NIL10: A New IL10-Receptor Binding Nanoparticle That Induces Cardiac Protection in Mice and Pigs Subjected to Acute Myocardial Infarction through STAT3/NF-κB Activation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Equipment
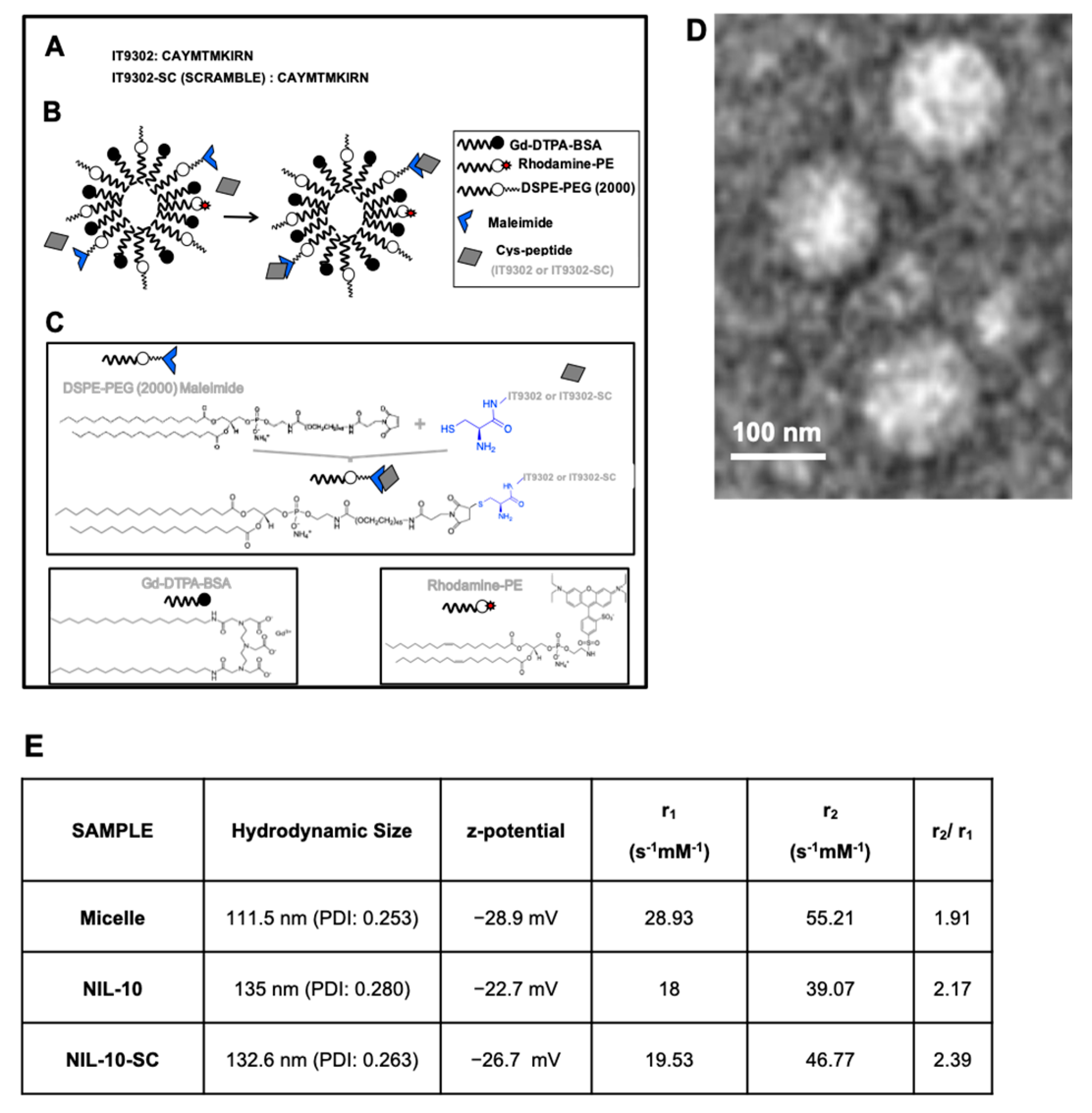
2.2. Peptide and Nanoprobe Composition
2.3. Animal Studies
2.3.1. Porcine Model of Coronary Ischemia/Reperfusion
2.3.2. Murine Model of Coronary Ischemia/Reperfusion
2.4. Echocardiography
2.5. Histology
2.6. Confocal Microscopy
2.7. Immunoblotting
2.8. Blood Collection and Plasma Isolation
2.9. Cytokine and Chemokine Determinations
2.10. Single-Cell Suspension for Flow Cytometry
2.11. Statistical Analysis
3. Results
3.1. NIL10 Improves Cardiac Function in Mice Subjected to IR
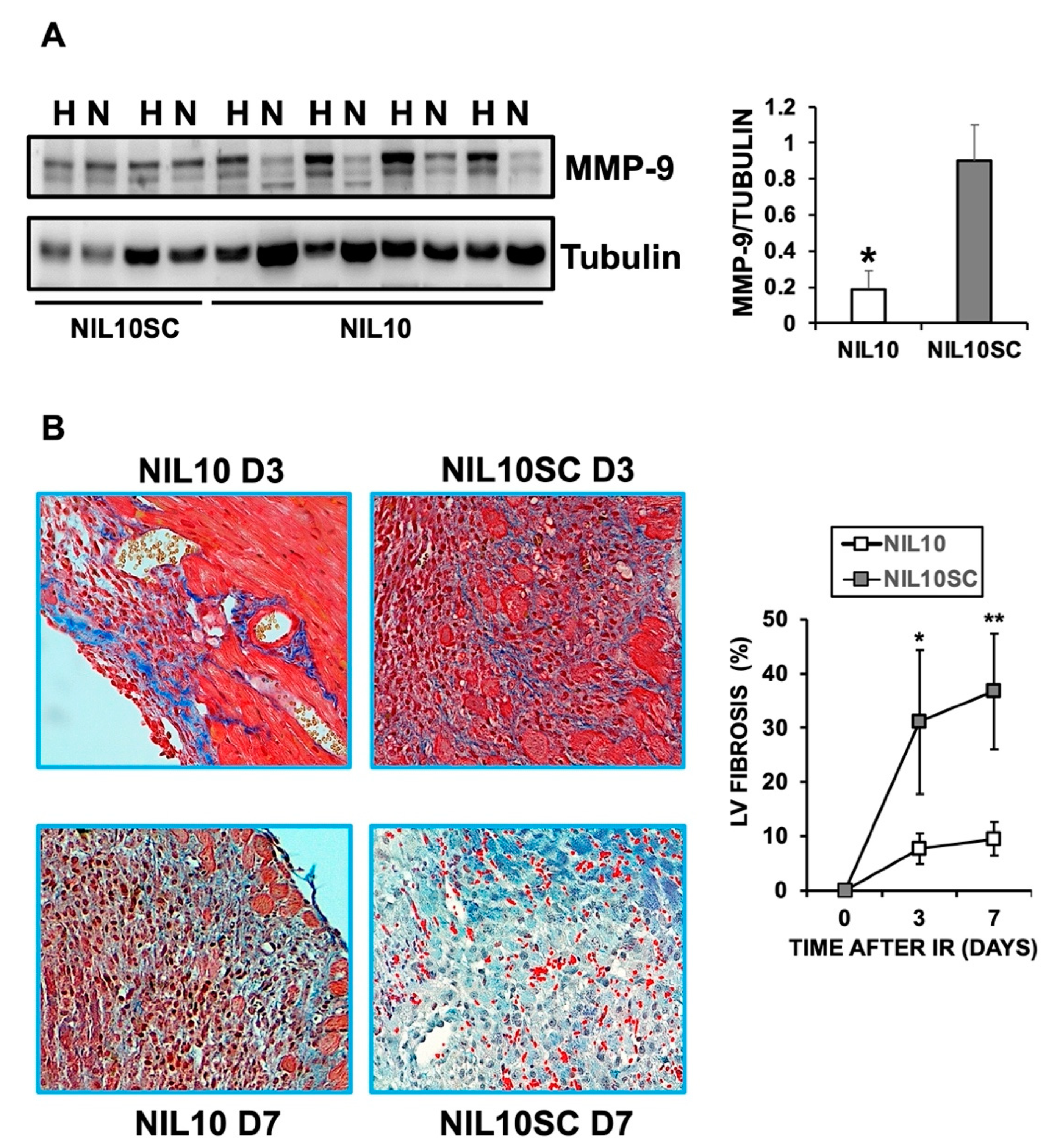
3.2. Administration of NIL10 Reduces Necrosis and Fibrosis in Mouse Hearts Subjected to IR
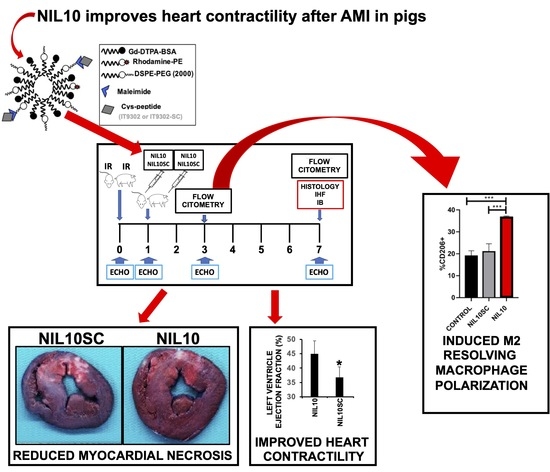
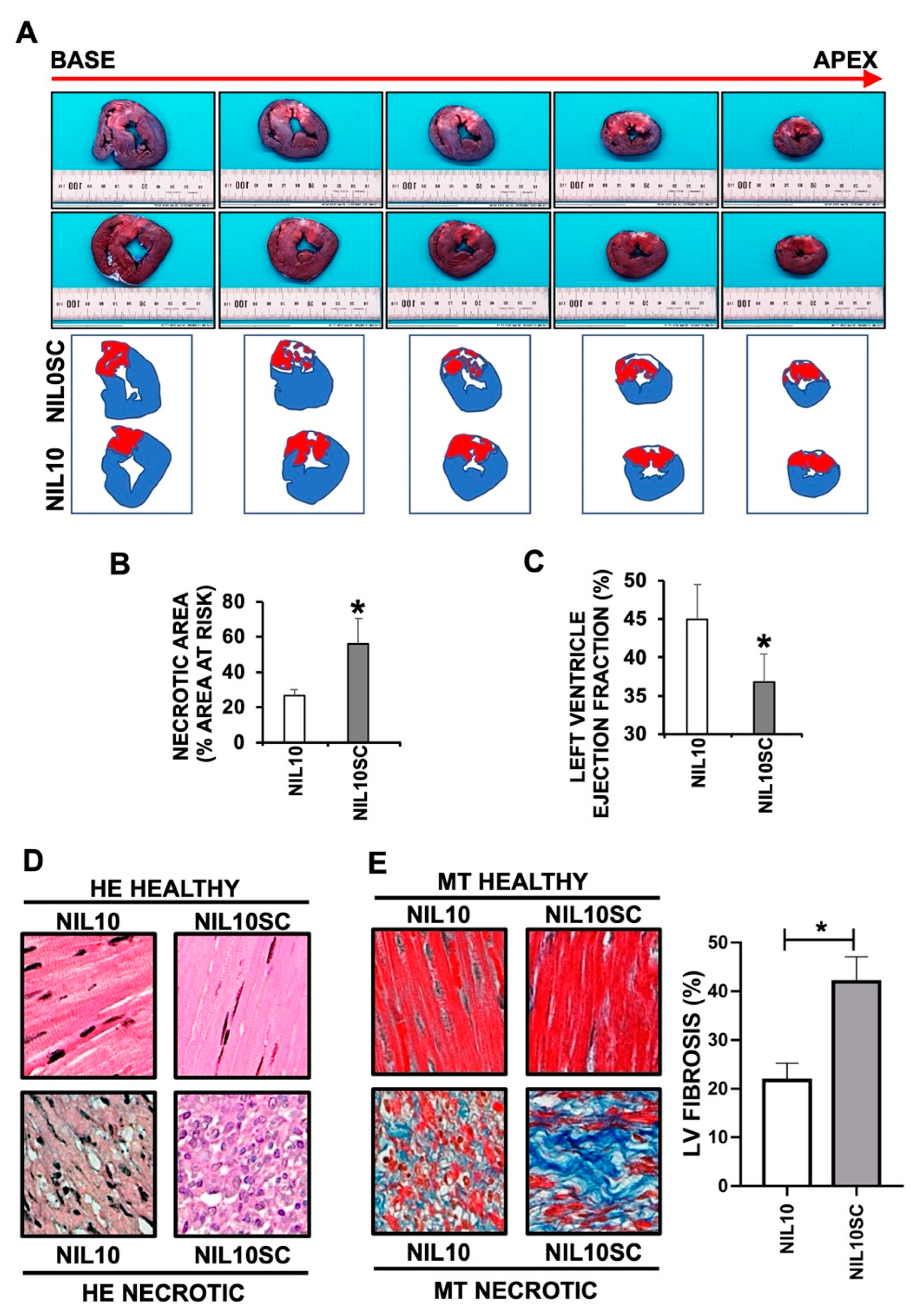
3.3. NIL10 Improves Cardiac Function in Pigs Subjected to IR
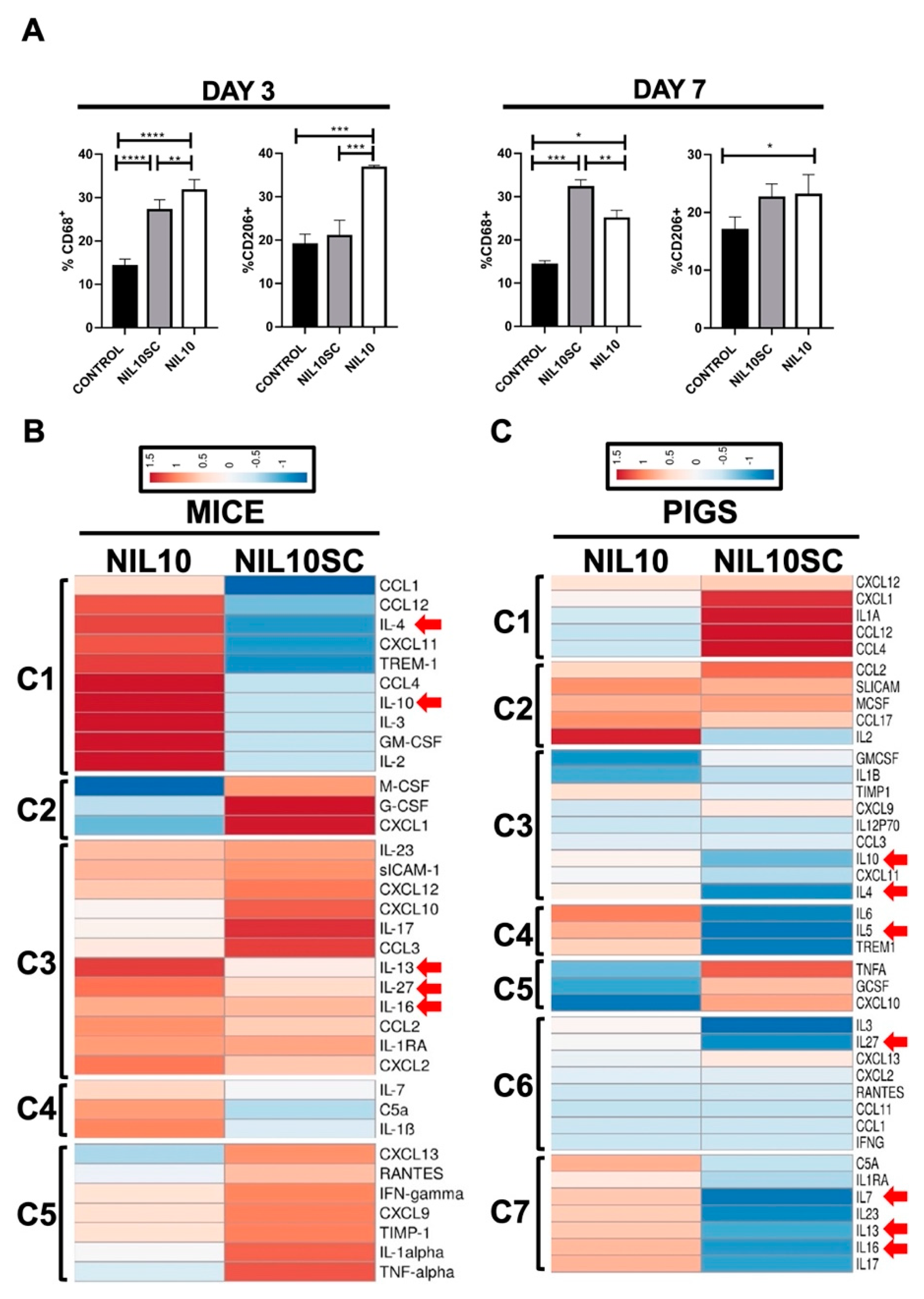
3.4. NIL10 Has an Impact on Macrophage Polarization
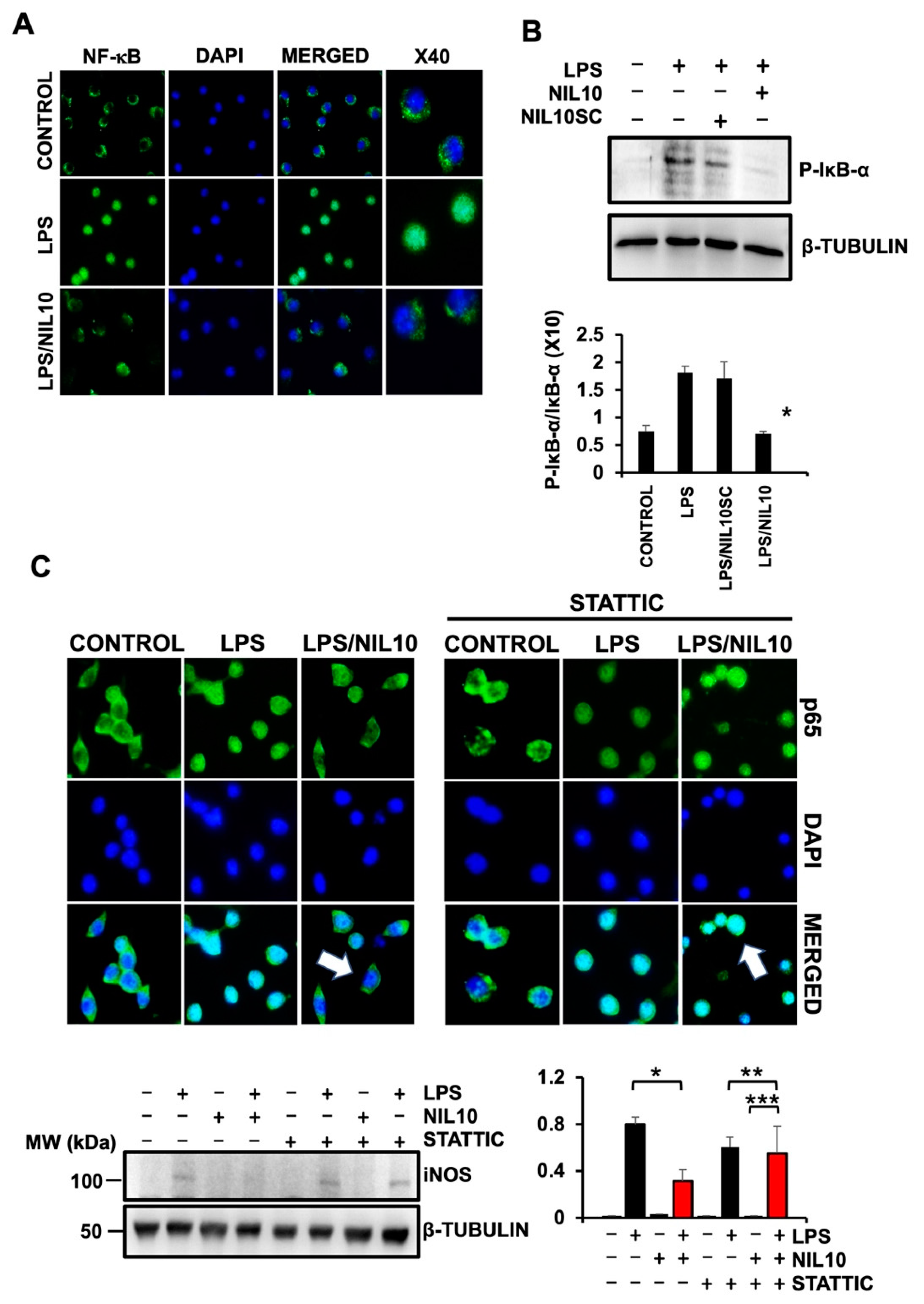
3.5. NIL10 Induces Phosphorylation of STAT3 in Pigs Subjected to IR
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Virani, S.S.; Alonso, A.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Delling, F.N.; et al. Heart disease and stroke statistics—2020 update: A report from the American Heart Association. Circulation 2020, 141, e139–e596. [Google Scholar] [CrossRef] [PubMed]
- Velagaleti, R.S.; Pencina, M.J.; Murabito, J.M.; Wang, T.J.; Parikh, N.I.; D’Agostino, R.B.; Levy, D.; Kannel, W.B.; Vasan, R.S. Long-term trends in the incidence of heart failure after myocardial infarction. Circulation 2008, 118, 2057–2062. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Hsieh, A.F.C.; Dharmarajan, K.; Masoudi, F.A.; Krumholz, H.M. National trends in heart failure hospitalization after acute myocardial infarction for medicare beneficiaries 1998–2010. Circulation 2013, 128, 2577–2584. [Google Scholar] [CrossRef] [PubMed]
