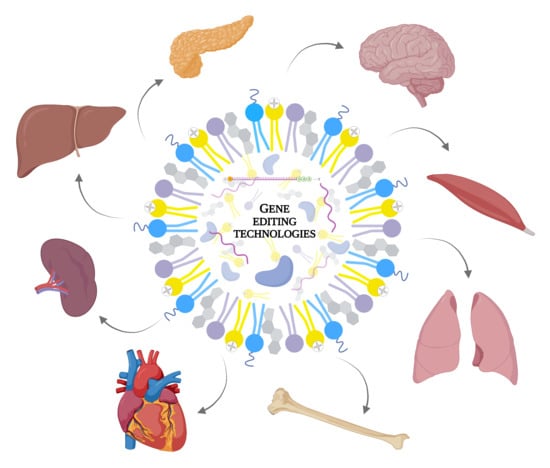
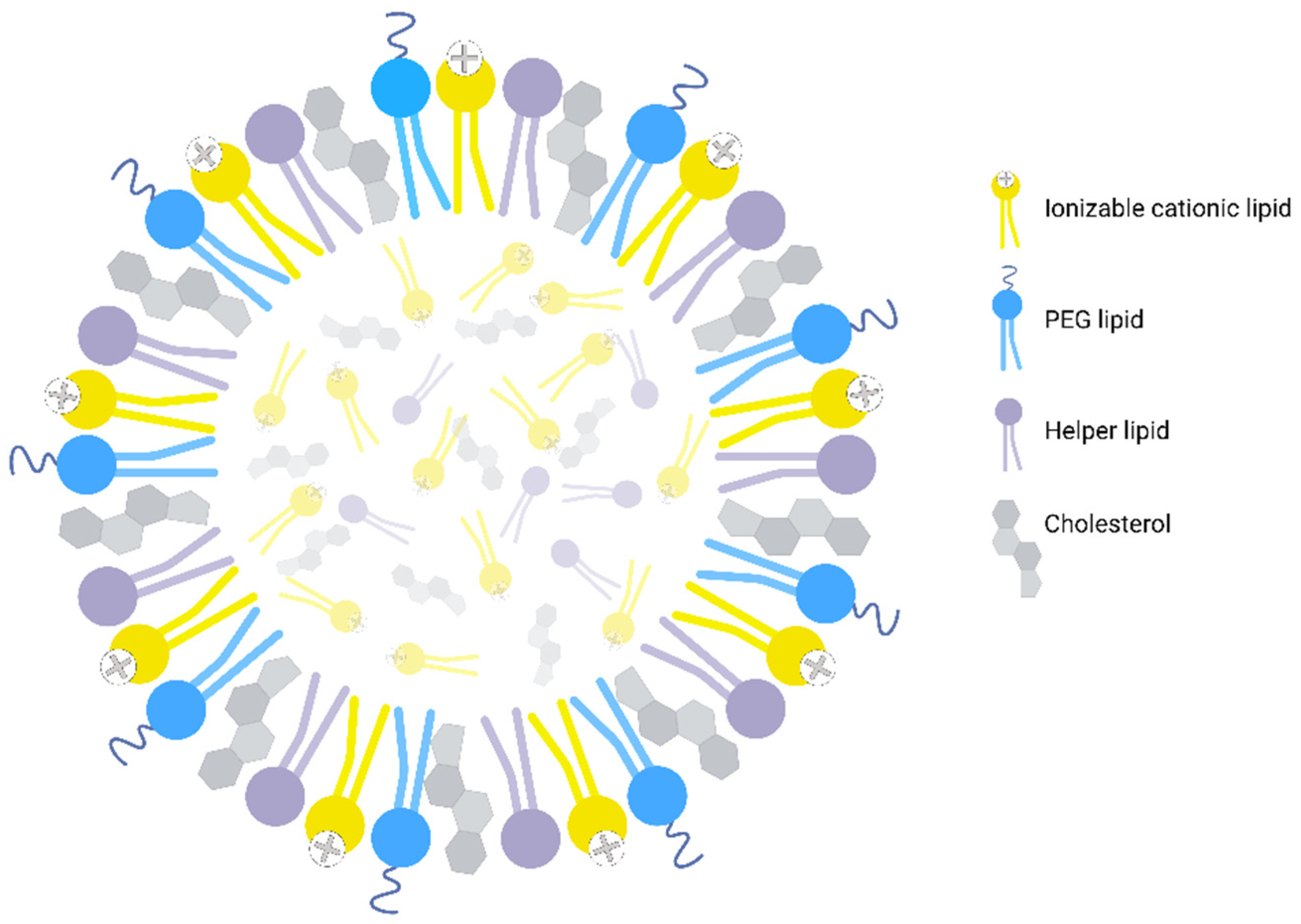
Delivery of RNAs to Specific Organs by Lipid Nanoparticles for Gene Therapy
Abstract
:1. Introduction
2. Targeting the Muscles
3. Targeting the Brain
4. Targeting the Lungs
5. Targeting the Liver
6. Targeting the Heart
7. Targeting the Spleen
8. Targeting the Bones
9. Perspectives
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Sudhakar, V.; Richardson, R.M. Gene Therapy for Neurodegenerative Diseases. Neurotherapeutics 2019, 16, 166–175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manghwar, H.; Lindsey, K.; Zhang, X.; Jin, S. CRISPR/Cas System: Recent Advances and Future Prospects for Genome Editing. Trends Plant Sci. 2019, 24, 1102–1125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karimian, A.; Azizian, K.; Parsian, H.; Rafieian, S.; Shafiei-Irannejad, V.; Kheyrollah, M.; Yousefi, M.; Majidinia, M.; Yousefi, B. CRISPR/Cas9 Technology as a Potent Molecular Tool for Gene Therapy. J. Cell. Physiol. 2019, 234, 12267–12277. [Google Scholar] [CrossRef] [PubMed]
- Torres-Ruiz, R.; Rodriguez-Perales, S. CRISPR-Cas9 Technology: Applications and Human Disease Modelling. Brief. Funct. Genom. 2017, 16, 4–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kantor, A.; McClements, M.; MacLaren, R. CRISPR-Cas9 DNA Base-Editing and Prime-Editing. Int. J. Mol. Sci. 2020, 21, 6240. [Google Scholar] [CrossRef]
- Anzalone, A.V.; Koblan, L.W.; Liu, D.R. Genome Editing with CRISPR–Cas Nucleases, Base Editors, Transposases and Prime Editors. Nat. Biotechnol. 2020, 38, 824–844. [Google Scholar] [CrossRef]
- Lei, S.; Zhang, X.; Li, J.; Gao, Y.; Wu, J.; Duan, X.; Men, K. Current Progress in Messenger RNA-Based Gene Therapy. J. Biomed. Nanotechnol. 2020, 16, 1018–1044. [Google Scholar] [CrossRef]
- Colella, P.; Ronzitti, G.; Mingozzi, F. Emerging Issues in AAV-Mediated In Vivo Gene Therapy. Mol. Ther. Methods Clin. Dev. 2018, 8, 87–104. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Samulski, R.J. Engineering Adeno-Associated Virus Vectors for Gene Therapy. Nat. Rev. Genet. 2020, 21, 255–272. [Google Scholar] [CrossRef]
- Naso, M.F.; Tomkowicz, B.; Perry, W.L.; Strohl, W.R. Adeno-Associated Virus (AAV) as a Vector for Gene Therapy. BioDrugs 2017, 31, 317–334. [Google Scholar] [CrossRef]
- Wu, P.; Zhang, B.; Ocansey, D.K.W.; Xu, W.; Qian, H. Extracellular Vesicles: A Bright Star of Nanomedicine. Biomaterials 2021, 269, 120467. [Google Scholar] [CrossRef] [PubMed]
- Villata, S.; Canta, M.; Cauda, V. EVs and Bioengineering: From Cellular Products to Engineered Nanomachines. Int. J. Mol. Sci. 2020, 21, 6048. [Google Scholar] [CrossRef] [PubMed]
- Samaridou, E.; Heyes, J.; Lutwyche, P. Lipid Nanoparticles for Nucleic Acid Delivery: Current Perspectives. Adv. Drug Deliv. Rev. 2020, 154–155, 37–63. [Google Scholar] [CrossRef] [PubMed]
- Swingle, K.L.; Hamilton, A.G.; Mitchell, M.J. Lipid Nanoparticle-Mediated Delivery of MRNA Therapeutics and Vaccines. Trends Mol. Med. 2021, 27, 616–617. [Google Scholar] [CrossRef]
- Kulkarni, J.A.; Witzigmann, D.; Chen, S.; Cullis, P.R.; van der Meel, R. Lipid Nanoparticle Technology for Clinical Translation of SiRNA Therapeutics. Acc. Chem. Res. 2019, 52, 2435–2444. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, Y.; Dong, Y. Lipid Nanoparticle–MRNA Formulations for Therapeutic Applications. Acc. Chem. Res. 2021, 54, 4283–4293. [Google Scholar] [CrossRef]
