Toward Optimized 89Zr-Immuno-PET: Side-by-Side Comparison of [89Zr]Zr-DFO-, [89Zr]Zr-3,4,3-(LI-1,2-HOPO)- and [89Zr]Zr-DFO*-Cetuximab for Tumor Imaging: Which Chelator Is the Most Suitable?
Abstract
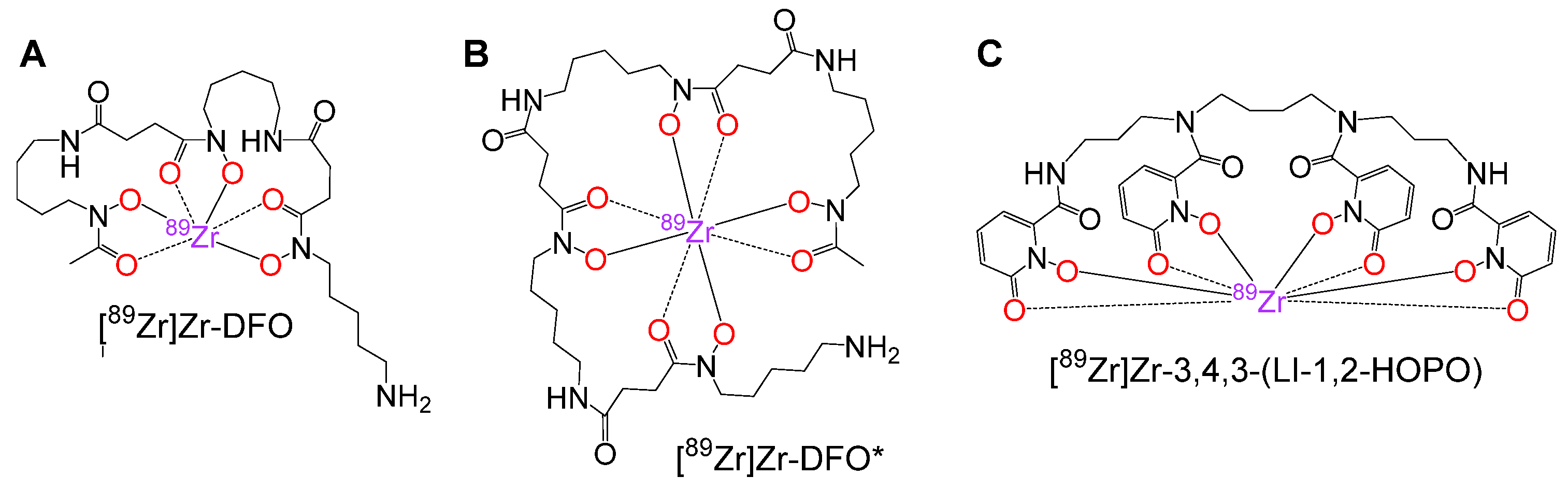
1. Introduction
2. Materials and Methods
2.1. General
2.2. Bradford Assay
2.3. Preparation of TCO-Cetuximab 2
2.4. Determination of the TCO-to-Cetuximab Ratio of 2
2.5. Preparation of Chelator-Cetuximab-Bioconjugates 7–9
2.6. 89Zr-Radiolabeling of 7–9 Yielding [89Zr]Zr-7–[89Zr]Zr-9
2.7. Cell Culture
2.8. Determination of the Immunoreactive Fraction of [89Zr]Zr-7–[89Zr]Zr-9
2.9. In Vivo PET/CT Imaging and Ex Vivo Biodistribution of [89Zr]Zr-7–[89Zr]Zr-9
3. Results and Discussion
3.1. Development of an Antibody Conjugation Approach Enabling the Exact Quantification of the Number of Derivatization Sites per Antibody
3.2. Preparation of Cetuximab-Chelator-Conjugates 7–9
3.3. 89Zr-Radiolabeling of Cetuximab-Chelator-Conjugates 7–9
3.4. Determination of the Immunoreactive Fraction of [89Zr]Zr-7–[89Zr]Zr-9
3.5. Immuno-PET/CT Imaging of [89Zr]Zr-7–[89Zr]Zr-9
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Deri, M.A.; Zeglis, B.M.; Francesconi, L.C.; Lewis, J.S. PET imaging with 89Zr: From radiochemistry to the clinic. Nucl. Med. Biol. 2013, 40, 3–14. [Google Scholar] [CrossRef] [PubMed]
- van Dongen, G.A.; Beaino, W.; Windhorst, A.D.; Zwezerijnen, G.J.; Oprea-Lager, D.E.; Hendrikse, N.H.; Van Kuijk, C.C.; Boellaard, R.; Huisman, M.C.; Vugts, D.J. The Role of (89)Zr-Immuno-PET in Navigating and Derisking the Development of Biopharmaceuticals. J. Nucl. Med. 2021, 62, 438–445. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.-K.; Park, B.-N.; Ryu, E.-K.; An, Y.-S.; Lee, S.-J. Current Perspectives on (89)Zr-PET Imaging. Int. J. Mol. Sci. 2020, 21, 4309. [Google Scholar] [CrossRef] [PubMed]
- Chomet, M.; van Dongen, G.A.M.S.; Vugts, D.J. State of the Art in Radiolabeling of Antibodies with Common and Uncommon Radiometals for Preclinical and Clinical Immuno-PET. Bioconj. Chem. 2021, 32, 1315–1330. [Google Scholar] [CrossRef]
- Feiner, I.V.J.; Brandt, M.; Cowell, J.; Demuth, T.; Vugts, D.; Gasser, G.; Mindt, T.L. The Race for Hydroxamate-Based Zirconium-89 Chelators. Cancers 2021, 13, 4466. [Google Scholar] [CrossRef] [PubMed]
- Fischer, G.; Seibold, U.; Schirrmacher, R.; Wängler, B.; Wängler, C. Zr-89, a Radiometal Nuclide with High Potential for Molecular Imaging with PET: Chemistry, Applications and Remaining Challenges. Molecules 2013, 18, 6469–6490. [Google Scholar] [CrossRef]
- Heskamp, S.; Raavé, R.; Boerman, O.; Rijpkema, M.; Goncalves, V.; Denat, F. Zr-89-Immuno-Positron Emission Tomography in Oncology: State-of-the-Art Zr-89 Radiochemistry. Bioconj. Chem. 2017, 28, 2211–2223. [Google Scholar] [CrossRef]