- Ezekowitz, J.A.; Kaul, P.; Bakal, J.A.; Armstrong, P.W.; Welsh, R.C.; McAlister, F.A. Declining In-Hospital Mortality and Increasing Heart Failure Incidence in Elderly Patients with First Myocardial Infarction. J. Am. Coll. Cardiol. 2009, 53, 13–20. [Google Scholar] [CrossRef]
- Prabhu, S.D.; Frangogiannis, N.G. The biological basis for cardiac repair after myocardial infarction. Circ. Res. 2016, 119, 91–112. [Google Scholar] [CrossRef]
- Frangogiannis, N. Targeting the Inflammatory Response in Healing Myocardial Infarcts. Curr. Med. Chem. 2006, 13, 1877–1893. [Google Scholar] [CrossRef]
- Westman, P.C.; Lipinski, M.J.; Luger, D.; Waksman, R.; Bonow, R.O.; Wu, E.; Epstein, S.E. Inflammation as a Driver of Adverse Left Ventricular Remodeling after Acute Myocardial Infarction. J. Am. Coll. Cardiol. 2016, 67, 2050–2060. [Google Scholar] [CrossRef]
- Peet, C.; Ivetic, A.; Bromage, D.I.; Shah, A.M. Cardiac monocytes and macrophages after myocardial infarction. Cardiovasc. Res. 2020, 116, 1101–1112. [Google Scholar] [CrossRef]
- Ramirez-Carracedo, R.; Tesoro, L.; Hernandez, I.; Diez-Mata, J.; Piñeiro, D.; Hernandez-Jimenez, M.; Zamorano, J.L.; Zaragoza, C. TargetingTLR4 with aptoll improves heart function in response to coronary ischemia reperfusion in pigs undergoing acute myocardial infarction. Biomolecules 2020, 10, 1167. [Google Scholar] [CrossRef]
- Fujiu, K.; Wang, J.; Nagai, R. Cardioprotective function of cardiac macrophages. Cardiovasc. Res. 2014, 102, 232–239. [Google Scholar] [CrossRef] [Green Version]
- Martinez, F.O.; Gordon, S. The M1 and M2 paradigm of macrophage activation: Time for reassessment. F1000Prime Rep. 2014, 6, 13. [Google Scholar] [CrossRef] [PubMed]
