Initial Steps towards Spatiotemporal Signaling through Biomaterials Using Click-to-Release Chemistry
Abstract
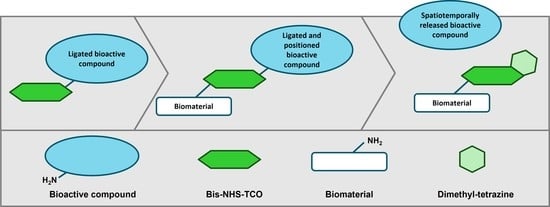
1. Introduction
2. Materials and Methods
2.1. Preparation of Type I Collagen Scaffolds
2.2. Reaction Components and Conditions
2.3. Ligation of hEGF to TCO and Release of hEGF following Tetrazine Exposure
2.4. Ligation of hEGF–TCO to Collagen Scaffolds and Release of hEGF following Tetrazine Exposure
2.4.1. SDS-PAGE and Western Blotting
2.4.2. Immunofluorescence Staining
2.5. Ligation of hEGF to BSA and Release following Tetrazine Exposure
3. Results
3.1. hEGF Ligates to TCO to Form an ‘hEGF–TCO’ Complex
3.2. The hEGF–TCO Complex Is Able to Ligate to Collagen Scaffolds
3.3. Establishing Click-to-Release of hEGF from BSA–TCO–hEGF
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Deflorin, C.; Hohenauer, E.; Stoop, R.; van Daele, U.; Clijsen, R.; Taeymans, J. Physical Management of Scar Tissue: A Systematic Review and Meta-Analysis. J. Altern. Complement. Med. 2020, 26, 854–865. [Google Scholar] [CrossRef]
- Tredget, E.E.; Shupp, J.W.; Schneider, J.C. Scar Management Following Burn Injury. J. Burn Care Res. 2017, 38, 146–147. [Google Scholar] [CrossRef]
- Ngaage, M.; Agius, M. The Psychology of Scars: A Mini-Review. Psychiatr. Danub. 2018, 30, 633–638. [Google Scholar] [PubMed]
- Gicquel, C.; Le Bouc, Y. Hormonal Regulation of Retal Growth. Horm. Res. 2006, 65, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Barrientos, S.; Stojadinovic, O.; Golinko, M.S.; Brem, H.; Tomic-Canic, M. Growth factors and cytokines in wound healing. Wound Repair Regen. 2008, 16, 585–601. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Owen, M.R.; Mao, Y. The spatiotemporal order of signaling events unveils the logic of development signaling. Bioinformatics 2016, 32, 2313–2320. [Google Scholar] [CrossRef] [PubMed]
- Burgy, O.; Konigshöff, M. The WNT signaling pathways in wound healing and fibrosis. Matrix Biol. 2018, 68–69, 67–80. [Google Scholar] [CrossRef]
- Mascharak, S.; Talbott, H.E.; Januszyk, M.; Griffin, M.; Chen, K.; Davitt, M.F.; Demeter, J.; Henn, D.; Bonham, C.A.; Foster, D.S.; et al. Multi-omic analysis reveals divergent molecular events in scarring and regenerative wound healing. Cell Stem Cell 2022, 29, 315–327.e316. [Google Scholar] [CrossRef]
- Werner, S.; Grose, R. Regulation of wound healing by growth factors and cytokines. Physiol. Rev. 2003, 83, 835–870. [Google Scholar] [CrossRef] [PubMed]
- Profyris, C.; Tziotzios, C.; Do Vale, I. Cutaneous scarring: Pathophysiology, molecular mechanisms, and scar reduction therapeutics Part I. The molecular basis of scar formation. J. Am. Acad. Dermatol. 2012, 66, 1–10; quiz 11–12. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.; Kosaric, N.; Bonham, C.A.; Gurtner, G.C. Wound Healing: A Cellular Perspective. Physiol. Rev. 2019, 99, 665–706. [Google Scholar] [CrossRef]
- Ridiandries, A.; Tan, J.T.M.; Bursill, C.A. The Role of Chemokines in Wound Healing. Int. J. Mol. Sci. 2018, 19, 3217. [Google Scholar] [CrossRef]
