Extracts of Rheum palmatum and Aloe vera Show Beneficial Properties for the Synergistic Improvement of Oral Wound Healing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Isolation and Cell Culture
2.2. Microorganisms and Culture Conditions
2.3. Preparation of RPR/AV Solutions for Cell Culture Experiments
2.4. HPLC (High-Performance Liquid Chromatography) Analysis
2.5. NMR (Nuclear Magnetic Resonance) Analysis
2.6. Cellular Viability
2.7. Migration Assay
2.8. Calculation of the Phenomenological Combination Index (pCI)
2.9. Cell Cycle Analysis
2.10. Actin Staining
2.11. Broth Microdilution Assay
2.12. Calculation of the Fractional Inhibitory Concentration Index (FICI)
2.13. Statistical Analysis
3. Results
3.1. Chemical Analysis of AV and RPR
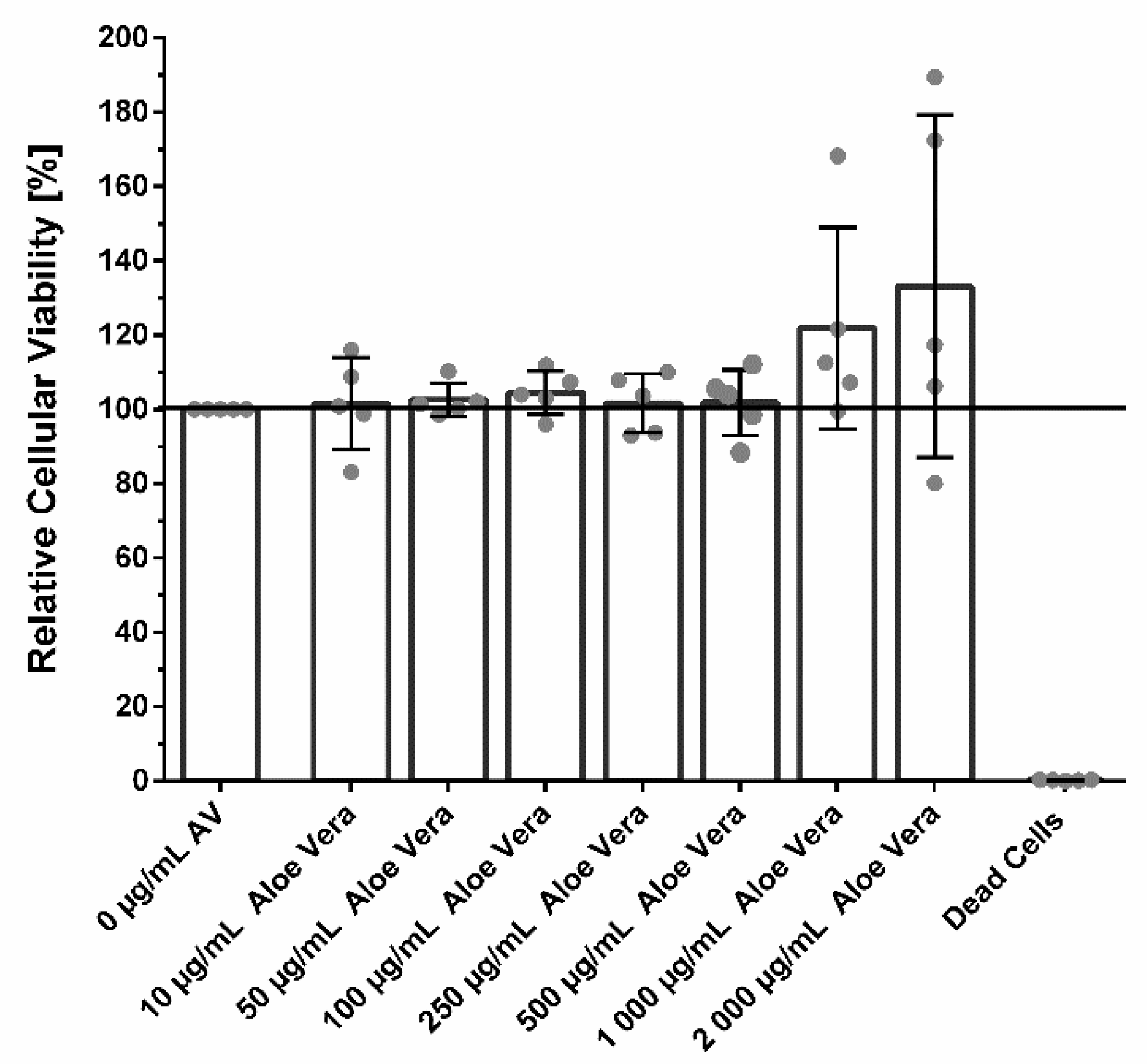
3.2. Cellular Viability
3.3. Cell Migration
Calculation of the Synergistic Action of AV and RPR on Fibroblast Migration
3.4. Cell Cycle Analysis
3.5. Actin Staining
3.6. Broth Microdilution Assay
Calculation of the Synergistic Antimicrobial Action of AVRPR
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Politis, C.; Schoenaers, J.; Jacobs, R.; Agbaje, J.O. Wound healing problems in the mouth. Front. Physiol. 2016, 7, 507. [Google Scholar] [CrossRef] [PubMed]
- Sculean, A.; Gruber, R.; Bosshardt, D.D. Soft tissue wound healing around teeth and dental implants. J. Clin. Periodontol. 2014, 41, S6–S22. [Google Scholar] [CrossRef] [PubMed]
- Costalonga, M.; Herzberg, M.C. The oral microbiome and the immunobiology of periodontal disease and caries. Immunol. Lett. 2014, 162, 22–38. [Google Scholar] [CrossRef]
- Maruyama, N.; Maruyama, F.; Takeuchi, Y.; Aikawa, C.; Izumi, Y.; Nakagawa, I. Intraindividual variation in core microbiota in peri-implantitis and periodontitis. Sci. Rep. 2014, 4, 6602. [Google Scholar] [CrossRef]
- Velnar, T.; Bailey, T.; Smrkolj, V. The wound healing process: An overview of the cellular and molecular mechanisms. J. Int. Med. Res. 2009, 37, 1528–1542. [Google Scholar] [CrossRef] [PubMed]
- Bainbridge, P. Wound healing and the role of fibroblasts. J. Wound Care 2013, 22, 407–408, 410–412. [Google Scholar] [CrossRef]
- Szpaderska, A.M.; Zuckerman, J.D.; DiPietro, L.A. Differential injury responses in oral mucosal and cutaneous wounds. J. Dent. Res. 2003, 82, 621–626. [Google Scholar] [CrossRef] [PubMed]
- Waasdorp, M.; Krom, B.P.; Bikker, F.J.; van Zuijlen, P.P.M.; Niessen, F.B.; Gibbs, S. The bigger picture: Why oral mucosa heals better than skin. Biomolecules 2021, 11, 1165. [Google Scholar] [CrossRef]
