 44, 191–208. [Google Scholar] [CrossRef]
- Repetto, M.G.; Ferrarotti, N.F.; Boveris, A. The involvement of transition metal ions on iron-dependent lipid peroxidation. Arch. Toxicol. 2010, 84, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Dodson, M.; Castro-Portuguez, R.; Zhang, D.D. NRF2 plays a critical role in mitigating lipid peroxidation and ferroptosis. Redox Biol. 2019, 23, 101107. [Google Scholar] [CrossRef] [PubMed]
- Ayala, A.; Munoz, M.F.; Arguelles, S. Lipid peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid. Med. Cell Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef]
- Rodriguez-Martinez, M.A.; Martinez-Orgado, J.; Salaices, M.; Marin, J. Impairment of acetylcholine relaxations by malondialdehyde, a marker of lipid peroxidation. J. Vasc. Res. 1996, 33, 463–470. [Google Scholar] [CrossRef]
- D’Arcy, M.S. Cell death: A review of the major forms of apoptosis, necrosis and autophagy. Cell Biol. Int. 2019, 43, 582–592. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Liu, J.; Kang, R.; Klionsky, D.J.; Kroemer, G.; Tang, D. Ferroptosis is a type of autophagy-dependent cell death. Semin. Cancer Biol. 2020, 66, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Que, K.T.; Zhang, Z.; Yi, Z.J.; Zhao, P.X.; You, Y.; Gong, J.P.; Liu, Z.J. Iron overloaded polarizes macrophage to proinflammation phenotype through ROS/acetyl-p53 pathway. Cancer Med. 2018, 7, 4012–4022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boedtkjer, E.; Pedersen, S.F. The Acidic Tumor Microenvironment as a Driver of Cancer. Annu. Rev. Physiol. 2020, 82, 103–126. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, Y.; Mochida, A.; Choyke, P.L.; Kobayashi, H. Nanodrug Delivery: Is the Enhanced Permeability and Retention Effect Sufficient for Curing Cancer? Bioconjug. Chem. 2016, 27, 2225–2238. [Google Scholar] [CrossRef]
- Robe, P.A.; Martin, D.H.; Nguyen-Khac, M.T.; Artesi, M.; Deprez, M.; Albert, A.; Vanbelle, S.; Califice, S.; Bredel, M.; Bours, V. Early termination of ISRCTN45828668, a phase 1/2 prospective, randomized study of sulfasalazine for the treatment of progressing malignant gliomas in adults. BMC Cancer 2009, 9, 372. [Google Scholar] [CrossRef] [Green Version]
- Liu, P.; Ma, D.; Wang, P.; Pan, C.; Fang, Q.; Wang, J. Nrf2 overexpression increases risk of high tumor mutation burden in acute myeloid leukemia by inhibiting MSH2. Cell Death Dis. 2021, 12, 20. [Google Scholar] [CrossRef]
- Dagogo-Jack, I.; Shaw, A.T. Tumour heterogeneity and resistance to cancer therapies. Nat. Rev. Clin. Oncol. 2018, 15, 81–94. [Google Scholar] [CrossRef]
- Anselmo, A.C.; Mitragotri, S. Nanoparticles in the clinic: An update. Bioeng. Transl. Med. 2019, 4, e10143. [Google Scholar] [CrossRef] [Green Version]
- Lin, C.H.; Chen, Y.C.; Huang, P.I. Preparation of Multifunctional Dopamine-Coated Zerovalent Iron/Reduced Graphene Oxide for Targeted Phototheragnosis in Breast Cancer. Nanomaterials 2020, 10, 1957. [Google Scholar] [CrossRef] [PubMed]