- Bailly, C.; Gouard, S.; Guérard, F.; Chalopin, B.; Carlier, T.; Faivre-Chauvet, A.; Saëc, P.R.-L.; Bourgeois, M.; Chouin, N.; Rbah-Vidal, L.; et al. What is the Best Radionuclide for Immuno-PET of Multiple Myeloma? A Comparison Study Between Zr-89- and Cu-64-Labeled Anti-CD138 in a Preclinical Syngeneic Model. Int. J. Mol. Sci. 2019, 20, 2564. [Google Scholar] [CrossRef] [PubMed]
- Damerow, H.; Hübner, R.; Judmann, B.; Schirrmacher, R.; Wängler, B.; Fricker, G.; Wängler, C. Side-by-Side Comparison of Five Chelators for Zr-89-Labeling of Biomolecules: Investigation of Chemical/Radiochemical Properties and Complex Stability. Cancers 2021, 13, 6349. [Google Scholar] [CrossRef]
- Roy, J.; Jagoda, E.M.; Basuli, F.; Vasalatiy, O.; Phelps, T.E.; Wong, K.; Ton, A.T.; Hagemann, U.B.; Cuthbertson, A.S.; Cole, P.E.; et al. In Vitro and In Vivo Comparison of 3,2-HOPO Versus Deferoxamine-Based Chelation of Zirconium-89 to the Antimesothelin Antibody Anetumab. Cancer Biother. Radiopharm. 2021, 36, 316–325. [Google Scholar] [CrossRef]
- Chomet, M.; Schreurs, M.; Bolijn, M.J.; Verlaan, M.; Beaino, W.; Brown, K.; Poot, A.J.; Windhorst, A.D.; Gill, H.; Marik, J.; et al. Head-to-head comparison of DFO* and DFO chelators: Selection of the best candidate for clinical (89)Zr-immuno-PET. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 694–707. [Google Scholar] [CrossRef] [PubMed]
- Berg, E.; Gill, H.; Marik, J.; Ogasawara, A.; Williams, S.; van Dongen, G.; Vugts, D.; Cherry, S.R.; Tarantal, A.F. Total-Body PET and Highly Stable Chelators Together Enable Meaningful (89)Zr-Antibody PET Studies up to 30 Days After Injection. J. Nucl. Med. 2020, 61, 453–460. [Google Scholar] [CrossRef]
- Deri, M.A.; Ponnala, S.; Kozlowski, P.; Burton-Pye, B.P.; Cicek, H.T.; Hu, C.; Lewis, J.S.; Francesconi, L.C. p-SCN-Bn-HOPO: A Superior Bifunctional Chelator for Zr-89 ImmunoPET. Bioconj. Chem. 2015, 26, 2579–2591. [Google Scholar] [CrossRef] [PubMed]
- Raavé, R.; Sandker, G.; Adumeau, P.; Jacobsen, C.B.; Mangin, F.; Meyer, M.; Moreau, M.; Bernhard, C.; Da Costa, L.; Dubois, A.; et al. Direct comparison of the in vitro and in vivo stability of DFO, DFO* and DFOcyclo* for Zr-89-immunoPET. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 1966–1977. [Google Scholar] [CrossRef] [PubMed]
- Rousseau, J.; Zhang, Z.; Wang, X.; Zhang, C.; Lau, J.; Rousseau, E.; Čolović, M.; Hundal-Jabal, N.; Bénard, F.; Lin, K.-S. Synthesis and evaluation of bifunctional tetrahydroxamate chelators for labeling antibodies with Zr-89 for imaging with positron emission tomography. Bioorg. Med. Chem. Lett. 2018, 28, 899–905. [Google Scholar] [CrossRef]
- Tinianow, J.N.; Pandya, D.N.; Pailloux, S.L.; Ogasawara, A.; Vanderbilt, A.N.; Gill, H.S.; Williams, S.-P.; Wadas, T.J.; Magda, D.; Marik, J. Evaluation of a 3-hydroxypyridin-2-one (2,3-HOPO) Based Macrocyclic Chelator for Zr-89(4+) and Its Use for ImmunoPET Imaging of HER2 Positive Model of Ovarian Carcinoma in Mice. Theranostics 2016, 6, 511–521. [Google Scholar] [CrossRef]
