Hyaluronic Acid-Based Nanomaterials Applied to Cancer: Where Are We Now?
Abstract
:1. Introduction
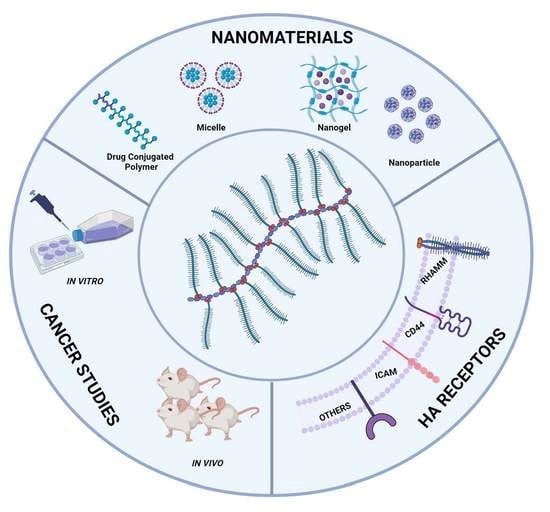
2. Hyaluronic Acid
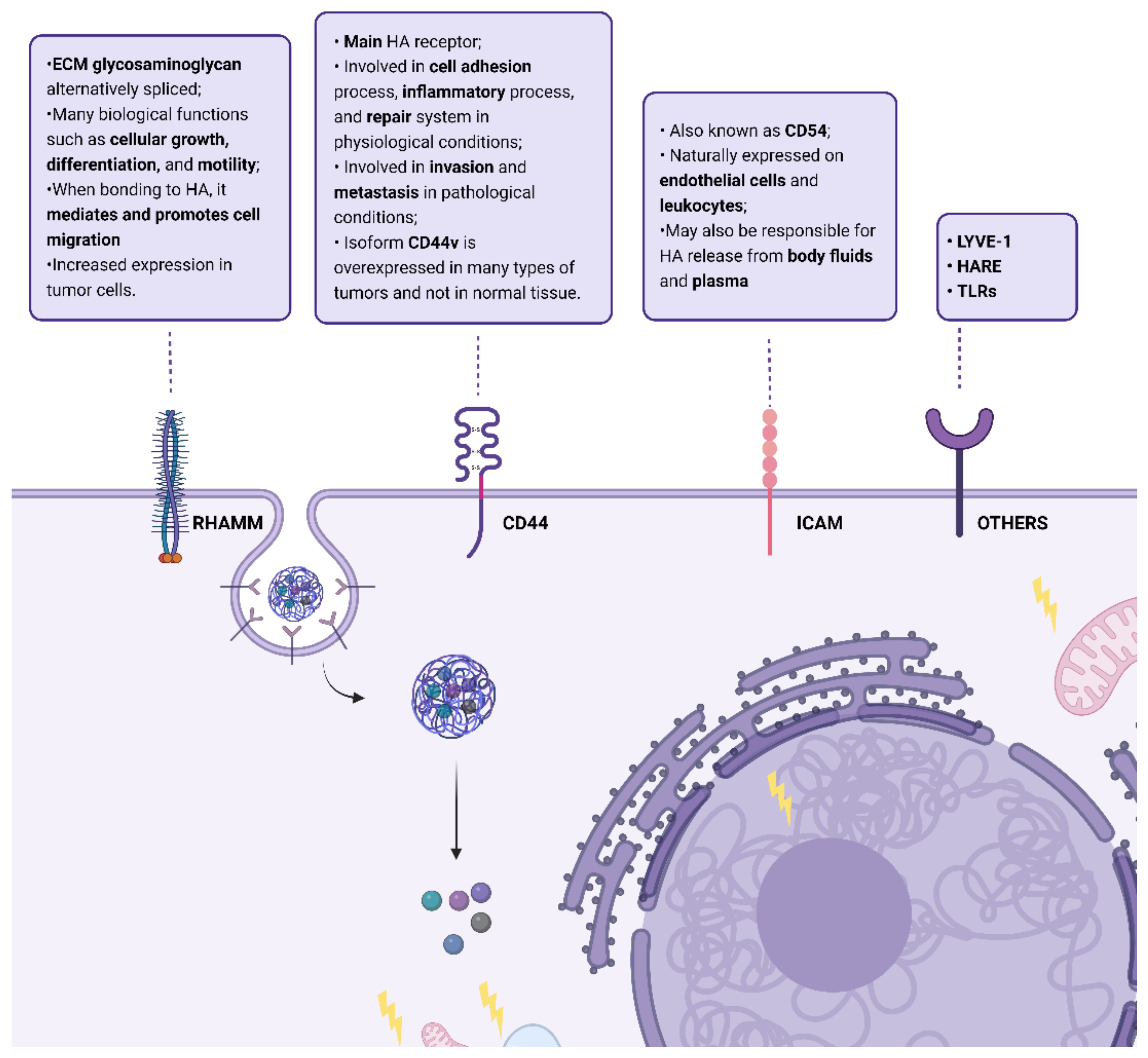
3. Hyaluronic Acid Receptors
4. Therapeutic Applications of HA in Cancer
5. Evidence Acquisition
6. HA–Drug Conjugates
7. HA-Based Hydrogel
8. HA Micelles
9. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hofmarcher, T.; Lindgren, P.; Wilking, N.; Jönsson, B. The cost of cancer in Europe 2018. Eur. J. Cancer 2020, 129, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Gavas, S.; Quazi, S.; Karpiński, T.M. Nanoparticles for Cancer Therapy: Current Progress and Challenges. Nanoscale Res. Lett. 2021, 16, 173. [Google Scholar] [CrossRef] [PubMed]
- Morais, M.; Teixeira, A.L.; Dias, F.; Machado, V.; Medeiros, R.; Prior, J.A.V. Cytotoxic Effect of Silver Nanoparticles Synthesized by Green Methods in Cancer. J. Med. Chem. 2020, 63, 14308–14335. [Google Scholar] [CrossRef]
- Morais, M.; Machado, V.; Dias, F.; Palmeira, C.; Martins, G.; Fonseca, M.; Martins, C.; Teixeira, A.; Prior, J.; Medeiros, R. Starch-Capped AgNPs’ as Potential Cytotoxic Agents against Prostate Cancer Cells. Nanomaterials 2021, 11, 256. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, P.; Bauleth-Ramos, T.; Hirvonen, J.; Sarmento, B.; Santos, H.A. Chapter 1—The emerging role of multifunctional theranostic materials in cancer nanomedicine. In Handbook of Nanomaterials for Cancer Theranostics; Conde, J., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 1–31. [Google Scholar]
- Zhang, M.; Qin, X.; Zhao, Z.; Du, Q.; Li, Q.; Jiang, Y.; Luan, Y. A self-amplifying nanodrug to manipulate the Janus-faced nature of ferroptosis for tumor therapy. Nanoscale Horiz. 2022, 7, 198–210. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, N.; Li, Q.; Zhou, Y.; Luan, Y. A two-pronged photodynamic nanodrug to prevent metastasis of basal-like breast cancer. Chem. Commun. 2021, 57, 2305–2308. [Google Scholar] [CrossRef]
- Chiang, C.-L.; Cheng, M.-H.; Lin, C.-H. From Nanoparticles to Cancer Nanomedicine: Old Problems with New Solutions. Nanomaterials 2021, 11, 1727. [Google Scholar] [CrossRef]
- Lee, S.Y.; Kang, M.S.; Jeong, W.Y.; Han, D.-W.; Kim, K.S. Hyaluronic Acid-Based Theranostic Nanomedicines for Targeted Cancer Therapy. Cancers 2020, 12, 940. [Google Scholar] [CrossRef]
- Hisada, N.; Satsu, H.; Mori, A.; Totsuka, M.; Kamei, J.-I.; Nozawa, T.; Shimizu, M. Low-Molecular-Weight Hyaluronan Permeates through Human Intestinal Caco-2 Cell Monolayers via the Paracellular Pathway. Biosci. Biotechnol. Biochem. 2008, 72, 1111–1114. [Google Scholar] [CrossRef]
- Fallacara, A.; Baldini, E.; Manfredini, S.; Vertuani, S. Hyaluronic Acid in the Third Millennium. Polymers 2018, 10, 701. [Google Scholar] [CrossRef] [Green Version]
- McAtee, C.O.; Barycki, J.J.; Simpson, M.A. Emerging Roles for Hyaluronidase in Cancer Metastasis and Therapy. Adv. Cancer Res. 2014, 123, 1–34. [Google Scholar] [CrossRef] [PubMed]
- Valachová, K.; Šoltés, L. Hyaluronan as a Prominent Biomolecule with Numerous Applications in Medicine. Int. J. Mol. Sci. 2021, 22, 7077. [Google Scholar] [CrossRef]
- Marcotti, S.; Maki, K.; Reilly, G.C.; Lacroix, D.; Adachi, T. Hyaluronic acid selective anchoring to the cytoskeleton: An atomic force microscopy study. PLoS ONE 2018, 13, e0206056. [Google Scholar] [CrossRef]
- Lapcik, L., Jr.; Lapcik, L.; De Smedt, S.; Demeester, J.; Chabreček, P. Hyaluronan: Preparation, Structure, Properties, and Applications. Chem. Rev. 1998, 98, 2663–2684. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Dai, Y.; Gao, H. Development and application of hyaluronic acid in tumor targeting drug delivery. Acta Pharm. Sin. B 2019, 9, 1099–1112. [Google Scholar] [CrossRef] [PubMed]
- Cichy, J.; Puré, E. The liberation of CD44. J. Cell Biol. 2003, 161, 839–843. [Google Scholar] [CrossRef] [PubMed]
- Dosio, F.; Arpicco, S.; Stella, B.; Fattal, E. Hyaluronic acid for anticancer drug and nucleic acid delivery. Adv. Drug Deliv. Rev. 2016, 97, 204–236. [Google Scholar] [CrossRef]
- Misra, S.; Heldin, P.; Hascall, V.C.; Karamanos, N.K.; Skandalis, S.S.; Markwald, R.R.; Ghatak, S. Hyaluronan-CD44 interactions as potential targets for cancer therapy. FEBS J. 2011, 278, 1429–1443. [Google Scholar] [CrossRef]
- Turley, E.A.; Noble, P.W.; Bourguignon, L.Y. Signaling Properties of Hyaluronan Receptors. J. Biol. Chem. 2002, 277, 4589–4592. [Google Scholar] [CrossRef]
- Günthert, U.; Hofmann, M.; Rudy, W.; Reber, S.; Zöller, M.; Hauβmann, I.; Matzku, S.; Wenzel, A.; Ponta, H.; Herrlich, P. A new variant of glycoprotein CD44 confers metastatic potential to rat carcinoma cells. Cell 1991, 65, 13–24. [Google Scholar] [CrossRef]
- Zöller, M. CD44: Can a cancer-initiating cell profit from an abundantly expressed molecule? Nat. Rev. Cancer 2011, 11, 254–267. [Google Scholar] [CrossRef] [PubMed]
- Jaggupilli, A.; Elkord, E. Significance of CD44 and CD24 as Cancer Stem Cell Markers: An Enduring Ambiguity. Clin. Dev. Immunol. 2012, 2012, 708036. [Google Scholar] [CrossRef] [PubMed]
- Crainie, M.; Belch, A.R.; Mant, M.J.; Pilarski, L.M. Overexpression of the receptor for hyaluronan-mediated motility (RHAMM) characterizes the malignant clone in multiple myeloma: Identification of three distinct RHAMM variants. Blood 1999, 93, 1684–1696. [Google Scholar] [CrossRef] [PubMed]
- Gust, K.M.; Hofer, M.D.; Perner, S.R.; Kim, R.; Chinnaiyan, A.M.; Varambally, S.; Moller, P.; Rinnab, L.; Rubin, M.; Greiner, J.; et al. RHAMM (CD168) Is Overexpressed at the Protein Level and May Constitute an Immunogenic Antigen in Advanced Prostate Cancer Disease. Neoplasia 2009, 11, 956–963. [Google Scholar] [CrossRef]
- Vigetti, D.; Karousou, E.; Viola, M.; Deleonibus, S.; De Luca, G.; Passi, A. Hyaluronan: Biosynthesis and signaling. Biochim. Biophys. Acta Gen. Subj. 2014, 1840, 2452–2459. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, P.; Brown, T.J.; Ng, R.; Jennens, R.; Cinc, E.; Pho, M.; Michael, M.; Fox, R.M. A Pilot Human Evaluation of a Formulation of Irinotecan and Hyaluronic Acid in 5-Fluorouracil-Refractory Metastatic Colorectal Cancer Patients. Chemotherapy 2009, 55, 49–59. [Google Scholar] [CrossRef]
- Augustin, F.; Fiegl, M.; Schmid, T.; Pomme, G.; Sterlacci, W.; Tzankov, A. Receptor for hyaluronic acid-mediated motility (RHAMM, CD168) expression is prognostically important in both nodal negative and nodal positive large cell lung cancer. J. Clin. Pathol. 2015, 68, 368–373. [Google Scholar] [CrossRef]
- Misra, S.; Hascall, V.C.; Markwald, R.R.; Ghatak, S. Interactions between Hyaluronan and Its Receptors (CD44, RHAMM) Regulate the Activities of Inflammation and Cancer. Front. Immunol. 2015, 6, 201. [Google Scholar] [CrossRef]
- Marinho, A.; Nunes, C.; Reis, S. Hyaluronic Acid: A Key Ingredient in the Therapy of Inflammation. Biomolecules 2021, 11, 1518. [Google Scholar] [CrossRef]
- Gupta, R.C.; Lall, R.; Srivastava, A.; Sinha, A. Hyaluronic Acid: Molecular Mechanisms and Therapeutic Trajectory. Front. Vet. Sci. 2019, 6, 192. [Google Scholar] [CrossRef] [Green Version]
- Lokeshwar, V.B.; Mirza, S.; Jordan, A. Targeting Hyaluronic Acid Family for Cancer Chemoprevention and Therapy. Adv. Cancer Res. 2014, 123, 35–65. [Google Scholar] [CrossRef] [PubMed]
- Zhong, W.; Pang, L.; Feng, H.; Dong, H.; Wang, S.; Cong, H.; Shen, Y.; Bing, Y. Recent advantage of hyaluronic acid for anti-cancer application: A review of “3S” transition approach. Carbohydr. Polym. 2020, 238, 116204. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wang, J.; Wang, Y.; Gao, H.; Wei, G.; Huang, Y.; Yu, H.; Gan, Y.; Wang, Y.; Mei, L. Recent progress in drug delivery. Acta Pharm. Sin. B 2019, 9, 1145–1162. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.-J.; De Geest, B.G. Nanomedicine and cancer immunotherapy. Acta Pharmacol. Sin. 2020, 41, 879–880. [Google Scholar] [CrossRef]
- Belete, T.M. The Current Status of Gene Therapy for the Treatment of Cancer. Biol. Targets Ther. 2021, 15, 67–77. [Google Scholar] [CrossRef]
- Keles, E.; Song, Y.; Du, D.; Dong, W.-J.; Lin, Y. Recent progress in nanomaterials for gene delivery applications. Biomater. Sci. 2016, 4, 1291–1309. [Google Scholar] [CrossRef]
- Chen, B.; Miller, R.J.; Dhal, P.K. Hyaluronic Acid-Based Drug Conjugates: State-of-the-Art and Perspectives. J. Biomed. Nanotechnol. 2014, 10, 4–16. [Google Scholar] [CrossRef]
- Lai, H.; Ding, X.; Ye, J.; Deng, J.; Cui, S. pH-responsive hyaluronic acid-based nanoparticles for targeted curcumin delivery and enhanced cancer therapy. Colloids Surf. B Biointerfaces 2021, 198, 111455. [Google Scholar] [CrossRef]
- Kim, D.E.; Kim, C.W.; Lee, H.J.; Min, K.H.; Kwack, K.H.; Lee, H.-W.; Bang, J.; Chang, K.; Lee, S.C. Intracellular NO-Releasing Hyaluronic Acid-Based Nanocarriers: A Potential Chemosensitizing Agent for Cancer Chemotherapy. ACS Appl. Mater. Interfaces 2018, 10, 26870–26881. [Google Scholar] [CrossRef]
- Xu, X.; Huang, B.; Zeng, Z.; Chen, J.; Huang, Z.; Guan, Z.; Chen, M.; Huang, Y.; Zhao, C. Broaden sources and reduce expenditure: Tumor-specific transformable oxidative stress nanoamplifier enabling economized photodynamic therapy for reinforced oxidation therapy. Theranostics 2020, 10, 10513–10530. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Qian, Y.; Xu, L.; Shao, Y.; Zhang, H.; Shi, F.; Chen, J.; Cui, S.; Chen, X.; Zhu, D.; et al. Hyaluronic acid-shelled, peptide drug conjugate-cored nanomedicine for the treatment of hepatocellular carcinoma. Mater. Sci. Eng. C 2020, 117, 111261. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.T.; Du, J.-K.; Yang, Y.-Q.; Li, L.; Zhang, D.-W.; Liang, C.-L.; Wang, J.; Mei, J.; Wang, G.-H. pH and redox dual stimulate-responsive nanocarriers based on hyaluronic acid coated mesoporous silica for targeted drug delivery. Mater. Sci. Eng. C 2017, 81, 478–484. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Yang, Z.; Chen, S.; Wu, H.; Liu, Y.; Wright, A.; Lu, J.-W.; Xia, X.; Lee, A.; Zhang, J.; et al. Bioreducible Zinc(II)–Dipicolylamine Functionalized Hyaluronic Acid Mediates Safe siRNA Delivery and Effective Glioblastoma RNAi Therapy. ACS Appl. Bio Mater. 2019, 2, 362–369. [Google Scholar] [CrossRef]
- Highley, C.B.; Prestwich, G.D.; Burdick, J.A. Recent advances in hyaluronic acid hydrogels for biomedical applications. Curr. Opin. Biotechnol. 2016, 40, 35–40. [Google Scholar] [CrossRef]
- Hoffman, A.S. Hydrogels for biomedical applications. Adv. Drug Deliv. Rev. 2012, 64, 18–23. [Google Scholar] [CrossRef]
- Mendes, B.B.; Daly, A.C.; Reis, R.L.; Domingues, R.M.; Gomes, M.E.; Burdick, J.A. Injectable hyaluronic acid and platelet lysate-derived granular hydrogels for biomedical applications. Acta Biomater. 2021, 119, 101–113. [Google Scholar] [CrossRef]
- Liu, C.; Bae, K.H.; Yamashita, A.; Chung, J.E.; Kurisawa, M. Thiol-Mediated Synthesis of Hyaluronic Acid–Epigallocatechin-3-O-Gallate Conjugates for the Formation of Injectable Hydrogels with Free Radical Scavenging Property and Degradation Resistance. Biomacromolecules 2017, 18, 3143–3155. [Google Scholar] [CrossRef]
- Seliktar, D. Designing Cell-Compatible Hydrogels for Biomedical Applications. Science 2012, 336, 1124–1128. [Google Scholar] [CrossRef]
- Barbarisi, M.; Iaffaioli, R.V.; Armenia, E.; Schiavo, L.; De Sena, G.; Tafuto, S.; Barbarisi, A.; Quagliariello, V. Novel nanohydrogel of hyaluronic acid loaded with quercetin alone and in combination with temozolomide as new therapeutic tool, CD44 targeted based, of glioblastoma multiforme. J. Cell. Physiol. 2018, 233, 6550–6564. [Google Scholar] [CrossRef]
