Gold Nanoparticles Synthesized with Common Mullein (Verbascum thapsus) and Castor Bean (Ricinus communis) Ethanolic Extracts Displayed Antiproliferative Effects and Induced Caspase 3 Activity in Human HT29 and SW480 Cancer Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Extract Obtention and Characterization
2.3. Green (AuNPsME and AuNPsCE) and Chemical (AuNPsCS) Synthesis of Gold Nanoparticles
2.4. AuNPs Characterization
2.5. Cell Culture
2.5.1. Cell Viability Assay by 3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyl Tetrazolium Bromide (MTT) Assay
2.5.2. Intracellular Reactive Oxygen Species (ROS) Quantification
2.5.3. Caspase 3/7 Activity Assay
2.6. Statistical Analysis
3. Results and Discussion
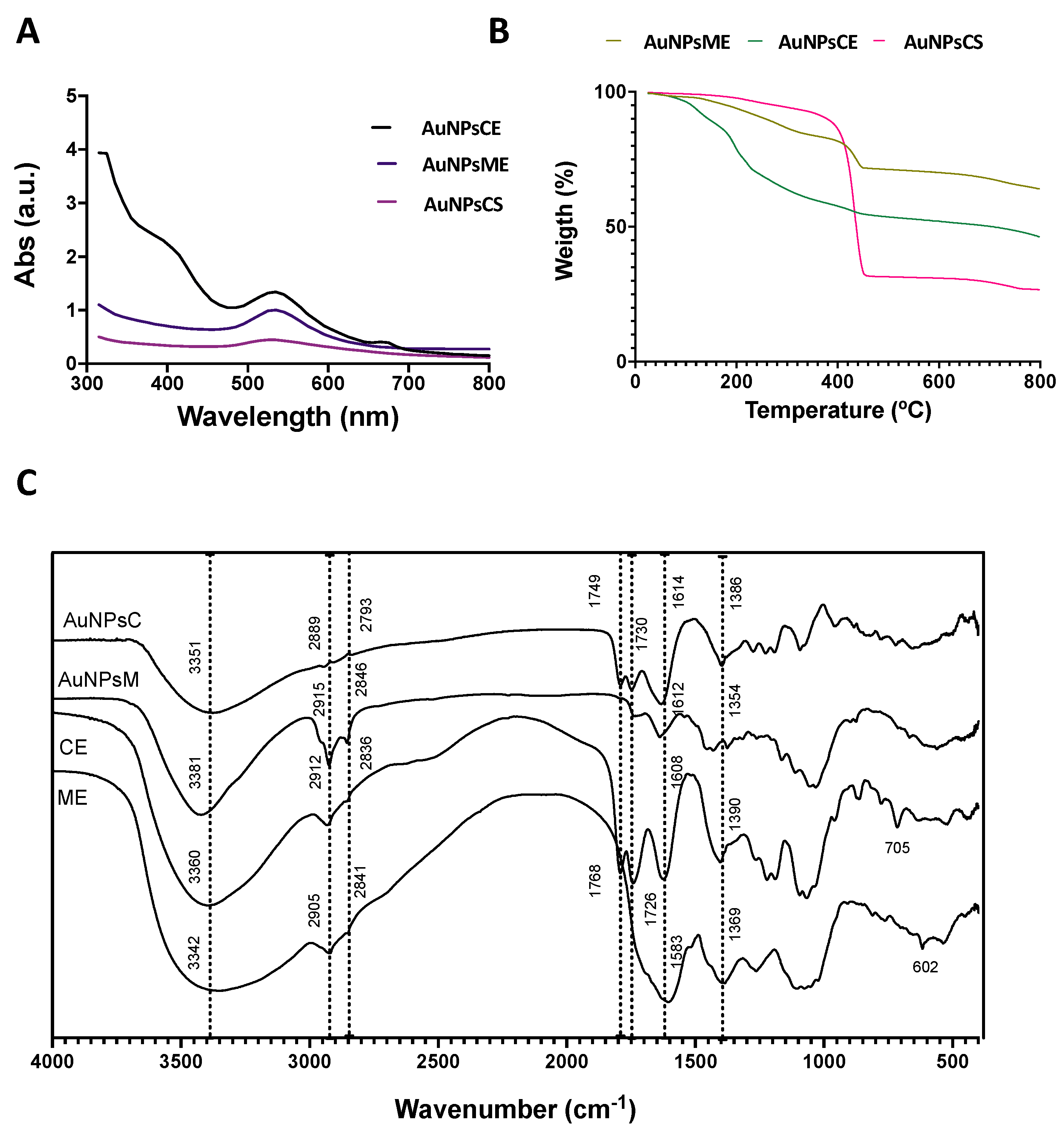
3.1. Extraction and Characterization of Ethanolic Extracts
3.2. Synthesis and Characterization of AuNPs
3.2.1. Thermogravimetric Analysis
3.2.2. Infrared Spectroscopy
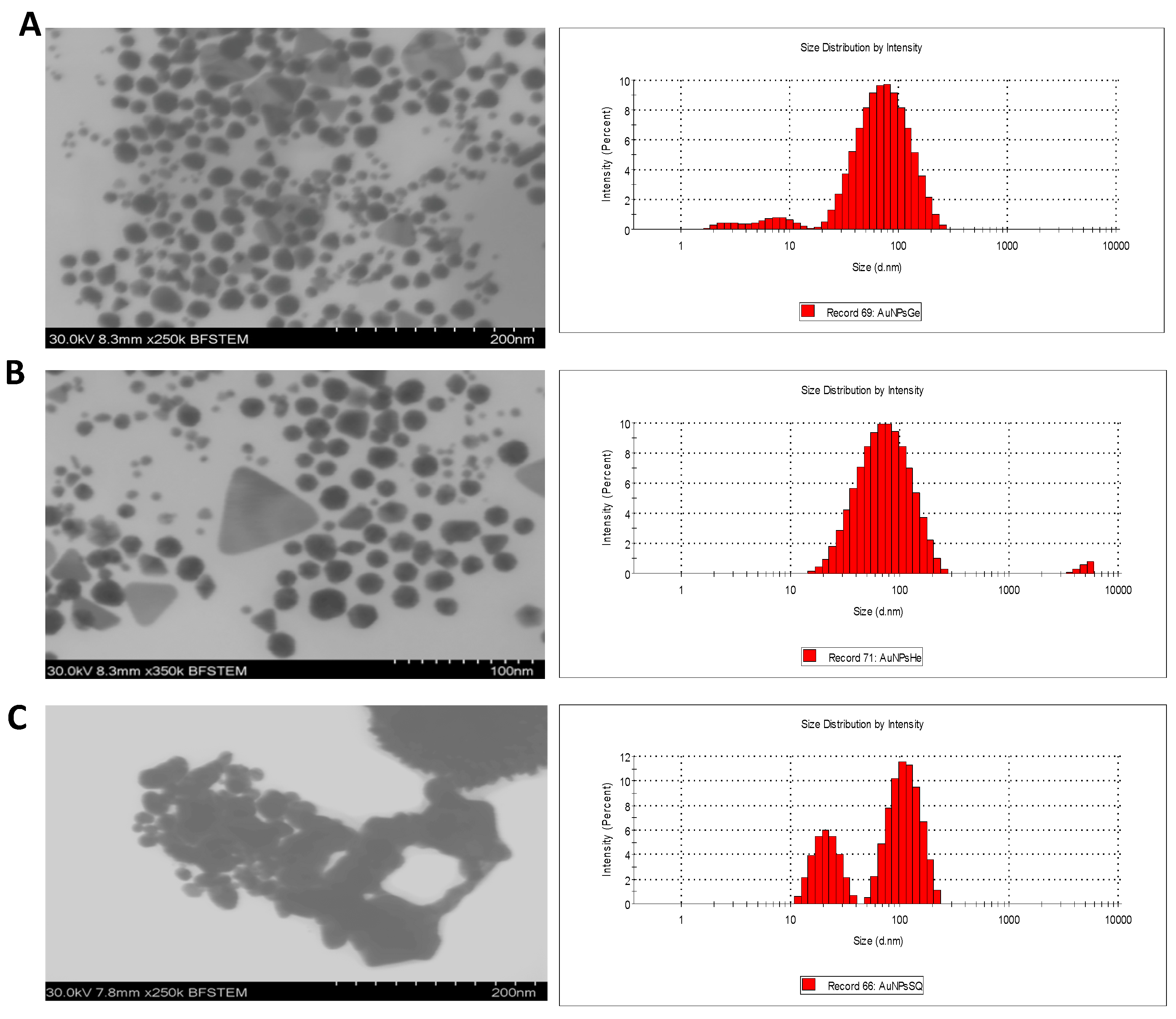
3.2.3. Morphology and Size Distribution
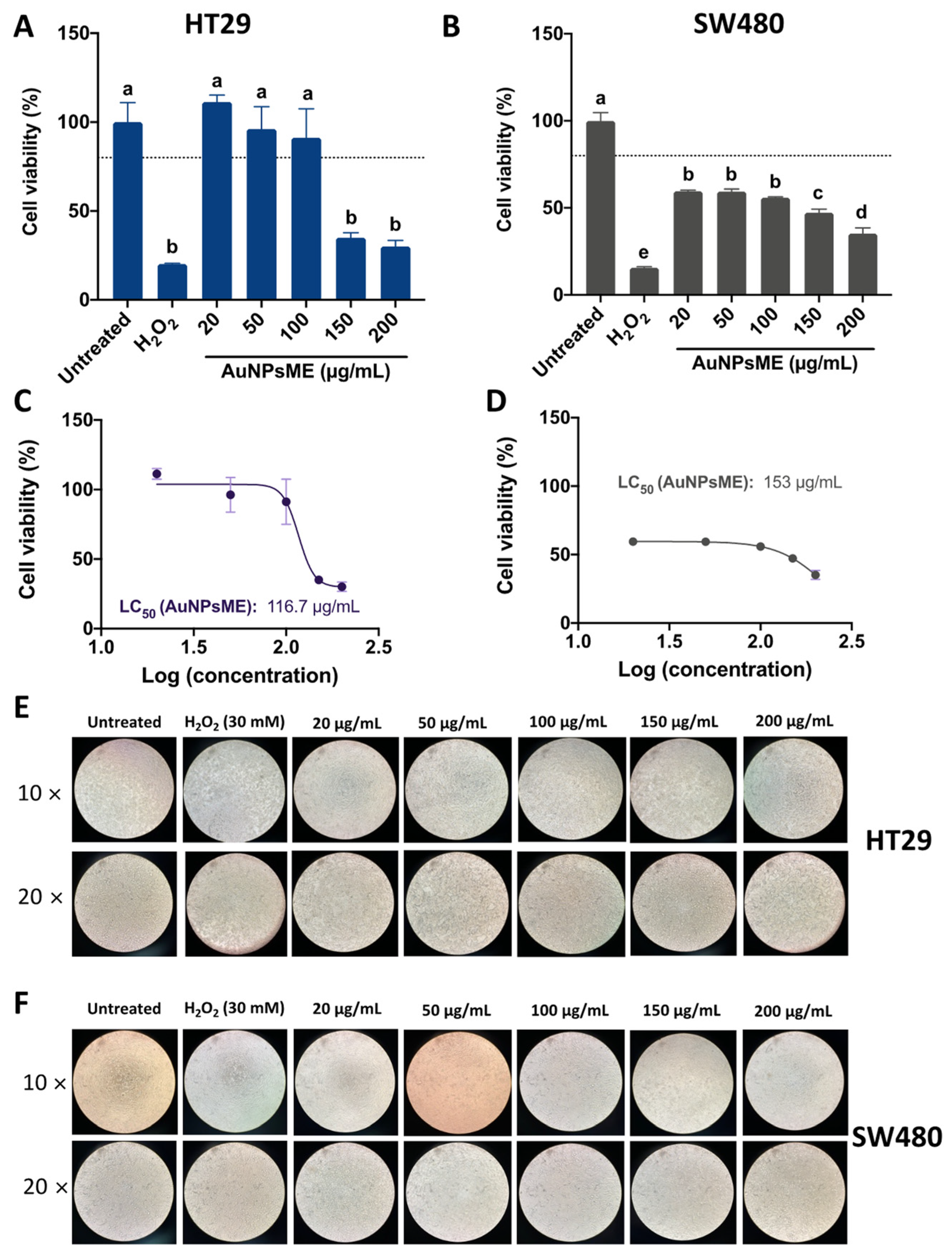
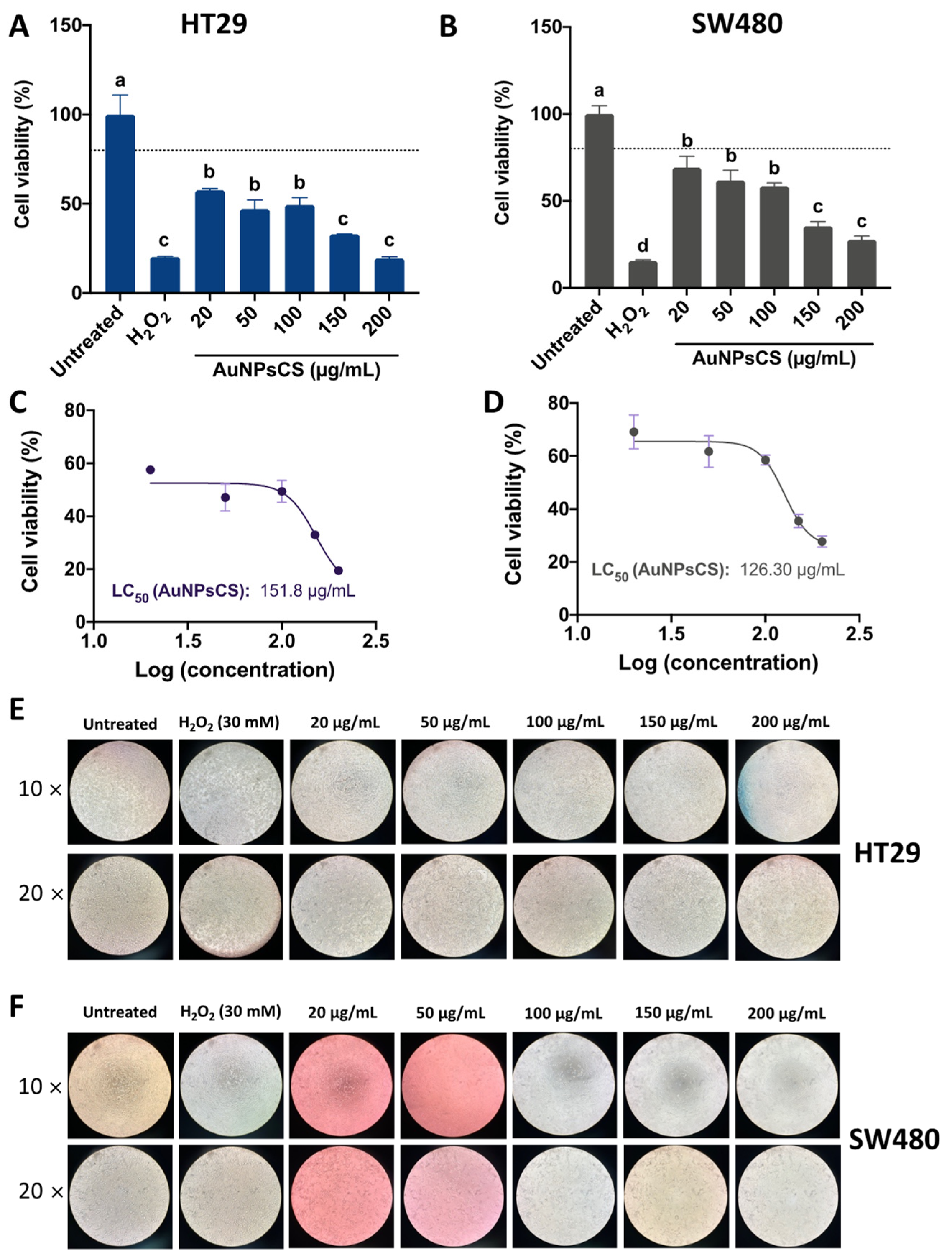
3.3. Ethanolic Extracts-Based AuNPs Are More Cytotoxic than AuNPsCS
3.3.1. Cellular Viability of AuNPsME
3.3.2. Cellular Viability of AuNPsCE
3.3.3. Cellular Viability of AuNPsCS
3.4. A Stronger ROS Generation Was Shown in the Ethanolic-Based AuNPs Compared to AuNPsCS
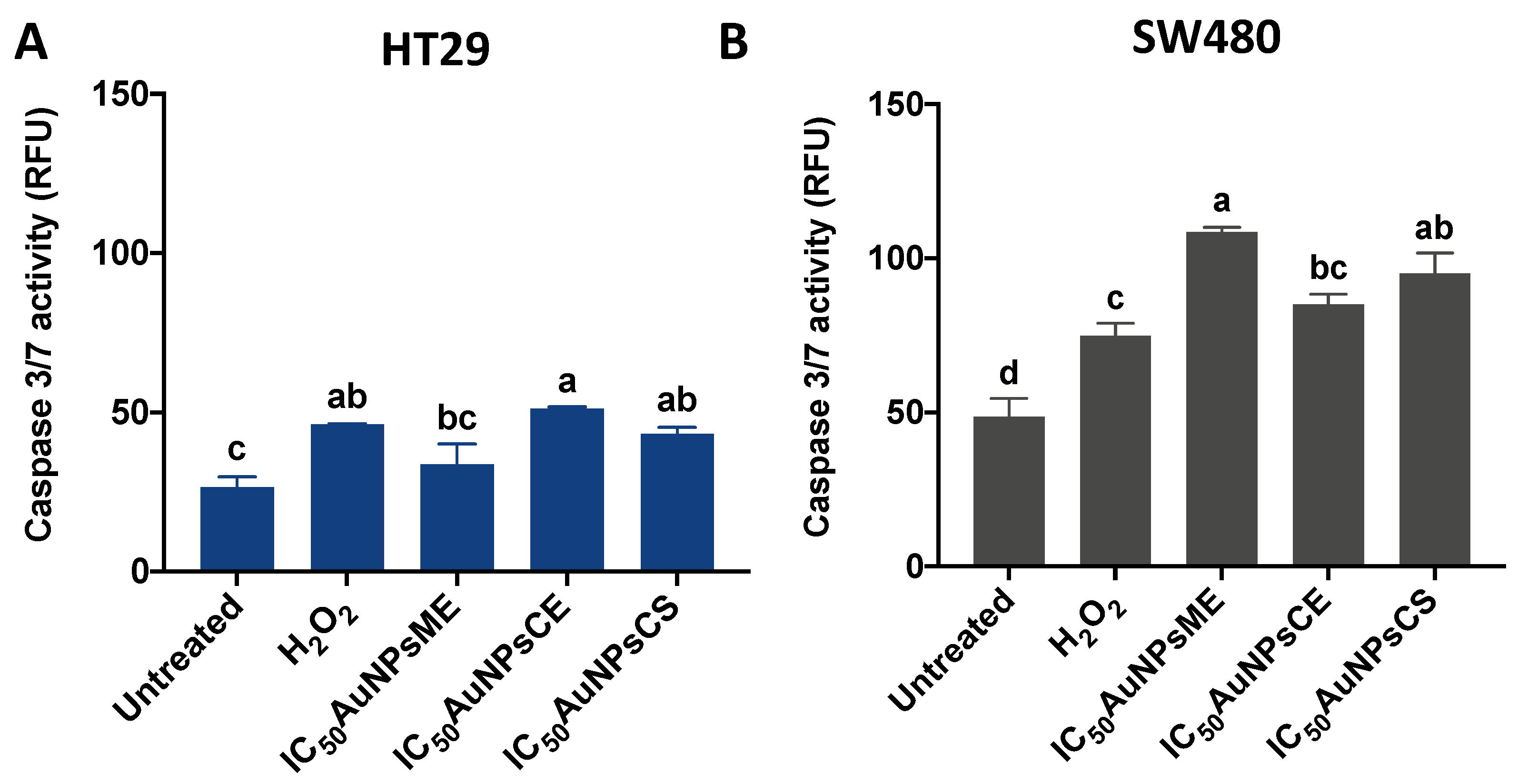
3.5. Caspase-3/7 Activity Is Increased Due to AuNPs Treatments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kyzioł, A.; Łukasiewicz, S.; Sebastian, V.; Kuśtrowski, P.; Kozieł, M.; Majda, D.; Cierniak, A. Towards Plant-Mediated Chemistry–Au Nanoparticles Obtained Using Aqueous Extract of Rosa damascena and Their Biological Activity in Vitro. J. Inorg. Biochem. 2021, 214, 111300. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Feng, X. Gold Nanoparticles for Skin Drug Delivery. Int. J. Pharm. 2022, 625, 122122. [Google Scholar] [CrossRef] [PubMed]
- Mikhailova, E.O. Gold Nanoparticles: Biosynthesis and Potential of Biomedical Application. J. Funct. Biomater. 2021, 12, 70. [Google Scholar] [CrossRef] [PubMed]
- Medici, S.; Peana, M.; Coradduzza, D.; Zoroddu, M.A. Gold Nanoparticles and Cancer: Detection, Diagnosis and Therapy. Semin. Cancer Biol. 2021, 76, 27–37. [Google Scholar] [CrossRef]
