Theranostic Properties of Crystalline Aluminum Phthalocyanine Nanoparticles as a Photosensitizer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation and Characterization of Nanoparticle Colloids
2.2. Assessment of Intracellular Accumulation and Localization
2.3. Estimation of Fluorescence Energy Yield and Photodynamic Efficiency
- Photosens + cells (HeLa or THP-1) + erythrocytes. The final concentration of Photosens in the medium was 20 mg/L (0.23 μM).
- NP-AlPc + cells (HeLa or THP-1) + erythrocytes. The final concentration of NPs in the medium was 25 mg/L (the total molar concentration of AlPc in the sample was 0.43 μM).
3. Results
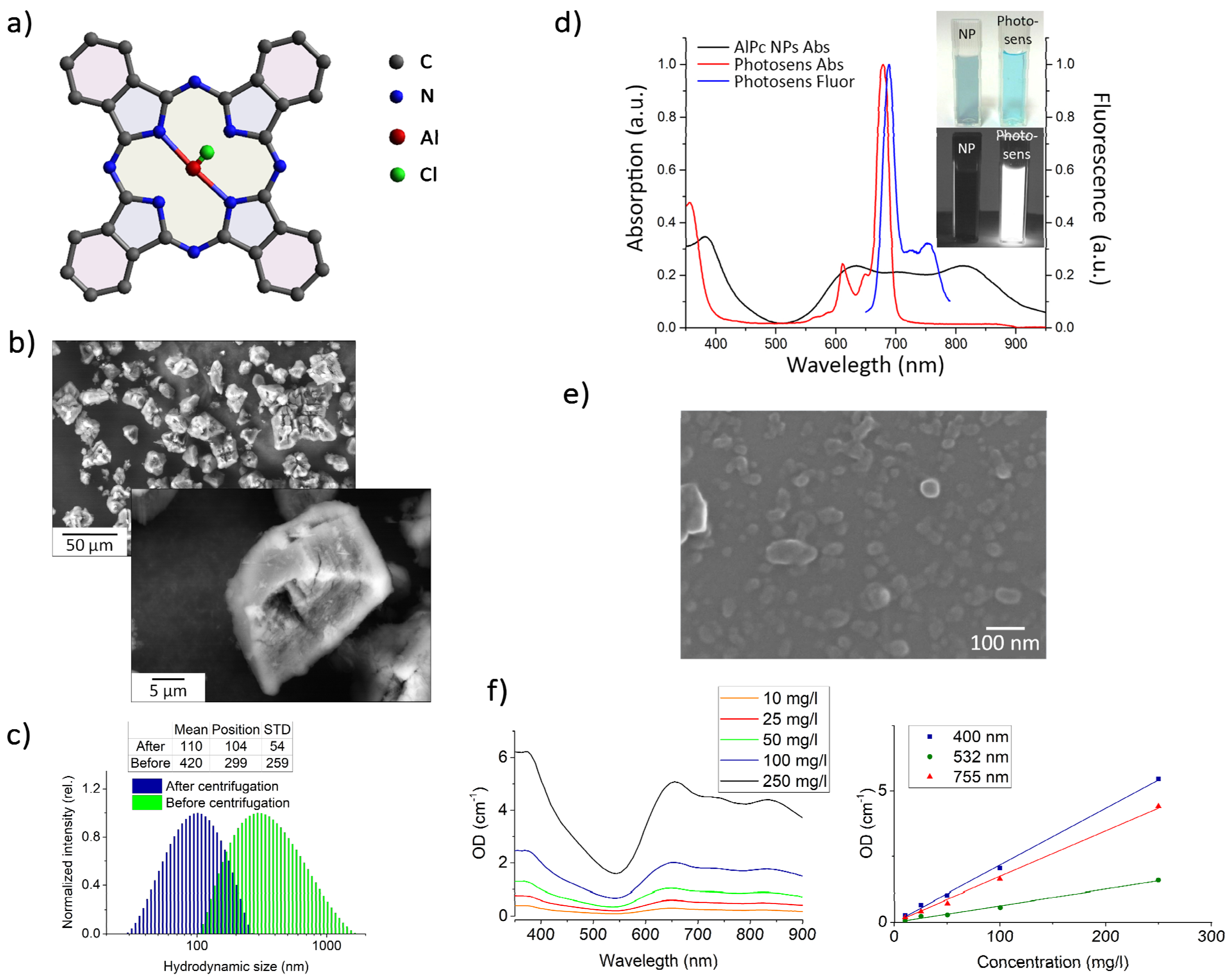
3.1. Dimensional and Spectroscopic Characteristics of the Obtained AlPc NP Colloids
3.2. Changes in the Spectral Characteristics of AlPc NPs Depending on the pH
3.3. AlPc NP Accumulation, Localization, and Fluorescence in Cells
3.4. Photodynamic Efficiency and Fluorescence Energy Yield of AlPc NPs upon Interaction with Cells
3.5. AlPc NP Fluorescence and Phototoxicity Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sosnik, A. Drug Self-Assembly: A Phenomenon at the Nanometer Scale with Major Impact in the Structure–Biological Properties Relationship and the Treatment of Disease. Prog. Mater. Sci. 2016, 82, 39–82. [Google Scholar] [CrossRef]
- Lamch, Ł.; Gancarz, R.; Tsirigotis-Maniecka, M.; Moszyńska, I.M.; Ciejka, J.; Wilk, K.A. Studying the “Rigid–Flexible” Properties of Polymeric Micelle Core-Forming Segments with a Hydrophobic Phthalocyanine Probe Using NMR and UV Spectroscopy. Langmuir 2021, 37, 4316–4330. [Google Scholar] [CrossRef] [PubMed]
- Snow, A.W. Phthalocyanine Aggregation. In The Porphyrin Handbook; Kadish, K.M., Smith, K.M., Guilard, R., Eds.; Academic Press: Cambridge, MA, USA, 2003; Volume 17, pp. 129–176. [Google Scholar]
- Li, X.; Zheng, B.-D.; Peng, X.-H.; Li, S.-Z.; Ying, J.-W.; Zhao, Y.; Huang, J.-D.; Yoon, J. Phthalocyanines as Medicinal Photosensitizers: Developments in the Last Five Years. Coord. Chem. Rev. 2019, 379, 147–160. [Google Scholar] [CrossRef]
- Chan, W.-S.; Marshall, J.F.; Svensen, R.; Bedwell, J.; Hart, I.R. Effect of Sulfonation on the Cell and Tissue Distribution of the Photosensitizer Aluminum Phthalocyanine. Cancer Res. 1990, 50, 4533–4538. [Google Scholar]
- Chen, D.; Song, M.; Huang, J.; Chen, N.; Xue, J.; Huang, M. Photocyanine: A Novel and Effective Phthalocyanine-Based Photosensitizer for Cancer Treatment. J. Innov. Opt. Health Sci. 2020, 13, 2030009. [Google Scholar] [CrossRef]