- Brusselaers, N.; Monstrey, S.; Vogelaers, D.; Hoste, E.; Blot, S. Severe burn injury in Europe: A systematic review of the incidence, etiology, morbidity, and mortality. Crit. Care 2010, 14, R188. [Google Scholar] [CrossRef] [PubMed]
- Esselman, P.C. Burn Rehabilitation: An Overview. Arch. Phys. Med. Rehabil. 2007, 88, S3–S6. [Google Scholar] [CrossRef] [PubMed]
- Bochaton-Piallat, M.L.; Gabbiani, G.; Hinz, B. The myofibroblast in wound healing and fibrosis: Answered and unanswered questions. F1000Research 2016, 5, 752. [Google Scholar] [CrossRef]
- Young, A.W.; Dewey, W.S.; King, B.T. Rehabilitation of Burn Injuries: An Update. Phys. Med. Rehabil. Clin. N. Am. 2019, 30, 111–132. [Google Scholar] [CrossRef]
- Tai, Y.; Woods, E.L.; Dally, J.; Kong, D.; Steadman, R.; Moseley, R.; Midgley, A.C. Myofibroblasts: Function, Formation, and Scope of Molecular Therapies for Skin Fibrosis. Biomolecules 2021, 11, 1095. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Wang, X.F.; Wang, Z.C.; Lou, D.; Fang, Q.Q.; Hu, Y.Y.; Zhao, W.Y.; Zhang, L.Y.; Wu, L.H.; Tan, W.Q. Current potential therapeutic strategies targeting the TGF-beta/Smad signaling pathway to attenuate keloid and hypertrophic scar formation. Biomed. Pharmacother. 2020, 129, 110287. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Guo, S.; Wang, C.C.; Sun, X.; Wang, D.; Xu, N.; Jin, S.F.; Li, K.Z. Cross-talk between TGF-beta/Smad pathway and Wnt/beta-catenin pathway in pathological scar formation. Int. J. Clin. Exp. Pathol. 2015, 8, 7631–7639. [Google Scholar] [PubMed]
- Akita, S.; Akino, K.; Imaizumi, T.; Hirano, A. Basic fibroblast growth factor accelerates and improves second-degree burn wound healing. Wound Repair Regen. 2008, 16, 635–641. [Google Scholar] [CrossRef] [PubMed]
- Dolivo, D.M.; Larson, S.A.; Dominko, T. FGF2-mediated attenuation of myofibroblast activation is modulated by distinct MAPK signaling pathways in human dermal fibroblasts. J. Dermatol. Sci. 2017, 88, 339–348. [Google Scholar] [CrossRef]
- Oostendorp, C.; Geutjes, P.J.; Smit, F.; Tiemessen, D.M.; Polman, S.; Abbawi, A.; Brouwer, K.M.; Eggink, A.J.; Feitz, W.F.J.; Daamen, W.F.; et al. Sustained Postnatal Skin Regeneration Upon Prenatal Application of Functionalized Collagen Scaffolds. Tissue Eng. Part A 2020, 27, 10–25. [Google Scholar] [CrossRef] [PubMed]
- Nillesen, S.T.; Geutjes, P.J.; Wismans, R.; Schalkwijk, J.; Daamen, W.F.; van Kuppevelt, T.H. Increased angiogenesis and blood vessel maturation in acellular collagen-heparin scaffolds containing both FGF2 and VEGF. Biomaterials 2007, 28, 1123–1131. [Google Scholar] [CrossRef]
- Hardwicke, J.; Schmaljohann, D.; Boyce, D.; Thomas, D. Epidermal growth factor therapy and wound healing—Past, present and future perspectives. Surgeon 2008, 6, 172–177. [Google Scholar] [CrossRef]
- Kim, H.; Kong, W.H.; Seong, K.Y.; Sung, D.K.; Jeong, H.; Kim, J.K.; Yang, S.Y.; Hahn, S.K. Hyaluronate-Epidermal Growth Factor Conjugate for Skin Wound Healing and Regeneration. Biomacromolecules 2016, 17, 3694–3705. [Google Scholar] [CrossRef]
- Li, Y.; Leng, Q.; Pang, X.; Shi, H.; Liu, Y.; Xiao, S.; Zhao, L.; Zhou, P.; Fu, S. Therapeutic effects of EGF-modified curcumin/chitosan nano-spray on wound healing. Regen. Biomater. 2021, 8, rbab009. [Google Scholar] [CrossRef] [PubMed]