- Sica, A.; Mantovani, A. Macrophage plasticity and polarization: In vivo veritas. J. Clin. Investig. 2012, 122, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Murray, P.J.; Allen, J.E.; Biswas, S.K.; Fisher, E.A.; Gilroy, D.W.; Goerdt, S.; Gordon, S.; Hamilton, J.A.; Ivashkiv, L.B.; Lawrence, T.; et al. Macrophage Activation and Polarization: Nomenclature and Experimental Guidelines. Immunity 2014, 41, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Josephson, K.; Logsdon, N.J.; Walter, M.R. Crystal structure of the IL-10/IL-10R1 complex reveals a shared receptor binding site. Immunity 2001, 15, 35–46. [Google Scholar] [CrossRef]
- Tedgui, A.; Mallat, Z. Anti-inflammatory mechanisms in the vascular wall. Circ. Res. 2001, 88, 877–887. [Google Scholar] [CrossRef]
- Van Der Meeren, A.; Squiban, C.; Gourmelon, P.; Lafont, H.; Gaugler, M.H. Differential regulation by IL-4 and IL-10 of radiation-induced IL-6 and IL-8 production and ICAM-1 expression by human endothelial cells. Cytokine 1999, 11, 831–838. [Google Scholar] [CrossRef]
- Selzman, C.H.; McIntyre, R.C., Jr.; Shames, B.D.; Whitehill, T.A.; Banerjee, A.; Harken, A.H. Interleukin-10 inhibits human vascular smooth muscle proliferation. J. Mol. Cell. Cardiol. 1998, 30, 889–896. [Google Scholar] [CrossRef]
- Weber-Nordt, R.M.; Riley, J.K.; Greenlund, A.C.; Moore, K.W.; Darnell, J.E.; Schreiber, R.D. Stat3 recruitment by two distinct ligand-induced, tyrosine- phosphorylated docking sites in the interleukin-10 receptor intracellular domain. J. Biol. Chem. 1996, 271, 27954–27961. [Google Scholar] [CrossRef]
- Riley, J.K.; Takeda, K.; Akira, S.; Schreiber, R.D. Interleukin-10 Receptor Signaling through the JAK-STAT Pathway. J. Biol. Chem. 1999, 274, 16513–16521. [Google Scholar] [CrossRef]
