High Incidence of Intracerebral Hemorrhaging Associated with the Application of Low-Intensity Focused Ultrasound Following Acute Cerebrovascular Injury by Intracortical Injection
Abstract
:1. Introduction
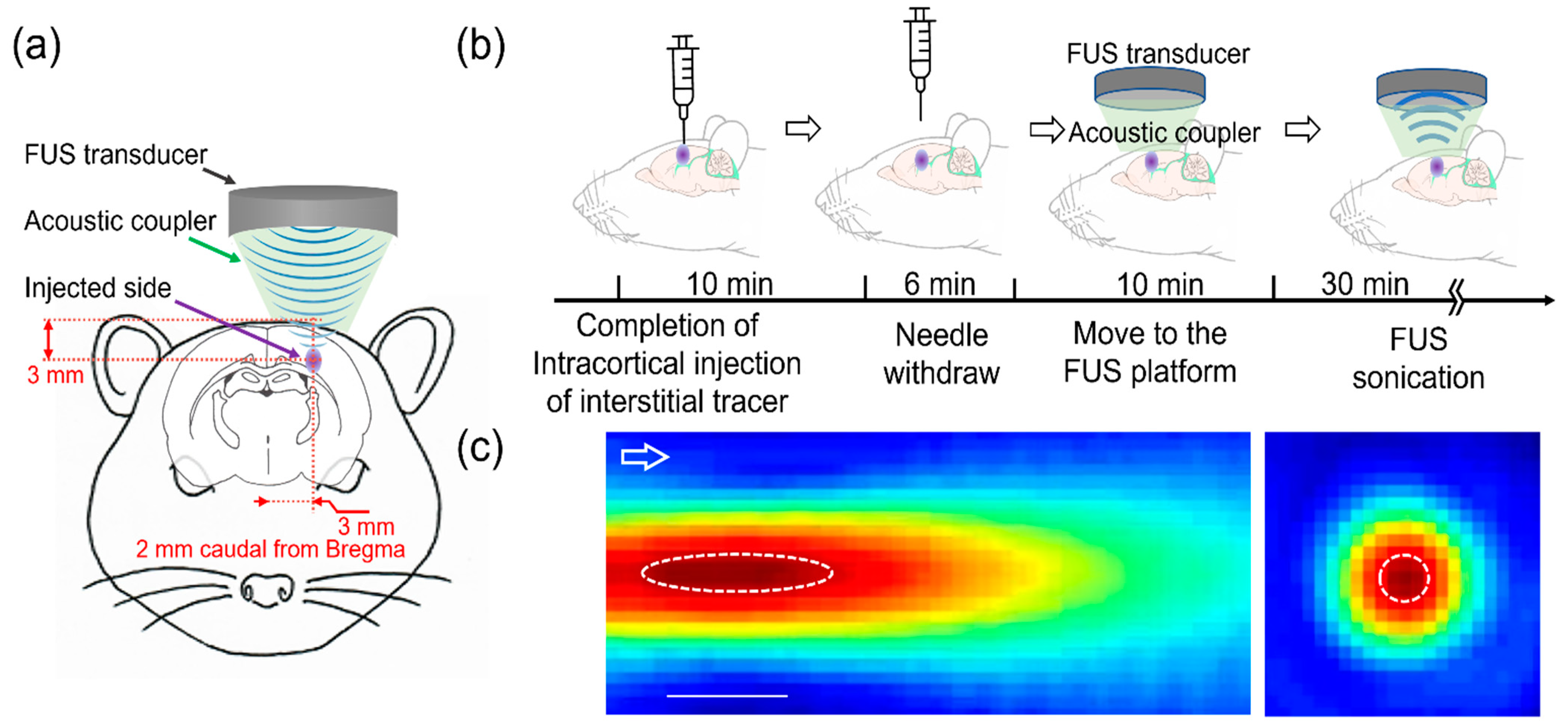
2. Materials and Methods
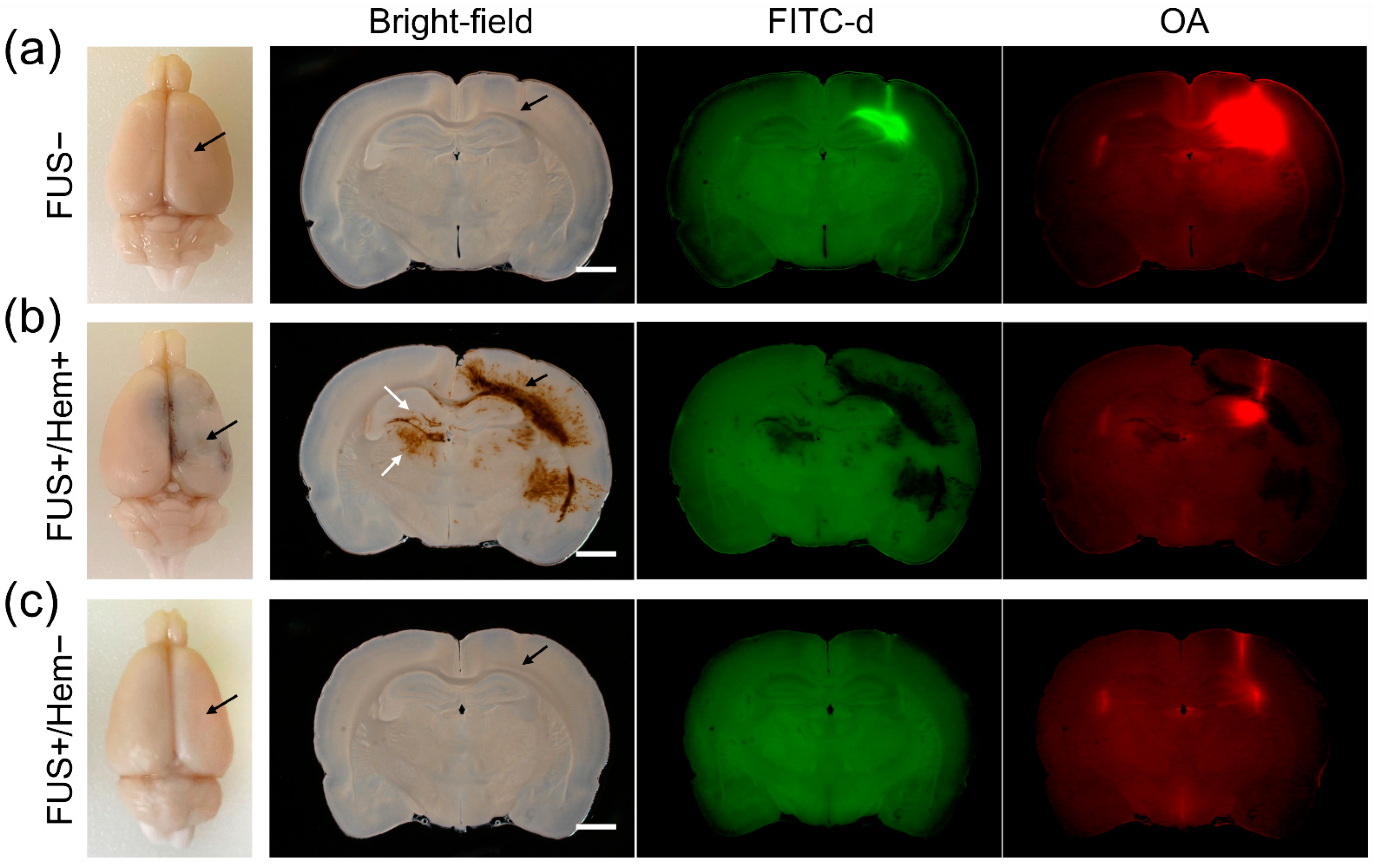
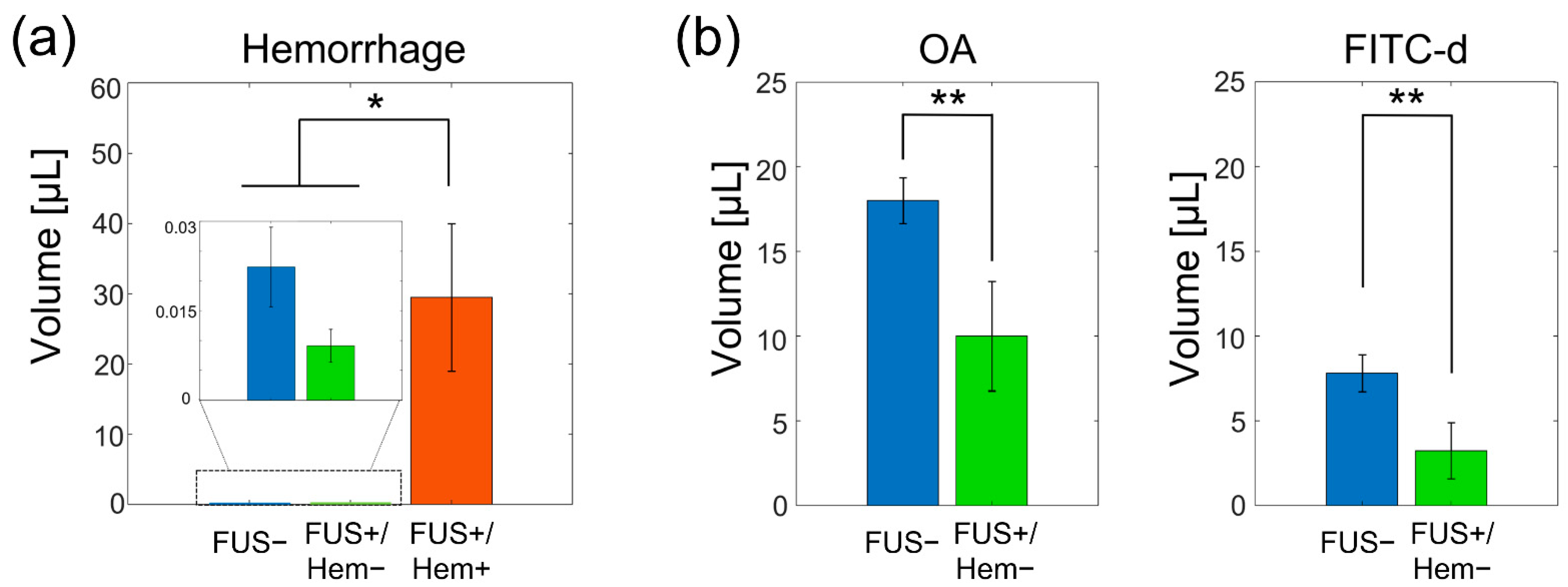
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pachter, J.S.; de Vries, H.E.; Fabry, Z. The blood-brain barrier and its role in immune privilege in the central nervous system. J. Neuropathol. Exp. Neurol. 2003, 62, 593–604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Sorge, N.M.; Doran, K.S. Defense at the border: The blood-brain barrier versus bacterial foreigners. Future Microbiol. 2012, 7, 383–394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banks, W.A. Characteristics of compounds that cross the blood-brain barrier. BMC Neurol. 2009, 9 (Suppl. 1), S3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mehta, A.M.; Sonabend, A.M.; Bruce, J.N. Convection-Enhanced Delivery. Neurotherapeutics 2017, 14, 358–371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jahangiri, A.; Chin, A.T.; Flanigan, P.M.; Chen, R.; Bankiewicz, K.; Aghi, M.K. Convection-enhanced delivery in glioblastoma: A review of preclinical and clinical studies. J. Neurosurg. 2017, 126, 191–200. [Google Scholar] [CrossRef] [Green Version]
- Lidar, Z.; Mardor, Y.; Jonas, T.; Pfeffer, R.; Faibel, M.; Nass, D.; Hadani, M.; Ram, Z. Convection-enhanced delivery of paclitaxel for the treatment of recurrent malignant glioma: A phase I/II clinical study. J. Neurosurg. 2004, 100, 472–479. [Google Scholar] [CrossRef]
- Ung, T.H.; Malone, H.; Canoll, P.; Bruce, J.N. Convection-enhanced delivery for glioblastoma: Targeted delivery of antitumor therapeutics. CNS Oncol. 2015, 4, 225–234. [Google Scholar] [CrossRef]
- Lun, X.; Alain, T.; Zemp, F.J.; Zhou, H.; Rahman, M.M.; Hamilton, M.G.; McFadden, G.; Bell, J.; Senger, D.L.; Forsyth, P.A. Myxoma virus virotherapy for glioma in immunocompetent animal models: Optimizing administration routes and synergy with rapamycin. Cancer Res. 2010, 70, 598–608. [Google Scholar] [CrossRef] [Green Version]
- Ren, H.; Boulikas, T.; Lundstrom, K.; Söling, A.; Warnke, P.C.; Rainov, N.G. Immunogene therapy of recurrent glioblastoma multiforme with a liposomally encapsulated replication-incompetent Semliki forest virus vector carrying the human interleukin-12 gene—A phase I/II clinical protocol. J. Neuro-Oncol. 2003, 64, 147–154. [Google Scholar] [CrossRef]