- Cullis, P.R.; Hope, M.J. Lipid Nanoparticle Systems for Enabling Gene Therapies. Mol. Ther. 2017, 25, 1467–1475. [Google Scholar] [CrossRef] [Green Version]
- Walther, J.; Wilbie, D.; Tissingh, V.S.J.; Öktem, M.; van der Veen, H.; Lou, B.; Mastrobattista, E. Impact of Formulation Conditions on Lipid Nanoparticle Characteristics and Functional Delivery of CRISPR RNP for Gene Knock-Out and Correction. Pharmaceutics 2022, 14, 213. [Google Scholar] [CrossRef]
- Arteta, M.Y.; Kjellman, T.; Bartesaghi, S.; Wallin, S.; Wu, X.; Kvist, A.J.; Dabkowska, A.; Székely, N.; Radulescu, A.; Bergenholtz, J.; et al. Successful Reprogramming of Cellular Protein Production through MRNA Delivered by Functionalized Lipid Nanoparticles. Proc. Natl. Acad. Sci. USA 2018, 115, E3351–E3360. [Google Scholar] [CrossRef] [Green Version]
- Semple, S.C.; Leone, R.; Barbosa, C.J.; Tam, Y.K.; Lin, P.J.C. Lipid Nanoparticle Delivery Systems to Enable MRNA-Based Therapeutics. Pharmaceutics 2022, 14, 398. [Google Scholar] [CrossRef]
- Suzuki, Y.; Ishihara, H. Difference in the Lipid Nanoparticle Technology Employed in Three Approved SiRNA (Patisiran) and MRNA (COVID-19 Vaccine) Drugs. Drug. Metab. Pharmacokinet. 2021, 41, 100424. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chang, J.; Jiang, Y.; Meng, X.; Sun, T.; Mao, L.; Xu, Q.; Wang, M. Fast and Efficient CRISPR/Cas9 Genome Editing In Vivo Enabled by Bioreducible Lipid and Messenger RNA Nanoparticles. Adv. Mater. 2019, 31, 1902575. [Google Scholar] [CrossRef] [PubMed]
- Mendell, J.R.; Al-Zaidy, S.A.; Rodino-Klapac, L.R.; Goodspeed, K.; Gray, S.J.; Kay, C.N.; Boye, S.L.; Boye, S.E.; George, L.A.; Salabarria, S.; et al. Current Clinical Applications of In Vivo Gene Therapy with AAVs. Mol. Ther. 2021, 29, 464–488. [Google Scholar] [CrossRef] [PubMed]
- Kazemian, P.; Yu, S.Y.; Thomson, S.B.; Birkenshaw, A.; Leavitt, B.R.; Ross, C.J.D. Lipid-Nanoparticle-Based Delivery of CRISPR/Cas9 Genome-Editing Components. Mol. Pharm. 2022, 19, 1669–1686. [Google Scholar] [CrossRef] [PubMed]
- Blömer, U.; Ganser, A.; Scherr, M. Invasive Drug Delivery. In Molecular and Cellular Biology of Neuroprotection in the CNS; Springer: Berlin/Heidelberg, Germany, 2003; pp. 431–451. [Google Scholar]
- Fenton, O.S.; Kauffman, K.J.; McClellan, R.L.; Appel, E.A.; Dorkin, J.R.; Tibbitt, M.W.; Heartlein, M.W.; DeRosa, F.; Langer, R.; Anderson, D.G. Bioinspired Alkenyl Amino Alcohol Ionizable Lipid Materials for Highly Potent In Vivo MRNA Delivery. Adv. Mater. 2016, 28, 2939–2943. [Google Scholar] [CrossRef] [Green Version]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering Precision Nanoparticles for Drug Delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef]
- Kon, E.; Elia, U.; Peer, D. Principles for Designing an Optimal MRNA Lipid Nanoparticle Vaccine. Curr. Opin. Biotechnol. 2022, 73, 329–336. [Google Scholar] [CrossRef]
- Hou, X.; Zaks, T.; Langer, R.; Dong, Y. Lipid Nanoparticles for MRNA Delivery. Nat. Rev. Mater. 2021, 6, 1078–1094. [Google Scholar] [CrossRef]
- Behr, M.; Zhou, J.; Xu, B.; Zhang, H. In Vivo Delivery of CRISPR-Cas9 Therapeutics: Progress and Challenges. Acta Pharm. Sin. B 2021, 11, 2150–2171. [Google Scholar] [CrossRef]