- Götze, A.M.; Schubert, C.; Jung, G.; Dörr, O.; Liebetrau, C.; Hamm, C.W.; Schmitz-Rixen, T.; Troidl, C.; Troidl, K. IL10 Alters Peri-Collateral Macrophage Polarization and Hind-Limb Reperfusion in Mice after Femoral Artery Ligation. Int. J. Mol. Sci. 2020, 21, 2821. [Google Scholar] [CrossRef] [Green Version]
- Zaringhalam, J.; Akhtari, Z.; Eidi, A.; Ruhani, A.H.; Tekieh, E. Relationship between serum IL10 level and p38MAPK enzyme activity on behavioral and cellular aspects of variation of hyperalgesia during different stages of arthritis in rats. Inflammopharmacology 2014, 22, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Qian, Q.; Wu, C.; Chen, J.; Wang, W. Relationship between IL10 and PD-L1 in Liver Hepatocellular Carcinoma Tissue and Cell Lines. BioMed Res. Int. 2020, 16, 8910183. [Google Scholar] [CrossRef] [PubMed]
- Hsu, T.I.; Wang, Y.C.; Hung, C.Y.; Yu, C.H.; Su, W.C.; Chang, W.C.; Hung, J.-J. Positive feedback regulation between IL10 and EGFR promotes lung cancer formation. Oncotarget 2016, 7, 20840–20854. [Google Scholar] [CrossRef] [PubMed]
- McKelvey, R.; Berta, T.; Old, E.; Ji, R.R.; Fitzgerald, M. Neuropathic pain is constitutively suppressed in early life by anti-inflammatory neuroimmune regulation. J. Neurosci. 2015, 35, 457–466. [Google Scholar] [CrossRef]
- López, M.N.; Pesce, B.; Kurte, M.; Pérez, C.; Segal, G.; Roa, J.; Aguillón, J.C.; Mendoza-Naranjo, A.; Gesser, B.; Larsen, C.; et al. A synthetic peptide homologous to IL-10 functional domain induces monocyte differentiation to TGF-β+ tolerogenic dendritic cells. Immunobiology 2011, 216, 1117–1126. [Google Scholar] [CrossRef]
- Yurchenko, V.; Pushkarsky, T.; Li, J.H.; Dai, W.W.; Sherry, B.; Bukrinsky, M. Regulation of CD147 cell surface expression: Involvement of the proline residue in the CD147 transmembrane domain. J. Biol. Chem. 2005, 280, 17013–17019. [Google Scholar] [CrossRef]