- Souweidane, M.M.; Kramer, K.; Pandit-Taskar, N.; Zhou, Z.; Zanzonico, P.; Donzelli, M.; Lyashchenko, S.K.; Haque, S.; Thakur, S.B.; Cheung, N.-K.V.; et al. A phase I study of convection-enhanced delivery of 124I-8H9 radio-labeled monoclonal antibody in children with diffuse intrinsic pontine glioma: An update with dose-response assessment. J. Clin. Oncol. 2019, 37, 2008. [Google Scholar] [CrossRef]
- Shoji, T.; Saito, R.; Chonan, M.; Shibahara, I.; Sato, A.; Kanamori, M.; Sonoda, Y.; Kondo, T.; Ishii, N.; Tominaga, T. Local convection-enhanced delivery of an anti-CD40 agonistic monoclonal antibody induces antitumor effects in mouse glioma models. Neuro-Oncology 2016, 18, 1120–1128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morrison, P.F.; Chen, M.Y.; Chadwick, R.S.; Lonser, R.R.; Oldfield, E.H. Focal delivery during direct infusion to brain: Role of flow rate, catheter diameter, and tissue mechanics. Am. J. Physiol. 1999, 277, R1218–R1229. [Google Scholar] [CrossRef] [PubMed]
- Debinski, W.; Tatter, S.B. Convection-enhanced delivery for the treatment of brain tumors. Expert Rev. Neurother. 2009, 9, 1519–1527. [Google Scholar] [CrossRef] [Green Version]
- Olbricht, W.; Sistla, M.; Ghandi, G.; Lewis, G., Jr.; Sarvazyan, A. Time-reversal acoustics and ultrasound-assisted convection-enhanced drug delivery to the brain. J. Acoust. Soc. Am. 2013, 134, 1569–1575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lewis, G.K., Jr.; Schulz, Z.R.; Pannullo, S.C.; Southard, T.L.; Olbricht, W.L. Ultrasound-assisted convection-enhanced delivery to the brain in vivo with a novel transducer cannula assembly: Laboratory investigation. J. Neurosurg. 2012, 117, 1128–1140. [Google Scholar] [CrossRef]
- Mano, Y.; Saito, R.; Haga, Y.; Matsunaga, T.; Zhang, R.; Chonan, M.; Haryu, S.; Shoji, T.; Sato, A.; Sonoda, Y.; et al. Intraparenchymal ultrasound application and improved distribution of infusate with convection-enhanced delivery in rodent and nonhuman primate brain. J. Neurosurg. 2016, 124, 1490–1500. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Paliwal, S.; Bankiewicz, K.S.; Bringas, J.R.; Heart, G.; Mitragotri, S.; Prausnitz, M.R. Ultrasound-enhanced drug transport and distribution in the brain. AAPS PharmSciTech 2010, 11, 1005–1017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xue, M.; Del Bigio, M.R. Intracortical hemorrhage injury in rats: Relationship between blood fractions and brain cell death. Stroke 2000, 31, 1721–1727. [Google Scholar] [CrossRef] [Green Version]