- Benedec, D.; Oniga, I.; Cuibus, F.; Sevastre, B.; Stiufiuc, G.; Duma, M.; Hanganu, D.; Iacovita, C.; Stiufiuc, R.; Lucaciu, C.M. Origanum Vulgare Mediated Green Synthesis of Biocompatible Gold Nanoparticles Simultaneously Possessing Plasmonic, Antioxidant and Antimicrobial Properties. Int. J. Nanomed. 2018, 13, 1041–1058. [Google Scholar] [CrossRef]
- Kalimuthu, K.; Lubin, B.C.; Bazylevich, A.; Gellerman, G.; Shpilberg, O.; Luboshits, G.; Firer, M.A. Gold Nanoparticles Stabilize Peptide-Drug-Conjugates for Sustained Targeted Drug Delivery to Cancer Cells. J. Nanobiotechnology 2018, 16, 34. [Google Scholar] [CrossRef]
- Wang, L.; Xu, J.; Yan, Y.; Liu, H.; Karunakaran, T.; Li, F. Green Synthesis of Gold Nanoparticles from Scutellaria Barbata and Its Anticancer Activity in Pancreatic Cancer Cell (PANC-1). Artif. Cells Nanomed. Biotechnol. 2019, 47, 1617–1627. [Google Scholar] [CrossRef]
- Ferreira, D.; Fontinha, D.; Martins, C.; Pires, D.; Fernandes, A.R.; Baptista, P.V. Gold Nanoparticles for Vectorization of Nucleic Acids for Cancer Therapeutics. Molecules 2020, 25, 3489. [Google Scholar] [CrossRef]
- Donga, S.; Bhadu, G.R.; Chanda, S. Antimicrobial, Antioxidant and Anticancer Activities of Gold Nanoparticles Green Synthesized Using Mangifera Indica Seed Aqueous Extract. Artif. Cells Nanomed. Biotechnol. 2020, 48, 1315–1325. [Google Scholar] [CrossRef]
- Soto, K.M.; Mendoza, S.; López-Romero, J.M.; Gasca-Tirado, J.R.; Manzano-Ramírez, A. Gold Nanoparticles: Synthesis, Application in Colon Cancer Therapy and New Approaches-Review. Green Chem. Lett. Rev. 2021, 14, 663–676. [Google Scholar] [CrossRef]
- Nalli, M.; Puxeddu, M.; la Regina, G.; Gianni, S.; Silvestri, R. Emerging Therapeutic Agents for Colorectal Cancer. Molecules 2021, 26, 7463. [Google Scholar] [CrossRef] [PubMed]
- Gala de Pablo, J.; Armistead, F.J.; Peyman, S.A.; Bonthron, D.; Lones, M.; Smith, S.; Evans, S.D. Biochemical Fingerprint of Colorectal Cancer Cell Lines Using Label-Free Live Single-Cell Raman Spectroscopy. J. Raman Spectrosc. 2018, 49, 1323–1332. [Google Scholar] [CrossRef] [PubMed]
- Thipe, V.C.; Karikachery, A.R.; Çakılkaya, P.; Farooq, U.; Genedy, H.H.; Kaeokhamloed, N.; Phan, D.-H.; Rezwan, R.; Tezcan, G.; Roger, E.; et al. Green Nanotechnology—An Innovative Pathway towards Biocompatible and Medically Relevant Gold Nanoparticles. J. Drug Deliv. Sci. Technol. 2022, 70, 103256. [Google Scholar] [CrossRef]