- Han, X.; Zhang, H.; Butowska, K.; Swingle, K.L.; Alameh, M.-G.; Weissman, D.; Mitchell, M.J. An Ionizable Lipid Toolbox for RNA Delivery. Nat. Commun. 2021, 12, 7233. [Google Scholar] [CrossRef]
- Schlich, M.; Palomba, R.; Costabile, G.; Mizrahy, S.; Pannuzzo, M.; Peer, D.; Decuzzi, P. Cytosolic Delivery of Nucleic Acids: The Case of Ionizable Lipid Nanoparticles. Bioeng. Transl. Med. 2021, 6, e10213. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, J.A.; Witzigmann, D.; Leung, J.; Tam, Y.Y.C.; Cullis, P.R. On the Role of Helper Lipids in Lipid Nanoparticle Formulations of SiRNA. Nanoscale 2019, 11, 21733–21739. [Google Scholar] [CrossRef]
- Cheng, X.; Lee, R.J. The Role of Helper Lipids in Lipid Nanoparticles (LNPs) Designed for Oligonucleotide Delivery. Adv. Drug Deliv. Rev. 2016, 99, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Suk, J.S.; Xu, Q.; Kim, N.; Hanes, J.; Ensign, L.M. PEGylation as a Strategy for Improving Nanoparticle-Based Drug and Gene Delivery. Adv. Drug Deliv. Rev. 2016, 99, 28–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nunes, S.S.; Fernandes, R.S.; Cavalcante, C.H.; da Costa César, I.; Leite, E.A.; Lopes, S.C.A.; Ferretti, A.; Rubello, D.; Townsend, D.M.; de Oliveira, M.C.; et al. Influence of PEG Coating on the Biodistribution and Tumor Accumulation of PH-Sensitive Liposomes. Drug Deliv. Transl. Res. 2019, 9, 123–130. [Google Scholar] [CrossRef]
- Menon, I.; Zaroudi, M.; Zhang, Y.; Aisenbrey, E.; Hui, L. Fabrication of Active Targeting Lipid Nanoparticles: Challenges and Perspectives. Mater. Today Adv. 2022, 16, 100299. [Google Scholar] [CrossRef]
- DMG-PEG 2000 Powder 99 (TLC) Avanti Polar Lipids. Available online: https://www.sigmaaldrich.com/CA/en/product/avanti/880151p (accessed on 18 August 2022).
- TCL053 (CAS Number: 2361162-70-9) | Cayman Chemical. Available online: https://www.caymanchem.com/product/37045/tcl053 (accessed on 18 August 2022).
- 18:1 PA Avanti CAS No.108392-02-5. Available online: https://www.sigmaaldrich.com/CA/en/product/avanti/840875p (accessed on 18 August 2022).
- DOTMA Powder Cationic Lipid Avanti Polar Lipids. Available online: https://www.sigmaaldrich.com/CA/en/product/avanti/890898p?gclid=Cj0KCQjwxveXBhDDARIsAI0Q0x2VnmS_XNjE_ySu2V9q7i4B5v7ZrIc8zycWR2rqXynN9ClTAWSTV70aAgvQEALw_wcB (accessed on 18 August 2022).
- 9A1P9 (CAS Number: 2760467-57-8) | Cayman Chemical. Available online: https://www.caymanchem.com/product/37276/helping-make-research-possible (accessed on 18 August 2022).
- YSK05 (CAS Number: 1318793-78-0) | Cayman Chemical. Available online: https://www.caymanchem.com/product/35786/helping-make-research-possible (accessed on 18 August 2022).
- D-Lin-MC3-DMA | SiRNA Delivery Vehicle | MedChemExpress. Available online: https://www.medchemexpress.com/D-Lin-MC3-DMA.html?utm_source=google&utm_medium=CPC&utm_campaign=France&utm_term=HY-112251&utm_content=D-Lin-MC3-DMA&gclid=Cj0KCQjwxveXBhDDARIsAI0Q0x3ERWC1tASBjIJxnT5HSlxAen-lr88x-wjKlbEW3gBJv9C2nmQWH-UaApzaEALw_wcB (accessed on 18 August 2022).
- NT1-O14B (CAS Number: 2739805-64-0) | Cayman Chemical. Available online: https://www.caymanchem.com/product/37095/nt1-o14b (accessed on 18 August 2022).
- PEG Lipid | BroadPharm. Available online: https://broadpharm.com/product-categories/lipid/peg-lipid?gclid=Cj0KCQjwxveXBhDDARIsAI0Q0x0ZfLzMcO0iyr0-2BxrNVFUmhiIqp2LzecM9EJW3Q-3i3ZwWZwE-BAaAgC7EALw_wcB (accessed on 18 August 2022).