- Del Bigio, M.R.; Yan, H.J.; Buist, R.; Peeling, J. Experimental intracerebral hemorrhage in rats. Magnetic resonance imaging and histopathological correlates. Stroke 1996, 27, 2312–2319; discussion 2319–2320. [Google Scholar] [CrossRef]
- Mestre, H.; Hablitz, L.M.; Xavier, A.L.; Feng, W.; Zou, W.; Pu, T.; Monai, H.; Murlidharan, G.; Castellanos Rivera, R.M.; Simon, M.J.; et al. Aquaporin-4-dependent glymphatic solute transport in the rodent brain. eLife 2018, 7, e40070. [Google Scholar] [CrossRef]
- Yoo, S.S.; Kim, H.C.; Kim, J.; Kim, E.; Kowsari, K.; Van Reet, J.; Yoon, K. Enhancement of cerebrospinal fluid tracer movement by the application of pulsed transcranial focused ultrasound. Sci. Rep. 2022, 12, 12940. [Google Scholar] [CrossRef] [PubMed]
- Santana-Gomez, C.E.; Medel-Matus, J.S.; Rundle, B.K. Animal models of post-traumatic epilepsy and their neurobehavioral comorbidities. Seizure 2021, 90, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.C.; Jeong, J.Y.; Jin, B.K. Interleukin-4-Mediated Oxidative Stress Is Harmful to Hippocampal Neurons of Prothrombin Kringle-2-Lesioned Rat In Vivo. Antioxidants 2020, 9, 1068. [Google Scholar] [CrossRef] [PubMed]
- Triggs, W.J.; Willmore, L.J. In vivo lipid peroxidation in rat brain following intracortical Fe2+ injection. J. Neurochem. 1984, 42, 976–980. [Google Scholar] [CrossRef] [PubMed]
- Mandel, R.J.; Spratt, S.K.; Snyder, R.O.; Leff, S.E. Midbrain injection of recombinant adeno-associated virus encoding rat glial cell line-derived neurotrophic factor protects nigral neurons in a progressive 6-hydroxydopamine-induced degeneration model of Parkinson’s disease in rats. Proc. Natl. Acad. Sci. USA 1997, 94, 14083–14088. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paumier, K.L.; Luk, K.C.; Manfredsson, F.P.; Kanaan, N.M.; Lipton, J.W.; Collier, T.J.; Steece-Collier, K.; Kemp, C.J.; Celano, S.; Schulz, E.; et al. Intrastriatal injection of pre-formed mouse α-synuclein fibrils into rats triggers α-synuclein pathology and bilateral nigrostriatal degeneration. Neurobiol. Dis. 2015, 82, 185–199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeong, M.; Kim, Y.; Kim, J.; Ferrante, D.D.; Mitra, P.P.; Osten, P.; Kim, D. Comparative three-dimensional connectome map of motor cortical projections in the mouse brain. Sci. Rep. 2016, 6, 20072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, Y.G.; Kim, S.E.; Lee, J.; Hwang, S.; Yoo, S.S.; Lee, H.W. Neuromodulation Using Transcranial Focused Ultrasound on the Bilateral Medial Prefrontal Cortex. J. Clin. Med. 2022, 11, 3809. [Google Scholar] [CrossRef]