- Soto, K.M.; Quezada-Cervantes, C.T.; Hernández-Iturriaga, M.; Luna-Bárcenas, G.; Vazquez-Duhalt, R.; Mendoza, S. Fruit Peels Waste for the Green Synthesis of Silver Nanoparticles with Antimicrobial Activity against Foodborne Pathogens. LWT 2019, 103, 293–300. [Google Scholar] [CrossRef]
- Vasco-Leal, J.F.; Cuellar-Nuñez, M.L.; Luzardo-Ocampo, I.; Ventura-Ramos, E.; Loarca-Piña, G.; Rodriguez-García, M.E. Valorization of Mexican Ricinus communis L. Leaves as a Source of Minerals and Antioxidant Compounds. Waste Biomass Valorization 2020, 12, 2071–2088. [Google Scholar] [CrossRef]
- Luzardo-Ocampo, I.; Vasco-Leal, J.; Cuellar-Nuñez, M.L.; Quintero-Castaño, V.; Pérez-Serrano, R.; Luzardo-Ocampo, I. Castor Bean (Ricinus communis L.) Polyphenolic Extracts Exhibited Anti-Inflammatory Effects on LPS-Stimulated RAW 264.7 Macrophages. Curr Dev. Nutr. 2022, 6, 309. [Google Scholar] [CrossRef]
- Abdul, W.M.; Hajrah, N.H.; Sabir, J.S.M.; Al-Garni, S.M.; Sabir, M.J.; Kabli, S.; Saini, K.S.; Bora, R.S. Therapeutic Role of Ricinus communis L. and Its Bioactive Compounds in Disease Prevention and Treatment. Asian Pac. J. Trop. Med. 2018, 11, 177. [Google Scholar] [CrossRef]
- Turker, A.U.; Gurel, E. Common Mullein (Verbascum thapsus L.): Recent Advances in Research. Phytother. Res. 2005, 19, 733–739. [Google Scholar] [CrossRef]
- Ghramh, H.A.; Khan, K.A.; Ibrahim, E.H.; Setzer, W.N. Synthesis of Gold Nanoparticles (AuNPs) Using Ricinus Communis Leaf Ethanol Extract, Their Characterization, and Biological Applications. Nanomaterials 2019, 9, 765. [Google Scholar] [CrossRef]
- Gonca, S.; Arslan, H.; Isik, Z.; Özdemir, S.; Dizge, N. The Surface Modification of Ultrafiltration Membrane with Silver Nanoparticles Using Verbascum thapsus Leaf Extract Using Green Synthesis Phenomena. Surf. Interfaces 2021, 26, 101291. [Google Scholar] [CrossRef]
- Weldegebrieal, G.K. Photocatalytic and Antibacterial Activityof CuO Nanoparticles Biosynthesized Using Verbascum thapsus Leaves Extract. Optik 2020, 204, 164230. [Google Scholar] [CrossRef]
- Mohamed, S.; Zela, I.; Yusuf, A.; Hudaverdi, A.; Mutlu, Y.; Dizge, N. Green Synthesis of Zero Valent Iron Nanoparticles Using Vervascum thapsus and Its Cr (VI) Reduction Activity. Bioresour. Technol. Rep. 2022, 13, 100637. [Google Scholar] [CrossRef]