- Kuznetsova, N.A.; Gretsova, N.S.; Derkacheva, V.M.; Mikhalenko, S.A.; Solov’eva, L.I.; Yuzhakova, O.A.; Kaliya, O.L.; Luk’yanets, E.A. Generation of Singlet Oxygen with Anionic Aluminum Phthalocyanines in Water. Russ. J. Gen. Chem. 2002, 72, 300–306. [Google Scholar] [CrossRef]
- Jia, X.; Jia, L. Nanoparticles Improve Biological Functions of Phthalocyanine Photosensitizers Used for Photodynamic Therapy. Curr. Drug Metab. 2012, 13, 1119–1122. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-Y.; Sharma, S.K.; Dai, T.; Chung, H.; Yaroslavsky, A.; Garcia-Diaz, M.; Chang, J.; Chiang, L.Y.; Hamblin, M.R. Can Nanotechnology Potentiate Photodynamic Therapy? Nanotechnol. Rev. 2012, 1, 111–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rak, J.; Pouckova, P.; Benes, J.; Vetvicka, D. Drug Delivery Systems for Phthalocyanines for Photodynamic Therapy. Anticancer Res. 2019, 39, 3323–3339. [Google Scholar] [CrossRef] [Green Version]
- Muehlmann, L.A.; Ma, B.C.; Longo, J.P.F.; Santos, M.d.F.M.A.; Azevedo, R.B. Aluminum–Phthalocyanine Chloride Associated to Poly (Methyl Vinyl Ether-Co-Maleic Anhydride) Nanoparticles as a New Third-Generation Photosensitizer for Anticancer Photodynamic Therapy. Int. J. Nanomed. 2014, 9, 1199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herlambang, S.; Kumagai, M.; Nomoto, T.; Horie, S.; Fukushima, S.; Oba, M.; Miyazaki, K.; Morimoto, Y.; Nishiyama, N.; Kataoka, K. Disulfide Crosslinked Polyion Complex Micelles Encapsulating Dendrimer Phthalocyanine Directed to Improved Efficiency of Photodynamic Therapy. J. Control. Release 2011, 155, 449–457. [Google Scholar] [CrossRef]
- Ribeiro, J.B.P.; Miranda-Vilela, A.L.; Amorim, A.A.S.; Garcia, R.D.; Moreira, J.R.; Gomes, C.M.; Takano, G.H.S.; de Oliveira, G.M.F.; Lima, A.V.; da Silva, I.C.R. Study of the Efficacy of N-Methyl Glucamine Antimoniate (SbV) Associated with Photodynamic Therapy Using Liposomal Chloroaluminium Phthalocyanine in the Treatment of Cutaneous Leishmaniasis Caused by Leishmania (L.) Amazonensis in C57BL6 Mice. Photodiagnosis Photodyn. Ther. 2019, 26, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Garcia, A.M.; Alarcon, E.; Muñoz, M.; Scaiano, J.C.; Edwards, A.M.; Lissi, E. Photophysical Behaviour and Photodynamic Activity of Zinc Phthalocyanines Associated to Liposomes. Photochem. Photobiol. Sci. 2011, 10, 507–514. [Google Scholar] [CrossRef]
- Lin, A.-L.; Fan, P.-P.; Liu, S.-F.; Chen, J.-H.; Zhao, Y.-Y.; Zheng, B.-Y.; Ke, M.-R.; Huang, J.-D. A Phthalocyanine-Based Liposomal Nanophotosensitizer with Highly Efficient Tumor-Targeting and Photodynamic Activity. Dye. Pigment. 2020, 180, 108455. [Google Scholar] [CrossRef]