- 306-O12B-3 | Cayman Chemical. Available online: https://www.caymanchem.com/product/37096/306-o12b-3 (accessed on 18 August 2022).
- C12-200 | Cationic Lipidoid | MedChemExpress. Available online: https://www.medchemexpress.com/c12-200.html (accessed on 18 August 2022).
- DLin-KC2-DMA (KC2, CAS Number: 1190197-97-7) | Cayman Chemical. Available online: https://www.caymanchem.com/product/34363/dlin-kc2-dma (accessed on 18 August 2022).
- 18:1 TAP (DOTAP) | Avanti Chloroform, Powder Chloride Salt. Available online: https://avantilipids.com/product/890890 (accessed on 18 August 2022).
- DOPE | 5/1/4004 | BroadPharm. Available online: https://broadpharm.com/product/bp-25709?gclid=Cj0KCQjwxveXBhDDARIsAI0Q0x3CILN3p4cbhqDm6cxSeM_CkiOualbwh12PwbuvipElgnjaVHd1HooaApTvEALw_wcB (accessed on 18 August 2022).
- 5A2-SC8 | MedChemExpress. Available online: https://www.medchemexpress.com/5a2-sc8.html (accessed on 18 August 2022).
- Cholesterol SigmaGrade, =99 57-88-5. Available online: https://www.sigmaaldrich.com/CA/en/product/sigma/c8667 (accessed on 18 August 2022).
- 16:0 PC (DPPC). Available online: https://avantilipids.com/product/850355 (accessed on 18 August 2022).
- Stephenson, A.A.; Flanigan, K.M. Gene Editing and Modulation for Duchenne Muscular Dystrophy. In Progress in Molecular Biology and Translational Science; Elsevier: Amsterdam, The Netherlands, 2021; Volume 182, pp. 225–255. ISBN 9780323853019. [Google Scholar]
- Fortunato, F.; Farnè, M.; Ferlini, A. The DMD Gene and Therapeutic Approaches to Restore Dystrophin. Neuromusc. Disord. 2021, 31, 1013–1020. [Google Scholar] [CrossRef]
- Gutiérrez Gutiérrez, G.; Díaz-Manera, J.; Almendrote, M.; Azriel, S.; Eulalio Bárcena, J.; Cabezudo García, P.; Camacho Salas, A.; Casanova Rodríguez, C.; Cobo, A.M.; Díaz Guardiola, P.; et al. Guía Clínica Para El Diagnóstico y Seguimiento de La Distrofia Miotónica Tipo 1, DM1 o Enfermedad de Steinert. Neurología 2020, 35, 185–206. [Google Scholar] [CrossRef]
- Betzenhauser, M.J.; Marks, A.R. Ryanodine Receptor Channelopathies. Pfl. Arch. 2010, 460, 467–480. [Google Scholar] [CrossRef] [Green Version]
- Mary, P.; Servais, L.; Vialle, R. Neuromuscular Diseases: Diagnosis and Management. Orthopaed. Traumatol. Surg. Res. 2018, 104, S89–S95. [Google Scholar] [CrossRef]
- Shieh, P.B. Muscular Dystrophies and Other Genetic Myopathies. Neurol. Clin. 2013, 31, 1009–1029. [Google Scholar] [CrossRef]
- Ravenscroft, G.; Bryson-Richardson, R.J.; Nowak, K.J.; Laing, N.G. Recent Advances in Understanding Congenital Myopathies. F1000Research 2018, 7, 1921. [Google Scholar] [CrossRef] [PubMed]
- Beaufils, M.; Travard, L.; Rendu, J.; Marty, I. Therapies for RYR1-Related Myopathies: Where We Stand and the Perspectives. Curr. Pharm. Des. 2022, 28, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Kenjo, E.; Hozumi, H.; Makita, Y.; Iwabuchi, K.A.; Fujimoto, N.; Matsumoto, S.; Kimura, M.; Amano, Y.; Ifuku, M.; Naoe, Y.; et al. Low Immunogenicity of LNP Allows Repeated Administrations of CRISPR-Cas9 MRNA into Skeletal Muscle in Mice. Nat. Commun. 2021, 12, 7101. [Google Scholar] [CrossRef] [PubMed]
- Blakney, A.K.; McKay, P.F.; Yus, B.I.; Aldon, Y.; Shattock, R.J. Inside out: Optimization of Lipid Nanoparticle Formulations for Exterior Complexation and in Vivo Delivery of SaRNA. Gene Ther. 2019, 26, 363–372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carrasco, M.J.; Alishetty, S.; Alameh, M.G.; Said, H.; Wright, L.; Paige, M.; Soliman, O.; Weissman, D.; Cleveland, T.E.; Grishaev, A.; et al. Ionization and Structural Properties of MRNA Lipid Nanoparticles Influence Expression in Intramuscular and Intravascular Administration. Commun. Biol. 2021, 4, 956. [Google Scholar] [CrossRef]
- Wei, T.; Cheng, Q.; Min, Y.L.; Olson, E.N.; Siegwart, D.J. Systemic Nanoparticle Delivery of CRISPR-Cas9 Ribonucleoproteins for Effective Tissue Specific Genome Editing. Nat. Commun. 2020, 11, 3232. [Google Scholar] [CrossRef]
- Guimaraes, P.P.G.; Zhang, R.; Spektor, R.; Tan, M.; Chung, A.; Billingsley, M.M.; El-Mayta, R.; Riley, R.S.; Wang, L.; Wilson, J.M.; et al. Ionizable Lipid Nanoparticles Encapsulating Barcoded MRNA for Accelerated in Vivo Delivery Screening. J. Control. Release 2019, 316, 404–417. [Google Scholar] [CrossRef] [PubMed]