- Lee, W.; Kim, H.; Jung, Y.; Song, I.U.; Chung, Y.A.; Yoo, S.S. Image-guided transcranial focused ultrasound stimulates human primary somatosensory cortex. Sci. Rep. 2015, 5, 8743. [Google Scholar] [CrossRef] [Green Version]
- Jeong, H.; Song, I.U.; Chung, Y.A.; Park, J.S.; Na, S.H.; Im, J.J.; Bikson, M.; Lee, W.; Yoo, S.S. Short-Term Efficacy of Transcranial Focused Ultrasound to the Hippocampus in Alzheimer’s Disease: A Preliminary Study. J. Pers. Med. 2022, 12, 250. [Google Scholar] [CrossRef]
- Huang, A.P.; Lai, D.M.; Hsu, Y.H.; Kung, Y.; Lan, C.; Yeh, C.S.; Tsai, H.H.; Lin, C.F.; Chen, W.S. Cavitation-induced traumatic cerebral contusion and intracerebral hemorrhage in the rat brain by using an off-the-shelf clinical shockwave device. Sci. Rep. 2019, 9, 15614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Food and Drug Administration. Guidance for Industry and FDA Staff Information for Manufacturers Seeking Marketing Clearance of Diagnostic Ultrasound Systems and Transducers; FDA: Rockville, MD, USA, 2008.
- Claes, L.; Willie, B. The enhancement of bone regeneration by ultrasound. Prog. Biophys. Mol. Biol. 2007, 93, 384–398. [Google Scholar] [CrossRef] [PubMed]
- Enwemeka, C.S.; Rodriguez, O.; Mendosa, S. The biomechanical effects of low-intensity ultrasound on healing tendons. Ultrasound Med. Biol. 1990, 16, 801–807. [Google Scholar] [CrossRef]
- Jagannathan, J.; Sanghvi, N.T.; Crum, L.A.; Yen, C.P.; Medel, R.; Dumont, A.S.; Sheehan, J.P.; Steiner, L.; Jolesz, F.; Kassell, N.F. High-intensity focused ultrasound surgery of the brain: Part 1—A historical perspective with modern applications. Neurosurgery 2009, 64, 201–211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hynynen, K.; Clement, G. Clinical applications of focused ultrasound-the brain. Int. J. Hyperth. 2007, 23, 193–202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schroeder, A.; Kost, J.; Barenholz, Y. Ultrasound, liposomes, and drug delivery: Principles for using ultrasound to control the release of drugs from liposomes. Chem. Phys. Lipids 2009, 162, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, A.; Liu, M.; Ojha, T.; Storm, G.; Kiessling, F.; Lammers, T. Ultrasound-mediated drug delivery to the brain: Principles, progress and prospects. Drug Discov. Today Technol. 2016, 20, 41–48. [Google Scholar] [CrossRef] [Green Version]
- Molina, C.A.; Ribo, M.; Rubiera, M.; Montaner, J.; Santamarina, E.; Delgado-Mederos, R.; Arenillas, J.F.; Huertas, R.; Purroy, F.; Delgado, P.; et al. Microbubble administration accelerates clot lysis during continuous 2-MHz ultrasound monitoring in stroke patients treated with intravenous tissue plasminogen activator. Stroke 2006, 37, 425–429. [Google Scholar] [CrossRef]