- Stuchinskaya, T.; Moreno, M.; Cook, M.J.; Edwards, D.R.; Russell, D.A. Targeted Photodynamic Therapy of Breast Cancer Cells Using Antibody-Phthalocyanine-Gold Nanoparticle Conjugates. Photochem. Photobiol. Sci. 2011, 10, 822–831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poiroux, G.; Barre, A.; Rougé, P.; Benoist, H. Targeting Glycosylation Aberrations to Improve the Efficiency of Cancer Phototherapy. Curr. Cancer Drug Targets 2019, 19, 349–359. [Google Scholar] [CrossRef]
- Wang, Z.; Gai, S.; Wang, C.; Yang, G.; Zhong, C.; Dai, Y.; He, F.; Yang, D.; Yang, P. Self-Assembled Zinc Phthalocyanine Nanoparticles as Excellent Photothermal/Photodynamic Synergistic Agent for Antitumor Treatment. Chem. Eng. J. 2019, 361, 117–128. [Google Scholar] [CrossRef]
- Kasha, M.; Rawls, H.R.; El-Bayoumi, M.A. The Exciton Model in Molecular Spectroscopy. Pure Appl. Chem. 1965, 11, 371–392. [Google Scholar] [CrossRef] [Green Version]
- Dini, D.; Hanack, M. The Porphyrin Handbook: Phthalocyanines: Properties and Materials; Academic Press: Cambridge, MA, USA, 2003; Volume 17, pp. 1–36. [Google Scholar]
- Antunes, E.; Rapulenyane, N.; Ledwaba, M.; Litwinski, C.; Chidawanyika, W.; Nyokong, T. The Synthesis and Characterisation of Magnetic Nanoparticles and Their Interaction with a Zinc Phthalocyanine. Inorg. Chem. Commun. 2013, 29, 60–64. [Google Scholar] [CrossRef]
- Lacey, J.A.; Phillips, D. Fluorescence Lifetime Measurements of Disulfonated Aluminium Phthalocyanine in the Presence of Microbial Cells. Photochem. Photobiol. Sci. 2002, 1, 378–383. [Google Scholar] [CrossRef]
- Yaghini, E.; Giuntini, F.; Eggleston, I.M.; Suhling, K.; Seifalian, A.M.; MacRobert, A.J. Fluorescence Lifetime Imaging and FRET-induced Intracellular Redistribution of Tat-conjugated Quantum Dot Nanoparticles through Interaction with a Phthalocyanine Photosensitiser. Small 2014, 10, 782–792. [Google Scholar] [CrossRef] [PubMed]
- Dhami, S. Photophysics of Phthalocyanines in Microheterogeneous Systems. Ph.D. Thesis, Imperial College London (University of London), London, UK, 1996. [Google Scholar]
- Garcia, A.M.; de Alwis Weerasekera, H.; Pitre, S.P.; McNeill, B.; Lissi, E.; Edwards, A.M.; Alarcon, E.I. Photodynamic Performance of Zinc Phthalocyanine in HeLa Cells: A Comparison between DPCC Liposomes and BSA as Delivery Systems. J. Photochem. Photobiol. B Biol. 2016, 163, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Makarov, D.A.; Kuznetsova, N.A.; Yuzhakova, O.A.; Savvina, L.P.; Kaliya, O.L.; Lukyanets, E.A.; Negrimovskii, V.M.; Strakhovskaya, M.G. Effects of the Degree of Substitution on the Physicochemical Properties and Photodynamic Activity of Zinc and Aluminum Phthalocyanine Polycations. Russ. J. Phys. Chem. A 2009, 83, 1044–1050. [Google Scholar] [CrossRef]
