In Vitro and In Vivo Relevant Antineoplastic Activity of Platinum(II) Complexes toward Triple-Negative MDA-MB-231 Breast Cancer Cell Line
Abstract
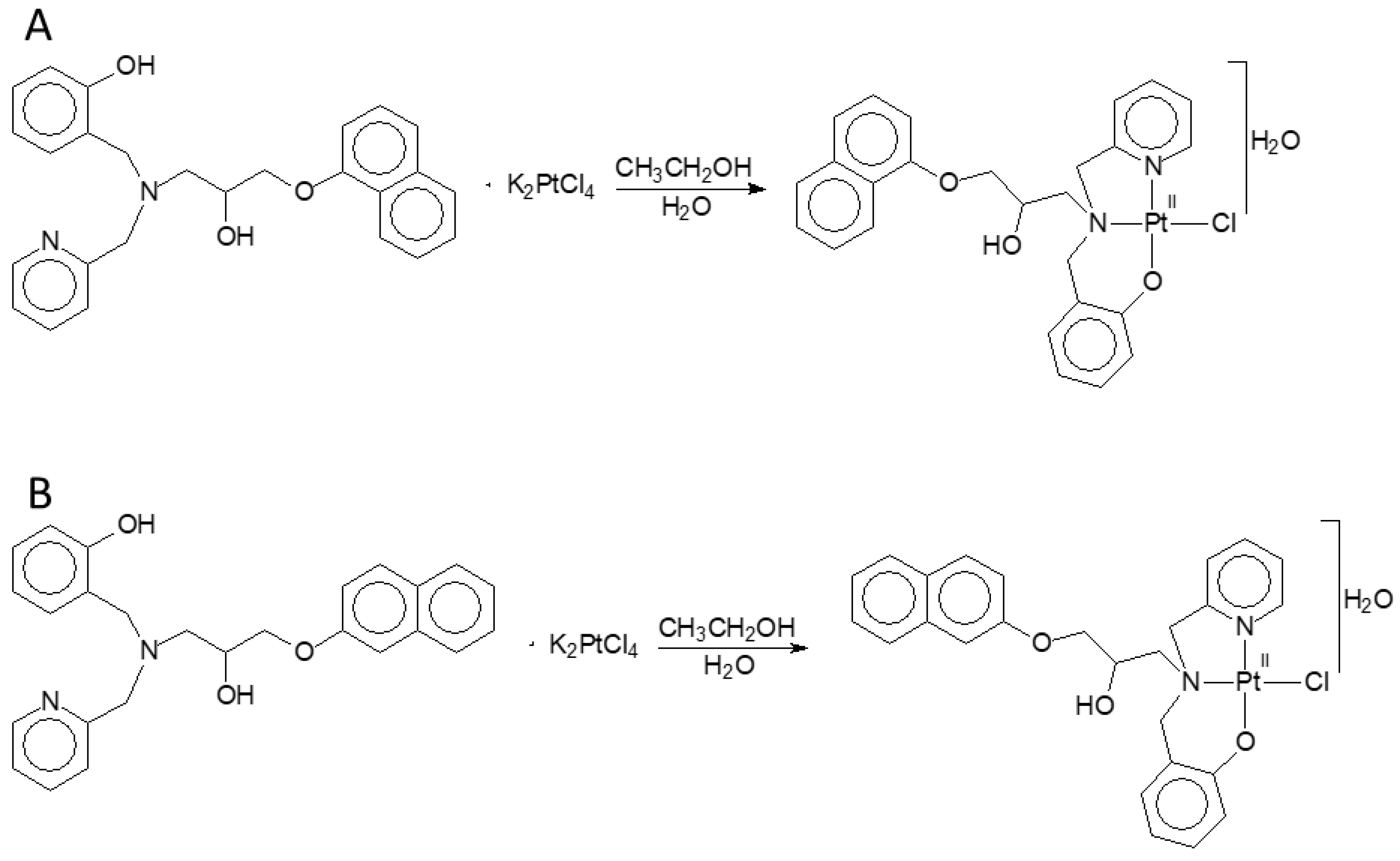
1. Introduction
2. Materials and Methods
2.1. Compounds
2.2. Cell Lines
2.3. Cell Viability Assay
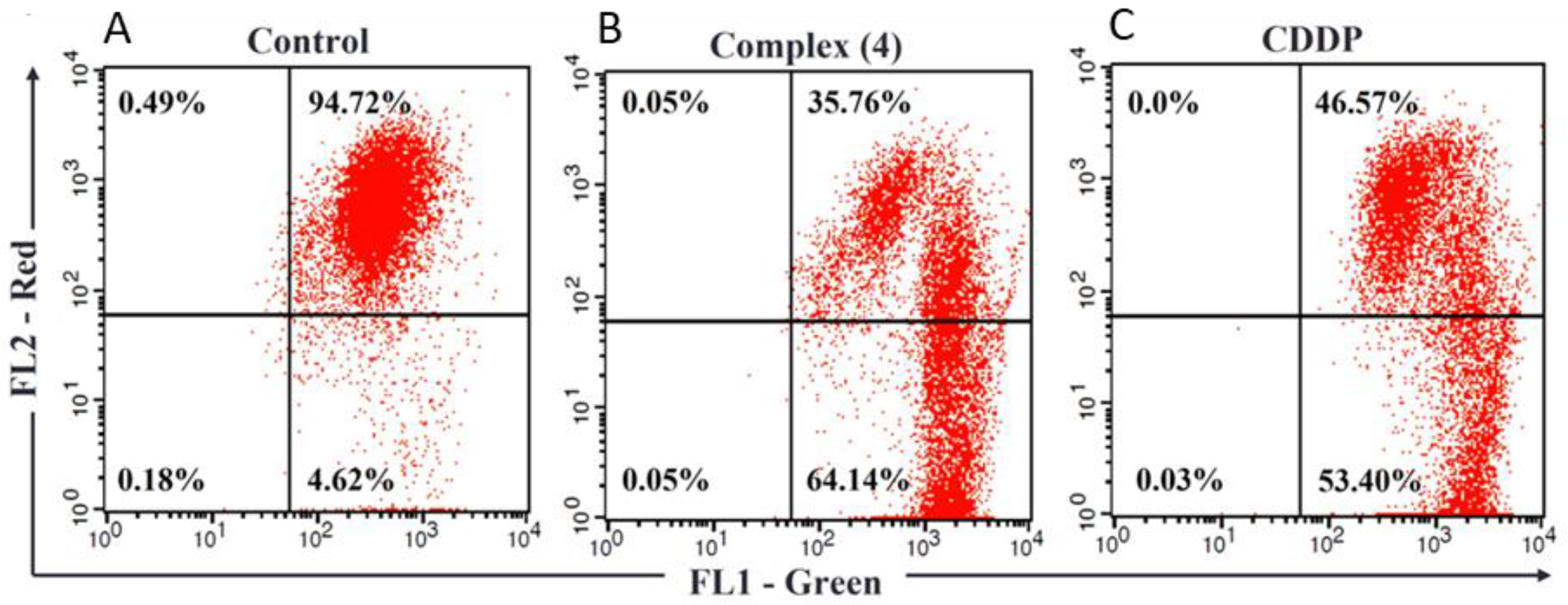
2.4. Apoptosis Evaluation by Cell Cycle Analysis
2.5. Determination of Mitochondrial Membrane Potential
2.6. Measurement of Reactive Oxygen Species (ROS)
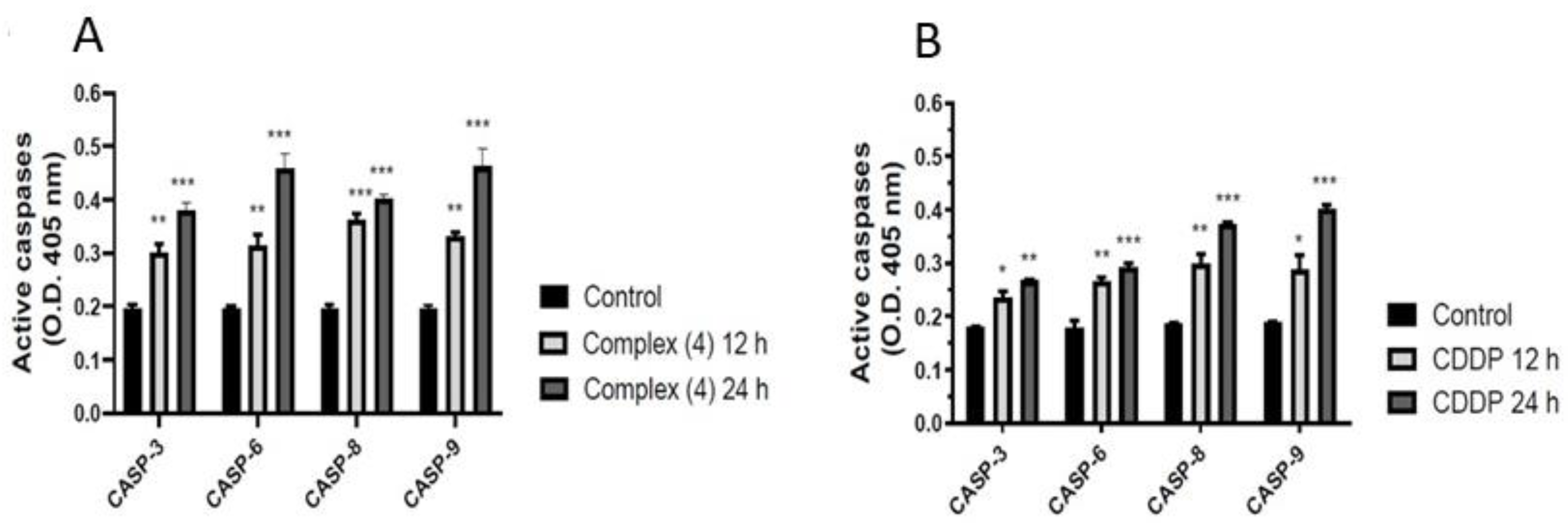
2.7. Caspases Activity Assay
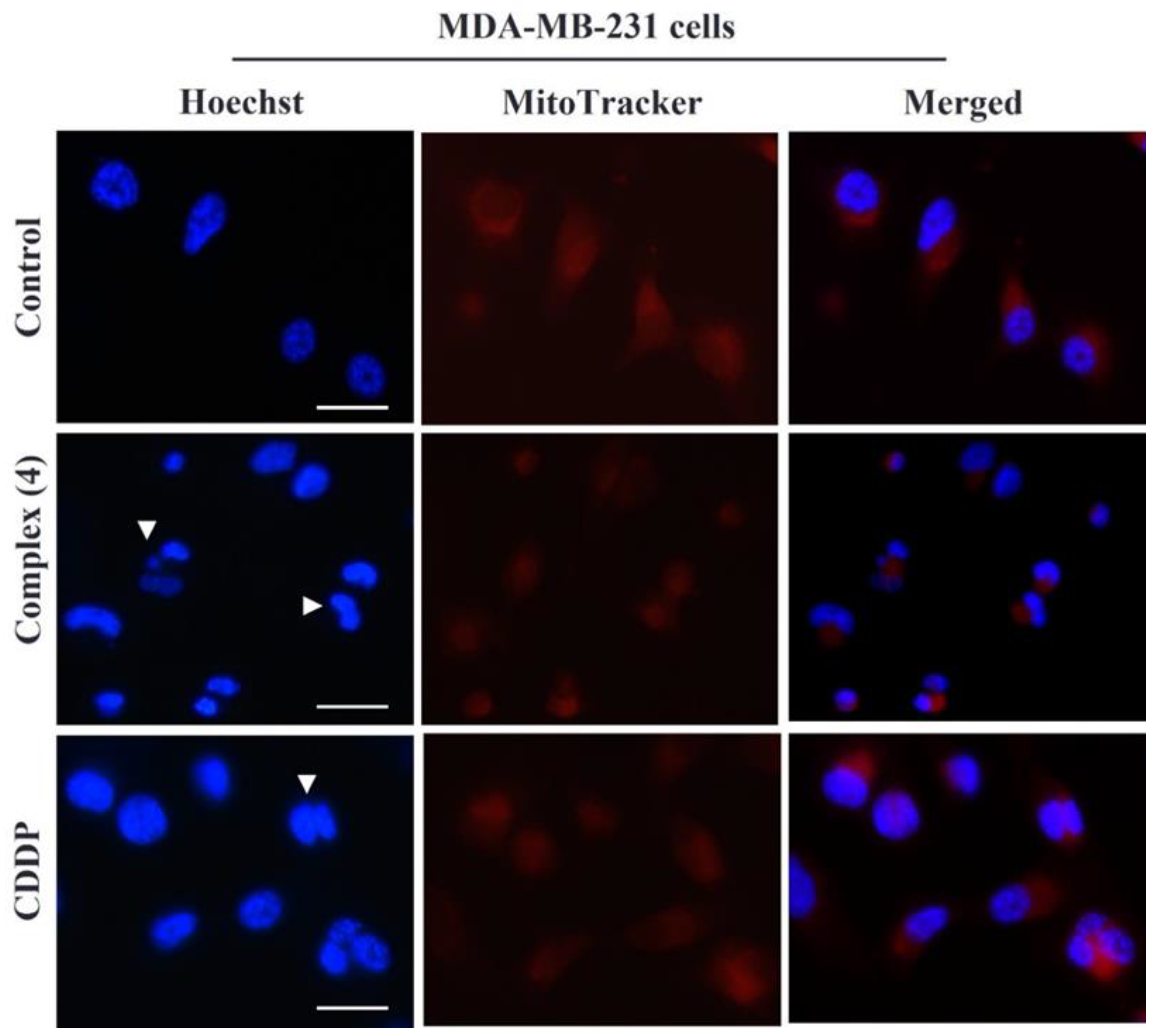
2.8. Morphological Assessment by Hoechst 33342/MitoTracker Double Staining
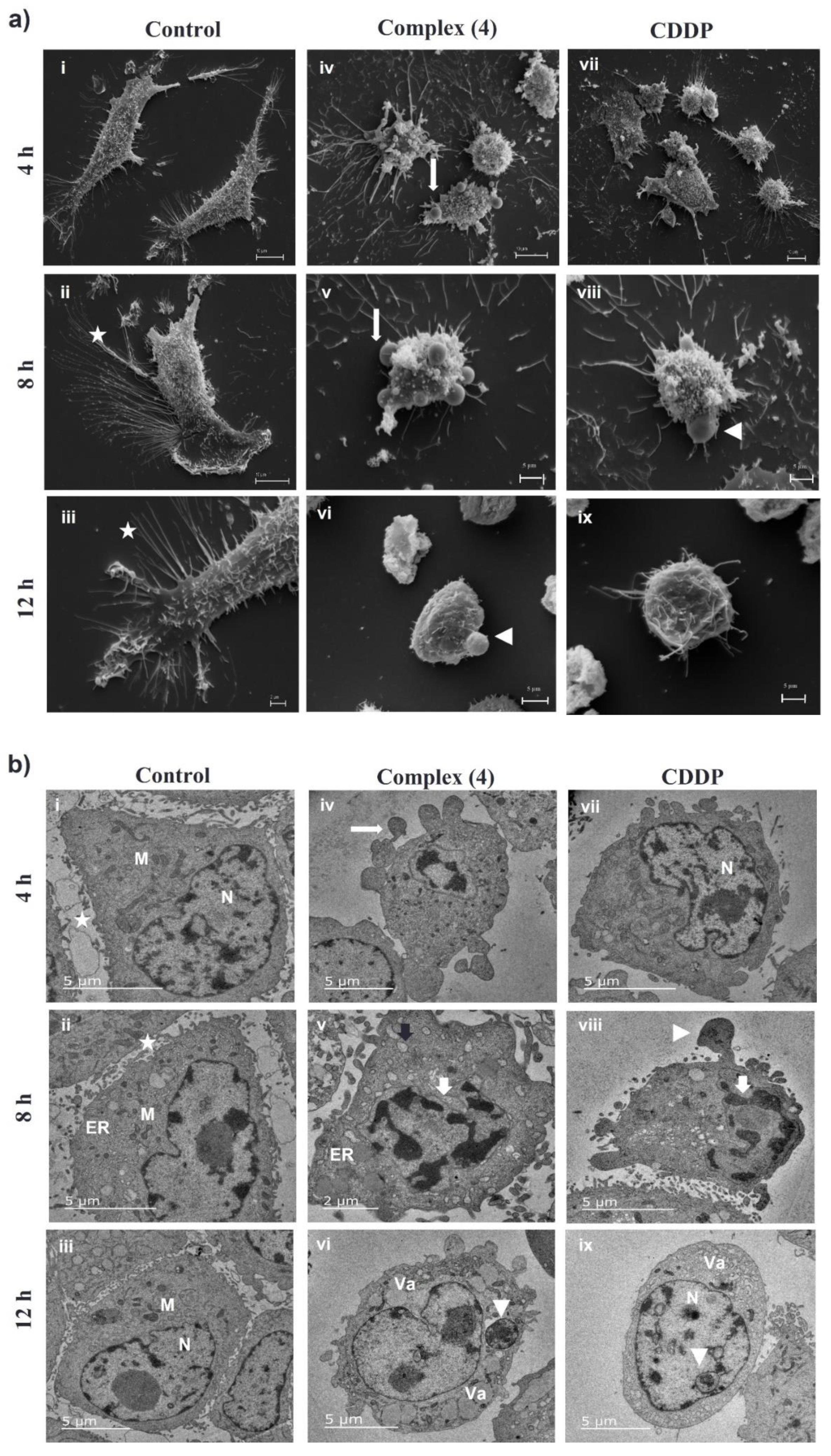
2.9. Transmission Electron Microscopy
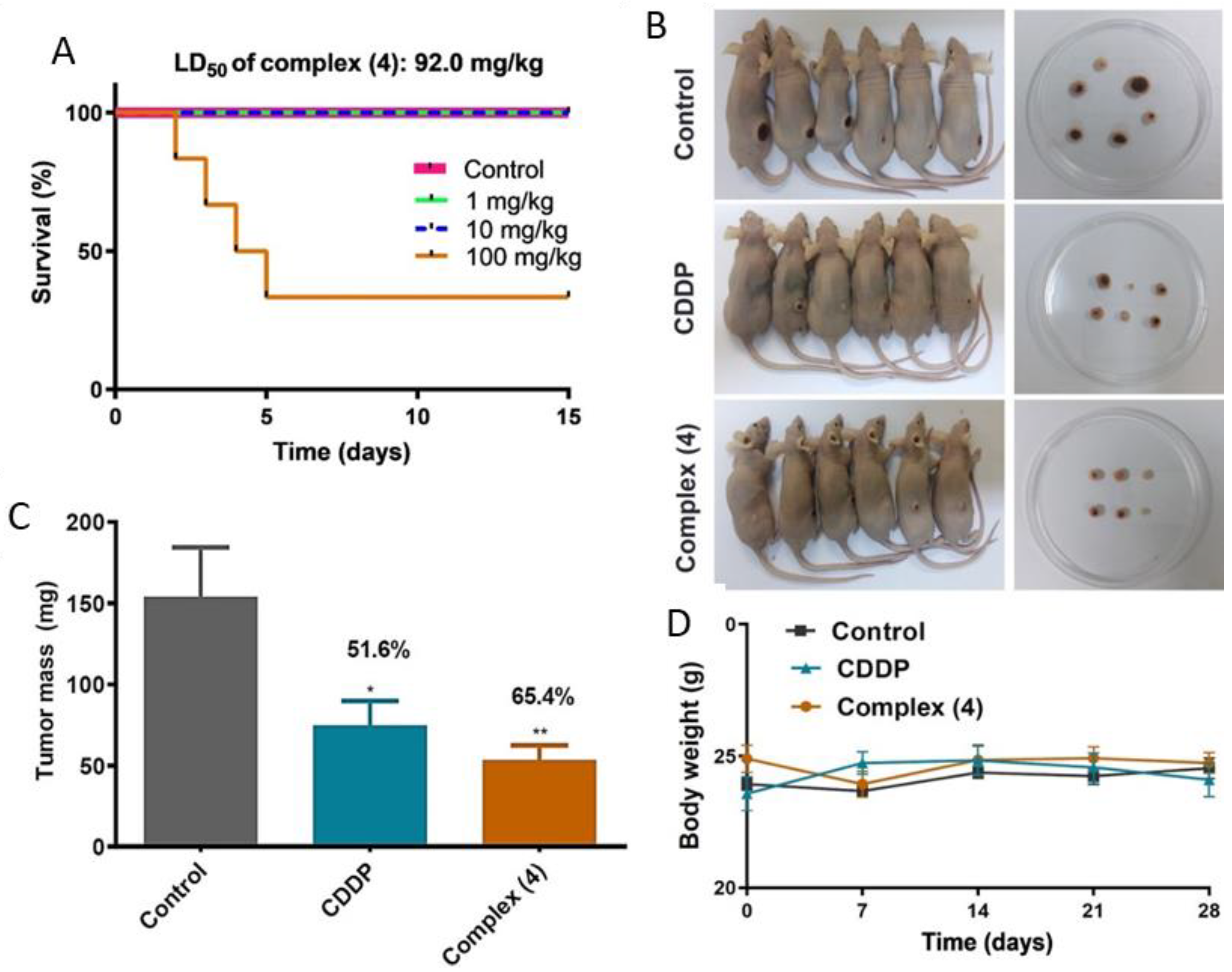
2.10. Determination of Acute Toxicity of Complex (4)
2.11. Analysis of Antitumor Efficacy In Vivo
2.12. Statistical Analysis
3. Results
3.1. Assessment of Cell Viability by MTT Assay
| IC50 (μM) | ||||||
|---|---|---|---|---|---|---|
| Compounds | MDA-MB-231 | MCF-7 | A549 | PC-3 | BXPC-3 | PBMC a |
| Complex (3) | 14.4 ± 1.1 | 20.5 ± 1.1 | 35.3 ± 1 | 23 ± 1.1 | 16.3 ± 1.1 | 22.0 ± 1.0 |
| Complex (4) | 8.0 ± 1.1 | 16.2 ± 1.2 | 18 ± 1.1 | 14.4 ± 1.2 | 13.7 ± 1.1 | 42.0 ± 1.0 |
| (H2L4) | >100 | >100 | >100 | >100 | >100 | >100 |
| CDDP | 63 ± 1.2 | 20.7 ± 1.1 | 70 ± 1.2 | 45.6 ± 1.0 | 12.7 ± 1.2 | 44.0 ± 2.0 |
| Selectivity Index (SI b) | |||||
|---|---|---|---|---|---|
| Compounds | MDA-MB-2311 | MCF-7 | A549 | PC-3 | BXPC-3 |
| Complex (3) | 1.5 | 1.1 | 0.6 | 0.95 | 1.3 |
| Complex (4) | 5.2 | 2.6 | 2.3 | 3 | 3.1 |
| CDDP | 0.7 | 2.1 | 0.6 | 1 | 3.5 |
3.2. Complex (4) Induces Apoptosis in Human Breast Cancer Cells
3.3. Complex (4) Decreases the Mitochondrial Transmembrane Potential
3.4. Alteration in Nuclear and Mitochondrial Morphology
3.5. Complex (4) Induces Cellular Cytotoxicity by ROS Production
