Continuous NPWT Regulates Fibrosis in Murine Diabetic Wound Healing
Abstract
1. Introduction
2. Materials and Methods
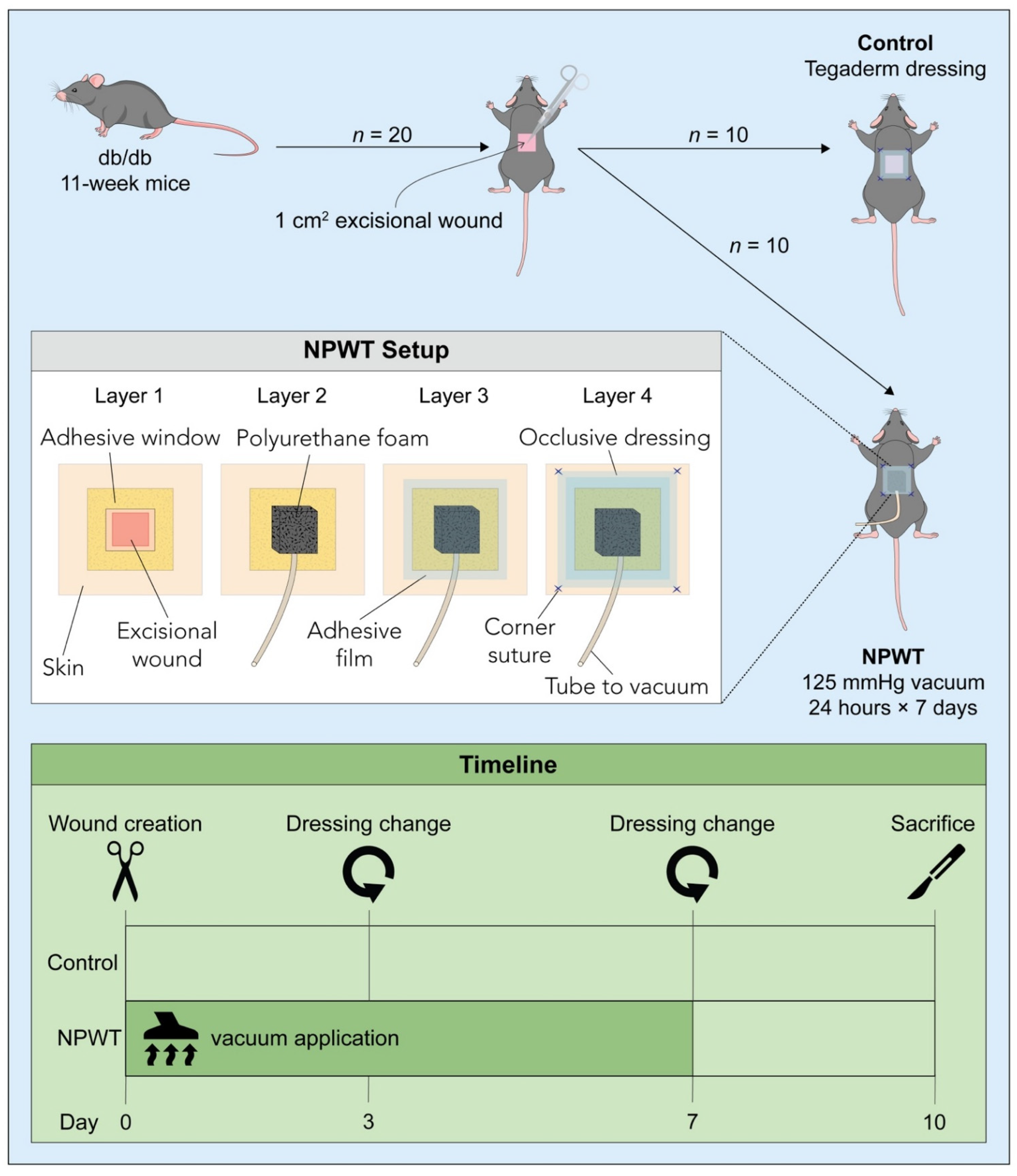
2.1. Animals
2.2. Surgery and Post-Surgical Monitoring
2.3. Histology
2.4. Western Blotting
2.5. Statistical Analysis
3. Results
3.1. NPWT Was Associated with an Increase in YAP but a Decrease in Pro-Fibrotic Fibroblasts
3.2. NPWT Modified Collagen Deposition
3.3. NPWT Was Associated with Increased Fibronectin, but Decreased αSMA, Vimentin and Hsp47
3.4. NPWT Affected Cellular Turnover
3.5. NPWT Upregulated RhoA and YAP, while Downregulating Caspase-3
4. Discussion
Innovation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gurtner, G.C.; Werner, S.; Barrandon, Y.; Longaker, M.T. Wound repair and regeneration. Nature 2008, 453, 314–321. [Google Scholar] [CrossRef]
- Walmsley, G.G.; Maan, Z.N.; Wong, V.W.; Duscher, D.; Hu, M.S.; Zielins, E.R.; Wearda, T.; Muhonen, E.; McArdle, A.; Tevlin, R.M.B.; et al. Scarless wound healing: Chasing the holy grail. Plast. Reconstr. Surg. 2015, 135, 907–917. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, H.P.; Longaker, M.; Perkocha, L.; Jennings, R.; Harrison, M.; Adzick, N. Scarless wound repair: A human fetal skin model. Development 1992, 114, 253–259. [Google Scholar] [CrossRef]
- Ud-Din, S.; Volk, S.W.; Bayat, A. Regenerative healing, scar-free healing and scar formation across the species: Current concepts and future perspectives. Exp. Dermatol. 2014, 23, 615–619. [Google Scholar] [CrossRef]
- Armstrong, J.R.; Ferguson, M.W.J. Ontogeny of the skin and the transition from scar-free to scarring phenotype during wound healing in the pouch young of a marsupial, Monodelphis domestica. Dev. Biol. 1995, 169, 242–260. [Google Scholar] [CrossRef] [PubMed]
- Ihara, S.; Motobayashi, Y.; Nagao, E.; Kistler, A. Ontogenetic transition of wound healing pattern in rat skin occurring at the fetal stage. Development 1990, 110, 671–680. [Google Scholar] [CrossRef] [PubMed]
- Rinkevich, Y.; Walmsley, G.G.; Hu, M.S.; Maan, Z.N.; Newman, A.M.; Drukker, M.; Januszyk, M.; Krampitz, G.W.; Gurtner, G.C.; Lorenz, H.P.; et al. Identification and isolation of a dermal lineage with intrinsic fibrogenic potential. Science 2015, 348, aaa2151. [Google Scholar] [CrossRef]
- Driskell, R.R.; Lichtenberger, B.M.; Hoste, E.; Kretzschmar, K.; Simons, B.D.; Charalambous, M.; Ferron, S.R.; Herault, Y.; Pavlovic, G.; Ferguson-Smith, A.C.; et al. Distinct fibroblast lineages determine dermal architecture in skin development and repair. Nature 2013, 504, 277–281. [Google Scholar] [CrossRef]