- Dahlman, J.E.; Kauffman, K.J.; Xing, Y.; Shaw, T.E.; Mir, F.F.; Dlott, C.C.; Langer, R.; Anderson, D.G.; Wang, E.T. Barcoded Nanoparticles for High Throughput in Vivo Discovery of Targeted Therapeutics. Proc. Natl. Acad. Sci. USA 2017, 114, 2060–2065. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferlini, A.; Sabatelli, P.; Fabris, M.; Bassi, E.; Falzarano, S.; Vattemi, G.; Perrone, D.; Gualandi, F.; Maraldi, N.M.; Merlini, L.; et al. Dystrophin Restoration in Skeletal, Heart and Skin Arrector Pili Smooth Muscle of Mdx Mice by ZM2 NP-AON Complexes. Gene Ther. 2010, 17, 432–438. [Google Scholar] [CrossRef]
- Van Haute, D.; Berlin, J.M. Challenges in Realizing Selectivity for Nanoparticle Biodistribution and Clearance: Lessons from Gold Nanoparticles. Ther. Deliv. 2017, 8, 763–774. [Google Scholar] [CrossRef]
- Horodecka, K.; Düchler, M. CRISPR/Cas9: Principle, Applications, and Delivery through Extracellular Vesicles. Int. J. Mol. Sci. 2021, 22, 6072. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zheng, W.; Liu, S.; Li, B.; Jiang, X. Delivery of CRISPR/Cas9 by Novel Strategies for Gene Therapy. ChemBioChem 2018, 20, 634–643. [Google Scholar] [CrossRef]
- Xu, C.-F.; Chen, G.-J.; Luo, Y.-L.; Zhang, Y.; Zhao, G.; Lu, Z.-D.; Czarna, A.; Gu, Z.; Wang, J. Rational Designs of in Vivo CRISPR-Cas Delivery Systems. Adv. Drug Deliv. Rev. 2021, 168, 3–29. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Hu, S.; Chen, X. Non-Viral Delivery Systems for CRISPR/Cas9-Based Genome Editing: Challenges and Opportunities. Biomaterials 2018, 171, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Mout, R.; Ray, M.; Lee, Y.-W.; Scaletti, F.; Rotello, V.M. In Vivo Delivery of CRISPR/Cas9 for Therapeutic Gene Editing: Progress and Challenges. Bioconjug. Chem. 2017, 28, 880–884. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lattanzi, A.; Meneghini, V.; Pavani, G.; Amor, F.; Ramadier, S.; Felix, T.; Antoniani, C.; Masson, C.; Alibeu, O.; Lee, C.; et al. Optimization of CRISPR/Cas9 Delivery to Human Hematopoietic Stem and Progenitor Cells for Therapeutic Genomic Rearrangements. Mol. Ther. 2019, 27, 137–150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, X.; Potter, J.; Kumar, S.; Zou, Y.; Quintanilla, R.; Sridharan, M.; Carte, J.; Chen, W.; Roark, N.; Ranganathan, S.; et al. Rapid and Highly Efficient Mammalian Cell Engineering via Cas9 Protein Transfection. J. Biotechnol. 2015, 208, 44–53. [Google Scholar] [CrossRef] [Green Version]
- Ramakrishna, S.; Kwaku Dad, A.-B.; Beloor, J.; Gopalappa, R.; Lee, S.-K.; Kim, H. Gene Disruption by Cell-Penetrating Peptide-Mediated Delivery of Cas9 Protein and Guide RNA. Genome Res. 2014, 24, 1020–1027. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Zhang, L.; Liu, H.; Cheng, K. Delivery Strategies of the CRISPR-Cas9 Gene-Editing System for Therapeutic Applications. J. Control. Release 2017, 266, 17–26. [Google Scholar] [CrossRef]
- Gerring, Z.F.; Gamazon, E.R.; White, A.; Derks, E.M. Integrative Network-Based Analysis Reveals Gene Networks and Novel Drug Repositioning Candidates for Alzheimer Disease. Neurol. Genet. 2021, 7, e622. [Google Scholar] [CrossRef] [PubMed]
- Thorley, E.M.; Iyer, R.G.; Wicks, P.; Curran, C.; Gandhi, S.K.; Abler, V.; Anderson, K.E.; Carlozzi, N.E. Understanding How Chorea Affects Health-Related Quality of Life in Huntington Disease: An Online Survey of Patients and Caregivers in the United States. Pat. Pat. Cent. Outcomes Res. 2018, 11, 547–559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turner, T.J.; Zourray, C.; Schorge, S.; Lignani, G. Recent Advances in Gene Therapy for Neurodevelopmental Disorders with Epilepsy. J. Neurochem. 2021, 157, 229–262. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Liu, Y.; Ding, G.; Qu, D.; Qu, H. Online Database for Brain Cancer-Implicated Genes: Exploring the Subtype-Specific Mechanisms of Brain Cancer. BMC Genom. 2021, 22, 458. [Google Scholar] [CrossRef]
- Li, J.; Zheng, M.; Shimoni, O.; Banks, W.A.; Bush, A.I.; Gamble, J.R.; Shi, B. Development of Novel Therapeutics Targeting the Blood–Brain Barrier: From Barrier to Carrier. Adv. Sci. 2021, 8, 2101090. [Google Scholar] [CrossRef] [PubMed]
- Dunton, A.D.; Göpel, T.; Ho, D.H.; Burggren, W. Form and Function of the Vertebrate and Invertebrate Blood-Brain Barriers. Int. J. Mol. Sci. 2021, 22, 12111. [Google Scholar] [CrossRef]