3.6. Effect of Complex (4) Treatment on Caspases Activation
3.7. Complex (4) Affects the Ultrastructure of Breast Cancer Cells
3.8. Complex (4) Reduces Tumor Growth in a Murine Model of Breast Cancer
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- DeSantis, C.E.; Ma, J.; Gaudet, M.M.; Newman, L.A.; Miller, K.D.; Goding Sauer, A.; Jemal, A.; Siegel, R.L. Breast Cancer Statistics, 2019. CA Cancer J. Clin. 2019, 69, 438–451. [Google Scholar] [CrossRef] [PubMed]
- Manjunath, M.; Choudhary, B. Triple-Negative Breast Cancer: A Run-through of Features, Classification and Current Therapies (Review). Oncol. Lett. 2021, 22, 512. [Google Scholar] [CrossRef] [PubMed]
- Popolin, C.P.; Reis, J.P.B.; Becceneri, A.B.; Graminha, A.E.; Almeida, M.A.P.; Corrêa, R.S.; Colina-Vegas, L.A.; Ellena, J.; Batista, A.A.; Cominetti, M.R. Cytotoxicity and Anti-Tumor Effects of New Ruthenium Complexes on Triple Negative Breast Cancer Cells. PLoS ONE 2017, 12, e0183275. [Google Scholar] [CrossRef]
- Ghosh, S. Cisplatin: The First Metal Based Anticancer Drug. Bioorg. Chem. 2019, 88, 102925. [Google Scholar] [CrossRef] [PubMed]
- Dasari, S.; Bernard Tchounwou, P. Cisplatin in Cancer Therapy: Molecular Mechanisms of Action. Eur. J. Pharmacol. 2014, 740, 364–378. [Google Scholar] [CrossRef] [PubMed]
- Czarnomysy, R.; Radomska, D.; Szewczyk, O.K.; Roszczenko, P.; Bielawski, K. Platinum and Palladium Complexes as Promising Sources for Antitumor Treatments. Int. J. Mol. Sci. 2021, 22, 8271. [Google Scholar] [CrossRef]
- Cai, L.; Yu, C.; Ba, L.; Liu, Q.; Qian, Y.; Yang, B.; Gao, C. Anticancer Platinum-Based Complexes with Non-Classical Structures. Appl. Organomet. Chem. 2018, 32, e4228. [Google Scholar] [CrossRef]
- Lazarević, T.; Rilak, A.; Bugarčić, Ž.D. Platinum, Palladium, Gold and Ruthenium Complexes as Anticancer Agents: Current Clinical Uses, Cytotoxicity Studies and Future Perspectives. Eur. J. Med. Chem. 2017, 142, 8–31. [Google Scholar] [CrossRef]