- Henderson, B.W.; Dougherty, T.J. How Does Photodynamic Therapy Work? Photochem. Photobiol. 1992, 55, 145–157. [Google Scholar] [CrossRef] [PubMed]
- Dube, E.; Oluwole, D.O.; Prinsloo, E.; Nyokong, T. A Gold–Chitosan Composite with Low Symmetry Zinc Phthalocyanine for Enhanced Singlet Oxygen Generation and Improved Photodynamic Therapy Activity. New J. Chem. 2018, 42, 10214–10225. [Google Scholar] [CrossRef]
- Zasedatelev, A.V.; Dubinina, T.V.; Krichevsky, D.M.; Krasovskii, V.I.; Gak, V.Y.; Pushkarev, V.E.; Tomilova, L.G.; Chistyakov, A.A. Plasmon-Induced Light Absorption of Phthalocyanine Layer in Hybrid Nanoparticles: Enhancement Factor and Effective Spectra. J. Phys. Chem. C 2016, 120, 1816–1823. [Google Scholar] [CrossRef]
- Li, L.; Chen, J.-Y.; Wu, X.; Wang, P.-N.; Peng, Q. Plasmonic Gold Nanorods Can Carry Sulfonated Aluminum Phthalocyanine to Improve Photodynamic Detection and Therapy of Cancers. J. Phys. Chem. B 2010, 114, 17194–17200. [Google Scholar] [CrossRef] [PubMed]
- Rakov, I.I.; Pridvorova, S.M.; Shafeev, G.A. Interaction of Gold and Phthalocyanines Nanoparticles Generated by Laser Radiation in Water. Colloids Surfaces A Physicochem. Eng. Asp. 2021, 618, 126491. [Google Scholar] [CrossRef]
- Ke, X.; Wang, D.; Chen, C.; Yang, A.; Han, Y.; Ren, L.; Li, D.; Wang, H. Co-Enhancement of Fluorescence and Singlet Oxygen Generation by Silica-Coated Gold Nanorods Core-Shell Nanoparticle. Nanoscale Res. Lett. 2014, 9, 2492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, X.; Xie, J.; Li, Z.; Chen, M.; Wang, M.; Wang, P.-N.; Chen, L.; Mi, L. Enhancement of the Photokilling Effect of Aluminum Phthalocyanine in Photodynamic Therapy by Conjugating with Nitrogen-Doped TiO2 Nanoparticles. Colloids Surf. B Biointerfaces 2015, 130, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Goto, P.L.; Siqueira-Moura, M.P.; Tedesco, A.C. Application of Aluminum Chloride Phthalocyanine-Loaded Solid Lipid Nanoparticles for Photodynamic Inactivation of Melanoma Cells. Int. J. Pharm. 2017, 518, 228–241. [Google Scholar] [CrossRef] [PubMed]
- Farrakhova, D.S.; Makarov, V.I.; Loschenov, V.B. The Engraftment Dynamics Evaluation of Skin Grafts via Aluminium Phthalocyanine Nanoparticles Using Spectroscopic Methods. J. Phys. Conf. Ser. 2019, 1238, 012026. [Google Scholar] [CrossRef] [Green Version]
- Bystrov, F.G.; Makarov, V.I.; Pominova, D.V.; Ryabova, A.V.; Loschenov, V.B. Analysis of Photoluminescence Decay Kinetics of Aluminum Phthalocyanine Nanoparticles Interacting with Immune Cells. Biomed. Photonics 2016, 5, 3–8. [Google Scholar] [CrossRef]