- Cao, Z.; Li, W.; Liu, R.; Li, C.; Song, Y.; Liu, G.; Chen, Y.; Lu, C.; Lu, A.; Liu, Y. pH-Responsive Fluorescence Enhanced Nanogel for Targeted Delivery of AUR and CDDP Against Breast Cancer. Int. J. Nanomed. 2020, 15, 8369–8382. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.T.; Ding, Y.-F.; Han, Z.-H.; Yuwen, L.; Ye, Z.; Mok, G.S.; Li, S.; Wang, L.-H. Hyaluronic acid-based nanogels derived from multicomponent self-assembly for imaging-guided chemo-photodynamic cancer therapy. Carbohydr. Polym. 2021, 268, 118257. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.; Iscaro, A.; Zambito, G.; Mijiti, Y.; Minicucci, M.; Essand, M.; Lowik, C.; Muthana, M.; Censi, R.; Mezzanotte, L.; et al. Development of a New Hyaluronic Acid Based Redox-Responsive Nanohydrogel for the Encapsulation of Oncolytic Viruses for Cancer Immunotherapy. Nanomaterials 2021, 11, 144. [Google Scholar] [CrossRef] [PubMed]
- Stefanello, T.F.; Couturaud, B.; Szarpak-Jankowska, A.; Fournier, D.; Louage, B.; Garcia, F.P.; Nakamura, C.V.; De Geest, B.G.; Woisel, P.; van der Sanden, B.; et al. Coumarin-containing thermoresponsive hyaluronic acid-based nanogels as delivery systems for anticancer chemotherapy. Nanoscale 2017, 9, 12150–12162. [Google Scholar] [CrossRef]
- Onder, F.C.; Suner, S.S.; Sahiner, N.; Ay, M.; Ozpolat, B. Delivery of Small Molecule EF2 Kinase Inhibitor for Breast and Pancreatic Cancer Cells Using Hyaluronic Acid Based Nanogels. Pharm. Res. 2020, 37, 63. [Google Scholar] [CrossRef]
- Quagliariello, V.; Iaffaioli, R.V.; Armenia, E.; Clemente, O.; Barbarisi, M.; Nasti, G.; Berretta, M.; Ottaiano, A.; Barbarisi, A. Hyaluronic Acid Nanohydrogel Loaded with Quercetin Alone or in Combination to a Macrolide Derivative of Rapamycin RAD001 (Everolimus) as a New Treatment for Hormone-Responsive Human Breast Cancer. J. Cell. Physiol. 2017, 232, 2063–2074. [Google Scholar] [CrossRef]
- Xiao, T.; Hu, W.; Fan, Y.; Shen, M.; Shi, X. Macrophage-mediated tumor homing of hyaluronic acid nanogels loaded with polypyrrole and anticancer drug for targeted combinational photothermo-chemotherapy. Theranostics 2021, 11, 7057–7071. [Google Scholar] [CrossRef]
- Faraji, N.; Esrafili, A.; Esfandiari, B.; Abednezhad, A.; Naghizadeh, M.; Arasteh, J. Synthesis of pH-sensitive hyaluronic acid nanogels loaded with paclitaxel and interferon gamma: Characterization and effect on the A549 lung carcinoma cell line. Colloids Surf. B Biointerfaces 2021, 205, 111845. [Google Scholar] [CrossRef]
- Ma, Y.; Zhou, H.; Hu, F.; Pei, Z.; Xu, Y.; Shuai, Q. Multifunctional nanogel engineering with redox-responsive and AIEgen features for the targeted delivery of doxorubicin hydrochloride with enhanced antitumor efficiency and real-time intracellular imaging. Artif. Cells Nanomed. Biotechnol. 2018, 46, S900–S910. [Google Scholar] [CrossRef]
- Štaka, I.; Cadete, A.; Surikutchi, B.T.; Abuzaid, H.; Bradshaw, T.D.; Alonso, M.J.; Marlow, M. A novel low molecular weight nanocomposite hydrogel formulation for intra-tumoural delivery of anti-cancer drugs. Int. J. Pharm. 2019, 565, 151–161. [Google Scholar] [CrossRef]
- Biswas, S.; Vaze, O.S.; Movassaghian, S.; Torchilin, V.P. Polymeric micelles for the delivery of poorly soluble drugs. In Drug Delivery Strategies for Poorly Water-Soluble Drugs; Douroumis, D., Fahr, A., Eds.; Wiley: Hoboken, NJ, USA, 2013; pp. 411–476. [Google Scholar] [CrossRef]
- Hwang, D.; Ramsey, J.D.; Kabanov, A.V. Polymeric micelles for the delivery of poorly soluble drugs: From nanoformulation to clinical approval. Adv. Drug Deliv. Rev. 2020, 156, 80–118. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Li, Y.; Gu, X.; Li, Q. Development of a Hyaluronic Acid-Based Nanocarrier Incorporating Doxorubicin and Cisplatin as a pH-Sensitive and CD44-Targeted Anti-Breast Cancer Drug Delivery System. Front. Pharmacol. 2020, 11, 532457. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Feng, Z.; Liu, J.; Hou, X.; Zhou, X.; Gao, J.; Wang, W.; Zhang, Y.; Li, G.; Liu, J. γ-Ray-Triggered Drug Release of Reactive Oxygen Species-Sensitive Nanomedicine for Enhanced Concurrent Chemoradiation Therapy. ACS Omega 2021, 6, 19445–19457. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Zhang, W.; Zhou, X.; Liu, M.; Hou, X.; Cheng, Z.; Chen, D. Development of dual-targeted nano-dandelion based on an oligomeric hyaluronic acid polymer targeting tumor-associated macrophages for combination therapy of non-small cell lung cancer. Drug Deliv. 2019, 26, 1265–1279. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Wang, S.; Zhang, T.; He, D.; Tu, J.; Shen, Y. Enhanced cytotoxicity of a redox-sensitive hyaluronic acid-based nanomedicine toward different oncocytes via various internalization mechanisms. Drug Deliv. 2020, 27, 128–136. [Google Scholar] [CrossRef]
- Bae, K.H.; Tan, S.; Yamashita, A.; Ang, W.X.; Gao, S.J.; Wang, S.; Chung, J.E.; Kurisawa, M. Hyaluronic acid-green tea catechin micellar nanocomplexes: Fail-safe cisplatin nanomedicine for the treatment of ovarian cancer without off-target toxicity. Biomaterials 2017, 148, 41–53. [Google Scholar] [CrossRef]
- Xu, W.; Wang, H.; Dong, L.; Zhang, P.; Mu, Y.; Cui, X.; Zhou, J.; Huo, M.; Yin, T. Hyaluronic acid-decorated redox-sensitive chitosan micelles for tumor-specific intracellular delivery of gambogic acid. Int. J. Nanomed. 2019, 14, 4649–4666. [Google Scholar] [CrossRef]
- Liu, X.; Li, W.; Chen, T.; Yang, Q.; Huang, T.; Fu, Y.; Gong, T.; Zhang, Z. Hyaluronic Acid-Modified Micelles Encapsulating Gem-C12 and HNK for Glioblastoma Multiforme Chemotherapy. Mol. Pharm. 2018, 15, 1203–1214. [Google Scholar] [CrossRef]
- Sun, F.; Zhang, P.; Liu, Y.; Lu, C.; Qiu, Y.; Mu, H.; Duan, J. A photo-controlled hyaluronan-based drug delivery nanosystem for cancer therapy. Carbohydr. Polym. 2018, 206, 309–318. [Google Scholar] [CrossRef]
- Xia, J.; Du, Y.; Huang, L.; Chaurasiya, B.; Tu, J.; Webster, T.J.; Sun, C. Redox-responsive micelles from disulfide bond-bridged hyaluronic acid-tocopherol succinate for the treatment of melanoma. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 713–723. [Google Scholar] [CrossRef]
- Su, Y.; Liu, Y.; Xu, X.; Zhou, J.; Xu, L.; Xu, X.; Wang, D.; Li, M.; Chen, K.; Wang, W. On-Demand Versatile Prodrug Nanomicelle for Tumor-Specific Bioimaging and Photothermal-Chemo Synergistic Cancer Therapy. ACS Appl. Mater. Interfaces 2018, 10, 38700–38714. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Sutrisno, L.; Hou, Y.; Fei, Y.; Xue, C.; Hu, Y.; Li, M.; Luo, Z. A redox-activatable biopolymer-based micelle for sequentially enhanced mitochondria-targeted photodynamic therapy and hypoxia-dependent chemotherapy. Chem. Commun. 2020, 56, 9978–9981. [Google Scholar] [CrossRef] [PubMed]
- Batool, A.; Arshad, R.; Razzaq, S.; Nousheen, K.; Kiani, M.H.; Shahnaz, G. Formulation and evaluation of hyaluronic acid-based mucoadhesive self nanoemulsifying drug delivery system (SNEDDS) of tamoxifen for targeting breast cancer. Int. J. Biol. Macromol. 2020, 152, 503–515. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xue, Y.; Tian, J.; Liu, Z.; Zhuang, A.; Gu, P.; Zhou, H.; Zhang, W.; Fan, X. Fluorinated-functionalized hyaluronic acid nanoparticles for enhanced photodynamic therapy of ocular choroidal melanoma by ameliorating hypoxia. Carbohydr. Polym. 2020, 237, 116119. [Google Scholar] [CrossRef]
- Sun, C.-Y.; Zhang, B.-B.; Zhou, J.-Y. Light-activated drug release from a hyaluronic acid targeted nanoconjugate for cancer therapy. J. Mater. Chem. B 2019, 7, 4843–4853. [Google Scholar] [CrossRef]
- Gao, Q.-Q.; Zhang, C.-M.; Zhang, E.-X.; Chen, H.-Y.; Zhen, Y.-H.; Zhang, S.-B. Zwitterionic pH-responsive hyaluronic acid polymer micelles for delivery of doxorubicin. Colloids Surf. B Biointerfaces 2019, 178, 412–420. [Google Scholar] [CrossRef] [PubMed]
- Chai, Z.; Teng, C.; Yang, L.; Ren, L.; Yuan, Z.; Xu, S.; Cheng, M.; Wang, Y.; Yan, Z.; Qin, C.; et al. Doxorubicin delivered by redox-responsive Hyaluronic Acid–Ibuprofen prodrug micelles for treatment of metastatic breast cancer. Carbohydr. Polym. 2020, 245, 116527. [Google Scholar] [CrossRef]
- Li, M.; Sun, J.; Zhang, W.; Zhao, Y.; Zhang, S.; Zhang, S. Drug delivery systems based on CD44-targeted glycosaminoglycans for cancer therapy. Carbohydr. Polym. 2021, 251, 117103. [Google Scholar] [CrossRef]
- Huang, G.; Huang, H. Application of hyaluronic acid as carriers in drug delivery. Drug Deliv. 2018, 25, 766–772. [Google Scholar] [CrossRef]
- Serri, C.; Quagliariello, V.; Iaffaioli, R.V.; Fusco, S.; Botti, G.; Mayol, L.; Biondi, M. Combination therapy for the treatment of pancreatic cancer through hyaluronic acid-decorated nanoparticles loaded with quercetin and gemcitabine: A preliminary in vitro study. J. Cell. Physiol. 2019, 234, 4959–4969. [Google Scholar] [CrossRef]
- Yu, S.; Wang, S.; Xie, Z.; Yu, S.; Li, L.; Xiao, H.; Song, Y. Hyaluronic acid coating on the surface of curcumin-loaded ZIF-8 nanoparticles for improved breast cancer therapy: An in vitro and in vivo study. Colloids Surf. B Biointerfaces 2021, 203, 111759. [Google Scholar] [CrossRef] [PubMed]
- Naz, S.; Wang, M.; Han, Y.; Hu, B.; Teng, L.; Zhou, J.; Zhang, H.; Chen, J. Enzyme-responsive mesoporous silica nanoparticles for tumor cells and mitochondria multistage-targeted drug delivery. Int. J. Nanomed. 2019, 14, 2533–2542. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Wei, W.; Chen, X.; Xu, X.; Zhang, X.; Zhang, X. pH and redox dual responsive carrier-free anticancer drug nanoparticles for targeted delivery and synergistic therapy. Nanomed. Nanotechnol. Biol. Med. 2019, 20, 102008. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Yang, H.; Khan, A.R.; Yang, X.; Xu, J.; Ji, J.; Zhai, G. Redox-responsive hyaluronic acid-based nanoparticles for targeted photodynamic therapy/chemotherapy against breast cancer. J. Colloid Interface Sci. 2021, 598, 213–228. [Google Scholar] [CrossRef]
- Carton, F.; Chevalier, Y.; Nicoletti, L.; Tarnowska, M.; Stella, B.; Arpicco, S.; Malatesta, M.; Jordheim, L.P.; Briançon, S.; Lollo, G. Rationally designed hyaluronic acid-based nano-complexes for pentamidine delivery. Int. J. Pharm. 2019, 568, 118526. [Google Scholar] [CrossRef]
- Jeong, J.Y.; Hong, E.-H.; Lee, S.Y.; Lee, J.-Y.; Song, J.-H.; Ko, S.-H.; Shim, J.-S.; Choe, S.; Kim, D.-D.; Ko, H.-J.; et al. Boronic acid-tethered amphiphilic hyaluronic acid derivative-based nanoassemblies for tumor targeting and penetration. Acta Biomater. 2017, 53, 414–426. [Google Scholar] [CrossRef]
- Alves, C.G.; de Melo-Diogo, D.; Sousa, A.R.L.; Costa, E.C.; Correia, I.J. Hyaluronic acid functionalized nanoparticles loaded with IR780 and DOX for cancer chemo-photothermal therapy. Eur. J. Pharm. Biopharm. 2019, 137, 86–94. [Google Scholar] [CrossRef]
- Phua, S.Z.F.; Yang, G.; Lim, W.Q.; Verma, A.; Chen, H.; Thanabalu, T.; Zhao, Y. Catalase-Integrated Hyaluronic Acid as Nanocarriers for Enhanced Photodynamic Therapy in Solid Tumor. ACS Nano 2019, 13, 4742–4751. [Google Scholar] [CrossRef]
- Lu, G.; Cao, L.; Zhu, C.; Xie, H.; Hao, K.; Xia, N.; Wang, B.; Zhang, Y.; Liu, F. Improving lung cancer treatment: Hyaluronic acid-modified and glutathione-responsive amphiphilic TPGS-doxorubicin prodrug-entrapped nanoparticles. Oncol. Rep. 2019, 42, 361–369. [Google Scholar] [CrossRef]
- Ghosh, S.; Dutta, S.; Sarkar, A.; Kundu, M.; Sil, P.C. Targeted delivery of curcumin in breast cancer cells via hyaluronic acid modified mesoporous silica nanoparticle to enhance anticancer efficiency. Colloids Surf. B Biointerfaces 2021, 197, 111404. [Google Scholar] [CrossRef]
- Wang, Y.; Qian, J.; Yang, M.; Xu, W.; Wang, J.; Hou, G.; Ji, L.; Suo, A. Doxorubicin/cisplatin co-loaded hyaluronic acid/chitosan-based nanoparticles for in vitro synergistic combination chemotherapy of breast cancer. Carbohydr. Polym. 2019, 225, 115206. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Luo, B.; Chen, Z.; Yuan, Y.; Kuang, Y.; Wan, L.; Yao, L.; Chen, X.; Jiang, B.; Liu, J.; et al. Host-guest fabrication of dual-responsive hyaluronic acid/mesoporous silica nanoparticle based drug delivery system for targeted cancer therapy. Int. J. Biol. Macromol. 2020, 146, 363–373. [Google Scholar] [CrossRef] [PubMed]