- Jin, S.; Guo, Y.; Guo, Z.; Wang, X. Monofunctional Platinum(II) Anticancer Agents. Pharmaceuticals 2021, 14, 133. [Google Scholar] [CrossRef]
- Ndagi, U.; Mhlongo, N.; Soliman, M.E. Metal Complexes in Cancer Therapy—An Update from Drug Design Perspective. Drug Des. Devel. Ther. 2017, 11, 599–616. [Google Scholar] [CrossRef] [PubMed]
- Medici, S.; Peana, M.; Nurchi, V.M.; Lachowicz, J.I.; Crisponi, G.; Zoroddu, M.A. Noble Metals in Medicine: Latest Advances. Coord. Chem. Rev. 2015, 284, 329–350. [Google Scholar] [CrossRef]
- Xue, Y.; Gao, S.; Gou, J.; Yin, T.; He, H.; Wang, Y.; Zhang, Y.; Tang, X.; Wu, R. Platinum-Based Chemotherapy in Combination with PD-1/PD-L1 Inhibitors: Preclinical and Clinical Studies and Mechanism of Action. Expert Opin. Drug Deliv. 2021, 18, 187–203. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, M.C.; Resasco, A.; Di Virgilio, A.L.; Ayala, M.; Cavaco, I.; Cabrera, S.; Aleman, J.; León, I.E. In Vitro and in Vivo Anticancer Effects of Two Quinoline–Platinum(II) Complexes on Human Osteosarcoma Models. Cancer Chemother Pharm. 2019, 83, 681–692. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Gao, C.; Liu, Q.; Yu, C.; Zhang, Z.; Cai, L.; Yang, B.; Qian, Y.; Yang, J.; Liao, X. Research Progress in Modern Structure of Platinum Complexes. Eur. J. Med. Chem. 2017, 140, 349–382. [Google Scholar] [CrossRef]
- Huang, X.; Liu, Z.; Wang, M.; Yin, X.; Wang, Y.; Dai, L.; Wang, H. Platinum(IV) Complexes Conjugated with Chalcone Analogs as Dual Targeting Anticancer Agents: In Vitro and in Vivo Studies. Bioorg. Chem. 2020, 105, 104430. [Google Scholar] [CrossRef]
- Zhong, Y.; Jia, C.; Zhang, X.; Liao, X.; Yang, B.; Cong, Y.; Pu, S.; Gao, C. Targeting Drug Delivery System for Platinum(Ⅳ)-Based Antitumor Complexes. Eur. J. Med. Chem. 2020, 194, 112229. [Google Scholar] [CrossRef]