- Konieczny, P.; Naik, S. Healing without scarring. Science 2021, 372, 346–347. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Correa-Gallegos, D.; Christ, S.; Stefanska, A.; Liu, J.; Ramesh, P.; Rajendran, V.; De Santis, M.M.; Wagner, D.E.; Rinkevich, Y. Two succeeding fibroblastic lineages drive dermal development and the transition from regeneration to scarring. Nat. Cell Biol. 2018, 20, 422–431. [Google Scholar] [CrossRef] [PubMed]
- Mascharak, S.; Desjardins-Park, H.E.; Davitt, M.F.; Griffin, M.; Borrelli, M.R.; Moore, A.L.; Chen, K.; Duoto, B.; Chinta, M.; Foster, D.S.; et al. Preventing Engrailed-1 activation in fibroblasts yields wound regeneration without scarring. Science 2021, 372, eaba2374. [Google Scholar] [CrossRef] [PubMed]
- Provenzano, P.P.; Keely, P.J. Mechanical signaling through the cytoskeleton regulates cell proliferation by coordinated focal adhesion and Rho GTPase signaling. J. Cell Sci. 2011, 124, 1195–1205. [Google Scholar] [CrossRef]
- Zwanenburg, P.R.; Timmermans, F.W.; Timmer, A.S.; Middelkoop, E.; Tol, B.T.; Lapid, O.; Obdeijn, M.C.; Gans, S.L.; Boermeester, M.A. A systematic review evaluating the influence of incisional Negative Pressure Wound Therapy on scarring. Wound Repair Regen. 2020, 29, 8–19. [Google Scholar] [CrossRef]
- Labib, A.; Salfity, L.; Powell, B. Acne Keloidalis Nuchae: A Staged Reconstruction. Cureus 2021, 13, e18173. [Google Scholar] [CrossRef] [PubMed]
- Fraccalvieri, M.; Sarno, A.; Gasperini, S.; Zingarelli, E.; Fava, R.; Salomone, M.; Bruschi, S. Can single use negative pressure wound therapy be an alternative method to manage keloid scarring? A preliminary report of a clinical and ultrasound/colour-power-doppler study. Int. Wound J. 2012, 10, 340–344. [Google Scholar] [CrossRef] [PubMed]
- Tanaydin, V.; Beugels, J.; Andriessen, A.; Sawor, J.H.; van der Hulst, R.R.W.J. Randomized Controlled Study Comparing Disposable Negative-Pressure Wound Therapy with Standard Care in Bilateral Breast Reduction Mammoplasty Evaluating Surgical Site Complications and Scar Quality. Aesthetic Plast. Surg. 2018, 42, 927–935. [Google Scholar] [CrossRef] [PubMed]
- Rashed, A.; Csiszar, M.; Beledi, A.; Gombocz, K. The impact of incisional negative pressure wound therapy on the wound healing process after midline sternotomy. Int. Wound J. 2020, 18, 95–102. [Google Scholar] [CrossRef]
- Nagata, T.; Miura, K.; Homma, Y.; Fukamizu, H. Comparison between negative-pressure fixation and film dressing in wound management after tissue expansion: A randomized controlled trial. Plastic Reconstr. Surg. 2018, 142, 37–41. [Google Scholar] [CrossRef]
- Alas, H.; Fernando, H.; Baker, J.F.; Brown, A.E.; Bortz, C.; Naessig, S.; Pierce, K.E.; Ahmad, W.; Diebo, B.G.; Passias, P.G. Comparative outcomes of operative relative to medical management of spondylodiscitis accounting for frailty status at presentation. J. Clin. Neurosci. 2020, 75, 134–138. [Google Scholar] [CrossRef] [PubMed]