- Wong, J.W.; Gallant-Behm, C.; Wiebe, C.; Mak, K.; Hart, D.A.; Larjava, H.; Häkkinen, L. Wound healing in oral mucosa results in reduced scar formation as compared with skin: Evidence from the red Duroc pig model and humans. Wound Repair Regen. 2009, 17, 717–729. [Google Scholar] [CrossRef]
- Sørensen, L.T. Wound healing and Iifection in surgery: The pathophysiological impact of smoking, smoking cessation, and nicotine replacement therapy: A systematic review. Ann. Surg. 2012, 255, 1069–1079. [Google Scholar] [CrossRef]
- Dormand, E.L.; Banwell, P.E.; Goodacre, T.E. Radiotherapy and wound healing. Int. Wound J. 2005, 2, 112–127. [Google Scholar] [CrossRef] [PubMed]
- Haubner, F.; Ohmann, E.; Pohl, F.; Strutz, J.; Gassner, H.G. Wound healing after radiation therapy: Review of the literature. Radiat. Oncol. 2012, 7, 162. [Google Scholar] [CrossRef] [PubMed]
- Gieringer, M.; Gosepath, J.; Naim, R. Radiotherapy and wound healing: Principles, management and prospects (review). Oncol. Rep. 2011, 26, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Karamanos, E.; Osgood, G.; Siddiqui, A.; Rubinfeld, I. Wound healing in plastic surgery: Does age matter? An american college of surgeons national surgical quality improvement program study. Plast. Reconstr. Surg. 2015, 135, 876–881. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.S.; Armstrong, E.J.; Armstrong, A.W. Corticosteroids and wound healing: Clinical considerations in the perioperative period. Am. J. Surg. 2013, 206, 410–417. [Google Scholar] [CrossRef] [PubMed]
- Broughton, G.I.; Janis, J.E.; Attinger, C.E. Wound Healing: An Overview. Plast. Reconstr. Surg. 2006, 117, 1e-S–32e-S. [Google Scholar] [CrossRef]
- Lu, R.; Zhang, J.; Sun, W.; Du, G.; Zhou, G. Inflammation-related cytokines in oral lichen planus: An overview. J. Oral Pathol. Med. 2015, 44, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Halonen, P.; Jakobsson, M.; Heikinheimo, O.; Riska, A.; Gissler, M.; Pukkala, E. Cancer risk of lichen planus: A cohort study of 13,100 women in Finland. Int. J. Cancer 2018, 142, 18–22. [Google Scholar] [CrossRef]
- Le Cleach, L.; Chosidow, O. Clinical practice. Lichen planus. N. Engl. J. Med. 2012, 366, 723–732. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.Y.; Liu, W.; Zhu, L.K.; Feng, J.Q.; Tang, G.Y.; Zhou, Z.T. A retrospective clinicopathological study on oral lichen planus and malignant transformation: Analysis of 518 cases. Med. Oral Patol. Oral Cir. Bucal 2012, 17, e943–e947. [Google Scholar] [CrossRef]