- Auboire, L.; Sennoga, C.A.; Hyvelin, J.M.; Ossant, F.; Escoffre, J.M.; Tranquart, F.; Bouakaz, A. Microbubbles combined with ultrasound therapy in ischemic stroke: A systematic review of in-vivo preclinical studies. PLoS ONE 2018, 13, e0191788. [Google Scholar] [CrossRef] [Green Version]
- Xu, L.; Lee, W.; Rotenberg, A.; Böhlke, M.; Yoon, K.; Yoo, S.S. Localized Disruption of Blood Albumin-Phenytoin Binding Using Transcranial Focused Ultrasound. Ultrasound Med. Biol. 2020, 46, 1986–1997. [Google Scholar] [CrossRef]
- Niu, L.; Guo, Y.; Lin, Z.; Shi, Z.; Bian, T.; Qi, L.; Meng, L.; Grace, A.A.; Zheng, H.; Yuan, T.F. Noninvasive ultrasound deep brain stimulation of nucleus accumbens induces behavioral avoidance. Sci. China Life Sci. 2020, 63, 1328–1336. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Jo, Y.; Kook, G.; Pasquinelli, C.; Kim, H.; Kim, K.; Hoe, H.S.; Choe, Y.; Rhim, H.; Thielscher, A.; et al. Transcranial focused ultrasound stimulation with high spatial resolution. Brain Stimul. 2021, 14, 290–300. [Google Scholar] [CrossRef]
- Yoo, S.S.; Bystritsky, A.; Lee, J.H.; Zhang, Y.; Fischer, K.; Min, B.K.; McDannold, N.J.; Pascual-Leone, A.; Jolesz, F.A. Focused ultrasound modulates region-specific brain activity. Neuroimage 2011, 56, 1267–1275. [Google Scholar] [CrossRef] [Green Version]
- Yoon, K.; Lee, W.; Chen, E.; Lee, J.E.; Croce, P.; Cammalleri, A.; Foley, L.; Tsao, A.L.; Yoo, S.-S. Localized blood–brain barrier opening in ovine model using image-guided transcranial focused ultrasound. Ultrasound Med. Biol. 2019, 45, 2391–2404. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.L.; Wai, Y.Y.; Chen, W.S.; Chen, J.C.; Hsu, P.H.; Wu, X.Y.; Huang, W.C.; Yen, T.C.; Wang, J.J. Hemorrhage detection during focused-ultrasound induced blood-brain-barrier opening by using susceptibility-weighted magnetic resonance imaging. Ultrasound Med. Biol. 2008, 34, 598–606. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Lee, S.D.; Park, M.Y.; Foley, L.; Purcell-Estabrook, E.; Kim, H.; Fischer, K.; Maeng, L.S.; Yoo, S.S. Image-Guided Focused Ultrasound-Mediated Regional Brain Stimulation in Sheep. Ultrasound Med. Biol. 2016, 42, 459–470. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Chiu, A.; Lee, S.D.; Fischer, K.; Yoo, S.S. Focused ultrasound-mediated non-invasive brain stimulation: Examination of sonication parameters. Brain Stimul. 2014, 7, 748–756. [Google Scholar] [CrossRef] [Green Version]