- Alam, N.; Koul, M.; Mintoo, M.J.; Khare, V.; Gupta, R.; Rawat, N.; Sharma, P.R.; Singh, S.K.; Mondhe, D.M.; Gupta, P.N. Development and characterization of hyaluronic acid modified PLGA based nanoparticles for improved efficacy of cisplatin in solid tumor. Biomed. Pharmacother. 2017, 95, 856–864. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Cho, H.-J. Mitochondria Targeting and Destabilizing Hyaluronic Acid Derivative-Based Nanoparticles for the Delivery of Lapatinib to Triple-Negative Breast Cancer. Biomacromolecules 2019, 20, 835–845. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Chen, M.; Xie, Q.; Li, L.; Zhu, L.; Ma, Q.; Gao, S. Construction and evaluation of hyaluronic acid-based copolymers as a targeted chemotherapy drug carrier for cancer therapy. Nanotechnology 2020, 31, 305702. [Google Scholar] [CrossRef]
- Liang, J.; Yang, X.; Liu, D.; Cong, M.; Song, Y.; Bai, S. Lipid/Hyaluronic Acid–Coated Doxorubicin-Fe3O4 as a Dual-Targeting Nanoparticle for Enhanced Cancer Therapy. AAPS PharmSciTech 2020, 21, 1–9. [Google Scholar] [CrossRef]
- Sun, W.; Du, Y.; Liang, X.; Yua, C.; Fanga, J.; Lua, W.; Guoa, X.; Tianbcef, J.; Jina, Y.; Zhenga, J. Synergistic triple-combination therapy with hyaluronic acid-shelled PPy/CPT nanoparticles results in tumor regression and prevents tumor recurrence and metastasis in 4T1 breast cancer. Biomaterials 2019, 217, 119264. [Google Scholar] [CrossRef]
- Gao, D.; Wong, R.C.; Wang, Y.; Guo, X.; Yang, Z.; Lo, P.C. Shifting the absorption to the near-infrared region and inducing a strong photothermal effect by encapsulating zinc(II) phthalocyanine in poly(lactic-co-glycolic acid)-hyaluronic acid nanoparticles. Acta Biomater. 2020, 116, 329–343. [Google Scholar] [CrossRef]
- Saneja, A.; Nayak, D.; Srinivas, M.; Kumar, A.; Khare, V.; Katoch, A.; Goswami, A.; Vishwakarma, R.A.; Sawant, S.D.; Gupta, P.N. Development and mechanistic insight into enhanced cytotoxic potential of hyaluronic acid conjugated nanoparticles in CD44 overexpressing cancer cells. Eur. J. Pharm. Sci. 2017, 97, 79–91. [Google Scholar] [CrossRef]
- Chen, C.; Sun, W.; Wang, X.; Wang, Y.; Wang, P. pH-responsive nanoreservoirs based on hyaluronic acid end-capped mesoporous silica nanoparticles for targeted drug delivery. Int. J. Biol. Macromol. 2018, 111, 1106–1115. [Google Scholar] [CrossRef]
- Pereira, F.M.; Melo, M.N.; Santos, K.M.; Oliveira, K.V.; Diz, F.M.; Ligabue, R.A.; Morrone, F.B.; Severino, P.; Fricks, A.T. Hyaluronic acid-coated chitosan nanoparticles as carrier for the enzyme/prodrug complex based on horseradish peroxidase/indole-3-acetic acid: Characterization and potential therapeutic for bladder cancer cells. Enzym. Microb. Technol. 2021, 150, 109889. [Google Scholar] [CrossRef] [PubMed]
- Jeannot, V.; Gauche, C.; Mazzaferro, S.; Couvet, M.; Vanwonterghem, L.; Henry, M.; Didier, C.; Vollaire, J.; Josserand, V.; Coll, J.-L.; et al. Anti-tumor efficacy of hyaluronan-based nanoparticles for the co-delivery of drugs in lung cancer. J. Control. Release 2018, 275, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-J.; Perumalsamy, H.; Castro-Aceituno, V.; Kim, D.; Markus, J.; Lee, S.; Kim, S.; Liu, Y.; Yang, D.C. Photoluminescent And Self-Assembled Hyaluronic Acid-Zinc Oxide-Ginsenoside Rh2 Nanoparticles and Their Potential Caspase-9 Apoptotic Mechanism Towards Cancer Cell Lines. Int. J. Nanomed. 2019, 14, 8195–8208. [Google Scholar] [CrossRef]
- Xu, Y.; Asghar, S.; Yang, L.; Li, H.; Wang, Z.; Ping, Q.; Xiao, Y. Lactoferrin-coated polysaccharide nanoparticles based on chitosan hydrochloride/hyaluronic acid/PEG for treating brain glioma. Carbohydr. Polym. 2017, 157, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Hu, H.; Tang, W.; Zhang, Q.; Li, M.; Jin, H.; Huang, Z.; Cui, Z.; Xu, J.; Wang, K.; et al. A multifunctional magnetic nanosystem based on “two strikes” effect for synergistic anticancer therapy in triple-negative breast cancer. J. Control. Release 2020, 322, 401–415. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, J.; Liu, D.; Zhu, W.; Guan, S.; Fan, L.; Cai, D. Targeted delivery of honokiol by zein/hyaluronic acid core-shell nanoparticles to suppress breast cancer growth and metastasis. Carbohydr. Polym. 2020, 240, 116325. [Google Scholar] [CrossRef]
- Wang, S.; Shao, M.; Zhong, Z.; Wang, A.; Cao, J.; Lu, Y.; Wang, Y.; Zhang, J. Co-delivery of gambogic acid and TRAIL plasmid by hyaluronic acid grafted PEI-PLGA nanoparticles for the treatment of triple negative breast cancer. Drug Deliv. 2017, 24, 1791–1800. [Google Scholar] [CrossRef]
- Gupta, B.; Poudel, B.K.; Ruttala, H.B.; Regmi, S.; Pathak, S.; Gautam, M.; Jin, S.G.; Jeong, J.-H.; Choi, H.-G.; Ku, S.K.; et al. Hyaluronic acid-capped compact silica-supported mesoporous titania nanoparticles for ligand-directed delivery of doxorubicin. Acta Biomater. 2018, 80, 364–377. [Google Scholar] [CrossRef]
- Fang, Z.; Li, X.; Xu, Z.; Du, F.; Wang, W.; Shi, R.; Gao, D. Hyaluronic acid-modified mesoporous silica-coated superparamagnetic Fe3O4 nanoparticles for targeted drug delivery. Int. J. Nanomed. 2019, 14, 5785–5797. [Google Scholar] [CrossRef] [Green Version]
- Matha, K.; Lollo, G.; Taurino, G.; Respaud, R.; Marigo, I.; Shariati, M.; Bussolati, O.; Vermeulen, A.; Remaut, K.; Benoit, J.-P. Bioinspired hyaluronic acid and polyarginine nanoparticles for DACHPt delivery. Eur. J. Pharm. Biopharm. 2020, 150, 1–13. [Google Scholar] [CrossRef]
- Wang, X.; Cheng, R.; Zhong, Z. Facile fabrication of robust, hyaluronic acid-surfaced and disulfide-crosslinked PLGA nanoparticles for tumor-targeted and reduction-triggered release of docetaxel. Acta Biomater. 2021, 125, 280–289. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yuan, T.; Li, Z.; Luo, C.; Wu, Y.; Zhang, J.; Zhang, X.; Fan, W. Hyaluronate-Based Self-Stabilized Nanoparticles for Immunosuppression Reversion and Immunochemotherapy in Osteosarcoma Treatment. ACS Biomater. Sci. Eng. 2021, 7, 1515–1525. [Google Scholar] [CrossRef] [PubMed]
- Qiao, L.; Yuan, X.; Peng, H.; Shan, G.; Gao, M.; Yi, X.; He, X. Targeted delivery and stimulus-responsive release of anticancer drugs for efficient chemotherapy. Drug Deliv. 2021, 28, 2218–2228. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhao, M.; Cao, N.; Qin, W.; Zhao, M.; Wu, J.; Lin, D. Construction of a tumor microenvironment pH-responsive cleavable PEGylated hyaluronic acid nano-drug delivery system for colorectal cancer treatment. Biomater. Sci. 2020, 8, 1885–1896. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.-Y.; Lee, C.-C.; Lin, H.-M. Hyaluronidase-Responsive Mesoporous Silica Nanoparticles with Dual-Imaging and Dual-Target Function. Cancers 2019, 11, 697. [Google Scholar] [CrossRef] [PubMed]
- Ju, X.; Chen, H.; Miao, T.; Ni, J.; Han, L. Prodrug Delivery Using Dual-Targeting Nanoparticles To Treat Breast Cancer Brain Metastases. Mol. Pharm. 2021, 18, 2694–2702. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Q.; Cui, M.; Bai, Y.; Liu, Y.; Liu, D.; Song, T. A supramolecular nanoparticle system based on β-cyclodextrin-conjugated poly-l-lysine and hyaluronic acid for co-delivery of gene and chemotherapy agent targeting hepatocellular carcinoma. Colloids Surf. B Biointerfaces 2017, 155, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.S.; Shan, M.H.; Chu, C.C. Inclusion complex from cyclodextrin-grafted hyaluronic acid and pseudo protein as biodegradable nano-delivery vehicle for gambogic acid. Acta Biomater. 2017, 62, 234–245. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Jia, Y.; Zheng, C.W.; Shao, D.; Zhao, Y.; Wang, Z.; Dawulietia, J.; Liu, W.; Sun, M.; Sun, W.; et al. Janus nanocarrier-based co-delivery of doxorubicin and berberine weakens chemotherapy-exacerbated hepatocellular carcinoma recurrence. Acta Biomater. 2019, 100, 352–364. [Google Scholar] [CrossRef]
- Cerqueira, B.B.S.; Lasham, A.; Shelling, A.N.; Al-Kassas, R. Development of biodegradable PLGA nanoparticles surface engineered with hyaluronic acid for targeted delivery of paclitaxel to triple negative breast cancer cells. Mater. Sci. Eng. C 2017, 76, 593–600. [Google Scholar] [CrossRef]
- Sun, X.; Xu, Y.; Guo, Q.; Wang, N.; Wu, B.; Zhu, C.; Zhao, W.; Qiang, W.; Zheng, M. A Novel Nanoprobe for Targeted Imaging and Photothermal/Photodynamic Therapy of Lung Cancer. Langmuir 2022, 38, 1360–1367. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Park, J.-H.; Ko, S.-H.; Shim, J.-S.; Kim, D.-D.; Cho, H.-J. Mussel-Inspired Hyaluronic Acid Derivative Nanostructures for Improved Tumor Targeting and Penetration. ACS Appl. Mater. Interfaces 2017, 9, 22308–22320. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-Y.; Zhang, Y.; Ren, X.-H.; He, X.-W.; Li, W.-Y.; Zhang, Y.-K. HA targeted-biodegradable nanocomposites responsive to endogenous and exogenous stimulation for multimodal imaging and chemo-/photothermal therapy. Nanoscale 2021, 13, 886–900. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Gu, X.; Wang, H.; Yang, J.; Mao, S. Enhanced delivery of doxorubicin to the liver through self-assembled nanoparticles formed via conjugation of glycyrrhetinic acid to the hydroxyl group of hyaluronic acid. Carbohydr. Polym. 2018, 195, 170–179. [Google Scholar] [CrossRef]
- Sargazi, A.; Kamali, N.; Shiri, F.; Majd, M.H. Hyaluronic acid/polyethylene glycol nanoparticles for controlled delivery of mitoxantrone. Artif. Cells Nanomed. Biotechnol. 2018, 46, 500–509. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.; Zheng, H.; Fei, Z.; Lu, B.; Zheng, H.; Li, D.; Xiong, X.; Yi, Y. Tumor-targeting and pH-responsive nanoparticles from hyaluronic acid for the enhanced delivery of doxorubicin. Int. J. Biol. Macromol. 2018, 113, 737–747. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Niu, S.; Williams, G.R.; Wu, J.; Chen, X.; Zheng, H.; Zhu, L.-M. Dual-responsive nanoparticles based on chitosan for enhanced breast cancer therapy. Carbohydr. Polym. 2019, 221, 84–93. [Google Scholar] [CrossRef]
- Gaio, E.; Conte, C.; Esposito, D.; Miotto, G.; Quaglia, F.; Moret, F.; Reddi, E. Co-delivery of Docetaxel and Disulfonate Tetraphenyl Chlorin in One Nanoparticle Produces Strong Synergism between Chemo- and Photodynamic Therapy in Drug-Sensitive and -Resistant Cancer Cells. Mol. Pharm. 2018, 15, 4599–4611. [Google Scholar] [CrossRef]
- Huang, Y.; Xie, D.; Gou, S.; Canup, B.S.B.; Zhang, G.; Dai, F.; Li, C.; Xiao, B. Quadruple-responsive nanoparticle-mediated targeted combination chemotherapy for metastatic breast cancer. Nanoscale 2021, 13, 5765–5779. [Google Scholar] [CrossRef]
- Bhatnagar, P.; Kumari, M.; Pahuja, R.; Pant, A.B.; Shukla, Y.; Kumar, P.; Gupta, K.C. Hyaluronic acid-grafted PLGA nanoparticles for the sustained delivery of berberine chloride for an efficient suppression of Ehrlich ascites tumors. Drug Deliv. Transl. Res. 2018, 8, 565–579. [Google Scholar] [CrossRef]
- Shi, J.; Ren, Y.; Ma, J.; Luo, X.; Li, J.; Wu, Y.; Gu, H.; Fu, C.; Cao, Z.; Zhang, J. Novel CD44-targeting and pH/redox-dual-stimuli-responsive core–shell nanoparticles loading triptolide combats breast cancer growth and lung metastasis. J. Nanobiotechnol. 2021, 19, 188. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Zhu, R.; Sun, W.; Cai, K.; Chen, Y.; Yin, L. Selective cancer treatment via photodynamic sensitization of hypoxia-responsive drug delivery. Nanoscale 2018, 10, 2856–2865. [Google Scholar] [CrossRef] [PubMed]
- Zhu, R.; He, H.; Liu, Y.; Cao, D.; Yan, J.; Duan, S.; Chen, Y.; Yin, L. Cancer-Selective Bioreductive Chemotherapy Mediated by Dual Hypoxia-Responsive Nanomedicine upon Photodynamic Therapy-Induced Hypoxia Aggravation. Biomacromolecules 2019, 20, 2649–2656. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhou, Y.; Yang, D.; Gao, X.; Wen, T.; Fu, J.; Wen, X.; Quan, G.; Pan, X.; Wu, C. Intelligent and spatiotemporal drug release based on multifunctional nanoparticle-integrated dissolving microneedle system for synergetic chemo-photothermal therapy to eradicate melanoma. Acta Biomater. 2021, 135, 164–178. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xiong, T.; Cui, M.; Li, N.; Li, Q.; Zhu, L.; Duan, S.; Wang, Y.; Guo, Y. A novel targeted co-delivery nanosystem for enhanced ovarian cancer treatment via multidrug resistance reversion and mTOR-mediated signaling pathway. J. Nanobiotechnol. 2021, 19, 444. [Google Scholar] [CrossRef]