- Humblet, Y. Cetuximab: An IgG1 monoclonal antibody for the treatment of epidermal growth factor receptor-expressing tumours. Expert Opin. Pharmacother. 2004, 5, 1621–1633. [Google Scholar] [CrossRef]
- Delage, J.A.; Faivre-Chauvet, A.; Barbet, J.; Fierle, J.K.; Schaefer, N.; Coukos, G.; Viertl, D.; Dunn, S.M.; Gnesin, S.; Prior, J.O. Impact of DOTA Conjugation on Pharmacokinetics and Immunoreactivity of [Lu-177]Lu-1C1m-Fc, an Anti TEM-1 Fusion Protein Antibody in a TEM-1 Positive Tumor Mouse Model. Pharmaceutics 2021, 13, 96. [Google Scholar] [CrossRef]
- Wängler, C.; Moldenhauer, G.; Eisenhut, M.; Haberkorn, U.; Mier, W. Antibody−Dendrimer Conjugates: The Number, Not the Size of the Dendrimers, Determines the Immunoreactivity. Bioconj. Chem. 2008, 19, 813–820. [Google Scholar] [CrossRef]
- Lumen, D.; Vugts, D.; Chomet, M.; Imlimthan, S.; Sarparanta, M.; Vos, R.; Schreurs, M.; Verlaan, M.; Lang, P.A.; Hippeläinen, E.; et al. Pretargeted PET Imaging with a TCO-Conjugated Anti-CD44v6 Chimeric mAb U36 and [Zr-89]Zr-DFO-PEG(5)-Tz. Bioconj. Chem. 2022, 33, 956–968. [Google Scholar] [CrossRef]
- Zeglis, B.M.; Mohindra, P.; Weissmann, G.I.; Divilov, V.; Hilderbrand, S.A.; Weissleder, R.; Lewis, J.S. Modular Strategy for the Construction of Radiometalated Antibodies for Positron Emission Tomography Based on Inverse Electron Demand Diels–Alder Click Chemistry. Bioconj. Chem. 2011, 22, 2048–2059. [Google Scholar] [CrossRef] [PubMed]
- Meyer, J.-P.; Adumeau, P.; Lewis, J.S.; Zeglis, B.M. Click Chemistry and Radiochemistry: The First 10 Years. Bioconj. Chem. 2016, 27, 2791–2807. [Google Scholar] [CrossRef]
- Reiner, T.; Zeglis, B.M. The inverse electron demand Diels-Alder click reaction in radiochemistry. J. Label. Compd. Radiopharm. 2014, 57, 285–290. [Google Scholar] [CrossRef]
- Aerts, H.J.; Dubois, L.; Perk, L.; Vermaelen, P.; van Dongen, G.A.; Wouters, B.G.; Lambin, P. Disparity Between In Vivo EGFR Expression and (89)Zr-Labeled Cetuximab Uptake Assessed with PET. J. Nucl. Med. 2009, 50, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Prabakaran, P.; Chen, W.; Zhu, Z.; Feng, Y.; Dimitrov, D.S. Antibody Aggregation: Insights from Sequence and Structure. Antibodies 2016, 5, 19. [Google Scholar] [CrossRef] [PubMed]
- Lindmo, T.; Boven, E.; Cuttitta, F.; Fedorko, J.; Bunn, P. Determination of the immunoreactive function of radiolabeled monoclonal antibodies by linear extrapolation to binding at infinite antigen excess. J. Immunol. Methods 1984, 72, 77–89. [Google Scholar] [CrossRef]