- Han, C.M.; Cheng, B.; Wu, P.; Writing Group of Growth Factor Guideline on Behalf of Chinese Burn Association. Clinical guideline on topical growth factors for skin wounds. Burn. Trauma 2020, 8, tkaa035. [Google Scholar] [CrossRef]
- Dolati, S.; Yousefi, M.; Pishgahi, A.; Nourbakhsh, S.; Pourabbas, B.; Shakouri, S.K. Prospects for the application of growth factors in wound healing. Growth Factors 2020, 38, 25–34. [Google Scholar] [CrossRef]
- Monavarian, M.; Kader, S.; Moeinzadeh, S.; Jabbari, E. Regenerative Scar-Free Skin Wound Healing. Tissue Eng. Part B Rev. 2019, 25, 294–311. [Google Scholar] [CrossRef]
- Berry-Kilgour, C.; Cabral, J.; Wise, L. Advancements in the Delivery of Growth Factors and Cytokines for the Treatment of Cutaneous Wound Indications. Adv. Wound Care (New Rochelle) 2021, 10, 596–622. [Google Scholar] [CrossRef]
- Yamakawa, S.; Hayashida, K. Advances in surgical applications of growth factors for wound healing. Burn. Trauma 2019, 7, 10. [Google Scholar] [CrossRef]
- Nurkesh, A.; Jaguparov, A.; Jimi, S.; Saparov, A. Recent Advances in the Controlled Release of Growth Factors and Cytokines for Improving Cutaneous Wound Healing. Front. Cell Dev. Biol. 2020, 8, 638. [Google Scholar] [CrossRef]
- Epstein, N.E. Complications due to the use of BMP/INFUSE in spine surgery: The evidence continues to mount. Surg. Neurol. Int. 2013, 4, S343–S352. [Google Scholar] [CrossRef] [PubMed]
- Caballero Aguilar, L.M.; Silva, S.M.; Moulton, S.E. Growth factor delivery: Defining the next generation platforms for tissue engineering. J. Control. Release 2019, 306, 40–58. [Google Scholar] [CrossRef] [PubMed]
- Biondi, M.; Ungaro, F.; Quaglia, F.; Netti, P.A. Controlled drug delivery in tissue engineering. Adv. Drug Deliv. Rev. 2008, 60, 229–242. [Google Scholar] [CrossRef]
- Park, U.; Lee, M.S.; Jeon, J.; Lee, S.; Hwang, M.P.; Wang, Y.; Yang, H.S.; Kim, K. Coacervate-mediated exogenous growth factor delivery for scarless skin regeneration. Acta Biomater. 2019, 90, 179–191. [Google Scholar] [CrossRef]
- Dodero, A.; Alberti, S.; Gaggero, G.; Ferretti, M.; Botter, R.; Vicini, S.; Castellano, M. An Up-to-Date Review on Alginate Nanoparticles and Nanofibers for Biomedical and Pharmaceutical Applications. Adv. Mater. Interfaces 2021, 8, 2100809. [Google Scholar] [CrossRef]
- Khodadadi Yazdi, M.; Taghizadeh, A.; Taghizadeh, M.; Stadler, F.J.; Farokhi, M.; Mottaghitalab, F.; Zarrintaj, P.; Ramsey, J.D.; Seidi, F.; Saeb, M.R.; et al. Agarose-based biomaterials for advanced drug delivery. J. Control. Release 2020, 326, 523–543. [Google Scholar] [CrossRef]
- Nii, T. Strategies Using Gelatin Microparticles for Regenerative Therapy and Drug Screening Applications. Molecules 2021, 26, 6795. [Google Scholar] [CrossRef]
- Rizzo, F.; Kehr, N.S. Recent Advances in Injectable Hydrogels for Controlled and Local Drug Delivery. Adv. Healthc. Mater. 2021, 10, e2001341. [Google Scholar] [CrossRef]
- Van Gheluwe, L.; Chourpa, I.; Gaigne, C.; Munnier, E. Polymer-Based Smart Drug Delivery Systems for Skin Application and Demonstration of Stimuli-Responsiveness. Polymers 2021, 13, 1285. [Google Scholar] [CrossRef] [PubMed]