- Eskandari, A.; Kundu, A.; Ghosh, S.; Suntharalingam, K. A Triangular Platinum(II) Multinuclear Complex with Cytotoxicity Towards Breast Cancer Stem Cells. Angew. Chem.-Int. Ed. 2019, 58, 12059–12064. [Google Scholar] [CrossRef]
- Köberle, B.; Schoch, S. Platinum Complexes in Colorectal Cancer and Other Solid Tumors. Cancers 2021, 13, 2073. [Google Scholar] [CrossRef]
- Fernandes, C.; Horn, A.; Lopes, B.F.; Bull, E.S.; Azeredo, N.F.B.; Kanashiro, M.M.; Borges, F.V.; Bortoluzzi, A.J.; Szpoganicz, B.; Pires, A.B.; et al. Induction of Apoptosis in Leukemia Cell Lines by New Copper(II) Complexes Containing Naphthyl Groups via Interaction with Death Receptors. J. Inorg. Biochem. 2015, 153, 68–87. [Google Scholar] [CrossRef]
- Morcelli, S.R.; Bull, É.S.; Terra, W.S.; Moreira, R.O.; Borges, F.V.; Kanashiro, M.M.; Bortoluzzi, A.J.; Maciel, L.L.F.; de, A. Almeida, J.C.; Júnior, A.H.; et al. Synthesis, Characterization and Antitumoral Activity of New Cobalt(II)Complexes: Effect of the Ligand Isomerism on the Biological Activity of the Complexes. J. Inorg. Biochem. 2016, 161, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Maciel, L.L.F.; de Freitas, W.R.; Bull, E.S.; Fernandes, C.; Horn, A.; de Aquino Almeida, J.C.; Kanashiro, M.M. In Vitro and in Vivo Anti-Proliferative Activity and Ultrastructure Investigations of a Copper(II) Complex toward Human Lung Cancer Cell NCI-H460. J. Inorg. Biochem. 2020, 210, 111166. [Google Scholar] [CrossRef] [PubMed]
- Terra, W. da S.; Bull, É.S.; Morcelli, S.R.; Moreira, R.R.; Maciel, L.L.F.; Almeida, J.C. de A.; Kanashiro, M.M.; Fernandes, C.; Horn, A. Antitumor Activity via Apoptotic Cell Death Pathway of Water Soluble Copper(II) Complexes: Effect of the Diamino Unit on Selectivity against Lung Cancer NCI-H460 Cell Line. BioMetals 2021, 34, 661–674. [Google Scholar] [CrossRef] [PubMed]