- Boriani, F.; Margara, A.; Granchi, D.; Baldini, N. Negative pressure treatment for improvement of surgical wounds after circumferential thigh lift. Ann. Ital. Chir. 2018, 89, 261–265. [Google Scholar]
- Abatangelo, S.; Saporiti, E.; Giatsidis, G. Closed incision negative-pressure therapy (ciNPT) reduces minor local complications in post-bariatric abdominoplasty body contouring: A retrospective case-control series. Obes. Surg. 2018, 28, 2096–2104. [Google Scholar] [CrossRef] [PubMed]
- Falkner, F.; Thomas, B.; Mayer, S.; Haug, V.; Harhaus, L.; Nagel, S.; Kneser, U.; Bigdeli, A.K. The impact of closed incisional negative pressure therapy on anterior lateral thigh flap donor site healing and scarring: A retrospective case-control study. J. Plast. Reconstr. Aesthetic Surg. 2022, 75, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Galiano, R.D.; Hudson, D.; Shin, J.; van der Hulst, R.; Tanaydin, V.; Djohan, R.; Duteille, F.; Cockwill, J.; Megginson, S.; Huddleston, E. Incisional negative pressure wound therapy for prevention of wound healing complications following reduction mammaplasty. Plast. Reconstr. Surg. Glob. Open 2018, 6, e1560. [Google Scholar] [CrossRef]
- Shah, A.; Sumpio, B.J.; Tsay, C.; Swallow, M.; Dash, B.; Thorn, S.L.; Sinusas, A.J.; Koo, A.; Hsia, H.C.; Au, A. Incisional Negative Pressure Wound Therapy Augments Perfusion and Improves Wound Healing in a Swine Model Pilot Study. Ann. Plast. Surg. Ann. Plast. Surg. 2019, 82, S222–S227. [Google Scholar] [CrossRef]
- Kilpadi, D.V.; Lessing, C.; Derrick, K. Healed porcine incisions previously treated with a surgical incision management system: Mechanical, histomorphometric, and gene expression properties. Aesthetic Plast. Surg. 2014, 38, 767–778. [Google Scholar] [CrossRef]
- Glaser, D.; Farnsworth, C.L.; Varley, E.S.; Nunn, T.A.; Sayad-Shah, M.; Breisch, E.A.; Yaszay, B. Negative pressure therapy for closed spine incisions: A pilot study. Wounds 2012, 24, 308–316. [Google Scholar]
- Suh, H.; Lee, A.Y.; Park, E.J.; Hong, J.P. Negative pressure wound therapy on closed surgical wounds with dead space animal study using a swine model. Ann. Plast. Surg. 2016, 76, 717–722. [Google Scholar] [CrossRef]
- Paul, S.P. Biodynamic excisional skin tension lines for surgical excisions: Untangling the science. Ann. R. Coll. Surg. Engl. 2018, 100, 330–337. [Google Scholar] [CrossRef]
- Paul, S.P.; Matulich, J.; Charlton, N. A New Skin Tensiometer Device: Computational Analyses to Understand Biodynamic Excisional Skin Tension Lines. Sci. Rep. 2016, 6, 30117. [Google Scholar] [CrossRef]
- Han, G.; Ceilley, R. Chronic Wound Healing: A Review of Current Management and Treatments. Adv. Ther. 2017, 34, 599–610. [Google Scholar] [CrossRef]