- Ramirez-Carracedo, R.; Sanmartin, M.; Ten, A.; Hernandez, I.; Tesoro, L.; Diez-Mata, J.; Botana, L.; Ovejero-Paredes, K.; Filice, M.; Alberich-Bayarri, A.; et al. Theranostic Contribution of Extracellular Matrix Metalloprotease Inducer-Paramagnetic Nanoparticles Against Acute Myocardial Infarction in a Pig Model of Coronary Ischemia-Reperfusion. Circ. Cardiovasc. Imaging 2022, 15, e013379. [Google Scholar] [CrossRef]
- Babicki, S.; Arndt, D.; Marcu, A.; Liang, Y.; Grant, J.R.; Maciejewski, A.; Wishart, D.S. Heatmapper: Web-enabled heat mapping for all. Nucleic Acids Res. 2016, 44, W147–W153. [Google Scholar] [CrossRef]
- Shimamoto, A.; Chong, A.J.; Yada, M.; Shomura, S.; Takayama, H.; Fleisig, A.J.; Agnew, M.L.; Hampton, C.R.; Rothnie, C.L.; Spring, D.J.; et al. Inhibition of Toll-like receptor 4 with eritoran attenuates myocardial ischemia-reperfusion injury. Circulation 2006, 114 (Suppl. 1), I270–I274. [Google Scholar] [CrossRef]
- Saraiva, M.; Vieira, P.; O’Garra, A. Biology and therapeutic potential of interleukin-10. J. Exp. Med. 2020, 217, e20190418. [Google Scholar] [CrossRef] [Green Version]
- Ambrosius, W.; Kazmierski, R.; Michalak, S.; Kozubski, W. Anti-inflammatory cytokines in subclinical carotid atherosclerosis. Neurology 2006, 66, 1946–1948. [Google Scholar] [CrossRef] [PubMed]
- Heeschen, C.; Dimmeler, S.; Hamm, C.W.; Fichtlscherer, S.; Boersma, E.; Simoons, M.L.; Zeiher, A.M. CAPTURE Study Investigators. Serum level of the antiinflammatory cytokine interleukin-10 is an important prognostic determinant in patients with acute coronary syndromes. Circulation 2003, 107, 2109–2114. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.A.; Irving, S.D.; Sheldon, J.; Cole, D.; Kaski, J.C. Serum levels of the antiinflammatory cytokine interleukin-10 are decreased in patients with unstable angina. Circulation 2001, 104, 746–749. [Google Scholar] [CrossRef] [PubMed]