- Darweesh, R.S.; Ayoub, N.M.; Nazzal, S. Gold Nanoparticles and Angiogenesis: Molecular Mechanisms and Biomedical Applications. Int. J. Nanomed. 2019, 14, 7643–7663. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Gao, T.; Hong, H.; Sun, J. Applications of gold nanoparticles in cancer nanotechnology. Nanotechnol. Sci. Appl. 2008, 1, 17–32. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteu Reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Ramírez-Jiménez, A.K.; Reynoso-Camacho, R.; Mendoza-Díaz, S.; Loarca-Piña, G. Functional and Technological Potential of Dehydrated Phaseolus Vulgaris L. Flours. Food Chem. 2014, 161, 254–260. [Google Scholar] [CrossRef]
- Deraedt, C.; Salmon, L.; Gatard, S.; Ciganda, R.; Hernandez, R.; Ruiz, J.; Astruc, D. Sodium Borohydride Stabilizes Very Active Gold Nanoparticle Catalysts. Chem. Commun. 2014, 50, 14194–14196. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of Image Analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Torres-Vargas, O.L.; Luzardo-Ocampo, I.; Hernandez-Becerra, E.; Rodríguez-García, M.E. Physicochemical Characterization of Unripe and Ripe Chontaduro (Bactris Gasipaes Kunth) Fruit Flours and Starches. Starch Stärke 2021, 73, 2000242. [Google Scholar] [CrossRef]
- Lan, H.; Yuan, H.; Lin, C. Sulforaphane Induces P53-Deficient SW480 Cell Apoptosis via the ROS-MAPK Signaling Pathway. Mol. Med. Rep. 2017, 16, 7796–7804. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, B.Y.; Sarker, M.R.; Kamarudin, M.N.A.; Mohan, G. Antiproliferative and Apoptosis Inducing Effects of Citral via P53 and ROS-Induced Mitochondrial-Mediated Apoptosis in Human Colorectal HCT116 and HT29 Cell Lines. Biomed. Pharmacother. 2017, 96, 834–846. [Google Scholar] [CrossRef]
- Luzardo-Ocampo, I.; Loarca-Piña, G.; de Mejia, E.G. Gallic and Butyric Acids Modulated NLRP3 Inflammasome Markers in a Co-Culture Model of Intestinal Inflammation. Food Chem. Toxicol. 2020, 146, 111835. [Google Scholar] [CrossRef] [PubMed]
- Selseleh, M.; Ebrahimi, S.N.; Aliahmadi, A.; Sonboli, A.; Mirjalili, M.H. Metabolic Profiling, Antioxidant, and Antibacterial Activity of Some Iranian Verbascum L. Species. Ind. Crops. Prod. 2020, 153, 112609. [Google Scholar] [CrossRef]
- Taleb, S.; Saeedi, M. The Effect of the Verbascum thapsus on Episiotomy Wound Healing in Nulliparous Women: A Randomized Controlled Trial. BMC Complement. Med. Ther. 2021, 21, 166. [Google Scholar] [CrossRef]
- Wafa, G.; Amadou, D.; Larbi, K.M.; Héla, E.F.O. Larvicidal Activity, Phytochemical Composition, and Antioxidant Properties of Different Parts of Five Populations of Ricinus communis L. Ind. Crops. Prod. 2014, 56, 43–51. [Google Scholar] [CrossRef]