- Sezgin, Y.; Bilgin Çetin, M.; Bulut, Ş.; Alptekin, N.; Börçek, P. Evaluating the Effects of a Topical Preparation with Dexpanthenol, Silbiol, Undecylenic Acid, and Lidocaine on Palatal Mucosa Wound Healing in a Rat Model. Balk. Med. J. 2019, 36, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Dörr, W.; Schlichting, S.; Bray, M.A.; Flockhart, I.R.; Hopewell, J.W. Effects of dexpanthenol with or without Aloe vera extract on radiation-induced oral mucositis: Preclinical studies. Int. J. Radiat. Biol. 2005, 81, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Angmar, B.; Carlstrom, D.; Glas, J.E. Studies on the ultrastructure of dental enamel. IV. The mineralization of normal human enamel. J. Ultrastruct. Res. 1963, 8, 12–23. [Google Scholar] [CrossRef]
- Yilmaz, Ö. The chronicles of Porphyromonas gingivalis: The microbium, the human oral epithelium and their interplay. Microbiology 2008, 154, 2897–2903. [Google Scholar] [CrossRef]
- Bhattacharya, R.; Xu, F.; Dong, G.; Li, S.; Tian, C.; Ponugoti, B.; Graves, D.T. Effect of bacteria on the wound healing behavior of oral epithelial cells. PLoS ONE 2014, 9, e89475. [Google Scholar] [CrossRef]
- Laheij, A.M.; van Loveren, C.; Deng, D.; de Soet, J.J. The impact of virulence factors of Porphyromonas gingivalis on wound healing in vitro. J. Oral Microbiol. 2015, 7, 27543. [Google Scholar] [CrossRef]
- Song, L.T.; Tada, H.; Nishioka, T.; Nemoto, E.; Imamura, T.; Potempa, J.; Li, C.Y.; Matsushita, K.; Sugawara, S. Porphyromonas gingivalis gingipains-mediated degradation of plasminogen activator inhibitor-1 leads to delayed wound healing responses in human endothelial cells. J. Innate Immun. 2022, 14, 306–319. [Google Scholar] [CrossRef]
- Hekmatpou, D.; Mehrabi, F.; Rahzani, K.; Aminiyan, A. The Effect of Aloe vera clinical trials on prevention and healing of skin wound: A systematic review. Iran. J. Med. Sci. 2019, 44, 1–9. [Google Scholar]
- Burusapat, C.; Supawan, M.; Pruksapong, C.; Pitiseree, A.; Suwantemee, C. Topical Aloe vera gel for accelerated wound healing of split-thickness skin graft donor sites: S double-blind, randomized, controlled trial and systematic review. Plast. Reconstr. Surg. 2018, 142, 217–226. [Google Scholar] [CrossRef]
- Sánchez, M.; González-Burgos, E.; Iglesias, I.; Gómez-Serranillos, M.P. Pharmacological update properties of Aloe vera and its major active constituents. Molecules 2020, 25, 1324. [Google Scholar] [CrossRef]