- Miettinen, K.H.; Lassus, J.; Harjola, V.P.; Siirilä-Waris, K.; Melin, J.; Punnonen, K.R.; Nieminen, M.S.; Laakso, M.; Peuhkurinen, K.J. Prognostic role of pro- and anti-inflammatory cytokines and their polymorphisms in acute decompensated heart failure. Eur. J. Heart Fail. 2008, 10, 396–403. [Google Scholar] [CrossRef]
- Lakhani, H.V.; Khanal, T.; Gabi, A.; Yousef, G.; Alam, M.B.; Sharma, D.; Aljoudi, H.; Puri, N.; Thompson, E.; Shapiro, J.I.; et al. Developing a panel of biomarkers and miRNA in patients with myocardial infarction for early intervention strategies of heart failure in West Virginian population. PLoS ONE 2018, 13, e0205329. [Google Scholar] [CrossRef] [PubMed]
- Cuadrado, I.; Piedras, M.J.; Herruzo, I.; Turpin Mdel, C.; Castejón, B.; Reventun, P.; Martin, A.; Saura, M.; Zamorano, J.L.; Zaragoza, C. EMMPRIN-Targeted magnetic nanoparticles for in vivo visualization and regression of acute myocardial infarction. Theranostics 2016, 6, 545–557. [Google Scholar] [CrossRef]
- Kurte, M.; López, M.; Aguirre, A.; Escobar, A.; Aguillón, J.C.; Charo, J.; Larsen, C.G.; Kiessling, R.; Salazar-Onfray, F. A synthetic peptide homologous to functional domain of human IL-10 down-regulates expression of MHC class I and Transporter associated with Antigen Processing 1/2 in human melanoma cells. J. Immunol. 2004, 173, 1731–1737. [Google Scholar] [CrossRef]
- Osman, M.O.; Gesser, B.; Mortensen, J.T.; Matsushima, K.; Jensen, S.L.; Larsen, C.G. Profiles of pro-inflammatory cytokines in the serum of rabbits after experimentally induced acute pancreatitis. Cytokine 2002, 17, 53–59. [Google Scholar] [CrossRef]
- Osman, M.O.; Jacobsen, N.O.; Kristensen, J.U.; Deleuran, B.; Gesser, B.; Larsen, C.G.; Jensen, S.L. IT 9302, a synthetic interleukin-10 agonist, diminishes acute lung injury in rabbits with acute necrotizing pancreatitis. Surgery 1998, 124, 584–592. [Google Scholar] [CrossRef]