- Moreira, R.O.; Morcelli, S.R.; Kanashiro, M.M.; Resende, J.A.L.C.; Maciel, L.L.F.; João, J.C.; Gahan, L.R.; Horn, A.; Fernandes, C. Modulating the Antitumoral Activity by the Design of New Platinum(II) Compounds: Synthesis, Characterization, DFT, Ultrastructure and Mechanistic Studies. J. Inorg. Biochem. 2019, 194, 200–213. [Google Scholar] [CrossRef] [PubMed]
- Lasunskaia, E.B.; Fridlianskaia, I.I.; Darieva, Z.A.; Da Silva, M.S.R.; Kanashiro, M.M.; Margulis, B.A. Transfection of NS0 Myeloma Fusion Partner Cells with HSP70 Gene Results in Higher Hybridoma Yield by Improving Cellular Resistance to Apoptosis. Biotechnol. Bioeng. 2003, 81, 496–504. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid Colorimetric Assay for Cellular Growth and Survival: Application to Proliferation and Cytotoxicity Assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Fernandes, C.; Horn, A.; Vieira-Da-Motta, O.; Kanashiro, M.M.; Rocha, M.R.; Moreira, R.O.; Morcelli, S.R.; Lopes, B.F.; Mathias, L.D.S.; Borges, F.V.; et al. Synthesis, Characterization, Antibacterial and Antitumoral Activities of Mononuclear Zinc Complexes Containing Tridentate Amine Based Ligands with N3 or N2O Donor Groups. Inorganica. Chim. Acta. 2014, 416, 35–48. [Google Scholar] [CrossRef]
- Bézivin, C.; Tomasi, S.; Dévéhat, F.L.; Boustie, J. Cytotoxic Activity of Some Lichen Extracts on Murine and Human Cancer Cell Lines. Phytomedicine 2003, 10, 499–503. [Google Scholar] [CrossRef]
- Adhikari, S.; Hussain, O.; Phillips, R.M.; Kaminsky, W.; Kollipara, M.R. Neutral and Cationic Half-Sandwich Arene D6 Metal Complexes Containing Pyridyl and Pyrimidyl Thiourea Ligands with Interesting Bonding Modes: Synthesis, Structural and Anti-Cancer Studies. Appl. Organomet. Chem. 2018, 32, e4476. [Google Scholar] [CrossRef]
- Zorova, L.D.; Popkov, V.A.; Plotnikov, E.Y.; Silachev, D.N.; Pevzner, I.B.; Jankauskas, S.S.; Babenko, V.A.; Zorov, S.D.; Balakireva, A.V.; Juhaszova, M.; et al. Mitochondrial Membrane Potential. Anal. Biochem. 2018, 552, 50–59. [Google Scholar] [CrossRef]