- Sorg, H.; Tilkorn, D.J.; Hager, S.; Hauser, J.; Mirastschijski, U. Skin Wound Healing: An Update on the Current Knowledge and Concepts. Eur. Surg. Res. 2017, 58, 81–94. [Google Scholar] [CrossRef] [PubMed]
- Foot, N.C. The masson trichrome staining methods in routine laboratory use. Biotech. Histochem. 1933, 8, 101–110. [Google Scholar] [CrossRef]
- Cheng, K.Y.; Lin, Z.-H.; Cheng, Y.-P.; Chiu, H.-Y.; Yeh, N.-L.; Wu, T.-K.; Wu, J.-S. Wound Healing in Streptozotocin-Induced Diabetic Rats Using Atmospheric-Pressure Argon Plasma Jet. Sci. Rep. 2018, 8, 12214. [Google Scholar] [CrossRef]
- Akasaka, Y.; Ishikawa, Y.; Ono, I.; Fujita, K.; Masuda, T.; Asuwa, N.; Inuzuka, K.; Kiguchi, H.; Ishii, T. Enhanced expression of caspase-3 in hypertrophic scars and keloid: Induction of caspase-3 and apoptosis in keloid fibroblasts in vitro. Lab. Investig. 2000, 80, 345–357. [Google Scholar] [CrossRef]
- Akasaka, Y.; Fujita, K.; Ishikawa, Y.; Asuwa, N.; Inuzuka, K.; Ishihara, M.; Ito, M.; Masuda, T.; Akishima, Y.; Zhang, L.; et al. Detection of apoptosis in keloids and a comparative study on apoptosis between keloids, hypertrophic scars, normal healed flat scars, and dermatofibroma. Wound Repair Regen. 2001, 9, 501–506. [Google Scholar] [CrossRef]
- Bargagna-Mohan, P.; Paranthan, R.R.; Hamza, A.; Zhan, C.-G.; Lee, D.-M.; Kim, K.B.; Lau, D.L.; Srinivasan, C.; Nakayama, K.; Nakayama, K.I.; et al. Corneal antifibrotic switch identified in genetic and pharmacological deficiency of vimentin. J. Biol. Chem. 2012, 287, 989–1006. [Google Scholar] [CrossRef]
- Dos Santos, G.; Rogel, M.R.; Baker, M.A.; Troken, J.R.; Urich, D.; Morales-Nebreda, L.; Sennello, J.A.; Kutuzov, M.A.; Sitikov, A.; Davis, J.M.; et al. Vimentin regulates activation of the NLRP3 inflammasome. Nat. Commun. 2015, 6, 6574. [Google Scholar] [CrossRef]
- Walker, J.L.; Bleaken, B.M.; Romisher, A.R.; Alnwibit, A.A.; Menko, A.S. In wound repair vimentin mediates the transition of mesenchymal leader cells to a myofibroblast phenotype. Mol. Biol. Cell 2018, 29, 1555–1570. [Google Scholar] [CrossRef]
- El Agha, E.; Kramann, R.; Schneider, R.K.; Li, X.; Seeger, W.; Humphreys, B.D.; Bellusci, S. Mesenchymal Stem Cells in Fibrotic Disease. Cell Stem Cell 2017, 21, 166–177. [Google Scholar] [CrossRef]
- Yan, C.; Grimm, W.A.; Garner, W.L.; Qin, L.; Travis, T.; Tan, N.; Han, Y.-P. Epithelial to mesenchymal transition in human skin wound healing is induced by tumor necrosis factor-α through bone morphogenic protein-2. Am. J. Pathol. 2010, 176, 2247–2258. [Google Scholar] [CrossRef]
- Ohba, S.; Wang, Z.L.; Baba, T.T.; Nemoto, T.K.; Inokuchi, T. Antisense oligonucleotide against 47-kDa heat shock protein (Hsp47) inhibits wound-induced enhancement of collagen production. Arch. Oral Biol. 2003, 48, 627–633. [Google Scholar] [CrossRef]