- Kadry, H.; Noorani, B.; Cucullo, L. A Blood–Brain Barrier Overview on Structure, Function, Impairment, and Biomarkers of Integrity. Fluids Barriers CNS 2020, 17, 69. [Google Scholar] [CrossRef]
- Gernert, M.; Feja, M. Bypassing the Blood–Brain Barrier: Direct Intracranial Drug Delivery in Epilepsies. Pharmaceutics 2020, 12, 1134. [Google Scholar] [CrossRef]
- Ma, F.; Yang, L.; Sun, Z.; Chen, J.; Rui, X.; Glass, Z.; Xu, Q. Neurotransmitter-Derived Lipidoids (NT-Lipidoids) for Enhanced Brain Delivery through Intravenous Injection. Sci. Adv. 2022, 6, eabb4429. [Google Scholar] [CrossRef] [PubMed]
- Nabhan, J.F.; Wood, K.M.; Rao, V.P.; Morin, J.; Bhamidipaty, S.; Labranche, T.P.; Gooch, R.L.; Bozal, F.; Bulawa, C.E.; Guild, B.C. Intrathecal Delivery of Frataxin MRNA Encapsulated in Lipid Nanoparticles to Dorsal Root Ganglia as a Potential Therapeutic for Friedreich’s Ataxia. Sci. Rep. 2016, 6, 20019. [Google Scholar] [CrossRef]
- Tamaru, M.; Akita, H.; Nakatani, T.; Kajimoto, K.; Sato, Y.; Hatakeyama, H.; Harashima, H. Application of Apolipoprotein E-Modified Liposomal Nanoparticles as a Carrier for Delivering DNA and Nucleic Acid in the Brain. Int. J. Nanomed. 2014, 9, 4267–4276. [Google Scholar] [CrossRef] [Green Version]
- Bell, S.C.; Mall, M.A.; Gutierrez, H.; Macek, M.; Madge, S.; Davies, J.C.; Burgel, P.-R.; Tullis, E.; Castaños, C.; Castellani, C.; et al. The Future of Cystic Fibrosis Care: A Global Perspective. Lancet Respir. Med. 2020, 8, 65–124. [Google Scholar] [CrossRef] [Green Version]
- Cooney, A.; McCray, P.; Sinn, P. Cystic Fibrosis Gene Therapy: Looking Back, Looking Forward. Genes 2018, 9, 538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lebrett, M.B.; Crosbie, E.J.; Smith, M.J.; Woodward, E.R.; Evans, D.G.; Crosbie, P.A.J. Targeting Lung Cancer Screening to Individuals at Greatest Risk: The Role of Genetic Factors. J. Med. Genet. 2021, 58, 217–226. [Google Scholar] [CrossRef]
- Cheng, Q.; Wei, T.; Farbiak, L.; Johnson, L.T.; Dilliard, S.A.; Siegwart, D.J. Selective Organ Targeting (SORT) Nanoparticles for Tissue-Specific MRNA Delivery and CRISPR–Cas Gene Editing. Nat. Nanotechnol. 2020, 15, 313–320. [Google Scholar] [CrossRef]
- Robinson, E.; MacDonald, K.D.; Slaughter, K.; McKinney, M.; Patel, S.; Sun, C.; Sahay, G. Lipid Nanoparticle-Delivered Chemically Modified MRNA Restores Chloride Secretion in Cystic Fibrosis. Mol. Ther. 2018, 26, 2034–2046. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Zhao, W.; Nguyen, G.N.; Zhang, C.; Zeng, C.; Yan, J.; Du, S.; Hou, X.; Li, W.; Jiang, J.; et al. Functionalized Lipid-like Nanoparticles for in Vivo MRNA Delivery and Base Editing. Sci. Adv. 2020, 6, 34. [Google Scholar] [CrossRef]
- Paunovska, K.; Gil, C.J.; Lokugamage, M.P.; Sago, C.D.; Sato, M.; Lando, G.N.; Gamboa Castro, M.; Bryksin, A.V.; Dahlman, J.E. Analyzing 2000 in Vivo Drug Delivery Data Points Reveals Cholesterol Structure Impacts Nanoparticle Delivery. ACS Nano 2018, 12, 8341–8349. [Google Scholar] [CrossRef]
- Paunovska, K.; Sago, C.D.; Monaco, C.M.; Hudson, W.H.; Castro, M.G.; Rudoltz, T.G.; Kalathoor, S.; Vanover, D.A.; Santangelo, P.J.; Ahmed, R.; et al. A Direct Comparison of in Vitro and in Vivo Nucleic Acid Delivery Mediated by Hundreds of Nanoparticles Reveals a Weak Correlation. Nano Lett. 2018, 18, 2148–2157. [Google Scholar] [CrossRef]
- Sago, C.D.; Lokugamage, M.P.; Paunovska, K.; Vanover, D.A.; Monaco, C.M.; Shah, N.N.; Castro, M.G.; Anderson, S.E.; Rudoltz, T.G.; Lando, G.N.; et al. High-Throughput in Vivo Screen of Functional MRNA Delivery Identifies Nanoparticles for Endothelial Cell Gene Editing. Proc. Natl. Acad. Sci. USA 2018, 115, E9944–E9952. [Google Scholar] [CrossRef]
- Qiu, M.; Tang, Y.; Chen, J.; Muriph, R.; Ye, Z.; Huang, C.; Evans, J.; Henske, E.P.; Xu, Q. Lung-Selective MRNA Delivery of Synthetic Lipid Nanoparticles for the Treatment of Pulmonary Lymphangioleiomyomatosis. Proc. Natl. Acad. Sci. USA 2022, 119, e2116271119. [Google Scholar] [CrossRef]
- Liu, S.; Cheng, Q.; Wei, T.; Yu, X.; Johnson, L.T.; Farbiak, L.; Siegwart, D.J. Membrane-Destabilizing Ionizable Phospholipids for Organ-Selective MRNA Delivery and CRISPR–Cas Gene Editing. Nat. Mater. 2021, 20, 701–710. [Google Scholar] [CrossRef] [PubMed]
- Hagino, Y.; Khalil, I.A.; Kimura, S.; Kusumoto, K.; Harashima, H. GALA-Modified Lipid Nanoparticles for the Targeted Delivery of Plasmid DNA to the Lungs. Mol. Pharm. 2021, 18, 878–888. [Google Scholar] [CrossRef] [PubMed]