- Benedetto, S.; Pulito, R.; Crich, S.G.; Tarone, G.; Aime, S.; Silengo, L.; Hamm, J. Quantification of the expression level of integrin receptor αvβ3 in cell lines and MR imaging with antibody-coated iron oxide particles. Magn. Reson. Med. 2006, 56, 711–716. [Google Scholar] [CrossRef] [PubMed]
- Chang, A.J.; De Silva, R.A.; Lapi, S.E. Development and characterization of Zr-89-labeled panitumumab for immuno-positron emission tomographic imaging of the epidermal growth factor receptor. Mol. Imaging 2013, 12, 17–27. [Google Scholar]
- Achmad, A.; Hanaoka, H.; Yoshioka, H.; Yamamoto, S.; Tominaga, H.; Araki, T.; Ohshima, Y.; Oriuchi, N.; Endo, K. Predicting cetuximab accumulation in KRAS wild-type and KRAS mutant colorectal cancer using 64Cu-labeled cetuximab positron emission tomography. Cancer Sci. 2012, 103, 600–605. [Google Scholar] [CrossRef] [PubMed]
- Vugts, D.J.; Klaver, C.; Sewing, C.; Poot, A.J.; Adamzek, K.; Huegli, S.; Mari, C.; Visser, G.W.M.; Valverde, I.; Gasser, G.; et al. Comparison of the octadentate bifunctional chelator DFO*-pPhe-NCS and the clinically used hexadentate bifunctional chelator DFO-pPhe-NCS for Zr-89-immuno-PET. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 286–295. [Google Scholar] [CrossRef]
- Holland, J.P.; Divilov, V.; Bander, N.H.; Smith-Jones, P.M.; Larson, S.M.; Lewis, J.S. Zr-89-DFO-J591 for ImmunoPET of Prostate-Specific Membrane Antigen Expression In Vivo. J. Nucl. Med. 2010, 51, 1293–1300. [Google Scholar] [CrossRef] [PubMed]
- Laverman, P.; van der Geest, T.; Terry, S.Y.; Gerrits, D.; Walgreen, B.; Helsen, M.M.; Nayak, T.K.; Freimoser-Grundschober, A.; Waldhauer, I.; Hosse, R.J.; et al. Immuno-PET and Immuno-SPECT of Rheumatoid Arthritis with Radiolabeled Anti–Fibroblast Activation Protein Antibody Correlates with Severity of Arthritis. J. Nucl. Med. 2015, 56, 778–783. [Google Scholar] [CrossRef] [PubMed]
- Boswell, C.A.; Mundo, E.E.; Zhang, C.; Bumbaca, D.; Valle, N.R.; Kozak, K.R.; Fourie, A.; Chuh, J.; Koppada, N.; Saad, O.; et al. Impact of Drug Conjugation on Pharmacokinetics and Tissue Distribution of Anti-STEAP1 Antibody–Drug Conjugates in Rats. Bioconjug. Chem. 2011, 22, 1994–2004. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.-J.; Baik, I.H.; Ye, S.-K.; Lee, Y.-H. Molecular Targeted Therapy for Hepatocellular Carcinoma: Present Status and Future Directions. Biol. Pharm. Bull. 2015, 38, 986–991. [Google Scholar] [CrossRef]
- Cummings, M.C.; Simpson, P.T.; E Reid, L.; Jayanthan, J.; Skerman, J.; Song, S.; Reed, A.E.M.; Kutasovic, J.R.; Morey, A.L.; Marquart, L.; et al. Metastatic progression of breast cancer: Insights from 50 years of autopsies. J. Pathol. 2014, 232, 23–31. [Google Scholar] [CrossRef]
- Feng, Q.-Y.; Wei, Y.; Chen, J.-W.; Chang, W.-J.; Ye, L.-C.; Zhu, D.-X.; Xu, J.-M. Anti-EGFR and anti-VEGF agents: Important targeted therapies of colorectal liver metastases. World J. Gastroenterol. 2014, 20, 4263–4275. [Google Scholar] [CrossRef]