- Mukhtar, A.; Cao, X.M.; Mehmood, T.; Wang, D.S.; Wu, K.M. Structural characterization of self-assembled chain like Fe-FeOx Core shell nanostructure. Nanoscale Res. Lett. 2019, 14, 308. [Google Scholar] [CrossRef] [PubMed]
- Gloag, L.; Mehdipour, M.; Ulanova, M.; Mariandry, K.; Nichol, M.A.; Hernandez-Castillo, D.J.; Gaudet, J.; Qiao, R.; Zhang, J.; Nelson, M.; et al. Zero valent iron core-iron oxide shell nanoparticles as small magnetic particle imaging tracers. Chem. Commun. 2020, 56, 3504–3507. [Google Scholar] [CrossRef] [PubMed]
- Luo, P.; Bailey, E.H.; Mooney, S.J. Quantification of changes in zero valent iron morphology using X-ray computed tomography. J. Environ. Sci. 2013, 25, 2344–23451. [Google Scholar] [CrossRef]

| Year | Authors | Materials | Sizes (nm) | In Vitro Study (IC50), Cell Types 1, Treating Periods | In Vivo Study |
|---|---|---|---|---|---|
| 2021 [72] | Hsieh et al. | ZVI@Ag ZVI@CMC | 81 ± 14 70 ± 14 |
| 25 mg ZVI@Ag or ZVI@CMC/kg intravenous, once a week for 4 weeks |
| 2021 [67] | Liang et al. | Fe/Fe3O4 porous yolk shell NPs (PYSNPs) | 15 |
| 1, 10 mg iRGD-PYSNPs /kg, intravenous |
| 2020 [73] | Hashemi et al. | ZVI | 10–30 |
| NA |
| 2020 [68] | Yang et al. | ZVI@Ag | 85 ± 17 |
| 40 mg/kg, intravenous (single injection) |
| 2019 [69] | Yang et al. | ZVI@mSiO2 | 29 ± 7 |
| 40 mg/kg, intravenous |
| 2019 [74] | Huang et al. | ZVI@CMC | 50–100 |
When combined treatment with ferroptosis inducer:
| 25 mg/kg, intravenous (4 injections) |
| 2019 [76] | Anbouhi et al. | ZVI NPs | 37 |
| NA |
| 2016 [70] | Shevtsov et al. | Fe(0)@MCM-41 | 250 × 150 with 3 nm pores |
| 10 mg/kg, intravenous |
| 2016 [77] | Zhang et al. | Amorphous iron NPs | 10–15 |
| 15 mg/kg, intratumor, 75 mg/kg, intravenous |
| 2011 [43,71] | Wu et al. | Fe@Au | 10–20 |
| 50 mg/kg, intratumor |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.-N.; Yang, L.-X.; Wang, P.-W.; Braet, F.; Shieh, D.-B. From Microenvironment Remediation to Novel Anti-Cancer Strategy: The Emergence of Zero Valent Iron Nanoparticles. Pharmaceutics 2022, 14, 99. https://doi.org/10.3390/pharmaceutics14010099
Wu Y-N, Yang L-X, Wang P-W, Braet F, Shieh D-B. From Microenvironment Remediation to Novel Anti-Cancer Strategy: The Emergence of Zero Valent Iron Nanoparticles. Pharmaceutics. 2022; 14(1):99. https://doi.org/10.3390/pharmaceutics14010099
Chicago/Turabian StyleWu, Ya-Na, Li-Xing Yang, Pei-Wen Wang, Filip Braet, and Dar-Bin Shieh. 2022. "From Microenvironment Remediation to Novel Anti-Cancer Strategy: The Emergence of Zero Valent Iron Nanoparticles" Pharmaceutics 14, no. 1: 99. https://doi.org/10.3390/pharmaceutics14010099
APA StyleWu, Y.-N., Yang, L.-X., Wang, P.-W., Braet, F., & Shieh, D.-B. (2022). From Microenvironment Remediation to Novel Anti-Cancer Strategy: The Emergence of Zero Valent Iron Nanoparticles. Pharmaceutics, 14(1), 99. https://doi.org/10.3390/pharmaceutics14010099