- Vasilchenko, S.Y.; Volkova, A.I.; Ryabova, A.V.; Loschenov, V.B.; Konov, V.I.; Mamedov, A.A.; Kuzmin, S.G.; Lukyanets, E.A. Application of Aluminum Phthalocyanine Nanoparticles for Fluorescent Diagnostics in Dentistry and Skin Autotransplantology. J. Biophotonics 2010, 3, 336–346. [Google Scholar] [CrossRef]
- Breymayer, J.; Rück, A.; Ryabova, A.V.; Loschenov, V.B.; Steiner, R.W. Fluorescence Investigation of the Detachment of Aluminum Phthalocyanine Molecules from Aluminum Phthalocyanine Nanoparticles in Monocytes/Macrophages and Skin Cells and Their Localization in Monocytes/Macrophages. Photodiagnosis Photodyn. Ther. 2014, 11, 380–390. [Google Scholar] [CrossRef]
- Makarov, V.I.; Pominova, D.V.; Ryabova, A.V.; Saveleva, T.A.; Ignateva, I.V.; Reshetov, I.V.; Loschenov, V.B. Multispectral Imaging Technique for Skin Grafts’ Functional State Assessment. In Proceedings of the SPIE Photonics Europe, Strasbourg, France, 22–26 April 2018; Volume 10677. [Google Scholar] [CrossRef]
- Gratchev, A.; Kzhyshkowska, J.; Utikal, J.; Goerdt, S. Interleukin-4 and Dexamethasone Counterregulate Extracellular Matrix Remodelling and Phagocytosis in Type-2 Macrophages. Scand. J. Immunol. 2005, 61, 10–17. [Google Scholar] [CrossRef]
- Stratonnikov, A.A.; Douplik, A.Y.; Loschenov, V.B. Oxygen Consumption and Photobleaching in Whole Blood Incubated with Photosensitizer Induced by Laser Irradiation. Laser Phys. 2003, 13, 1–21. [Google Scholar]
- Ryabova, A.V.; Pominova, D.V.; Krut’ko, V.A.; Komova, M.G.; Loschenov, V.B. Spectroscopic research of upconversion nanomaterials based on complex oxide compounds doped with rare-earth ion pairs: Benefit for cancer diagnostics by upconversion fluorescence and radio sensitive methods. Photonics Lasers Med. 2013, 2, 117–128. [Google Scholar] [CrossRef]
- Canton, J.; Khezri, R.; Glogauer, M.; Grinstein, S. Contrasting Phagosome PH Regulation and Maturation in Human M1 and M2 Macrophages. Mol. Biol. Cell 2014, 25, 3330–3341. [Google Scholar] [CrossRef]
- Rosenthal, I.; Ben-Hur, E. Role of Oxygen in the Phototoxicity of Phthalocyanines. Int. J. Radiat. Biol. 1995, 67, 85–91. [Google Scholar] [CrossRef]
- Ambroz, M.; Beeby, A.; MacRobert, A.J.; Svensen, R.K.; Phillips, D. Preparative, Analytical and Fluorescence Spectroscopic Studies of Sulphonated Aluminium Phthalocyanine Photosensitizers. J. Photochem. Photobiol. B Biol. 1991, 9, 87–95. [Google Scholar] [CrossRef]
- Dhami, S.; Cosa, J.J.; Bishop, S.M.; Phillips, D. Photophysical Characterization of Sulfonated Aluminum Phthalocyanines in a Cationic Reversed Micellar System. Langmuir 1996, 12, 293–300. [Google Scholar] [CrossRef]