- Mihailović, V.; Kreft, S.; Benković, E.T.; Ivanović, N.; Stanković, M.S. Chemical Profile, Antioxidant Activity and Stability in Stimulated Gastrointestinal Tract Model System of Three Verbascum Species. Ind. Crops. Prod. 2016, 89, 141–151. [Google Scholar] [CrossRef]
- Nemudzivhadi, V.; Masoko, P. In Vitro Assessment of Cytotoxicity, Antioxidant, and Anti-Inflammatory Activities of Ricinus communis (Euphorbiaceae) Leaf Extracts. Evid. Based Complementary Altern. Med. 2014, 2014, 1–8. [Google Scholar] [CrossRef]
- Alegria, E.; Ribeiro, A.; Mendes, M.; Ferraria, A.; do Rego, A.; Pombeiro, A. Effect of Phenolic Compounds on the Synthesis of Gold Nanoparticles and Its Catalytic Activity in the Reduction of Nitro Compounds. Nanomaterials 2018, 8, 320. [Google Scholar] [CrossRef] [Green Version]
- Amendola, V.; Pilot, R.; Frasconi, M.; Maragò, O.M.; Iatì, M.A. Surface Plasmon Resonance in Gold Nanoparticles: A Review. J. Phys. Condens. Matter 2017, 29, 203002. [Google Scholar] [CrossRef] [PubMed]
- Rahman, T.J.; Anwar, M.; Zeb, M.A.; Liaqat, W. Green synthesis, characterization, antibacterial activity of metal nanoparticles and composite oxides using leaves extract of Ocimum basilicum L. Microsc. Res. Tech. 2022, 85, 2857–2865. [Google Scholar] [CrossRef] [PubMed]
- Botteon, C.E.A.; Silva, L.B.; Ccana-Ccapatinta, G.V.; Silva, T.S.; Ambrosio, S.R.; Veneziani, R.C.S.; Bastos, J.K.; Marcato, P.D. Biosynthesis and Characterization of Gold Nanoparticles Using Brazilian Red Propolis and Evaluation of Its Antimicrobial and Anticancer Activities. Sci. Rep. 2021, 11, 1974. [Google Scholar] [CrossRef] [PubMed]
- Aljabali, A.; Akkam, Y.; Al Zoubi, M.; Al-Batayneh, K.; Al-Trad, B.; Abo Alrob, O.; Alkilany, A.; Benamara, M.; Evans, D. Synthesis of Gold Nanoparticles Using Leaf Extract of Ziziphus Zizyphus and Their Antimicrobial Activity. Nanomaterials 2018, 8, 174. [Google Scholar] [CrossRef]
- Quilaqueo, M.; Millao, S.; Luzardo-Ocampo, I.; Campos-Vega, R.; Acevedo, F.; Shene, C.; Rubilar, M. Inclusion of Piperine in β-Cyclodextrin Complexes Improves Their Bioaccessibility and in Vitro Antioxidant Capacity. Food Hydrocoll. 2019, 91, 143–152. [Google Scholar] [CrossRef]
- Alghuthaymi, M.A.; Rajkuberan, C.; Santhiya, T.; Krejcar, O.; Kuča, K.; Periakaruppan, R.; Prabukumar, S. Green Synthesis of Gold Nanoparticles Using Polianthes Tuberosa L. Floral Extract. Plants 2021, 10, 2370. [Google Scholar] [CrossRef]