- Nair, G.R.; Naidu, G.S.; Jain, S.; Nagi, R.; Makkad, R.S.; Jha, A. Clinical effectiveness of Aloe vera in the management of oral mucosal diseases—A systematic review. J. Clin. Diagn. Res. 2016, 10, ZE01–ZE07. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.H.; Wei, F.; Vaziri, N.D.; Cheng, X.L.; Bai, X.; Lin, R.C.; Zhao, Y.Y. Metabolomics insights into chronic kidney disease and modulatory effect of rhubarb against tubulointerstitial fibrosis. Sci. Rep. 2015, 5, 14472. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, E.; Abou Baker, D.; El-Baz, F. Anti-inflammatory and antioxidant activities of rhubarb roots extract. Int. J. Pharm. Sci. Rev. Res. 2016, 39, 93–99. [Google Scholar]
- Müller-Heupt, L.K.; Vierengel, N.; Groß, J.; Opatz, T.; Deschner, J.; von Loewenich, F.D. Antimicrobial activity of Eucalyptus globulus, Azadirachta indica, Glycyrrhiza glabra, Rheum palmatum extracts and Rhein against Porphyromonas gingivalis. Antibiotics 2022, 11, 186. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.; Zhao, L.; Yoshioka, M.; Hinode, D.; Grenier, D. Effects of Japanese traditional herbal medicines (Kampo) on growth and virulence properties of Porphyromonas gingivalis and viability of oral epithelial cells. Pharm. Biol. 2013, 51, 1538–1544. [Google Scholar] [CrossRef]
- Kommerein, N.; Vierengel, N.; Groß, J.; Opatz, T.; Al-Nawas, B.; Müller-Heupt, L.K. Antiplanktonic and antibiofilm activity of Rheum palmatum against Streptococcus oralis and Porphyromonas gingivalis. Microorganisms 2022, 10, 965. [Google Scholar] [CrossRef]
- Fani, M.; Kohanteb, J. Inhibitory activity of Aloe vera gel on some clinically isolated cariogenic and periodontopathic bacteria. J. Oral Sci. 2012, 54, 15–21. [Google Scholar] [CrossRef]
- Rampersad, S.N. Multiple applications of Alamar Blue as an indicator of metabolic function and cellular health in cell viability bioassays. Sensors 2012, 12, 12347–12360. [Google Scholar] [CrossRef]
- Chou, T.C.; Talalay, P. Quantitative analysis of dose-effect relationships: The combined effects of multiple drugs or enzyme inhibitors. Adv. Enzym. Regul. 1984, 22, 27–55. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- CLSI. M11: Methods for Antimicrobial Susceptibility Testing of Anaerobic Bacteria, 9th ed.; Clinical & Laboratory Standards Institute: Wayne, PA, USA, 2018; Available online: https://clsi.org/media/2577/m11-ed9_sample.pdf (accessed on 21 September 2022).