- Dai, X.; Zhang, B.; Zhou, W.; Liu, Y. High-Efficiency Synergistic Effect of Supramolecular Nanoparticles Based on Cyclodextrin Prodrug on Cancer Therapy. Biomacromolecules 2020, 21, 4998–5007. [Google Scholar] [CrossRef]
- Yan, J.; Su, T.; Cheng, F.; Cao, J.; Zhang, H.; He, B. Multifunctional nanoparticles self-assembled from polyethylenimine-based graft polymers as efficient anticancer drug delivery. Colloids Surf. B Biointerfaces 2017, 155, 118–127. [Google Scholar] [CrossRef]
- Qiao, H.; Fang, D.; Zhang, L.; Gu, X.; Lu, Y.; Sun, M.; Sun, C.; Ping, Q.; Li, J.; Chen, Z.; et al. Nanostructured Peptidotoxins as Natural Pro-Oxidants Induced Cancer Cell Death via Amplification of Oxidative Stress. ACS Appl. Mater. Interfaces 2018, 10, 4569–4581. [Google Scholar] [CrossRef]
- Wang, L.; Hu, Y.; Hao, Y.; Li, L.; Zheng, C.; Zhao, H.; Niu, M.; Yin, Y.; Zhang, Z.; Zhang, Y. Tumor-targeting core-shell structured nanoparticles for drug procedural controlled release and cancer sonodynamic combined therapy. J. Control. Release 2018, 286, 74–84. [Google Scholar] [CrossRef]
- Li, Y.; Shi, S.; Ming, Y.; Wang, L.; Li, C.; Luo, M.; Li, Z.; Li, B.; Chen, J. Specific cancer stem cell-therapy by albumin nanoparticles functionalized with CD44-mediated targeting. J. Nanobiotechnol. 2018, 16, 99. [Google Scholar] [CrossRef]
- Li, T.; Geng, Y.; Zhang, H.; Wang, J.; Feng, Y.; Chen, Z.; Xie, X.; Qin, X.; Li, S.; Wu, C.; et al. A versatile nanoplatform for synergistic chemo-photothermal therapy and multimodal imaging against breast cancer. Expert Opin. Drug Deliv. 2020, 17, 725–733. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Wang, Y.; Huang, X.; Liu, B.; Hu, L.; Ma, C.; Liu, J.; Xue, J.; Qu, W.; Liu, F.; et al. A stage-specific cancer chemotherapy strategy through flexible combination of reduction-activated charge-conversional core-shell nanoparticles. Theranostics 2019, 9, 6532–6549. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Qiao, L.; Zhang, S.; Wan, G.; Chen, B.; Zhou, P.; Zhang, N.; Wang, Y. Dual pH-responsive multifunctional nanoparticles for targeted treatment of breast cancer by combining immunotherapy and chemotherapy. Acta Biomater. 2018, 66, 310–324. [Google Scholar] [CrossRef]
- Hou, G.; Qian, J.; Guo, M.; Xu, W.; Wang, J.; Wang, Y.; Suo, A. Hydrazided hyaluronan/cisplatin/indocyanine green coordination nanoprodrug for photodynamic chemotherapy in liver cancer. Carbohydr. Polym. 2022, 276, 118810. [Google Scholar] [CrossRef] [PubMed]
- Qiu, W.; Chen, R.; Chen, X.; Zhang, H.; Song, L.; Cui, W.; Zhang, J.; Ye, D.; Zhang, Y.; Wang, Z. Oridonin-loaded and GPC1-targeted gold nanoparticles for multimodal imaging and therapy in pancreatic cancer. Int. J. Nanomed. 2018, 13, 6809–6827. [Google Scholar] [CrossRef]
- Kabary, D.M.; Helmy, M.W.; Abdelfattah, E.-Z.A.; Fang, J.-Y.; Elkhodairy, K.A.; Elzoghby, A.O. Inhalable multi-compartmental phospholipid enveloped lipid core nanocomposites for localized mTOR inhibitor/herbal combined therapy of lung carcinoma. Eur. J. Pharm. Biopharm. 2018, 130, 152–164. [Google Scholar] [CrossRef]
- Jin, R.; Xie, J.; Yang, X.; Tian, Y.; Yuan, P.; Bai, Y.; Liu, S.; Cai, B.; Chen, X. A tumor-targeted nanoplatform with stimuli-responsive cascaded activities for multiple model tumor therapy. Biomater. Sci. 2020, 8, 1865–1874. [Google Scholar] [CrossRef]
- Liu, J.; Zhao, X.; Nie, W.; Yang, Y.; Wu, C.; Liu, W.; Zhang, K.; Zhang, Z.; Shi, J. Tumor cell-activated “Sustainable ROS Generator” with homogeneous intratumoral distribution property for improved anti-tumor therapy. Theranostics 2021, 11, 379–396. [Google Scholar] [CrossRef]
- Seo, J.-H.; Lee, S.Y.; Hwang, C.; Yang, M.; Lee, J.; Lee, S.-H.; Cho, H.-J. Multi-layered cellulose nanocrystal system for CD44 receptor-positive tumor-targeted anticancer drug delivery. Int. J. Biol. Macromol. 2020, 162, 798–809. [Google Scholar] [CrossRef]
- Liu, T.; Huang, X.; Zhao, L.; Xiao, Z.; Li, Z.; Xin, Y.; Yang, S.; Guo, D.; Zhao, W.; Mi, Y.; et al. Distinguishable Targeting of Non-Small Cell Lung Cancer Using Hyaluronan Functionalized Platinum Nanoclusters and Their Inhibition Behaviors of Proliferation, Invasion, Migration. ChemistryOpen 2021, 10, 882–888. [Google Scholar] [CrossRef]
- Fan, R.; Chuan, D.; Hou, H.; Chen, H.; Han, B.; Zhang, X.; Zhou, L.; Tong, A.; Xu, J.; Guo, G. Development of a hybrid nanocarrier-recognizing tumor vasculature and penetrating the BBB for glioblastoma multi-targeting therapy. Nanoscale 2019, 11, 11285–11304. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.-L.; Lai, C.-J.; Lin, Y.-N.; Huang, C.-M.; Lin, Y.-H. Multifunctional nanoparticles for targeting the tumor microenvironment to improve synergistic drug combinations and cancer treatment effects. J. Mater. Chem. B 2020, 8, 10416–10427. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Liu, L.; Lu, Q.; Yang, S.; Yang, L.; Cheng, Y.; Wang, Y.; Wang, S.; Song, Y.; Tan, F.; et al. Ultrasmall MoS2 Nanodots-Doped Biodegradable SiO2 Nanoparticles for Clearable FL/CT/MSOT Imaging-Guided PTT/PDT Combination Tumor Therapy. ACS Appl. Mater. Interfaces 2019, 11, 5771–5781. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Lu, T.; Wang, Y.; Song, Y.; Wang, S.; Lu, Q.; Yang, L.; Tan, F.; Li, J.; Li, N. Glutathione-Mediated Clearable Nanoparticles Based on Ultrasmall Gd2O3 for MSOT/CT/MR Imaging Guided Photothermal/Radio Combination Cancer Therapy. Mol. Pharm. 2019, 16, 3489–3501. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Tan, X.; Wang, J.; Wang, Y.; Song, Y.; You, Q.; Sun, Q.; Liu, L.; Wang, S.; Tan, F.; et al. Polymer-based gadolinium oxide nanocomposites for FL/MR/PA imaging guided and photothermal/photodynamic combined anti-tumor therapy. J. Control. Release 2018, 277, 77–88. [Google Scholar] [CrossRef]
- Lin, X.; Cao, Y.; Xue, Y.; Wu, F.; Yu, F.; Wu, M.; Zhu, X. Multifunctional theranostic agents based on prussian blue nanoparticles for tumor targeted and MRI—Guided photodynamic/photothermal combined treatment. Nanotechnology 2020, 31, 135101. [Google Scholar] [CrossRef]
- Elsey, J.; Bubley, J.A.; Zhu, L.; Rao, S.; Sasaki, M.; Pollack, B.P.; Yang, L.; Arbiser, J.L. Palladium based nanoparticles for the treatment of advanced melanoma. Sci. Rep. 2019, 9, 3255. [Google Scholar] [CrossRef]
- Peng, Y.; Liu, P.; Meng, Y.; Hu, S.; Ding, J.; Zhou, W. Nanoscale Copper(II)–Diethyldithiocarbamate Coordination Polymer as a Drug Self-Delivery System for Highly Robust and Specific Cancer Therapy. Mol. Pharm. 2020, 17, 2864–2873. [Google Scholar] [CrossRef]
- Liao, J.; Zheng, H.; Hu, R.; Cao, J.; Wei, X.; Li, D.; Zheng, H.; Yin, Y. Hyaluronan Based Tumor-Targeting and pH-Responsive Shell Cross-Linkable Nanoparticles for the Controlled Release of Doxorubicin. J. Biomed. Nanotechnol. 2018, 14, 496–509. [Google Scholar] [CrossRef]
- Sheng, S.; Liu, F.; Lin, L.; Yan, N.; Wang, Y.; Xu, C.; Tian, H.; Chen, X. Nanozyme-mediated cascade reaction based on metal-organic framework for synergetic chemo-photodynamic tumor therapy. J. Control. Release 2020, 328, 631–639. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, H.; Chen, Y.; Ma, J.; Lin, J.; Zhang, Y.; Fan, Z.; Su, G.; Xie, L.; Zhu, X.; et al. Integration of phospholipid-hyaluronic acid-methotrexate nanocarrier assembly and amphiphilic drug-drug conjugate for synergistic targeted delivery and combinational tumor therapy. Biomater. Sci. 2018, 6, 1818–1833. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Yang, J.; Yan, X.; Li, C.; Elsabahy, M.; Chen, L.; Yang, Y.-W.; Gao, H. A dendritic polyamidoamine supramolecular system composed of pillar[5]arene and azobenzene for targeting drug-resistant colon cancer. J. Mater. Chem. B 2021, 9, 9594–9605. [Google Scholar] [CrossRef]
- Zeng, S.; Liu, S.; Lan, Y.; Qiu, T.; Zhou, M.; Gao, W.; Huang, W.; Ge, L.; Zhang, J. Combined Photothermotherapy and Chemotherapy of Oral Squamous Cell Carcinoma Guided by Multifunctional Nanomaterials Enhanced Photoacoustic Tomography. Int. J. Nanomed. 2021, 16, 7373–7390. [Google Scholar] [CrossRef]
- Xu, Y.; Asghar, S.; Gao, S.; Chen, Z.; Huang, L.; Yin, L.; Ping, Q.; Xiao, Y. Polysaccharide-based nanoparticles for co-loading mitoxantrone and verapamil to overcome multidrug resistance in breast tumor. Int. J. Nanomed. 2017, 12, 7337–7350. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.-Y.; Zhang, J.-J.; Zhang, Y.-X.; Wang, W.; Li, C.; Zhou, S.-Y.; Zhang, B.-L. Construction of redox-responsive tumor targeted cisplatin nano-delivery system for effective cancer chemotherapy. Int. J. Pharm. 2020, 580, 119190. [Google Scholar] [CrossRef] [PubMed]
- Qian, X.; Shi, Z.; Qi, H.; Zhao, M.; Huang, K.; Han, D.; Zhou, J.; Liu, C.; Liu, Y.; Lu, Y.; et al. A novel Granzyme B nanoparticle delivery system simulates immune cell functions for suppression of solid tumors. Theranostics 2019, 9, 7616–7627. [Google Scholar] [CrossRef]
- Cui, X.; Deng, X.; Liang, Z.; Lu, J.; Shao, L.; Wang, X.; Jia, F.; Pan, Z.; Hu, Q.; Xiao, X.; et al. Multicomponent-assembled nanodiamond hybrids for targeted and imaging guided triple-negative breast cancer therapy via a ternary collaborative strategy. Biomater. Sci. 2021, 9, 3838–3850. [Google Scholar] [CrossRef]
- Singh, B.; Maharjan, S.; Pan, D.C.; Zhao, Z.; Gao, Y.; Zhang, Y.S.; Mitragotri, S. Imiquimod-gemcitabine nanoparticles harness immune cells to suppress breast cancer. Biomaterials 2022, 280, 121302. [Google Scholar] [CrossRef]
- Gou, S.; Yang, J.; Ma, Y.; Zhang, X.; Zu, M.; Kang, T.; Liu, S.; Ke, B.; Xiao, B. Multi-responsive nanococktails with programmable targeting capacity for imaging-guided mitochondrial phototherapy combined with chemotherapy. J. Control. Release 2020, 327, 371–383. [Google Scholar] [CrossRef]
- Shen, S.; Wu, Y.; Li, K.; Wang, Y.; Wu, J.; Zeng, Y.; Wu, D. Versatile hyaluronic acid modified AQ4N-Cu(II)-gossypol infinite coordination polymer nanoparticles: Multiple tumor targeting, highly efficient synergistic chemotherapy, and real-time self-monitoring. Biomaterials 2018, 154, 197–212. [Google Scholar] [CrossRef]
- Zhang, Y.; Xia, Q.; Wu, T.; He, Z.; Li, Y.; Li, Z.; Hou, X.; He, Y.; Ruan, S.; Wang, Z.; et al. A novel multi-functionalized multicellular nanodelivery system for non-small cell lung cancer photochemotherapy. J. Nanobiotechnol. 2021, 19, 245. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Zhang, L.; Bai, X.; Cao, X.; Jiao, X.; Huang, Y.; Li, Y.; Qin, Y.; Wen, Y. Stimuli-Responsive Nanocarrier for Co-delivery of MiR-31 and Doxorubicin to Suppress High MtEF4 Cancer. ACS Appl. Mater. Interfaces 2018, 10, 22767–22775. [Google Scholar] [CrossRef] [PubMed]
- Cong, Z.; Zhang, L.; Ma, S.-Q.; Lam, K.S.; Yang, F.-F.; Liao, Y.-H. Size-Transformable Hyaluronan Stacked Self-Assembling Peptide Nanoparticles for Improved Transcellular Tumor Penetration and Photo–Chemo Combination Therapy. ACS Nano 2020, 14, 1958–1970. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Tao, Y.; Li, X.; Pang, Y.; Yang, C.; Jiang, G.; Liu, Y. CD44-Targeting Oxygen Self-Sufficient Nanoparticles for Enhanced Photodynamic Therapy Against Malignant Melanoma. Int. J. Nanomed. 2020, 15, 10401–10416. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; You, Q.; Wang, J.; Song, Y.; Cheng, Y.; Wang, Y.; Yang, S.; Yang, L.; Li, P.; Lu, Q.; et al. MSOT/CT/MR imaging-guided and hypoxia-maneuvered oxygen self-supply radiotherapy based on one-pot MnO2-mSiO2@Au nanoparticles. Nanoscale 2019, 11, 6270–6284. [Google Scholar] [CrossRef]
- Shen, J.; Ma, M.; Zhang, H.; Yu, H.; Xue, F.; Hao, N.; Chen, H. Microfluidics-Assisted Surface Trifunctionalization of a Zeolitic Imidazolate Framework Nanocarrier for Targeted and Controllable Multitherapies of Tumors. ACS Appl. Mater. Interfaces 2020, 12, 45838–45849. [Google Scholar] [CrossRef]