- Zamzami, N.; Larochette, N.; Kroemer, G. Mitochondrial Permeability Transition in Apoptosis and Necrosis. Cell Death Differ. 2005, 12, 1478–1480. [Google Scholar] [CrossRef] [PubMed]
- Terenzi, A.; Pirker, C.; Keppler, B.K.; Berger, W. Anticancer Metal Drugs and Immunogenic Cell Death. J. Inorg. Biochem. 2016, 165, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Aston, W.J.; Hope, D.E.; Nowak, A.K.; Robinson, B.W.; Lake, R.A.; Lesterhuis, W.J. A Systematic Investigation of the Maximum Tolerated Dose of Cytotoxic Chemotherapy with and without Supportive Care in Mice. BMC Cancer 2017, 17, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Bergamo, A.; Dyson, P.J.; Sava, G. The Mechanism of Tumour Cell Death by Metal-Based Anticancer Drugs Is Not Only a Matter of DNA Interactions. Coord Chem. Rev. 2018, 360, 17–33. [Google Scholar] [CrossRef]
- Zaki, M.; Arjmand, F.; Tabassum, S. Current and Future Potential of Metallo Drugs: Revisiting DNA-Binding of Metal Containing Molecules and Their Diverse Mechanism of Action. Inorganica. Chim. Acta 2016, 444, 1–22. [Google Scholar] [CrossRef]
- Tang, D.; Kang, R.; Berghe, T.V.; Vandenabeele, P.; Kroemer, G. The Molecular Machinery of Regulated Cell Death. Cell Res. 2019, 29, 347–364. [Google Scholar] [CrossRef]
- Wang, F.Y.; Tang, X.M.; Wang, X.; Huang, K.B.; Feng, H.W.; Chen, Z.F.; Liu, Y.N.; Liang, H. Mitochondria-Targeted Platinum(II) Complexes Induce Apoptosis-Dependent Autophagic Cell Death Mediated by ER-Stress in A549 Cancer Cells. Eur. J. Med. Chem. 2018, 155, 639–650. [Google Scholar] [CrossRef]