- Odds, F.C. Synergy, antagonism, and what the chequerboard puts between them. J. Antimicrob. Chemother. 2003, 52, 1. [Google Scholar] [CrossRef] [PubMed]
- Damour, O.; Hua, S.Z.; Lasne, F.; Villain, M.; Rousselle, P.; Collombel, C. Cytotoxicity evaluation of antiseptics and antibiotics on cultured human fibroblasts and keratinocytes. Burns 1992, 18, 479–485. [Google Scholar] [CrossRef]
- Müller, G.; Kramer, A. Biocompatibility index of antiseptic agents by parallel assessment of antimicrobial activity and cellular cytotoxicity. J. Antimicrob. Chemother. 2008, 61, 1281–1287. [Google Scholar] [CrossRef] [PubMed]
- Punjataewakupt, A.; Napavichayanun, S.; Aramwit, P. The downside of antimicrobial agents for wound healing. Eur. J. Clin. Microbiol. Infect. Dis. 2019, 38, 39–54. [Google Scholar] [CrossRef]
- Thomas, G.W.; Rael, L.T.; Bar-Or, R.; Shimonkevitz, R.; Mains, C.W.; Slone, D.S.; Craun, M.L.; Bar-Or, D. Mechanisms of delayed wound healing by commonly used antiseptics. J. Trauma 2009, 66, 82–90, discussion 81–90. [Google Scholar] [CrossRef]
- Cui, Y.; Chen, L.J.; Huang, T.; Ying, J.Q.; Li, J. The pharmacology, toxicology and therapeutic potential of anthraquinone derivative emodin. Chin. J. Nat. Med. 2020, 18, 425–435. [Google Scholar] [CrossRef]
- Teplicki, E.; Ma, Q.; Castillo, D.E.; Zarei, M.; Hustad, A.P.; Chen, J.; Li, J. The Effects of Aloe vera on wound healing in cell proliferation, migration, and viability. Wounds 2018, 30, 263–268. [Google Scholar]
- Shafaie, S.; Andalib, S.; Shafaei, H.; Montaseri, A.; Tavakolizadeh, M. Differential biological behavior of fibroblasts and endothelial cells under Aloe vera gel culturing. Int. J. Mol. Cell Med. 2020, 9, 234–246. [Google Scholar] [CrossRef]
- Abudayyak, M. In vitro evaluation of Rheum ribes induced genotoxicity in HepG2 cell lines. Istanb. J. Pharm. 2019, 49, 132–136. [Google Scholar] [CrossRef]
- Azadpour, M.; Farajollahi, M.; Varzi, A.; Hadipour, F.; Barati, M. The evaluation of cytotoxicity effects of Rheum ribes L. (rubarb) extract on cancer cell lines and its antibacterial and mutagenicity activity. Entomol. Appl. Sci. Lett. 2020, 7, 7–12. [Google Scholar]
- Hong, J.-Y.; Chung, H.-J.; Bae, S.; Trung, T.; Bae, K.; Lee, S. Induction of cell cycle arrest and apoptosis by physcion, an anthraquinone isolated from rhubarb (rhizomes of Rheum tanguticum), in MDA-MB-231 human breast bancer cells. J. Cancer Prev. 2014, 19, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Vancheri, C. Idiopathic pulmonary fibrosis: An altered fibroblast proliferation linked to cancer biology. Proc. Am. Thorac. Soc. 2012, 9, 153–157. [Google Scholar] [CrossRef]
- Roberts, M.J.; Broome, R.E.; Kent, T.C.; Charlton, S.J.; Rosethorne, E.M. The inhibition of human lung fibroblast proliferation and differentiation by Gs-coupled receptors is not predicted by the magnitude of cAMP response. Respir. Res. 2018, 19, 56. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Rathod, N.; Nagi, R.; Sur, J.; Laheji, A.; Gupta, N.; Agrawal, P.; Prasad, S. Antibacterial effect of Aloe vera gel against oral pathogens: An in-vitro study. J. Clin. Diagn. Res. 2016, 10, zc41–zc44. [Google Scholar] [CrossRef] [PubMed]
- Mandrioli, R.; Mercolini, L.; Ferranti, A.; Fanali, S.; Raggi, M.A. Determination of aloe emodin in Aloe vera extracts and commercial formulations by HPLC with tandem UV absorption and fluorescence detection. Food Chem. 2011, 126, 387–393. [Google Scholar] [CrossRef]