- Tamada, H.; Blanc, J.; Korogod, N.; Petersen, C.C.; Knott, G.W. Ultrastructural comparison of dendritic spine morphology preserved with cryo and chemical fixation. eLife 2020, 9, e56384. [Google Scholar] [CrossRef]
- Bruce, J.N.; Fine, R.L.; Canoll, P.; Yun, J.; Kennedy, B.C.; Rosenfeld, S.S.; Sands, S.A.; Surapaneni, K.; Lai, R.; Yanes, C.L.; et al. Regression of recurrent malignant gliomas with convection-enhanced delivery of topotecan. Neurosurgery 2011, 69, 1272–1279; discussion 1279–1280. [Google Scholar] [CrossRef] [Green Version]
- Raghavan, R.; Brady, M.L.; Rodríguez-Ponce, M.I.; Hartlep, A.; Pedain, C.; Sampson, J.H. Convection-enhanced delivery of therapeutics for brain disease, and its optimization. Neurosurg. Focus 2006, 20, E12. [Google Scholar] [CrossRef]
- Sullivan, T.P.; Eaglstein, W.H.; Davis, S.C.; Mertz, P. The pig as a model for human wound healing. Wound Repair Regen. 2001, 9, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Bechet, N.B.; Shanbhag, N.C.; Lundgaard, I. Glymphatic pathways in the gyrencephalic brain. J. Cereb. Blood Flow Metab. 2021, 41, 2264–2279. [Google Scholar] [CrossRef] [PubMed]
- Iliff, J.J.; Wang, M.; Liao, Y.; Plogg, B.A.; Peng, W.; Gundersen, G.A.; Benveniste, H.; Vates, G.E.; Deane, R.; Goldman, S.A.; et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci. Transl. Med. 2012, 4, 147ra111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, A.J.; Yao, X.; Dix, J.A.; Jin, B.J.; Verkman, A.S. Test of the ‘glymphatic’ hypothesis demonstrates diffusive and aquaporin-4-independent solute transport in rodent brain parenchyma. eLife 2017, 6, e27679. [Google Scholar] [CrossRef] [PubMed]
- Iliff, J.J.; Nedergaard, M. Is there a cerebral lymphatic system? Stroke 2013, 44, S93–S95. [Google Scholar] [CrossRef] [Green Version]
- Abbott, N.J.; Pizzo, M.E.; Preston, J.E.; Janigro, D.; Thorne, R.G. The role of brain barriers in fluid movement in the CNS: Is there a ‘glymphatic’ system? Acta Neuropathol. 2018, 135, 387–407. [Google Scholar] [CrossRef] [Green Version]
- Aryal, M.; Azadian, M.M.; Hart, A.R.; Macedo, N.; Zhou, Q.; Rosenthal, E.L.; Airan, R.D. Noninvasive ultrasonic induction of cerebrospinal fluid flow enhances intrathecal drug delivery. J. Control. Release 2022, 349, 434–442. [Google Scholar] [CrossRef]
- Mestre, H.; Tithof, J.; Du, T.; Song, W.; Peng, W.; Sweeney, A.M.; Olveda, G.; Thomas, J.H.; Nedergaard, M.; Kelley, D.H. Flow of cerebrospinal fluid is driven by arterial pulsations and is reduced in hypertension. Nat. Commun. 2018, 9, 4878. [Google Scholar] [CrossRef] [Green Version]