- Medina-Reyes, E.I.; Mancera-Rodríguez, M.A.; Delgado-Buenrostro, N.L.; Moreno-Rodríguez, A.; Bautista-Martínez, J.L.; Díaz-Velásquez, C.E.; Martínez-Alarcón, S.A.; Torrens, H.; de los Ángeles Godínez-Rodríguez, M.; Terrazas-Valdés, L.I.; et al. Novel Thiosemicarbazones Induce High Toxicity in Estrogen-Receptor-Positive Breast Cancer Cells (MCF7) and Exacerbate Cisplatin Effectiveness in Triple-Negative Breast (MDA-MB231) and Lung Adenocarcinoma (A549) Cells. Investig. N. Drugs 2020, 38, 558–573. [Google Scholar] [CrossRef]
- Horn, A.; Fernandes, C.; Parrilha, G.L.; Kanashiro, M.M.; Borges, F.V.; De Melo, E.J.T.; Schenk, G.; Terenzi, H.; Pich, C.T. Highly Efficient Synthetic Iron-Dependent Nucleases Activate Both Intrinsic and Extrinsic Apoptotic Death Pathways in Leukemia Cancer Cells. J. Inorg. Biochem. 2013, 128, 38–47. [Google Scholar] [CrossRef]
- Chen, Q.Y.; DesMarais, T.; Costa, M. Metals and Mechanisms of Carcinogenesis. Annu. Rev. Pharmacol. Toxicol. 2019, 59, 537–554. [Google Scholar] [CrossRef]
- Brozovic, A.; Ambriović-Ristov, A.; Osmak, M. The Relationship between Cisplatin-Induced Reactive Oxygen Species, Glutathione, and BCL-2 and Resistance to Cisplatin. Crit. Rev. Toxicol. 2010, 40, 347–359. [Google Scholar] [CrossRef] [PubMed]
- Mello-Andrade, F.; Guedes, A.P.M.; Pires, W.C.; Velozo-Sá, V.S.; Delmond, K.A.; Mendes, D.; Molina, M.S.; Matuda, L.; de Sousa, M.A.M.; Melo-Reis, P.; et al. Ru(II)/Amino Acid Complexes Inhibit the Progression of Breast Cancer Cells through Multiple Mechanism-Induced Apoptosis. J. Inorg. Biochem. 2022, 226, 111625. [Google Scholar] [CrossRef] [PubMed]
- Altaf, M.; Casagrande, N.; Mariotto, E.; Baig, N.; Kawde, A.N.; Corona, G.; Larcher, R.; Borghese, C.; Pavan, C.; Seliman, A.A.; et al. Potent in Vitro and in Vivo Anticancer Activity of New Bipyridine and Bipyrimidine Gold (III) Dithiocarbamate Derivatives. Cancers 2019, 11, 474. [Google Scholar] [CrossRef] [PubMed]