- Xiang, H.; Zuo, J.; Guo, F.; Dong, D. What we already know about rhubarb: A comprehensive review. Chin. Med. 2020, 15, 88. [Google Scholar] [CrossRef] [PubMed]
- Leung, M.Y.; Liu, C.; Zhu, L.F.; Hui, Y.Z.; Yu, B.; Fung, K.P. Chemical and biological characterization of a polysaccharide biological response modifier from Aloe vera L. var. chinensis (Haw.) Berg. Glycobiology 2004, 14, 501–510. [Google Scholar] [CrossRef]
- Liu, C.; Leung, M.Y.; Koon, J.C.; Zhu, L.F.; Hui, Y.Z.; Yu, B.; Fung, K.P. Macrophage activation by polysaccharide biological response modifier isolated from Aloe vera L. var. chinensis (Haw.) Berg. Int. Immunopharmacol. 2006, 6, 1634–1641. [Google Scholar] [CrossRef]
- Thunyakitpisal, P.; Ruangpornvisuti, V.; Kengkwasing, P.; Chokboribal, J.; Sangvanich, P. Acemannan increases NF-κB/DNA binding and IL-6/-8 expression by selectively binding Toll-like receptor-5 in human gingival fibroblasts. Carbohydr. Polym. 2017, 161, 149–157. [Google Scholar] [CrossRef]
- Jettanacheawchankit, S.; Sasithanasate, S.; Sangvanich, P.; Banlunara, W.; Thunyakitpisal, P. Acemannan Stimulates Gingival Fibroblast Proliferation; Expressions of Keratinocyte Growth Factor-1, Vascular Endothelial Growth Factor, and Type I Collagen; and Wound Healing. J. Pharmacol. Sci. 2009, 109, 525–531. [Google Scholar] [CrossRef]
- Boonyagul, S.; Banlunara, W.; Sangvanich, P.; Thunyakitpisal, P. Effect of acemannan, an extracted polysaccharide from Aloe vera, on BMSCs proliferation, differentiation, extracellular matrix synthesis, mineralization, and bone formation in a tooth extraction model. Odontology 2014, 102, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.J.; Pu, Z.J.; Tang, Y.P.; Shen, J.; Chen, Y.Y.; Kang, A.; Zhou, G.S.; Duan, J.A. Advances in bio-active constituents, pharmacology and clinical applications of rhubarb. Chin. Med. 2017, 12, 36. [Google Scholar] [CrossRef] [PubMed] [Green Version]






| Substance | MIC |
|---|---|
| AV | >2048 |
| RPR | 4 |
| AVRPR | 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Müller-Heupt, L.K.; Wiesmann, N.; Schröder, S.; Korkmaz, Y.; Vierengel, N.; Groß, J.; Dahm, R.; Deschner, J.; Opatz, T.; Brieger, J.; et al. Extracts of Rheum palmatum and Aloe vera Show Beneficial Properties for the Synergistic Improvement of Oral Wound Healing. Pharmaceutics 2022, 14, 2060. https://doi.org/10.3390/pharmaceutics14102060
Müller-Heupt LK, Wiesmann N, Schröder S, Korkmaz Y, Vierengel N, Groß J, Dahm R, Deschner J, Opatz T, Brieger J, et al. Extracts of Rheum palmatum and Aloe vera Show Beneficial Properties for the Synergistic Improvement of Oral Wound Healing. Pharmaceutics. 2022; 14(10):2060. https://doi.org/10.3390/pharmaceutics14102060
Chicago/Turabian StyleMüller-Heupt, Lena Katharina, Nadine Wiesmann, Sofia Schröder, Yüksel Korkmaz, Nina Vierengel, Jonathan Groß, Rolf Dahm, James Deschner, Till Opatz, Juergen Brieger, and et al. 2022. "Extracts of Rheum palmatum and Aloe vera Show Beneficial Properties for the Synergistic Improvement of Oral Wound Healing" Pharmaceutics 14, no. 10: 2060. https://doi.org/10.3390/pharmaceutics14102060
APA StyleMüller-Heupt, L. K., Wiesmann, N., Schröder, S., Korkmaz, Y., Vierengel, N., Groß, J., Dahm, R., Deschner, J., Opatz, T., Brieger, J., Al-Nawas, B., & Kämmerer, P. W. (2022). Extracts of Rheum palmatum and Aloe vera Show Beneficial Properties for the Synergistic Improvement of Oral Wound Healing. Pharmaceutics, 14(10), 2060. https://doi.org/10.3390/pharmaceutics14102060