- Hablitz, L.M.; Vinitsky, H.S.; Sun, Q.; Stæger, F.F.; Sigurdsson, B.; Mortensen, K.N.; Lilius, T.O.; Nedergaard, M. Increased glymphatic influx is correlated with high EEG delta power and low heart rate in mice under anesthesia. Sci. Adv. 2019, 5, eaav5447. [Google Scholar] [CrossRef] [Green Version]
- Start, R.D.; Layton, C.M.; Cross, S.S.; Smith, J.H. Reassessment of the rate of fixative diffusion. J. Clin. Pathol. 1992, 45, 1120–1121. [Google Scholar] [CrossRef]
- Shih, A.Y.; Driscoll, J.D.; Drew, P.J.; Nishimura, N.; Schaffer, C.B.; Kleinfeld, D. Two-photon microscopy as a tool to study blood flow and neurovascular coupling in the rodent brain. J. Cereb. Blood Flow Metab. 2012, 32, 1277–1309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Viswanathan, A.; Chabriat, H. Cerebral microhemorrhage. Stroke 2006, 37, 550–555. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; McCullough, L.D. Middle cerebral artery occlusion model in rodents: Methods and potential pitfalls. J. Biomed. Biotechnol. 2011, 2011, 464701. [Google Scholar] [CrossRef]
- Uzdensky, A.B. Photothrombotic Stroke as a Model of Ischemic Stroke. Transl. Stroke Res. 2018, 9, 437–451. [Google Scholar] [CrossRef] [PubMed]
- Konofagou, E.E.; Tung, Y.S.; Choi, J.; Deffieux, T.; Baseri, B.; Vlachos, F. Ultrasound-induced blood-brain barrier opening. Curr. Pharm. Biotechnol. 2012, 13, 1332–1345. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.Y.; Tung, Y.S.; Marquet, F.; Downs, M.; Sanchez, C.; Chen, C.; Ferrera, V.; Konofagou, E. Transcranial cavitation detection in primates during blood-brain barrier opening--a performance assessment study. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2014, 61, 966–978. [Google Scholar] [CrossRef] [Green Version]
- Lee, W.; Weisholtz, D.S.; Strangman, G.E.; Yoo, S.-S. Safety Review and Perspectives of Transcranial Focused Ultrasound Brain Stimulation. Brain Neurorehabil. 2021, 14, e4. [Google Scholar] [CrossRef]
- Das, P.; Puri, T.; Jha, P.; Pathak, P.; Joshi, N.; Suri, V.; Sharma, M.C.; Sharma, B.S.; Mahapatra, A.K.; Suri, A.; et al. A clinicopathological and molecular analysis of glioblastoma multiforme with long-term survival. J. Clin. Neurosci. 2011, 18, 66–70. [Google Scholar] [CrossRef]
- Xi, G.; Hua, Y.; Bhasin, R.R.; Ennis, S.R.; Keep, R.F.; Hoff, J.T. Mechanisms of edema formation after intracerebral hemorrhage: Effects of extravasated red blood cells on blood flow and blood-brain barrier integrity. Stroke 2001, 32, 2932–2938. [Google Scholar] [CrossRef] [Green Version]