- Al-Bahlani, S.; Al-Dhahli, B.; Al-Adawi, K.; Al-Nabhani, A.; Al-Kindi, M. Platinum-Based Drugs Differentially Affect the Ultrastructure of Breast Cancer Cell Types. Biomed. Res. Int. 2017, 2017, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Hall, A. The Cytoskeleton and Cancer. Cancer Metastasis Rev. 2009, 28, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.X.; Zhong, H.J.; Yang, G.; Vellaisamy, K.; Ma, D.L.; Leung, C.H. Recent Development of Transition Metal Complexes with in Vivo Antitumor Activity. J. Inorg. Biochem. 2017, 177, 276–286. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maciel, L.L.F.; Silva, M.B.; Moreira, R.O.; Cardoso, A.P.; Fernandes, C.; Horn, A., Jr.; de Aquino Almeida, J.C.; Kanashiro, M.M. In Vitro and In Vivo Relevant Antineoplastic Activity of Platinum(II) Complexes toward Triple-Negative MDA-MB-231 Breast Cancer Cell Line. Pharmaceutics 2022, 14, 2013. https://doi.org/10.3390/pharmaceutics14102013
Maciel LLF, Silva MB, Moreira RO, Cardoso AP, Fernandes C, Horn A Jr., de Aquino Almeida JC, Kanashiro MM. In Vitro and In Vivo Relevant Antineoplastic Activity of Platinum(II) Complexes toward Triple-Negative MDA-MB-231 Breast Cancer Cell Line. Pharmaceutics. 2022; 14(10):2013. https://doi.org/10.3390/pharmaceutics14102013
Chicago/Turabian StyleMaciel, Leide Laura Figueiredo, Marina Barreto Silva, Rafaela Oliveira Moreira, Ana Paula Cardoso, Christiane Fernandes, Adolfo Horn, Jr., João Carlos de Aquino Almeida, and Milton Masahiko Kanashiro. 2022. "In Vitro and In Vivo Relevant Antineoplastic Activity of Platinum(II) Complexes toward Triple-Negative MDA-MB-231 Breast Cancer Cell Line" Pharmaceutics 14, no. 10: 2013. https://doi.org/10.3390/pharmaceutics14102013
APA StyleMaciel, L. L. F., Silva, M. B., Moreira, R. O., Cardoso, A. P., Fernandes, C., Horn, A., Jr., de Aquino Almeida, J. C., & Kanashiro, M. M. (2022). In Vitro and In Vivo Relevant Antineoplastic Activity of Platinum(II) Complexes toward Triple-Negative MDA-MB-231 Breast Cancer Cell Line. Pharmaceutics, 14(10), 2013. https://doi.org/10.3390/pharmaceutics14102013