- Wang, B.; Yang, G.; Chen, J.; Fang, G. Green Synthesis and Characterization of Gold Nanoparticles Using Lignin Nanoparticles. Nanomaterials 2020, 10, 1869. [Google Scholar] [CrossRef]
- Fang, A.; Sun, Y.; Feng, D.; Ma, M.; Xu, Z.; Zhang, T.; Shi, F. Flower-like Gold Nanoparticles Labeled and Silver Deposition Rapid Vertical Flow Technology for Highly Sensitive Detection of Brucella Antibodies. Analyst 2021, 146, 5362–5368. [Google Scholar] [CrossRef]
- Clayton, K.N.; Salameh, J.W.; Wereley, S.T.; Kinzer-Ursem, T.L. Physical Characterization of Nanoparticle Size and Surface Modification Using Particle Scattering Diffusometry. Biomicrofluidics 2016, 10, 054107. [Google Scholar] [CrossRef]
- Takechi-Haraya, Y.; Ohgita, T.; Demizu, Y.; Saito, H.; Izutsu, K.I.; Sakai-Kato, K. Current Status and Challenges of Analytical Methods for Evaluation of Size and Surface Modification of Nanoparticle-Based Drug Formulations. AAPS PharmSciTech 2022, 23, 150. [Google Scholar] [CrossRef]
- Al Saqr, A.; Khafagy, E.-S.; Alalaiwe, A.; Aldawsari, M.; Alshahrani, S.; Anwer, K.; Khan, S.; Lila, A.; Arab, H.; Hegazy, W. Synthesis of Gold Nanoparticles by Using Green Machinery: Characterization and in Vitro Toxicity. Nanomaterials 2021, 11, 808. [Google Scholar] [CrossRef] [PubMed]
- Nowakowska, M.; Pospiech, K.; Lewandowska, U.; Piastowska-Ciesielska, A.W.; Bednarek, A.K. Diverse Effect of WWOX Overexpression in HT29 and SW480 Colon Cancer Cell Lines. Tumor Biology 2014, 35, 9291–9301. [Google Scholar] [CrossRef] [PubMed]
- Dragicevic, S.; Kovacevic, D.; Divac-Rankov, A.; Nikolic, A.; Radojkovic, D.; Radovic, S. Evaluation of Toxicity and Antioxidative Effects of Tussilago Farfara and Verbascum thapsus Water Extracts in Zebrafish and in Bronchial Epithelial Cells. Arch. Biol. Sci. 2019, 71, 409–416. [Google Scholar] [CrossRef]
- Calabrese, G.; Zappalà, A.; Dolcimascolo, A.; Acquaviva, R.; Parenti, R.; Malfa, G.A. Phytochemical Analysis and Anti-Inflammatory and Anti-Osteoarthritic Bioactive Potential of Verbascum thapsus L. (Scrophulariaceae) Leaf Extract Evaluated in Two In Vitro Models of Inflammation and Osteoarthritis. Molecules 2021, 26, 5392. [Google Scholar] [CrossRef] [PubMed]
- Escobar, F.M.; Sabini, M.C.; Zanon, S.M.; Cariddi, L.N.; Tonn, C.E.; Sabini, L.I. Genotoxic Evaluation of a Methanolic Extract of Verbascum thapsus Using Micronucleus Test in Mouse Bone Marrow. Nat. Prod. Commun. 2011, 6, 989–991. [Google Scholar] [CrossRef]