- Ostrowski, R.P.; He, Z.; Pucko, E.B.; Matyja, E. Hemorrhage in brain tumor–An unresolved issue. Brain Hemorrhages 2022, 3, 98–102. [Google Scholar] [CrossRef]
- Jickling, G.C.; Liu, D.; Stamova, B.; Ander, B.P.; Zhan, X.; Lu, A.; Sharp, F.R. Hemorrhagic transformation after ischemic stroke in animals and humans. J. Cereb. Blood Flow Metab. 2014, 34, 185–199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, W.R.; Thore, C.R. Review: Cerebral microvascular pathology in ageing and neurodegeneration. Neuropathol. Appl. Neurobiol. 2011, 37, 56–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taki, Y.; Goto, R.; Evans, A.; Zijdenbos, A.; Neelin, P.; Lerch, J.; Sato, K.; Ono, S.; Kinomura, S.; Nakagawa, M.; et al. Voxel-based morphometry of human brain with age and cerebrovascular risk factors. Neurobiol. Aging 2004, 25, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Awad, I.A.; Spetzler, R.F.; Hodak, J.A.; Awad, C.A.; Carey, R. Incidental subcortical lesions identified on magnetic resonance imaging in the elderly. I. Correlation with age and cerebrovascular risk factors. Stroke 1986, 17, 1084–1089. [Google Scholar] [CrossRef] [PubMed]
| Weight (g) | RR (Breaths per Minute) | HR (Beats per Minute) | SpO2 (%) | |||||
|---|---|---|---|---|---|---|---|---|
| Beginning | End | Beginning | End | Beginning | End | |||
| FUS+ | Mean | 290.0 | 55.6 | 55.1 | 206.1 | 196.4 | 85.2 | 87.3 |
| Std | 13.3 | 8.1 | 6.4 | 23.3 | 14.5 | 4.8 | 5.9 | |
| FUS− | Mean | 286.7 | 57.3 | 56.3 | 217.8 | 207.3 | 88.8 | 88.5 |
| Std | 17.4 | 4.8 | 3.2 | 36.3 | 29.8 | 4.4 | 2.7 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, E.; Van Reet, J.; Kim, H.-C.; Kowsari, K.; Yoo, S.-S. High Incidence of Intracerebral Hemorrhaging Associated with the Application of Low-Intensity Focused Ultrasound Following Acute Cerebrovascular Injury by Intracortical Injection. Pharmaceutics 2022, 14, 2120. https://doi.org/10.3390/pharmaceutics14102120
Kim E, Van Reet J, Kim H-C, Kowsari K, Yoo S-S. High Incidence of Intracerebral Hemorrhaging Associated with the Application of Low-Intensity Focused Ultrasound Following Acute Cerebrovascular Injury by Intracortical Injection. Pharmaceutics. 2022; 14(10):2120. https://doi.org/10.3390/pharmaceutics14102120
Chicago/Turabian StyleKim, Evgenii, Jared Van Reet, Hyun-Chul Kim, Kavin Kowsari, and Seung-Schik Yoo. 2022. "High Incidence of Intracerebral Hemorrhaging Associated with the Application of Low-Intensity Focused Ultrasound Following Acute Cerebrovascular Injury by Intracortical Injection" Pharmaceutics 14, no. 10: 2120. https://doi.org/10.3390/pharmaceutics14102120