- Kobayashi, H.; Watanabe, R.; Choyke, P.L. Improving Conventional Enhanced Permeability and Retention (EPR) Effects; What Is the Appropriate Target? Theranostics 2014, 4, 81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greish, K. Enhanced Permeability and Retention (EPR) Effect for Anticancer Nanomedicine Drug Targeting. In Cancer Nanotechnology; Humana Press: New York, NY, USA, 2010; Volume 624, pp. 25–37. [Google Scholar]
- Iyer, A.K.; Khaled, G.; Fang, J.; Maeda, H. Exploiting the Enhanced Permeability and Retention Effect for Tumor Targeting. Drug Discov. Today 2006, 11, 812–818. [Google Scholar] [CrossRef]
- Weissleder, R.; Nahrendorf, M.; Pittet, M.J. Imaging Macrophages with Nanoparticles. Nat. Mater. 2014, 13, 125–138. [Google Scholar] [CrossRef]
- Kuznetsova, J.O.; Makarov, V.I. Application of Nanophotosensitizers (Aluminum Phthalocyanine Nanoparticles) for Early Diagnosis and Prevention of Inflammatory Diseases. J. Phys. Conf. Ser. 2016, 737, 012049. [Google Scholar] [CrossRef] [Green Version]
- Xu, H.; Ren, D. Lysosomal Physiology. Annu. Rev. Physiol. 2015, 77, 57–80. [Google Scholar] [CrossRef] [Green Version]
- Saftig, P.; Haas, A. Turn up the Lysosome. Nat. Cell Biol. 2016, 18, 1025–1027. [Google Scholar] [CrossRef]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Makarov, V.I.; Pominova, D.V.; Ryabova, A.V.; Romanishkin, I.D.; Voitova, A.V.; Steiner, R.W.; Loschenov, V.B. Theranostic Properties of Crystalline Aluminum Phthalocyanine Nanoparticles as a Photosensitizer. Pharmaceutics 2022, 14, 2122. https://doi.org/10.3390/pharmaceutics14102122
Makarov VI, Pominova DV, Ryabova AV, Romanishkin ID, Voitova AV, Steiner RW, Loschenov VB. Theranostic Properties of Crystalline Aluminum Phthalocyanine Nanoparticles as a Photosensitizer. Pharmaceutics. 2022; 14(10):2122. https://doi.org/10.3390/pharmaceutics14102122
Chicago/Turabian StyleMakarov, Vladimir I., Daria V. Pominova, Anastasiya V. Ryabova, Igor D. Romanishkin, Arina V. Voitova, Rudolf W. Steiner, and Victor B. Loschenov. 2022. "Theranostic Properties of Crystalline Aluminum Phthalocyanine Nanoparticles as a Photosensitizer" Pharmaceutics 14, no. 10: 2122. https://doi.org/10.3390/pharmaceutics14102122
APA StyleMakarov, V. I., Pominova, D. V., Ryabova, A. V., Romanishkin, I. D., Voitova, A. V., Steiner, R. W., & Loschenov, V. B. (2022). Theranostic Properties of Crystalline Aluminum Phthalocyanine Nanoparticles as a Photosensitizer. Pharmaceutics, 14(10), 2122. https://doi.org/10.3390/pharmaceutics14102122