- Boisguérin, P.; Covinhes, A.; Gallot, L.; Barrère, C.; Vincent, A.; Busson, M.; Piot, C.; Nargeot, J.; Lebleu, B.; Barrère-Lemaire, S. A novel therapeutic peptide targeting myocardial reperfusion injury. Cardiovasc. Res. 2020, 116, 633–644. [Google Scholar] [CrossRef]
- Moreira, R.S.; Irigoyen, M.C.; Capcha, J.M.C.; Sanches, T.R.; Gutierrez, P.S.; Garnica, M.R.; Noronha, I.d.; Andrade, L. Synthetic apolipoprotein A-I mimetic peptide 4F protects hearts and kidneys after myocardial infarction. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2020, 318, R529–R544. [Google Scholar] [CrossRef] [PubMed]
- Peña, J.R.; Pinney, J.R.; Ayala, P.; Desai, T.A.; Goldspink, P.H. Localized delivery of mechano-growth factor E-domain peptide via polymeric microstructures improves cardiac function following myocardial infarction. Biomaterials 2015, 46, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Laeremans, H.; Hackeng, T.M.; van Zandvoort, M.A.; Thijssen, V.L.; Janssen, B.J.; Ottenheijm, H.C.J.; Smits, J.F.M.; Blankesteijn, W.M. Blocking of frizzled signaling with a homologous peptide fragment of wnt3a/wnt5a reduces infarct expansion and prevents the development of heart failure after myocardial infarction. Circulation 2011, 124, 1626–1635. [Google Scholar] [CrossRef] [PubMed]
- MacRitchie, N.; Di Francesco, V.; Ferreira, M.F.M.M.; Guzik, T.J.; Decuzzi, P.; Maffia, P. Nanoparticle theranostics in cardiovascular inflammation. Semin. Immunol. 2021, 30, 101536. [Google Scholar] [CrossRef] [PubMed]
- Suka, J.S.; Xua, O.; Kima, N.; Hanesa, J.; Ensigna, L.M. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv. Drug. Deliv. Rev. 2016, 99 Pt A, 28–51. [Google Scholar] [CrossRef]
- Zolnik, B.S.; González-Fernández, A.; Sadrieh, N.; Dobrovolskaia, M.A. Nanoparticles and the Immune System. Endocrinology 2010, 151, 458–465. [Google Scholar] [CrossRef]