- Torino, E.; Auletta, L.; Vecchione, D.; Orlandella, F.M.; Salvatore, G.; Iaccino, E.; Fiorenza, D.; Grimaldi, A.M.; Sandomenico, A.; Albanese, S.; et al. Multimodal imaging for a theranostic approach in a murine model of B-cell lymphoma with engineered nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 483–491. [Google Scholar] [CrossRef]
- Yalcin, E.; Kara, G.; Celik, E.; Pinarli, F.A.; Saylam, G.; Sucularli, C.; Ozturk, S.; Yilmaz, E.; Bayır, O.; Korkmaz, M.H.; et al. Preparation and characterization of novel albumin-sericin nanoparticles as siRNA delivery vehicle for laryngeal cancer treatment. Prep. Biochem. Biotechnol. 2019, 49, 659–670. [Google Scholar] [CrossRef]
- Xu, Y.; Shi, W.; Li, H.; Li, X.; Ma, H. H2O2 -Responsive Organosilica-Doxorubicin Nanoparticles for Targeted Imaging and Killing of Cancer Cells Based on a Synthesized Silane-Borate Precursor. ChemMedChem 2019, 14, 1079–1085. [Google Scholar] [CrossRef]
- Huang, S.; Li, C.; Wang, W.; Li, H.; Sun, Z.; Song, C.; Li, B.; Duan, S.; Hu, Y. A54 peptide-mediated functionalized gold nanocages for targeted delivery of DOX as a combinational photothermal-chemotherapy for liver cancer. Int. J. Nanomed. 2017, 12, 5163–5176. [Google Scholar] [CrossRef]
- Xie, R.; Lian, S.; Peng, H.; Ouyang, C.; Li, S.; Lu, Y.; Cao, X.; Zhang, C.; Xu, J.; Jia, L. Mitochondria and Nuclei Dual-Targeted Hollow Carbon Nanospheres for Cancer Chemophotodynamic Synergistic Therapy. Mol. Pharm. 2019, 16, 2235–2248. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Wang, J.; Liu, L.; Sun, Q.; You, Q.; Cheng, Y.; Wang, Y.; Wang, S.; Tan, F.; Li, N. One-Pot Synthesis of a Bismuth Selenide Hexagon Nanodish Complex for Multimodal Imaging-Guided Combined Antitumor Phototherapy. Mol. Pharm. 2018, 15, 1941–1953. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Sun, D.; Liao, H.; Wang, Y.; Zhao, S.; Zhang, Y.; Lv, G.; Ma, X.; Liu, Y.; Sun, G. Synthesis and characterization of a bimodal nanoparticle based on the host-guest self-assembly for targeted cellular imaging. Talanta 2017, 171, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Guo, Z.; Zheng, K.; Ma, K.; Cui, C.; Wang, L.; Yuan, Y.; Tang, Y. Dual targeting mesoporous silica nanoparticles for inhibiting tumour cell invasion and metastasis. Int. J. Pharm. 2017, 534, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Dong, Y.; Yue, S.; Qiu, X.; Sun, H.; Zhong, Z. Dually Active Targeting Nanomedicines Based on a Direct Conjugate of Two Purely Natural Ligands for Potent Chemotherapy of Ovarian Tumors. ACS Appl. Mater. Interfaces 2019, 11, 46548–46557. [Google Scholar] [CrossRef]
- Su, X.; Zhao, F.; Wang, Y.; Yan, X.; Jia, S.; Du, B. CuS as a gatekeeper of mesoporous upconversion nanoparticles-based drug controlled release system for tumor-targeted multimodal imaging and synergetic chemo-thermotherapy. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 1761–1772. [Google Scholar] [CrossRef]
- Feng, X.; Xiong, X.; Ma, S. Docetaxel-Loaded Novel Nano-Platform for Synergistic Therapy of Non-Small Cell Lung Cancer. Front. Pharmacol. 2022, 13, 832725. [Google Scholar] [CrossRef]
- Trivedi, M.; Singh, A.; Talekar, M.; Pawar, G.; Shah, P.; Amiji, M. MicroRNA-34a Encapsulated in Hyaluronic Acid Nanoparticles Induces Epigenetic Changes with Altered Mitochondrial Bioenergetics and Apoptosis in Non-Small-Cell Lung Cancer Cells. Sci. Rep. 2017, 7, 3636. [Google Scholar] [CrossRef]
- Li, H.; Zhuang, S.; Yang, Y.; Zhou, F.; Rong, J.; Zhao, J. ATP/Hyals dually responsive core-shell hyaluronan/chitosan-based drug nanocarrier for potential application in breast cancer therapy. Int. J. Biol. Macromol. 2021, 183, 839–851. [Google Scholar] [CrossRef]
- Yang, J.; Zhao, R.; Feng, Q.; Zhuo, X.; Wang, R. Development of a carrier system containing hyaluronic acid and protamine for siRNA delivery in the treatment of melanoma. Investig. New Drugs 2021, 39, 66–76. [Google Scholar] [CrossRef]
- Parayath, N.; Parikh, A.; Amiji, M.M. Repolarization of Tumor-Associated Macrophages in a Genetically Engineered Nonsmall Cell Lung Cancer Model by Intraperitoneal Administration of Hyaluronic Acid-Based Nanoparticles Encapsulating MicroRNA-125b. Nano Lett. 2018, 18, 3571–3579. [Google Scholar] [CrossRef] [PubMed]
- Parayath, N.N.; Gandham, S.K.; Leslie, F.; Amiji, M.M. Improved anti-tumor efficacy of paclitaxel in combination with MicroRNA-125b-based tumor-associated macrophage repolarization in epithelial ovarian cancer. Cancer Lett. 2019, 461, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Liang, G.-F.; Li, Y.; Li, J.-H.; Jing, A.-H.; Feng, W.-P.; Li, G.-D.; Du, J.-X.; Feng, S.-Y. Preparation and Evaluation of Upconversion Nanoparticles Based miRNA Delivery Carrier in Colon Cancer Mice Model. J. Biomed. Nanotechnol. 2019, 15, 2240–2250. [Google Scholar] [CrossRef] [PubMed]
- Aldawsari, H.M.; Dhaliwal, H.K.; Aljaeid, B.M.; Alhakamy, N.A.; Banjar, Z.M.; Amiji, M.M. Optimization of the Conditions for Plasmid DNA Delivery and Transfection with Self-Assembled Hyaluronic Acid-Based Nanoparticles. Mol. Pharm. 2019, 16, 128–140. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.-L.; Li, Y.; Zhao, L.-M.; Su, L.-W.; Ding, G. Delivery of MTH1 inhibitor (TH287) and MDR1 siRNA via hyaluronic acid-based mesoporous silica nanoparticles for oral cancers treatment. Colloids Surf. B Biointerfaces 2019, 173, 599–606. [Google Scholar] [CrossRef]
- Zhang, W.; Xu, W.; Lan, Y.; He, X.; Liu, K.; Liang, Y. Antitumor effect of hyaluronic-acid-modified chitosan nanoparticles loaded with siRNA for targeted therapy for non-small cell lung cancer. Int. J. Nanomed. 2019, 14, 5287–5301. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, Z.; Qiu, Y.; Liu, Y.; Ding, M.; Zhang, Y. Anti-miRNA21 and resveratrol-loaded polysaccharide-based mesoporous silica nanoparticle for synergistic activity in gastric carcinoma. J. Drug Target. 2019, 27, 1135–1143. [Google Scholar] [CrossRef]
- Luo, K.; Yin, S.; Zhang, R.; Yu, H.; Wang, G.; Li, J. Multifunctional composite nanoparticles based on hyaluronic acid-paclitaxel conjugates for enhanced cancer therapy. Int. J. Pharm. 2020, 589, 119870. [Google Scholar] [CrossRef]
- Edelman, R.; Assaraf, Y.G.; Levitzky, I.; Shahar, T.; Livney, Y.D. Hyaluronic acid-serum albumin conjugate-based nanoparticles for targeted cancer therapy. Oncotarget 2017, 8, 24337–24353. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Pan, J.; Yao, M.; Mendes, L.P.; Sarisozen, C.; Mao, S.; Torchilin, V.P. Charge reversible hyaluronic acid-modified dendrimer-based nanoparticles for siMDR-1 and doxorubicin co-delivery. Eur. J. Pharm. Biopharm. 2020, 154, 43–49. [Google Scholar] [CrossRef]
- Wu, J.; Hu, X.; Liu, R.; Zhang, J.; Song, A.; Luan, Y. pH-responsive and self-targeting assembly from hyaluronic acid-based conjugate toward all-in-one chemo-photodynamic therapy. J. Colloid Interface Sci. 2019, 547, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Rangasami, V.K.; Samanta, S.; Parihar, V.S.; Asawa, K.; Zhu, K.; Varghese, O.P.; Teramura, Y.; Nilsson, B.; Hilborn, J.; Harris, R.A.; et al. Harnessing hyaluronic acid-based nanoparticles for combination therapy: A novel approach for suppressing systemic inflammation and to promote antitumor macrophage polarization. Carbohydr. Polym. 2021, 254, 117291. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.; Xiao, F.; Wang, Z.; Wang, B.; Pan, Z.; Zhao, W.; Zhu, Z.; Zhang, J. Redox-Sensitive Hyaluronic Acid Polymer Prodrug Nanoparticles for Enhancing Intracellular Drug Self-Delivery and Targeted Cancer Therapy. ACS Biomater. Sci. Eng. 2020, 6, 4106–4115. [Google Scholar] [CrossRef] [PubMed]
- Janik-Hazuka, M.; Szafraniec-Szczęsny, J.; Kamiński, K.; Odrobińska, J.; Zapotoczny, S. Uptake and in vitro anticancer activity of oleic acid delivered in nanocapsules stabilized by amphiphilic derivatives of hyaluronic acid and chitosan. Int. J. Biol. Macromol. 2020, 164, 2000–2009. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Cai, H.; Yin, T.; Huo, M.; Ma, P.; Zhou, J.; Lai, W. Paclitaxel-loaded redox-sensitive nanoparticles based on hyaluronic acid-vitamin E succinate conjugates for improved lung cancer treatment. Int. J. Nanomed. 2018, 13, 1585–1600. [Google Scholar] [CrossRef] [PubMed]
- Molina-Crespo, A.; Cadete, A.; Sarrio, D.; Gámez-Chiachio, M.; Martinez, L.; Chao, K.; Olivera, A.; Gonella, A.; Díaz, E.; Palacios, J.; et al. Intracellular Delivery of an Antibody Targeting Gasdermin-B Reduces HER2 Breast Cancer Aggressiveness. Clin. Cancer Res. 2019, 25, 4846–4858. [Google Scholar] [CrossRef]
- Rata, D.M.; Cadinoiu, A.N.; Atanase, L.I.; Popa, M.; Mihai, C.-T.; Solcan, C.; Ochiuz, L.; Vochita, G. Topical formulations containing aptamer-functionalized nanocapsules loaded with 5-fluorouracil—An innovative concept for the skin cancer therapy. Mater. Sci. Eng. C 2021, 119, 111591. [Google Scholar] [CrossRef]
- Chen, Q.; Li, X.; Xie, Y.; Hu, W.; Cheng, Z.; Zhong, H.; Zhu, H. Azo modified hyaluronic acid based nanocapsules: CD44 targeted, UV-responsive decomposition and drug release in liver cancer cells. Carbohydr. Polym. 2021, 267, 118152. [Google Scholar] [CrossRef]


| Compound/Drug | Status | Model | Effect | Role of Nanomaterial | Ref. |
|---|---|---|---|---|---|
| Curcumin | In vitro In vivo | Breast cancer | Efficiently accumulates in tumor site via EPR effect and CD44-mediated endocytosis; Antitumor effect. | Nanocarrier | [40] |
| Doxorubicin | In vitro In vivo | Breast cancer | Efficient delivery into cancer cells; Increases the therapeutic and the apoptotic activity of DOX; Effectively suppress tumor growth in vivo. | Chemosensitizing agent | [41] |
| Cinnamaldehyde and protoporphyrin | In vitro In vivo | Melanoma | Improves bioavailability and selective tumor accumulation; Induces cytotoxic ROS generation; Improves antitumor performance. | Delivery system and photodynamic therapy | [42] |
| Doxorubicin | In vitro In vivo | Hepatocellular carcinoma | Excellent antitumor capability. | Drug delivery system | [43] |
| Doxorubicin | In vitro | Cervical cancer | Much better cellular uptake and higher cytotoxicity in tumor cells than normal ones. | Drug delivery system | [44] |
| siRNA | In vitro In vivo | Glioblastoma | Efficiently delivers into tumor cells/tissues and mediates less cytotoxicities in normal cells; Significantly enhances antitumor ability. | siRNA delivery | [45] |
| Compound/Drug | Status | Model | Effect | Role of Nanomaterial | Ref. |
|---|---|---|---|---|---|
| Quercetin combined with Temozolomide | In vitro | Brain cancer (Glioblastoma multiform) | Proficient in mediating site-specific delivery of quercetin via CD44 receptor; Improves the therapeutic efficacy of temozolomide by modulating brain tumor microenvironment. | Drug delivery system | [51] |
| Auraptene and Cisplatin | In vitro In vivo | Breast cancer | Excellent physiological stability and fluorescence effects; Selective internalization; Antitumor effects and lower systemic toxicity. | Dual-targeted delivery and synergistic therapy | [52] |
| Doxorubicin | In vitro In vivo | Melanoma | High biosafety; Tumor microenvironment responsiveness; Ability to target CD44 overexpressed in melanoma cells; Ability to suppress tumor growth in vivo. | Drug delivery system | [53] |
| Oncolytic viruses | In vitro | Colorectal Cancer Prostate Cancer | In vitro cytotoxicity assays demonstrate good oncolytic activity of OV-loaded nanohydrogel against cells. | Delivery system | [54] |
| Coumarin | In vitro In vivo | Cervical Cancer | The results provide novel insights into several aspects of the in vitro and in vivo behavior of nanogels. | Drug delivery system | [55] |
| EF2-Kinase inhibitor | In vitro | Breast cancer Pancreatic cancer | Inhibition of cell proliferation and colony formation of breast and pancreatic cancer cells. | Drug delivery system | [56] |
| Quercetin and Everolimus | In vitro | Breast cancer | Synergistic cytotoxic effects; Antitumor and anti-inflammatory properties. | Nanocarrier | [57] |