- Lu, K.; Ou, Y.; Jiang, L.; Xiao, Y.; Chinnathambi, A.; Alahmadi, T.A.; Wainwright, M. Novel Formulation, Cytotoxicity, Antioxidant, and Anti-Human Lung Cancer Properties of Gold Nanoparticles Containing Verbascum thapsus L. Leaf Aqueous Extract. Arch. Med. Sci. 2021, 1–24. [Google Scholar] [CrossRef]
- Majumder, M.; Debnath, S.; Gajbhiye, R.L.; Saikia, R.; Gogoi, B.; Samanta, S.K.; Das, D.K.; Biswas, K.; Jaisankar, P.; Mukhopadhyay, R. Ricinus communis L. Fruit Extract Inhibits Migration/Invasion, Induces Apoptosis in Breast Cancer Cells and Arrests Tumor Progression in Vivo. Sci. Rep. 2019, 9, 14493. [Google Scholar] [CrossRef]
- Darmanin, S.; Wismayer, P.S.; Camilleri Podesta, M.T.; Micallef, M.J.; Buhagiar, J.A. An Extract from Ricinus communis L. Leaves Possesses Cytotoxic Properties and Induces Apoptosis in SK-MEL-28 Human Melanoma Cells. Nat. Prod. Res. 2009, 23, 561–571. [Google Scholar] [CrossRef]
- Hosseinzadeh, H.; Atyabi, F.; Varnamkhasti, B.S.; Hosseinzadeh, R.; Ostad, S.N.; Ghahremani, M.H.; Dinarvand, R. SN38 Conjugated Hyaluronic Acid Gold Nanoparticles as a Novel System against Metastatic Colon Cancer Cells. Int. J. Pharm. 2017, 526, 339–352. [Google Scholar] [CrossRef]
- Tabatabaie, F.; Franich, R.; Feltis, B.; Geso, M. Oxidative Damage to Mitochondria Enhanced by Ionising Radiation and Gold Nanoparticles in Cancer Cells. Int. J. Mol. Sci. 2022, 23, 6887. [Google Scholar] [CrossRef]
- Minai, L.; Yeheskely-Hayon, D.; Yelin, D. High Levels of Reactive Oxygen Species in Gold Nanoparticle-Targeted Cancer Cells Following Femtosecond Pulse Irradiation. Sci. Rep. 2013, 3, srep02146. [Google Scholar] [CrossRef] [PubMed]
- Venugopal, K.; Rather, H.A.; Rajagopal, K.; Shanthi, M.P.; Sheriff, K.; Illiyas, M.; Rather, R.A.; Manikandan, E.; Uvarajan, S.; Bhaskar, M.; et al. Synthesis of Silver Nanoparticles (Ag NPs) for Anticancer Activities (MCF 7 Breast and A549 Lung Cell Lines) of the Crude Extract of Syzygium Aromaticum. J. Photochem. Photobiol. B 2017, 167, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Gulbagca, F.; Aygun, A.; Tiri, R.N.E.; Xia, C.; Van Le, Q.; Gur, T.; Sen, F.; Vasseghian, Y. Phyto-Mediated Synthesis of Nanoparticles and Their Applications on Hydrogen Generation on NaBH4, Biological Activities and Photodegradation on Azo Dyes: Development of Machine Learning Model. Food Chem. Toxicol. 2022, 163, 112972. [Google Scholar] [CrossRef]