- Xu, X.; Prens, E.; Florencia, E.; Leenen, P.; Boon, L.; Asmawidjaja, P.; Mus, A.M.; Lubberts, E. Interleukin-17A Drives IL-19 and IL-24 Expression in Skin Stromal Cells Regulating Keratinocyte Proliferation. Front. Immunol. 2021, 12, 719562. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tesoro, L.; Hernández, I.; Ramírez-Carracedo, R.; Díez-Mata, J.; Alcharani, N.; Jiménez-Guirado, B.; Ovejero-Paredes, K.; Filice, M.; Zamorano, J.L.; Saura, M.; et al. NIL10: A New IL10-Receptor Binding Nanoparticle That Induces Cardiac Protection in Mice and Pigs Subjected to Acute Myocardial Infarction through STAT3/NF-κB Activation. Pharmaceutics 2022, 14, 2044. https://doi.org/10.3390/pharmaceutics14102044
Tesoro L, Hernández I, Ramírez-Carracedo R, Díez-Mata J, Alcharani N, Jiménez-Guirado B, Ovejero-Paredes K, Filice M, Zamorano JL, Saura M, et al. NIL10: A New IL10-Receptor Binding Nanoparticle That Induces Cardiac Protection in Mice and Pigs Subjected to Acute Myocardial Infarction through STAT3/NF-κB Activation. Pharmaceutics. 2022; 14(10):2044. https://doi.org/10.3390/pharmaceutics14102044
Chicago/Turabian StyleTesoro, Laura, Ignacio Hernández, Rafael Ramírez-Carracedo, Javier Díez-Mata, Nunzio Alcharani, Beatriz Jiménez-Guirado, Karina Ovejero-Paredes, Marco Filice, Jose Luis Zamorano, Marta Saura, and et al. 2022. "NIL10: A New IL10-Receptor Binding Nanoparticle That Induces Cardiac Protection in Mice and Pigs Subjected to Acute Myocardial Infarction through STAT3/NF-κB Activation" Pharmaceutics 14, no. 10: 2044. https://doi.org/10.3390/pharmaceutics14102044
APA StyleTesoro, L., Hernández, I., Ramírez-Carracedo, R., Díez-Mata, J., Alcharani, N., Jiménez-Guirado, B., Ovejero-Paredes, K., Filice, M., Zamorano, J. L., Saura, M., Zaragoza, C., & Botana, L. (2022). NIL10: A New IL10-Receptor Binding Nanoparticle That Induces Cardiac Protection in Mice and Pigs Subjected to Acute Myocardial Infarction through STAT3/NF-κB Activation. Pharmaceutics, 14(10), 2044. https://doi.org/10.3390/pharmaceutics14102044