- Dimatteo, R.; Darling, N.J.; Segura, T. In situ forming injectable hydrogels for drug delivery and wound repair. Adv. Drug Deliv. Rev. 2018, 127, 167–184. [Google Scholar] [CrossRef]
- Park, J.W.; Hwang, S.R.; Yoon, I.S. Advanced Growth Factor Delivery Systems in Wound Management and Skin Regeneration. Molecules 2017, 22, 1259. [Google Scholar] [CrossRef] [PubMed]
- Battigelli, A.; Almeida, B.; Shukla, A. Recent Advances in Bioorthogonal Click Chemistry for Biomedical Applications. Bioconjugate Chem. 2022, 33, 263–271. [Google Scholar] [CrossRef]
- Oliveira, B.L.; Guo, Z.; Bernardes, G.J.L. Inverse electron demand Diels—Alder reactions in chemical biology. Chem. Soc. Rev. 2017, 46, 4895–4950. [Google Scholar] [CrossRef]
- Li, J.; Chen, P.R. Development and application of bond cleavage reactions in bioorthogonal chemistry. Nat. Chem. Biol. 2016, 12, 129–137. [Google Scholar] [CrossRef]
- Tu, J.; Xu, M.; Franzini, R.M. Dissociative Bioorthogonal Reactions. ChemBioChem 2019, 20, 1615–1627. [Google Scholar] [CrossRef]
- Versteegen, R.M.; Rossin, R.; ten Hoeve, W.; Janssen, H.M.; Robillard, M.S. Click to release: Instantaneous doxorubicin elimination upon tetrazine ligation. Angew. Chem. Int. Ed. Engl. 2013, 52, 14112–14116. [Google Scholar] [CrossRef]
- Rossin, R.; van Duijnhoven, S.M.; Ten Hoeve, W.; Janssen, H.M.; Kleijn, L.H.; Hoeben, F.J.; Versteegen, R.M.; Robillard, M.S. Triggered Drug Release from an Antibody-Drug Conjugate Using Fast “Click-to-Release” Chemistry in Mice. Bioconjugate Chem. 2016, 27, 1697–1706. [Google Scholar] [CrossRef]
- Rossin, R.; Versteegen, R.M.; Wu, J.; Khasanov, A.; Wessels, H.J.; Steenbergen, E.J.; Ten Hoeve, W.; Janssen, H.M.; van Onzen, A.; Hudson, P.J.; et al. Chemically triggered drug release from an antibody-drug conjugate leads to potent antitumour activity in mice. Nat. Commun. 2018, 9, 1484. [Google Scholar] [CrossRef]
- Versteegden, L.R.; Hoogenkamp, H.R.; Lomme, R.M.; van Goor, H.; Tiemessen, D.M.; Geutjes, P.J.; Oosterwijk, E.; Feitz, W.F.; Hafmans, T.G.; Verdonschot, N.; et al. Design of an elasticized collagen scaffold: A method to induce elasticity in a rigid protein. Acta Biomater. 2016, 44, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Li, J.; Xie, R.; Fan, X.; Liu, Y.; Zheng, S.; Ge, Y.; Chen, P.R. Bioorthogonal Chemical Activation of Kinases in Living Systems. ACS Cent. Sci. 2016, 2, 325–331. [Google Scholar] [CrossRef] [PubMed]
- van Onzen, A.; Versteegen, R.M.; Hoeben, F.J.M.; Filot, I.A.W.; Rossin, R.; Zhu, T.; Wu, J.; Hudson, P.J.; Janssen, H.M.; Ten Hoeve, W.; et al. Bioorthogonal Tetrazine Carbamate Cleavage by Highly Reactive trans-Cyclooctene. J. Am. Chem. Soc. 2020, 142, 10955–10963. [Google Scholar] [CrossRef]
- Wu, K.; Yee, N.A.; Srinivasan, S.; Mahmoodi, A.; Zakharian, M.; Mejia Oneto, J.M.; Royzen, M. Click activated protodrugs against cancer increase the therapeutic potential of chemotherapy through local capture and activation. Chem. Sci. 2021, 12, 1259–1271, Correction in Chem. Sci. 2021, 12, 7583. [Google Scholar] [CrossRef]