| Excess of 1 per Cetuximab [equiv.] | Resulting TCO/Cetuximab Ratio |
|---|---|
| 20 | 5.4 ± 0.3 (n = 3) |
| 7 | 2.8 ± 0.5 (n = 3) |
| 4 | 1.4 ± 0.3 (n = 6) |
| 3 | 0.9 ± 0.3 (n = 2) |
| Organ | [89Zr]Zr-7 | [89Zr]Zr-8 | [89Zr]Zr-9 |
|---|---|---|---|
| tumor | 7.23 ± 1.52 | 9.15 ± 1.88 | 9.31 ± 2.04 |
| blood | 4.95 ± 0.39 | 11.31 ± 1.23 | 8.83 ± 1.45 |
| muscle | 0.73 ± 0.04 | 0.59 ± 0.31 | 0.68 ± 0.23 |
| brain | 0.19 ± 0.05 | 0.27 ± 0.07 | 0.20 ± 0.04 |
| heart | 2.10 ± 0.41 | 2.73 ± 1.12 | 2.56 ± 1.20 |
| lung | 1.72 ± 1.42 | 4.91 ± 1.14 | 4.14 ± 1.02 |
| spleen | 3.29 ± 0.13 | 3.57 ± 1.02 | 5.54 ± 1.22 |
| pancreas | 0.42 ± 0.14 | 0.72 ± 0.17 | 0.61 ± 0.17 |
| kidney | 2.87 ± 0.16 | 4.08 ± 0.70 | 4.04 ± 0.95 |
| liver | 4.66 ± 0.49 | 4.11 ± 0.61 | 9.80 ± 2.58 |
| stomach | 0.74 ± 0.22 | 0.83 ± 0.18 | 1.07 ± 0.30 |
| small intestine | 0.84 ± 0.05 | 1.10 ± 0.34 | 1.31 ± 0.20 |
| large intestine | 0.51 ± 0.21 | 0.78 ± 0.21 | 0.82 ± 0.17 |
| right femur | 19.68 ± 4.35 | 2.82 ± 0.75 | 2.52 ± 0.60 |
| left femur | 18.62 ± 5.06 | 2.81 ± 0.96 | 2.64 ± 0.25 |
| sternum | 5.56 ± 2.33 | 2.82 ± 0.80 | 2.81 ± 0.39 |
| skull | 11.66 ± 0.14 | 2.76 ± 0.84 | 2.64 ± 0.25 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Damerow, H.; Cheng, X.; von Kiedrowski, V.; Schirrmacher, R.; Wängler, B.; Fricker, G.; Wängler, C. Toward Optimized 89Zr-Immuno-PET: Side-by-Side Comparison of [89Zr]Zr-DFO-, [89Zr]Zr-3,4,3-(LI-1,2-HOPO)- and [89Zr]Zr-DFO*-Cetuximab for Tumor Imaging: Which Chelator Is the Most Suitable? Pharmaceutics 2022, 14, 2114. https://doi.org/10.3390/pharmaceutics14102114
Damerow H, Cheng X, von Kiedrowski V, Schirrmacher R, Wängler B, Fricker G, Wängler C. Toward Optimized 89Zr-Immuno-PET: Side-by-Side Comparison of [89Zr]Zr-DFO-, [89Zr]Zr-3,4,3-(LI-1,2-HOPO)- and [89Zr]Zr-DFO*-Cetuximab for Tumor Imaging: Which Chelator Is the Most Suitable? Pharmaceutics. 2022; 14(10):2114. https://doi.org/10.3390/pharmaceutics14102114
Chicago/Turabian StyleDamerow, Helen, Xia Cheng, Valeska von Kiedrowski, Ralf Schirrmacher, Björn Wängler, Gert Fricker, and Carmen Wängler. 2022. "Toward Optimized 89Zr-Immuno-PET: Side-by-Side Comparison of [89Zr]Zr-DFO-, [89Zr]Zr-3,4,3-(LI-1,2-HOPO)- and [89Zr]Zr-DFO*-Cetuximab for Tumor Imaging: Which Chelator Is the Most Suitable?" Pharmaceutics 14, no. 10: 2114. https://doi.org/10.3390/pharmaceutics14102114
APA StyleDamerow, H., Cheng, X., von Kiedrowski, V., Schirrmacher, R., Wängler, B., Fricker, G., & Wängler, C. (2022). Toward Optimized 89Zr-Immuno-PET: Side-by-Side Comparison of [89Zr]Zr-DFO-, [89Zr]Zr-3,4,3-(LI-1,2-HOPO)- and [89Zr]Zr-DFO*-Cetuximab for Tumor Imaging: Which Chelator Is the Most Suitable? Pharmaceutics, 14(10), 2114. https://doi.org/10.3390/pharmaceutics14102114