| Polypyrrole and doxorubicin | In vitro In vivo | Breast cancer | Significant inhibition of a subcutaneous tumor model through combined photothermo-chemotherapy under laser irradiation. | Drug delivery system | [58] |
| Paclitaxel and interferon gamma | In vitro | Lung carcinoma | Positive effects on cancer cells and fewer side effects on healthy ones. | Drug delivery system | [59] |
| Doxorubicin | In vitro | Hepatocellular carcinoma | Excellent DOX-loading capacity; Cytotoxicity induction. | Drug delivery system | [60] |
| C14-Gemcitabine | In vitro | Colon and Pancreatic cancer | Controlled release of drug; Potential for intratumoral delivery of anticancer agents. | Drug delivery system | [61] |
| Compound/Drug | Status | Model | Effect | Role of Nanomaterial | Ref. |
|---|---|---|---|---|---|
| Doxorubicin and Cisplatin | In vitro In vivo | Breast Cancer | Enhanced drug release under acidic conditions and higher cellular uptake; Stronger cellular growth inhibition and lower systemic toxicity than free drugs. | Drug delivery systems | [64] |
| Doxorubicin | In vitro In vivo | Breast cancer | Combined with radiotherapy, ROS-sensitive micelles disintegrated and released great drug cargos, enhancing cytotoxicity; Prolonged circulation time and improved tumor accumulation. | ROS-sensitive drug delivery system | [65] |
| Curcumin and Baicalin | In vitro In vivo | Lung cancer | Good cellular penetration and tumor cytotoxicity; Effective antitumor activity and reduced side effects. | Drug delivery system | [66] |
| Vitamin E Paclitaxel | In vitro In vivo | Breast Cancer Melanoma | Strong antineoplastic effects due to redox responsiveness; Excellent tumor-targeting ability and prolonged retention time compared to Taxol in vivo. | Drug delivery system | [67] |
| Cisplatin | In vitro In vivo | Ovarian cancer | Prolonged blood circulation and preferential tumor accumulation; higher antitumor efficacy. | Drug delivery system | [68] |
| Gambogic acid | In vitro In vivo | Lung cancer | Higher apoptosis induction and cytotoxicity. | Drug delivery system | [69] |
| Lauroyl-gemcitabine and honokiol | In vitro In vivo | Glioblastoma multiforme | Stronger inhibition of glioma proliferation and apoptosis induction. | Delivery system | [70] |
| Doxorubicin | In vitro | Cervical cancer | Nanomicelles could be disassembled upon UV light; Inhibition of proliferation. | Drug delivery system | [71] |
| Tocopherol succinate | In vitro In vivo | Melanoma | Greater tumor accumulation; Higher antineoplastic responses. | Drug delivery system | [72] |
| Indocyanine green derivative and paclitaxel | In vitro In vivo | Breast cancer | Improved stability and reduced systemic toxicity; High stability, smart release behavior, and excellent tumor-targeting ability; Great synergy in tumor inhibition. | Delivery system | [73] |
| Tirapazamine | In vitro In vivo | Breast cancer | Efficient activation of mitochondrial apoptosis cascade and oxygen depletion in the tumor intracellular environment to amplify the hypoxia-dependent cytotoxic effect of TPZ. | Delivery system | [74] |
| Tamoxifen | In vitro Ex vivo | Breast cancer | Safe and compatible against macrophages; Efficiently kills cancer cells; non-toxic nature in contrast to pure TMX; Augmented intracellular uptake with strong targeting potential for anti-proliferative activity. | Drug delivery system | [75] |
| Oxygen | In vitro In vivo | Ocular choroidal melanoma | Increased generation of O2 and elevated phototoxicity. | Delivery system | [76] |
| Doxorubicin | In vivo | Breast cancer | Remarkable therapeutic effect and minimized toxicity in vivo. | Light-activated drug release | [77] |
| Compound/Drug | Status | Model | Effect | Role of Nanomaterial | Ref. |
|---|---|---|---|---|---|
| Gemcitabine and Quercetin | In vitro | Pancreatic ductal adenocarcinoma | Improved cytotoxicity and cellular uptake; Improved anti-inflammatory properties of quercetin and decrease in interleukin cellular levels. | Drug delivery system | [82] |
| Curcumin | In vitro In vivo | Breast cancer | Cellular uptake and higher cytotoxicity; Higher lactate dehydrogenase release, cell cycle arrest in G2/M, S phases, ROS generation, and apoptosis; Stronger inhibitory effect on tumor growth and pulmonary metastasis. | Drug delivery system | [83] |
| Doxorubicin | In vitro | Gastric cancer | Preferentially taken up by cancer cells; Mainly accumulated in mitochondria; Efficiently killed cancer cells. | Drug delivery system | [84] |
| Doxorubicin and paclitaxel | In vitro In vivo | Lung and Breast cancer | High stability, excellent active targeting effect and controllable intracellular drug release and, ultimately, better anticancer efficiency than individual drugs. | Co-delivery system | [85] |
| Docetaxel | In vitro In vivo | Breast cancer | Antitumor effect. | Drug delivery system | [86] |
| Pentamidine isethionate | In vitro | Lung Adenocarcinoma Breast cancer | More cytotoxic in comparison to the free drug, suggesting an enhanced internalization of encapsulated drug by cancer cells. | Drug delivery system | [87] |
| Hyaluronic acid-ceramide | In vitro In vivo | Breast cancer | Additional tumor-targeting and penetration potential together with enhanced permeability and retention (EPR) effect (passive tumor targeting) and HA–CD44 receptor interaction (active tumor targeting). | Nanocarrier for imaging and therapy | [88] |
| IR780Doxorubicin | In vitro | Breast cancer | Increased photothermal potential and cytocompatibility of IR780; Higher internalization by cancer cells than by normal ones; Decrease in spheroid cell viability. | Cancer chemo-phototherapy Co-delivery system | [89] |
| Catalase | In vitro In vivo | Breast cancer | Minimal cytotoxicity in the dark and high toxicity under 660 nm light irradiation at normoxic conditions; Selective tumor accumulation in tumor-bearing nude mice; Significant tumor regression after intravenous injection under light irradiation compared to control system without loading catalase. | Photodynamic therapy | [90] |
| Doxorubicin | In vitro In vivo | Lung Adenocarcinoma | Antitumor effects and minimal systemic toxicity. | Nanocarrier | [91] |
| Curcumin | In vitro In vivo | Breast cancer | Cell death by ROS induction, cell cycle arrest, and modulation of NF-κB and Bax-mediated apoptotic pathway; Decreased tumor volume in tumor-bearing mice due to increased bioavailability and higher cellular uptake in tumor tissue. | Drug delivery system | [92] |
| Doxorubicin and cisplatin | In vitro | Breast cancer | DOX and cisplatin exhibited a synergistic cell-killing effect in human breast cancer MCF-7 cells. | Synergetic targeted combination chemotherapy | [93] |
| Doxorubicin | In vitro | Breast Cancer | Excellent targeting of cancer cells. | Drug delivery system | [94] |
| Cisplatin | In vitro In vivo | Human ovarian cancer; Ehrlich tumor (solid)-bearing mice | Higher cytotoxicity than the free drug; in vivo antitumor activity. | Drug delivery system | [95] |
| Lapatinib | In vitro In vivo | Breast cancer | Improved antiproliferation potential, apoptotic efficacy, and mitochondrial destabilizing activity; tumor growth suppression. | Drug delivery system | [96] |
| Paclitaxel | In vitro In vivo | Colorectal and Breast cancer; Lung adenocarcinoma; Hepatocellular carcinoma; Melanoma | Effective tumor ablation with minimal adverse events; Significantly inhibited melanoma tumor growth. | Drug delivery system | [97] |
| Doxorubicin | In vitro In vivo | Breast cancer | Greater cellular uptake and cytotoxicity; Significant tumor-targeting capabilities and tumor growth inhibition activity with less cardiotoxicity. | Drug delivery system | [98] |
| IRDye800CW Camptothecin | In vitro In vivo | Breast cancer | High-precision tumor therapy with no tumor recurrence and metastasis. | Drug delivery system Chemo-photothermal therapy | [99] |
| Zinc(II) phthalocyanine-based photosensitizer | In vitro In vivo | Colorectal adenocarcinoma; Lung adenocarcinoma | Upon irradiation, NPs caused significant temperature increase at the tumor site and ablation of the tumor. Effective photothermal agent for targeted photothermal therapy. | Nanocarrier for photothermal therapy | [100] |
| Thio-tetrazolyl analog of a clinical candidate, IC87114 | In vitro | Pancreatic cancer Breast Cancer | Higher cytotoxicity and enhanced intracellular accumulation of NPs in high-CD44-expressing cells; Induction of premature senescence with increase in senescence-associated β-galactosidase activity and senescence-specific marker p21 expression through modulation of Pi3K/Akt/NF-kB. | Nanocarrier | [101] |
| Doxorubicin | In vitro | Cervical cancer | Higher cellular uptake via CD44 receptor-mediated endocytosis and higher cytotoxicity in Hela cells compared to normal ones. | Drug delivery system | [102] |
| Horseradish peroxidase or indole-3-acetic acid | In vitro | Bladder cancer | Reduction of the cell viability of human bladder carcinoma cell line. | Delivery of enzyme/prodrug systems | [103] |
| Gefitinib and Vorinostat | In vitro In vivo | Lung cancer (2D and 3D cultures) | Stronger inhibition of orthotopic lung tumor growth compared to free drugs. | Co-delivery system | [104] |
| Zinc oxideGinsenoside Rh2 | In vitro | Lung and Colorectal adenocarcinoma; Breast cancer | Induction of apoptosis through generation of ROS by activation of the Caspase-9/p38 MAPK pathway. | Drug delivery system | [105] |
| Curcuminoid | In vitro In vivo | Malignant glioma | Effectively targeted and accumulated within the gliomas after enhanced permeation through blood–brain barrier. | Drug delivery system | [106] |
| Olaparib | In vitro In vivo | Triple-negative breast cancer | Antitumor effect. | Drug delivery system | [107] |
| Honokiol | In vitro In vivo | Breast cancer | Improved antiproliferative and proapoptotic activities; Downregulation of the expressions of Vimentin and upregulation of E-cadherin. | Drug delivery system | [108] |
| TRAIL plasmid and gambogic acid | In vitro In vivo | Breast cancer | Significantly augmented apoptotic cell death; inhibited TNBC tumor growth; efficiently co-delivered GA and pTRAIL. | Co-delivery system | [109] |
| Doxorubicin | In vitro In vivo | Breast cancer | Improved the cellular uptake and cytotoxicity; Inhibited tumor growth. | Drug delivery system | [110] |
| Doxorubicin | In vitro In vivo | Breast cancer | Specific uptake by the tumor; Better therapeutic efficacy. | Drug delivery system | [111] |
| Diaminocyclohexane-platinum | In vitro In vivo | Lung cancer | Anticancer activity; Ability to modulate immunogenic cell death. | Drug delivery system | [112] |
| Docetaxel | In vitro In vivo | Lung cancer | Fast cellular uptake; Improved tumor accumulation and repression and lower side effects compared with free docetaxel. | Drug delivery system | [113] |
| Doxorubicin, cisplatin and resiquimod | In vivo | Osteosarcoma | The growth of tumors and lung metastasis was greatly inhibited. | Intelligent co-delivery platform | [114] |
| Doxorubicin | In vitro In vivo | Breast cancer | Mitochondrial destruction and nuclear DNA leakage led to cell cycle arrest and cell apoptosis; Effective tumor inhibition. | Drug delivery system | [115] |
| Doxorubicin | In vitro In vivo | Colorectal cancer | Significantly increased DOX circulation time by 12.5 times; Efficiently targeted tumor tissues; Antitumor effect. | Drug delivery system | [116] |
| Camptothecin | In vitro | Lung cancer | Recognizes normal cells and cancer cells and has good anticancer effects. | Drug delivery system | [117] |
| Doxorubicin | In vitro In vivo | Breast Cancer Brain Metastases | Selective cytotoxicity to metastatic breast cancer cells rather than astrocytes; Efficient loading into dual-targeting NPs; Significantly extended the median survival time of mice with intracranial metastatic breast cancer. | Delivery system | [118] |