- Kusumoto, K.; Akita, H.; Ishitsuka, T.; Matsumoto, Y.; Nomoto, T.; Furukawa, R.; El-Sayed, A.; Hatakeyama, H.; Kajimoto, K.; Yamada, Y.; et al. Lipid Envelope-Type Nanoparticle Incorporating a Multifunctional Peptide for Systemic SiRNA Delivery to the Pulmonary Endothelium. ACS Nano 2013, 7, 7534–7541. [Google Scholar] [CrossRef] [PubMed]
- van Ginkel, W.G.; Rodenburg, I.L.; Harding, C.O.; Hollak, C.E.M.; Heiner-Fokkema, M.R.; van Spronsen, F.J. Long-Term Outcomes and Practical Considerations in the Pharmacological Management of Tyrosinemia Type 1. Pediatr. Drugs 2019, 21, 413–426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chinsky, J.M.; Singh, R.; Ficicioglu, C.; van Karnebeek, C.D.M.; Grompe, M.; Mitchell, G.; Waisbren, S.E.; Gucsavas-Calikoglu, M.; Wasserstein, M.P.; Coakley, K.; et al. Diagnosis and Treatment of Tyrosinemia Type I: A US and Canadian Consensus Group Review and Recommendations. Genet. Med. 2017, 19, 1380–1395. [Google Scholar] [CrossRef] [Green Version]
- Czaja, A.J. Diagnosis and Management of Autoimmune Hepatitis: Current Status and Future Directions. Gut Liver 2016, 10, 177. [Google Scholar] [CrossRef] [Green Version]
- Akinc, A.; Maier, M.A.; Manoharan, M.; Fitzgerald, K.; Jayaraman, M.; Barros, S.; Ansell, S.; Du, X.; Hope, M.J.; Madden, T.D.; et al. The Onpattro Story and the Clinical Translation of Nanomedicines Containing Nucleic Acid-Based Drugs. Nat. Nanotechnol. 2019, 14, 1084–1087. [Google Scholar] [CrossRef]
- Akinc, A.; Querbes, W.; De, S.; Qin, J.; Frank-Kamenetsky, M.; Jayaprakash, K.N.; Jayaraman, M.; Rajeev, K.G.; Cantley, W.L.; Dorkin, J.R.; et al. Targeted Delivery of RNAi Therapeutics with Endogenous and Exogenous Ligand-Based Mechanisms. Mol. Ther. 2010, 18, 1357–1364. [Google Scholar] [CrossRef]
- Quiviger, M.; Giannakopoulos, A.; Verhenne, S.; Marie, C.; Stavrou, E.F.; Vanhoorelbeke, K.; Izsvák, Z.; de Meyer, S.F.; Athanassiadou, A.; Scherman, D. Improved Molecular Platform for the Gene Therapy of Rare Diseases by Liver Protein Secretion. Eur. J. Med. Genet. 2018, 61, 723–728. [Google Scholar] [CrossRef]
- Yonezawa, S.; Koide, H.; Asai, T. Recent Advances in SiRNA Delivery Mediated by Lipid-Based Nanoparticles. Adv. Drug Deliv. Rev. 2020, 154–155, 64–78. [Google Scholar] [CrossRef]
- Cui, L.; Hunter, M.R.; Sonzini, S.; Pereira, S.; Romanelli, S.M.; Liu, K.; Li, W.; Liang, L.; Yang, B.; Mahmoudi, N.; et al. Mechanistic Studies of an Automated Lipid Nanoparticle Reveal Critical Pharmaceutical Properties Associated with Enhanced MRNA Functional Delivery In Vitro and In Vivo. Small 2022, 18, 2105832. [Google Scholar] [CrossRef]
- Cui, L.; Pereira, S.; Sonzini, S.; van Pelt, S.; Romanelli, S.M.; Liang, L.; Ulkoski, D.; Krishnamurthy, V.R.; Brannigan, E.; Brankin, C.; et al. Development of a High-Throughput Platform for Screening Lipid Nanoparticles for MRNA Delivery. Nanoscale 2022, 14, 1480–1491. [Google Scholar] [CrossRef]
- Rothgangl, T.; Dennis, M.K.; Lin, P.J.C.; Oka, R.; Witzigmann, D.; Villiger, L.; Qi, W.; Hruzova, M.; Kissling, L.; Lenggenhager, D.; et al. In Vivo Adenine Base Editing of PCSK9 in Macaques Reduces LDL Cholesterol Levels. Nat. Biotechnol. 2021, 39, 949–957. [Google Scholar] [CrossRef] [PubMed]
- Prakash, T.P.; Lima, W.F.; Murray, H.M.; Elbashir, S.; Cantley, W.; Foster, D.; Jayaraman, M.; Chappell, A.E.; Manoharan, M.; Swayze, E.E.; et al. Lipid Nanoparticles Improve Activity of Single-Stranded SiRNA and Gapmer Antisense Oligonucleotides in Animals. ACS Chem. Biol. 2013, 8, 1402–1406. [Google Scholar] [CrossRef] [PubMed]
- Scalzo, S.; Santos, A.K.; Ferreira, H.A.; Costa, P.A.; Prazeres, P.H.; da Silva, N.J.; Guimarães, L.C.; de Silva, M.M.; Rodrigues Alves, M.T.; Viana, C.T.; et al. Ionizable Lipid Nanoparticle-Mediated Delivery of Plasmid DNA in Cardiomyocytes. Int. J. Nanomed. 2022, 17, 2865–2881. [Google Scholar] [CrossRef] [PubMed]
- Maugeri, M.; Nawaz, M.; Papadimitriou, A.; Angerfors, A.; Camponeschi, A.; Na, M.; Hölttä, M.; Skantze, P.; Johansson, S.; Sundqvist, M.; et al. Linkage between Endosomal Escape of LNP-MRNA and Loading into EVs for Transport to Other Cells. Nat. Commun. 2019, 10, 4333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rossi, V.; Lee, B.; Marom, R. Osteogenesis Imperfecta: Advancements in Genetics and Treatment. Curr. Opin. Pediatr. 2019, 31, 708–715. [Google Scholar] [CrossRef] [PubMed]