- Carlson, J.C.T.; Mikula, H.; Weissleder, R. Unraveling Tetrazine-Triggered Bioorthogonal Elimination Enables Chemical Tools for Ultrafast Release and Universal Cleavage. J. Am. Chem. Soc. 2018, 140, 3603–3612. [Google Scholar] [CrossRef]
- Fan, X.; Ge, Y.; Lin, F.; Yang, Y.; Zhang, G.; Ngai, W.S.; Lin, Z.; Zheng, S.; Wang, J.; Zhao, J.; et al. Optimized Tetrazine Derivatives for Rapid Bioorthogonal Decaging in Living Cells. Angew. Chem. Int. Ed. Engl. 2016, 55, 14046–14050. [Google Scholar] [CrossRef]
- Svatunek, D.; Wilkovitsch, M.; Hartmann, L.; Houk, K.N.; Mikula, H. Uncovering the Key Role of Distortion in Bioorthogonal Tetrazine Tools That Defy the Reactivity/Stability Trade-Off. J. Am. Chem. Soc. 2022, 144, 8171–8177. [Google Scholar] [CrossRef] [PubMed]
- Langle, D.; Halver, J.; Rathmer, B.; Willems, E.; Schade, D. Small Molecules Targeting in Vivo Tissue Regeneration. ACS Chem. Biol. 2014, 9, 57–71. [Google Scholar] [CrossRef]
- Horinouchi, C.D.S.; Oostendorp, C.; Schade, D.; van Kuppevelt, T.H.; Daamen, W.F. Growth factor mimetics for skin regeneration: In vitro profiling of primary human fibroblasts and keratinocytes. Arch. Pharm. (Weinh.) 2021, 354, e2100082. [Google Scholar] [CrossRef]
- Hermanson, G.T. Bioconjugate Techniques, 3rd ed.; Academic Press Is an Imprint of Elsevier: London, UK, 2013. [Google Scholar]
- Nii, T.; Makino, K.; Tabata, Y. Three-Dimensional Culture System of Cancer Cells Combined with Biomaterials for Drug Screening. Cancers 2020, 12, 2754. [Google Scholar] [CrossRef]
- Armiento, A.R.; Stoddart, M.J.; Alini, M.; Eglin, D. Biomaterials for articular cartilage tissue engineering: Learning from biology. Acta Biomater. 2018, 65, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Dong, R.; Ma, P.X.; Guo, B. Conductive biomaterials for muscle tissue engineering. Biomaterials 2020, 229, 119584. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gansevoort, M.; Merx, J.; Versteeg, E.M.M.; Vuckovic, I.; Boltje, T.J.; van Kuppevelt, T.H.; Daamen, W.F. Initial Steps towards Spatiotemporal Signaling through Biomaterials Using Click-to-Release Chemistry. Pharmaceutics 2022, 14, 1991. https://doi.org/10.3390/pharmaceutics14101991
Gansevoort M, Merx J, Versteeg EMM, Vuckovic I, Boltje TJ, van Kuppevelt TH, Daamen WF. Initial Steps towards Spatiotemporal Signaling through Biomaterials Using Click-to-Release Chemistry. Pharmaceutics. 2022; 14(10):1991. https://doi.org/10.3390/pharmaceutics14101991
Chicago/Turabian StyleGansevoort, Merel, Jona Merx, Elly M. M. Versteeg, Isidora Vuckovic, Thomas J. Boltje, Toin H. van Kuppevelt, and Willeke F. Daamen. 2022. "Initial Steps towards Spatiotemporal Signaling through Biomaterials Using Click-to-Release Chemistry" Pharmaceutics 14, no. 10: 1991. https://doi.org/10.3390/pharmaceutics14101991
APA StyleGansevoort, M., Merx, J., Versteeg, E. M. M., Vuckovic, I., Boltje, T. J., van Kuppevelt, T. H., & Daamen, W. F. (2022). Initial Steps towards Spatiotemporal Signaling through Biomaterials Using Click-to-Release Chemistry. Pharmaceutics, 14(10), 1991. https://doi.org/10.3390/pharmaceutics14101991