- M Amin, H.I.; Hussain, F.H.S.; Gilardoni, G.; Thu, Z.M.; Clericuzio, M.; Vidari, G. Phytochemistry of Verbascum Species Growing in Iraqi Kurdistan and Bioactive Iridoids from the Flowers of Verbascum Calvum. Plants 2020, 9, 1066. [Google Scholar] [CrossRef] [PubMed]
- Arango-Varela, S.S.; Luzardo-Ocampo, I.; Reyes-Dieck, C.; Yahia, E.M.; Maldonado-Celis, M.E. Antiproliferative Potential of Andean Berry (Vacciniu m Meridionale Swartz) Juice in Combination with Aspirin in Human SW480 Colon Adenocarcinoma Cells. J. Food Biochem. 2021, 45, e13760. [Google Scholar] [CrossRef] [PubMed]
- Alkilany, A.M.; Murphy, C.J. Toxicity and Cellular Uptake of Gold Nanoparticles: What We Have Learned so Far? J. Nanoparticle Res. 2010, 12, 2313–2333. [Google Scholar] [CrossRef] [PubMed]
- Aldahhan, R.; Almohazey, D.; Khan, F.A. Emerging Trends in the Application of Gold Nanoformulations in Colon Cancer Diagnosis and Treatment. Semin. Cancer Biol. 2021, in press. [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soto, K.M.; Luzardo-Ocampo, I.; López-Romero, J.M.; Mendoza, S.; Loarca-Piña, G.; Rivera-Muñoz, E.M.; Manzano-Ramírez, A. Gold Nanoparticles Synthesized with Common Mullein (Verbascum thapsus) and Castor Bean (Ricinus communis) Ethanolic Extracts Displayed Antiproliferative Effects and Induced Caspase 3 Activity in Human HT29 and SW480 Cancer Cells. Pharmaceutics 2022, 14, 2069. https://doi.org/10.3390/pharmaceutics14102069
Soto KM, Luzardo-Ocampo I, López-Romero JM, Mendoza S, Loarca-Piña G, Rivera-Muñoz EM, Manzano-Ramírez A. Gold Nanoparticles Synthesized with Common Mullein (Verbascum thapsus) and Castor Bean (Ricinus communis) Ethanolic Extracts Displayed Antiproliferative Effects and Induced Caspase 3 Activity in Human HT29 and SW480 Cancer Cells. Pharmaceutics. 2022; 14(10):2069. https://doi.org/10.3390/pharmaceutics14102069
Chicago/Turabian StyleSoto, Karen M., Ivan Luzardo-Ocampo, José M. López-Romero, Sandra Mendoza, Guadalupe Loarca-Piña, Eric M. Rivera-Muñoz, and Alejandro Manzano-Ramírez. 2022. "Gold Nanoparticles Synthesized with Common Mullein (Verbascum thapsus) and Castor Bean (Ricinus communis) Ethanolic Extracts Displayed Antiproliferative Effects and Induced Caspase 3 Activity in Human HT29 and SW480 Cancer Cells" Pharmaceutics 14, no. 10: 2069. https://doi.org/10.3390/pharmaceutics14102069