- Khan, E.S.; Sankaran, S.; Paez, J.I.; Muth, C.; Han, M.K.L.; Del Campo, A. Photoactivatable Hsp47: A Tool to Regulate Collagen Secretion and Assembly. Adv. Sci. 2019, 6, 1801982. [Google Scholar] [CrossRef] [PubMed]
- Khan, E.S.; Sankaran, S.; Llontop, L.; Del Campo, A. Exogenous supply of Hsp47 triggers fibrillar collagen deposition in skin cell cultures in vitro. BMC Mol. Cell Biol. 2020, 21, 22. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.L.; Inokuchi, T.; Ikeda, H.; Baba, T.; Uehara, M.; Kamasaki, N.; Sano, K.; Nemoto, T.K.; Taguchi, T. Collagen-binding heat shock protein HSP47 expression during healing of fetal skin wounds. Int. J. Oral Maxillofac. Surg. 2002, 31, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Razzaque, M.S. The possible role of colligin/HSP47, a collagen-binding protein, in the pathogenesis of human and experimental fibrotic diseases. Histol. Histopathol. 1999, 14, 1199–1212. [Google Scholar]
- Yosefzon, Y.; Soteriou, D.; Feldman, A.; Kostic, L.; Koren, E.; Brown, S.; Ankawa, R.; Sedov, E.; Glaser, F.; Fuchs, Y. Caspase-3 Regulates YAP-Dependent Cell Proliferation and Organ Size. Mol. Cell 2018, 70, 573–587.e4. [Google Scholar] [CrossRef]
- Clark, R.A.F. To Scar or Not to Scar. N. Engl. J. Med. 2021, 385, 469–471. [Google Scholar] [CrossRef]
- Chen, L.; Mirza, R.; Kwon, Y.; DiPietro, L.A.; Koh, T.J. The murine excisional wound model: Contraction revisited. Wound Repair Regen. 2015, 23, 874–877. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, M.; Matar, D.Y.; Yu, Z.; Chen, Z.; Knoedler, S.; Ng, B.; Darwish, O.A.; Sohrabi, S.; Friedman, L.; Haug, V.; et al. Continuous NPWT Regulates Fibrosis in Murine Diabetic Wound Healing. Pharmaceutics 2022, 14, 2125. https://doi.org/10.3390/pharmaceutics14102125
Wu M, Matar DY, Yu Z, Chen Z, Knoedler S, Ng B, Darwish OA, Sohrabi S, Friedman L, Haug V, et al. Continuous NPWT Regulates Fibrosis in Murine Diabetic Wound Healing. Pharmaceutics. 2022; 14(10):2125. https://doi.org/10.3390/pharmaceutics14102125
Chicago/Turabian StyleWu, Mengfan, Dany Y. Matar, Zhen Yu, Ziyu Chen, Samuel Knoedler, Brian Ng, Oliver A. Darwish, Sadaf Sohrabi, Leigh Friedman, Valentin Haug, and et al. 2022. "Continuous NPWT Regulates Fibrosis in Murine Diabetic Wound Healing" Pharmaceutics 14, no. 10: 2125. https://doi.org/10.3390/pharmaceutics14102125
APA StyleWu, M., Matar, D. Y., Yu, Z., Chen, Z., Knoedler, S., Ng, B., Darwish, O. A., Sohrabi, S., Friedman, L., Haug, V., Murphy, G. F., Rinkevich, Y., Orgill, D. P., & Panayi, A. C. (2022). Continuous NPWT Regulates Fibrosis in Murine Diabetic Wound Healing. Pharmaceutics, 14(10), 2125. https://doi.org/10.3390/pharmaceutics14102125