| OligoRNA and Doxorubicin | In vitro In vivo | Hepatocellular carcinoma | Effective delivery of doxorubicin and oligoRNA into cells via CD44 receptor-mediated endocytosis; Significantly inhibited cell proliferation; Efficient accumulation in tumor. | Co-delivery system | [119] |
| Gambogic acid | In vitro | Melanoma | Improved cytotoxicity; Induced apoptosis and mitochondrial depolarization; Inhibited tumor metastasis. | Drug delivery system | [120] |
| Berberine and Doxorubicin | In vitro In vivo | Hepatocellular carcinoma | Enhanced antitumor activity, tumor accumulation, and biocompatibility. | Co-delivery system | [121] |
| Paclitaxel | In vitro | Breast cancer | Improved cellular uptake. | Drug delivery system | [122] |
| Photosensitive drug indocyanine green | In vitro In vivo | Lung cancer | Excellent drug loading and stability; Significant uptake. | Photothermal/photodynamic therapy | [123] |
| Dopamine | In vitro In vivo | Breast cancer | Enhanced cellular accumulation efficiency, antiproliferation property, tumor penetration efficiency, and spheroid growth inhibitory effect. | Tumor-targetable and penetrable nano-system | [124] |
| Doxorubicin and photothermal reagent indocyanine green | In vitro In vivo | Cervical cancer | Improved effectiveness of photothermal therapy; Excellent synergistic therapy. | Bimodal imaging | [125] |
| Doxorubicin | In vitro In vivo | Liver cancer | Prolonged drug blood circulation time; Increased accumulation of drug in the liver and decreased cardiotoxicity and nephrotoxicity; Tumor targeting. | Drug delivery system | [126] |
| Mitoxantrone | In vitro | Breast cancer | Specifically bound to and significantly inhibited CD44 receptor-positive cells. | Drug delivery system | [127] |
| Doxorubicin | In vitro | Cervical cancer | Higher tumor cell inhibition ratio; Efficient cellular uptake. | Drug delivery system | [128] |
| Paclitaxel | In vitro In vivo | Breast cancer | Anticancer efficacy; NPs accumulated in tumor site; Enhanced apoptosis; Reduced tumor growth. | Drug delivery system | [129] |
| Docetaxel and Disulfonate Tetraphenyl Chlorin | In vitro | Breast cancer Cervical cancer | Synergistic drug/treatment interaction; Induced cell mortality. | Co-delivery system | [130] |
| Curcumin and 5-fluorouracil | In vitro In vivo | Breast cancer | Synergistic anticancer, proapoptotic, and anti-migration effects; Anticancer activity against metastatic breast cancer. | Co-delivery system | [131] |
| Berberine chloride | In vitro In vivo | Cervical and breast cancer Ehrlich Ascites Carcinoma | Faster release of BRB and increased cytotoxicity; Enhanced apoptosis, sub-G1 content, life span, mean survival time, and ROS levels with subsequent decrease in mitochondrial membrane potential and tumor burden. | Delivery system | [132] |
| Triptolide | In vitro In vivo | Breast cancer | High drug loading efficiency; Selective tumor cellular uptake and high tumor tissue accumulation capacity; Suppression of cell proliferation; Blockage of proapoptotic and cell cycle activities; Strong inhibition of cell migration and invasion. | Drug delivery system | [133] |
| Doxorubicin and Ce6 | In vitro In vivo | Lung carcinoma | Tumor site-specific light irradiation generated high levels of ROS and greatly enhanced the hypoxic levels to induce NP dissociation and drug release. A synergistic anticancer efficacy and reduced side effects to normal cells. | Co-delivery system | [134] |
| Tirapazamine and Ce6 | In vitro In vivo | Breast cancer | Effective tumor accumulation; High levels of ROS. | Drug delivery system (photodynamic therapy) | [135] |
| Dissolving microneedles and photothermal agent (CuS) | In vitro In vivo | Melanoma | Improved specific uptake and distribution of targeted tumor; Delivers drug locally; Releases drug intelligently and spatiotemporally. | Co-delivery system | [136] |
| Paclitaxel and lethal-7a (let-7a), a microRNA (miR) | In vitro In vivo | Ovarian cancer | Effective cellular uptake; Significant downregulation of P-glycoprotein; Efficient drug release and induction of apoptosis; Synergistic growth inhibition. | Co-delivery system | [137] |
| Camptothecin | In vitro | Lung cancer | Easily taken up by mitochondria; Severe mitochondrial dysfunction; Rising cell death rate. | Drug delivery system | [138] |
| Doxorubicin | In vitro | Breast and Liver cancers | Exhibited an endosomal escape function to accelerate drug release in cancer cells, leading to low IC50. | Drug delivery system | [139] |
| Melittin and condensed epigallocatechin gallate | In vitro In vivo | Melanoma | Synergistic amplification of oxidative stress and prolonged ROS retention in cancer cells; Enhanced anticancer efficacy. | Drug delivery system | [140] |
| 5-Amino levulinic acid and artemisinin | In vitro In vivo | Hepatoma | Tumor targeting; antitumor effect; Good multi-functional therapeutic delivery system. | Co-delivery system | [141] |
| All-trans-retinoic acid | In vitro In vivo | Lung cancer | Tumor growth inhibition; Efficient system for targeted delivery of antitumor drugs to eliminate cancer stem cells. | Drug delivery system | [142] |
| Doxorubicin and a near-infrared dye (indocyanine green) | In vitro In vivo | Breast cancer | Fluorescence imaging ability and release of the drug; Generation of high heat upon NIR irradiation and induction of apoptosis; Inhibition of tumor growth with minimal systemic toxicity upon NIR irradiation. | Multifunctional drug delivery system for cancer therapy and imaging | [143] |
| Gambogic acid | In vitro In vivo | Hepatocellular carcinoma | Induction of reduction-activated charge conversion from about -25 to +30 mV with up to 95% drug release within 48 h; Excellent tumor inhibition. | Delivery system | [144] |
| Antitumor immune regulator (R848) and Doxorubicin | In vitro In vivo | Immune cells and Breast cancer | Strong immunoregulatory activities; Inhibited the breast cancer cell growth; Excellent tumor-targeting ability and inhibition of tumor growth by regulation of tumor immunity. | Co-delivery system | [145] |
| Cisplatin–indocyanine green | In vitro In vivo | Hepatocellular carcinoma | Ultra-high drug loading efficiency and glutathione/NIR light dual-responsive drug release; Efficient internalization and apoptosis-inducing ability; Efficient tumor accumulation, biosafety, and synergistic effect of combined photodynamic chemotherapy on inhibiting tumor growth. | Co-delivery system | [146] |
| Anti-Glypican-1, oridonin, gadolinium, and Cy7 dye | In vitro In vivo | Pancreatic cancer | Long-time stability and fluorescent/MRI properties; Significant inhibition of viability and apoptosis enhancement; Enabled multimodal targeted imaging. | Theranostic platform for simultaneous diagnosis and effective treatment | [147] |
| Hydrophobic rapamycin and hydrophilic herbal drug, berberine | In vitro In vivo | Lung cancer | Enhanced internalization and cytotoxicity; Anticancer efficacy; Decreased lung weight and reduction in both number and diameters of lung adenomatous foci and angiogenic markers. | Drug delivery Inhalable nanocomposites | [148] |
| Gambogic acid and Doxorubicin | In vitro In vivo | Tongue squamous cell carcinoma | Gradual release of DOX and GA under different tumor-specific physiological conditions (low pH and rich HAase); Tumor growth inhibition and significantly prolonged survival rate. | Drug delivery system | [149] |
| Mn3O4–Ce6 | In vitro In vivo | Breast cancer | Homogeneously distributed in whole tumor and significantly reduced the level of intracellular GSH; Intracellular ROS production; Induction of cell death; Complete inhibition of tumor growth. | Sustainable ROS Generator | [150] |
| Doxorubicin | In vitro (3D) In vivo | Lung cancer | Higher cellular accumulation efficiency and antiproliferation potentials; Superior tumor penetration capability, ROS production level, and cancer cell-killing capacity; Higher tumor accumulation efficiency. | Drug delivery system | [151] |
| Platinum | In vitro | Lung cancer | Inhibited proliferation, migration and invasion, and induced apoptosis in comparison with cisplatin and carboplatin. | Drug delivery system | [152] |
| Docetaxel | In vitro | Glioblastoma | Multi-target capability and stronger penetration ability into 3D tumor spheroids’ core; Migrated efficiently across the BBB. | Drug delivery system | [153] |
| Epigallocatechin-3-gallate and Docetaxel | In vitro In vivo | Prostate cancer | Inhibition of cell growth via induced G2/M phase cell cycle arrest; Significantly attenuated tumor growth and increased M30 protein expression without causing organ damage. | Co-delivery system | [154] |
| MoS2 quantum dotsCe6 | In vitro In vivo | Breast cancer | Appropriate particle size can not only degrade and excrete in a reasonable period induced by redox responsiveness of glutathione but also exhibits a high tumor uptake due to the longer blood circulation time. | Delivery system | [155] |
| Ultra-small gadolinium oxide | In vitro In vivo | Breast cancer | Rapidly degraded and excreted after reacting with glutathione (GSH) by the redox response; high tumor uptake. | Multimodal imaging; photothermal/radio therapy | [156] |
| Ultra-small gadolinium oxide and aluminum phthalocyanine | In vitro In vivo | Breast cancer | Enhanced tumor uptake effect; photothermal effect. | Polymer-based multifunctional theranostic/fluorescence/magnetic resonance/photoacoustic imaging | [157] |
| Chlorin e6 (Ce6) | In vitro In vivo | Cervical cancer | High colloid stability, good biocompatibility, and suitable transverse relaxation rate; High photothermal conversion efficiency and excellent ROS generation efficiency under NIR light irradiation; Significantly high tumor growth inhibition. | Multifunctional nanotheranostic agent Photodynamic/photothermal combined therapy | [158] |
| Palladium | In vivo | Melanoma | Efficient targeting and effective therapy for CD44-positive tumors such as melanoma. | Drug delivery system | [159] |
| Disulfiram | In vitro In vivo | Breast cancer | Induces strong cytotoxicity; Passively accumulates in tumors and elicits potent tumor growth inhibition. | Drug delivery system | [160] |
| Doxorubicin | In vitro | Cervical cancer | Good stability in vitro; Drug release mediated by pH gradient; Lower cytotoxicity in normal cells and higher inhibition ratio in tumor cells; Efficient internalization. | Drug delivery system | [161] |
| Ce6 | In vitro In vivo | Breast cancer | Good biocompatibility; Inhibition of tumor growth. | Delivery system | [162] |
| Methotrexate and 10-hydroxycamptothecin | In vitro In vivo | Breast cancer | High drug entrapment efficiency and pH/esterase-controlled release behavior; Significant increase in efficiency of selective internalization; Highly synergetic tumor cell-killing and tumor growth inhibition. | Dual-targeting delivery system | [163] |
| Azobenzene; ammonium polyamidoamine and carboxylatopillar [5]arene | In vitro In vivo | Colon cancer | Good biocompatibility and CRC treatment capability with negligible side effects. | Delivery system | [164] |
| Doxorubicin | In vitro In vivo | Squamous cell carcinoma | Favorable biocompatibility; relatively low cytotoxicity; good drug loading capability and strong photoacoustic imaging signals; synergistic chemo-photothermal therapy; better therapeutic effects than chemotherapy alone; accumulates at the tumor sites and achieves complete ablation of tumors. | Multifunctional platform in photoacoustic imaging-guided photothermal chemotherapy | [165] |
| Mitoxantrone and verapamil | In vitro | Breast cancer | Significant cytotoxicity. | Drug delivery system | [166] |
| Cisplatin | In vitro In vivo | Lung cancer | Specific tumor-targeting ability and redox-responsive drug release manner; effective antitumor performance along with minor side effects and systemic toxicity. | Drug delivery system | [167] |
| Granzyme B protein | In vitro In vivo | Glioblastoma and Breast cancer | Induced cell apoptosis; accumulated in the solid tumor through enhanced permeability and retention (EPR) effect; Induced tumor cell apoptosis in vivo. | Delivery system | [168] |
| Curcumin and IR780 | In vitro In vivo | Breast cancer | Uniform size, high drug loading ability and excellent colloidal stability; under the NIR condition, IR780 could be triggered to exhibit both PTT/PDT dual-pattern therapy effects, leading to an enhanced therapy efficiency of Cur with good biocompatibility. | Delivery system | [169] |
| Gemcitabine and imiquimod | In vitro In vivo | Breast cancer | Anticancer activity; suppressed the volume of tumor; imiquimod potentiates the effect of gemcitabine by activating immune cells to suppress tumors. | Drug delivery system | [170] |
| Photosensitizer (NIR770) and doxorubicin | In vitro In vivo | Lung cancer | Specifically internalized by tumor cells; preferentially retained in mitochondria; highly efficient photothermal therapy and photodynamic therapy upon NIR irradiation; DOX molecules were mainly accumulated in the nucleus. | Synergistic treatment | [171] |
| Gossypol, Cu(II) and AQ4N | In vitro In vivo | Prostate cancer | Multiple-tumor-targeting ability; accumulates and significantly releases drugs at the tumor region; High antitumor efficiency with negligible side effects. | Delivery system | [172] |
| Paclitaxel and IR780 | In vitro In vivo | Lung cancer | Combinatorial antitumor effects of paclitaxel and IR780 associated with microtubule destruction and mitochondrial apoptotic pathway. | Drug delivery system | [173] |
| microRNA-31 and Doxorubicin | In vitro | Cervical and Lung cancer | Promoted intracellular accumulation of drugs via the active transport at tumor site; microRNA-31 directly targeted to mtEF4 to promote cell death; synergistic effects. | Co-delivery system | [174] |
| Folic acid and Dopamine | In vitro In vivo | Melanoma | Improved blood circulation half-life of the drug and prevented premature intravascular drug leakage from the nanocarrier; efficient tumor penetration has shown potential in improving anticancer efficacy. | Co-delivery system | [175] |
| R820 and Catalase | In vitro In vivo | Melanoma | Selectively targeted melanoma cells with high expression of CD44, and generated oxygen by catalyzing H2O2, inhibiting tumor growth significantly. | Nanotechnology-based photodynamic therapy | [176] |
| MnO2-mSiO2 | In vitro In vivo | Breast cancer | Almost total suppression of tumor growth without observable recurrence. | Multifunctional nanotheranostic | [177] |
| Doxorubicin and IR780 | In vitro In vivo | Cervical cancer | Selective tumor targeting; synergistic dual-mode chemo-photodynamic therapy against cancers. | Co-delivery system | [178] |
| Peptide A20-36 (selectively binds to the Ig-BCR of A20 lymphoma cells) | Ex vivo In vivo | B lymphoma | Targeting specificity and kinetics of the NPs; multimodal imaging contrast agents. | Imaging and theranostic applications | [179] |
| siRNA | In vitro | laryngeal cancer | Downregulation of genes was confirmed; entrapment efficiency of siRNA of 36.8-61.2; significant inhibition of cell growth and induction of apoptosis. | siRNA delivery system | [180] |
| Doxorubicin | In vitro | Hepatocellular carcinoma | Exhibited H2O2-responsive release of about 80% DOX and displayed sevenfold selectivity for killing cancer cells over normal cells. | Drug delivery system | [181] |
| Doxorubicin | In vitro In vivo | Hepatocellular carcinoma | Cellular uptake demonstrated that this system could bind specifically with cancer cells; excellent therapeutic effect by photothermal-chemotherapy. | Drug delivery system | [182] |
| Doxorubicin | In vitro In vivo | Lung cancer | Suitable drug loading efficiency, excellent solubility, very low hemolytic effect; induction of apoptosis; DNA intercalation, cell cycle arrest at the S phase, light-induced ROS production; inhibition of tumor growth with good safety. | Drug delivery system | [183] |
| Zinc phthalocyanine | In vitro In vivo | Breast cancer | Good ability for infrared thermal, photoacoustic, fluorescence, and X-ray computed tomography imaging, high photo-heat conversion efficiency for photothermal therapy; tumor growth inhibition; excellent combined therapeutic effect. | Smart theranostic nanoplatform multimodal imaging-guided combined phototherapy | [184] |
| Cyclodextrin and amantadine | In vitro | Breast cancer | Excellent fluorescence; internalized into tumor cells via HA receptor CD44-mediated endocytosis; effective targeted tumor cell imaging. | Cancer diagnosis and treatment | [185] |
| Doxorubicin | In vitro | Lung cancer | Higher cytotoxicity; inhibited tumor cell invasion and metastasis by downregulating N-cadherin expression. | Drug delivery system | [186] |
| Doxorubicin | In vitro In vivo | Ovarian cancer | High selectivity resulting in strong killing; long elimination half-life, elevated tumor accumulation and effective inhibition of the ovarian tumor. | Drug delivery system | [187] |
| Doxorubicin and CuS | In vitro In vivo | Breast cancer | Good biocompatibility; targeting effect; synergistic combination of chemo- and phototherapy; potential for tumor diagnosis and treatment. | Drug delivery system | [188] |
| Mn-modified phthalocyanine derivative and docetaxel | In vitro In vivo | Lung cancer | Activated tumor immunity through cGAS–STING and chemotherapy; effectively inhibited tumor cell growth. | Delivery system | [189] |
| MicroRNA-34a | In vitro | Lung cancer | Successful delivery and uptake resulted in altered ATP levels, decreased glycolytic flux, Nrf-2, and glutathione levels, ultimately resulting in caspase-3 activation and apoptosis; underlying molecular changes in epigenetic status of D loop on the mtDNA and transcription of mtDNA-encoded genes. | Delivery system | [190] |
| Chitosan | In vitro | Breast cancer | Low hemolysis; high resistance to bovine serum albumin adsorption; efficient internalization; non-toxic to human skin fibroblasts. | Drug Nanocarrier or drug delivery system | [191] |
| siRNA | In vitro In vivo | Melanoma | Significant inhibitory effect against melanoma cells; siRNA liposomes may inhibit tumor growth by downregulating surviving; survivin–siRNA cationic liposome nanoparticles were able to effectively inhibit proliferation and migration of melanoma cells in vitro and in vivo, probably by inhibiting survivin–mRNA and protein expression. | siRNA delivery | [192] |
| MicroRNA-125b | In vitro In vivo | Lung cancer | Increase in M1 to M2 macrophage ratio and 300-fold increase in the iNOS (M1 marker)/Arg-1 (M2 marker) ratio; intraperitoneally administered macrophage-specific NPs can successfully transfect tumor-associated macrophages (TAMs) in lung tissues of both naïve mice and a KP-GEM NSCLC mouse model; successful TAM repolarization toward M1 phenotype has significant implication in anticancer immunotherapy. | Transfection system | [193] |
| Paclitaxel in combination with MicroRNA-125b | In vitro In vivo | Ovarian cancer | Specifically targets TAMs in the peritoneal cavity and can repolarize macrophages to an immune-activating phenotype; enhances antitumor efficacy of paclitaxel during later stages of disease progression as seen by significant reduction in ascitic fluid and peritoneal VEGF levels; does not induce systemic toxicity. | Delivery system | [194] |
| miRNA 145 | In vitro In vivo | Colon cancer | High up-conversion emission and good monodispersity; Excellent biocompatibility; High level of cellular uptake and miR-145 expression, resulting in significant cell cycle arrest in G1 and inducing CCND1, CDK6, and CCNE2 protein downregulation; inhibition of tumor growth. | Delivery system | [195] |
| Plasmid DNA | In vitro | Cervical and Lung cancer | Higher transfection efficiency; stable up to a week at 4 degrees. | Delivery and transfection system | [196] |
| MTH1 inhibitor–TH287 and MDR1 siRNA | In vitro | Oral cancer | Effective in controlling drug release and internalization; reduced tumor burden; inhibited MDR1 function and enhanced cell-killing effect. | Delivery system | [197] |
| Cyanine 3 (Cy3)-labeled siRNA | In vitro In vivo | Lung cancer | Effectively delivered Cy3-labeled siRNA to cancer cells via receptor CD44 and inhibited cell proliferation by BCL2 downregulation; Inhibition of tumor growth by BCL2 downregulation. | Delivery system | [198] |
| Anti-miR21 and Resveratrol | Gastric carcinoma | Higher cellular internalization; anticancer effect of the optimized formulation and synergistic effects of anti-miR21 and RSV; induction of apoptosis and cell necrosis. | Delivery system | [199] | |
| Paclitaxel | In vitro In vivo | Lung cancer | Antitumor growth activity. | Nanocarrier | [200] |
| Paclitaxel | In vitro | Ovarian cancer | Selectively targeted and entered CD44-overexpressing cancer cells via receptor-mediated endocytosis. | Drug delivery system | [201] |
| siRNA Doxorubicin | In vitro | Ovarian Cancer Colorectal Cancer | Formation of stable complexes with siRNA; prevented RNase-mediated siRNA degradation; increased cancer cell specificity and enhanced cytotoxic effect in CD44+ cells. | Co-delivery system | [202] |
| Doxorubicin (DOX) and photosensitizer chlorin e6 (Ce6) | In vitro In vivo | Melanoma | Higher cellular uptake and remarkably better tumor-targeted accumulation than free drugs; with laser irradiation, anticancer activities were enhanced both in vitro and in vivo. | Chemo-photodynamic therapy | [203] |
| Dexamethasone and Doxorubicin | In vitro Ex vivo | Breast cancer Colorectal Cancer Human whole blood | DEX suppressed cytotoxicity of DOX; synergistically enhanced cytotoxicity;in an ex vivo human whole blood sample, found activation of complement and coagulation cascade in one group of donors. Encapsulation of DOX within the nanoparticle core eliminated such deleterious side effects. | Drug delivery system | [204] |
| Doxorubicin | In vitro In vivo | Breast cancer Colon cancer | High targeting and antitumor activity against CD44 receptors; longer circulation time and higher accumulation in 4T1 tumors. | Drug delivery system | [205] |
| Oleic acid | In vitro | Breast Cancer Melanoma | Efficient delivery of oleic acid; greater uptake by cancer cells (expressing CD44 receptors) than normal cells. | Drug delivery system | [206] |
| Paclitaxel | In vitro In vivo | Lung cancer | Greater in vitro cytotoxicity and apoptosis; much higher antitumor efficacy and improved safety profile. | Drug delivery system | [207] |
| anti-Gasdermin B antibody | In vitro In vivo | Breast cancer | Reduces diverse protumor functions (migration, metastasis, and resistance to therapy) | Delivery system | [208] |
| 5-Fluorouracil | In vitro In vivo | Skin cancer | Non-irritant; permeability properties; cytotoxic effect; favorable biosafety; good antitumor effects. | Topical gel for drug delivery | [209] |
| Doxorubicin | In vitro | Hepatocellular carcinoma | Effectively avoids biological barriers; provides long blood circulation and achieves high tumor accumulation; fast elimination from tumor and released the loaded drugs for chemotherapy after UV-induced dissociation; good targetability to CD44 receptors. | Drug delivery system | [210] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Machado, V.; Morais, M.; Medeiros, R. Hyaluronic Acid-Based Nanomaterials Applied to Cancer: Where Are We Now? Pharmaceutics 2022, 14, 2092. https://doi.org/10.3390/pharmaceutics14102092
Machado V, Morais M, Medeiros R. Hyaluronic Acid-Based Nanomaterials Applied to Cancer: Where Are We Now? Pharmaceutics. 2022; 14(10):2092. https://doi.org/10.3390/pharmaceutics14102092
Chicago/Turabian StyleMachado, Vera, Mariana Morais, and Rui Medeiros. 2022. "Hyaluronic Acid-Based Nanomaterials Applied to Cancer: Where Are We Now?" Pharmaceutics 14, no. 10: 2092. https://doi.org/10.3390/pharmaceutics14102092