- Appelman-Dijkstra, N.M.; Papapoulos, S.E. Paget’s Disease of Bone. Best Pract. Res. Clin. Endocrinol. Metab. 2018, 32, 657–668. [Google Scholar] [CrossRef]
- Chapurlat, R.D.; Meunier, P.J. Fibrous Dysplasia of Bone. Best Pract. Res. Clin. Rheumatol. 2000, 14, 385–398. [Google Scholar] [CrossRef]
- Srivastava, M.; Deal, C. Osteoporosis in Elderly: Prevention and Treatment. Clin. Geriatr. Med. 2002, 18, 529–555. [Google Scholar] [CrossRef]
- Chindamo, G.; Sapino, S.; Peira, E.; Chirio, D.; Gonzalez, M.C.; Gallarate, M. Bone Diseases: Current Approach and Future Perspectives in Drug Delivery Systems for Bone Targeted Therapeutics. Nanomaterials 2020, 10, 875. [Google Scholar] [CrossRef]
- Basha, G.; Ordobadi, M.; Scott, W.R.; Cottle, A.; Liu, Y.; Wang, H.; Cullis, P.R. Lipid Nanoparticle Delivery of SiRNA to Osteocytes Leads to Effective Silencing of SOST and Inhibition of Sclerostin in Vivo. Mol. Ther. Nucleic Acids 2016, 5, e363. [Google Scholar] [CrossRef] [Green Version]
- Kularatne, R.N.; Crist, R.M.; Stern, S.T. The Future of Tissue-Targeted Lipid Nanoparticle-Mediated Nucleic Acid Delivery. Pharmaceuticals 2022, 15, 897. [Google Scholar] [CrossRef] [PubMed]

| Reference | Delivered Cargo | Targeted Tissue/Gene | Route | No. of Doses | Dose | LNP Formulation | Lipids Molar Ratios | Lipid: RNA (or DNA) Ratio (w:w) | Results |
|---|---|---|---|---|---|---|---|---|---|
| CRISPR-Cas9 mRNA/sgRNA | IV | 1 | 1.0–10.0 mg/kg total RNA | 23:1 | Restoration of dystrophin | ||||
| Kenjo 2021 [63] | CRISPR-Cas9 mRNA/sgRNA | Muscles /DMD | IM | 1 | 10 μg Cas9 mRNA (with 10 μg sgRNA) | TCL053 | 60 | ∼10% exon skipping and ∼1.1% dystrophin recovery | |
| 2 | DPPC | 10.6 | ∼13% exon skipping and ∼2.6% dystrophin recovery | ||||||
| 3 | Cholesterol | 27.3 | ∼15% exon skipping and ∼4.0% dystrophin recovery | ||||||
| 6 | DMG-PEG | 2.1 | Restoration of dystrophin in 38.5% of muscle fibers | ||||||
| Wei 2020 [66] | RNP | Muscles /DMD | IM | 1 | 1 mg/kg sgRNA | 5A2-SC8 | 21.4 | 40:1 | td-Tom fluorescence near the injection site |
| DOPE | 21.4 | ||||||||
| Cholesterol | 42.8 | ||||||||
| 3 | DMG-PEG | 4.3 | 4.2% restoration of dystrophin | ||||||
| DOTAP | 10 | ||||||||
| Guimaraes 2019 [67] | b-mRNA | Muscles | IV | 1 | 0.25 μg de b-mRNA | C12–200 | 35 | 5:1 1 | |
| DOPE | 16 | ||||||||
| Cholesterol | 46.5 | 7.5:1 1 | |||||||
| DMG-PEG | 2.5 | ||||||||
| Carrasco 2021 [65] | FLuc mRNA | Muscles | IM | 5 μg | DLin-KC2-DMA | 50 | 4:1 1 (mol:mol) | ||
| DSPC | 10 | ||||||||
| Cholesterol | 38.5 | ||||||||
| DMG-PEG | 1.5 | ||||||||
| Blakney 2019 [64] | FLuc saRNA | Muscles | IM | 5 μg | C12-200 | 35 | 12:1 1 | ||
| DOPE | 16 | ||||||||
| Cholesterol | 49 |
| Reference | Delivered Cargo | Targeted Tissue/Gene | Route | No. of Doses | Dose | LNP Formulation | Lipids Molar Ratios | Lipid: RNA (or DNA) Ratio (w:w) | Results |
|---|---|---|---|---|---|---|---|---|---|
| Ma 2020 [88] | ASO targeting tau mRNA | Brain/tau | IV | 5 | 1 mg/kg | 306-O12B-3 | 67.2 (w) | ~50% reduction of tau mRNA and ~30% reduction of tau protein | |
| DSPE-PEG | 4 (w) | ||||||||
| NT1-O14B | 28.8 (w) | ||||||||
| (-27)GFP-Cre protein | Brain | IV | 4 | 50 μg per injection | PBA-Q76-O16B | 67.2 (w) | Strong tdTomato signals were observed in multiple regions of the brain, including the cerebral cortex, hippocampus, and cerebellum. | ||
| DSPE-PEG | 4 (w) | ||||||||
| NT1-O14B | 28.8 (w) | ||||||||
| Wei 2020 [66] | RNP | Brain | IC | 1 | 0.15 mg/kg sgRNA | 5A2-SC8 | 21.4 | 40:1 | |
| DOPE | 21.4 | ||||||||
| Cholesterol | 42.8 | ||||||||
| DMG-PEG | 4.3 | ||||||||
| DOTAP | 10 | ||||||||
| Nabhan 2016 [89] | mRNA | DRG/FXN | ICV can | 1 | 0.2 mg/kg | DLin-MC3-DMA DSPC Cholesterol DMG-PEG | 55 | 30:1 | Robust increase in mFXN levels. |
| 10 | |||||||||
| IT | 32.5 | LNP-derived human mFXN levels were ~3-fold higher than mouse mFXN in the control group | |||||||
| 2.5 | |||||||||
| Tamaru 2014 [90] | DNA encoding mCherry | BECs | ICV | YSK05 | 70 | ||||
| Cholesterol | 30 | ||||||||
| DMG-PEG | 3 |
| Reference | Delivered Cargo | Targeted Tissue/Gene | Route | No. of Doses | Dose | LNP Formulation | Lipids Molar Ratios | Lipid: RNA (or DNA) Ratio (w:w) | Results |
|---|---|---|---|---|---|---|---|---|---|
| Cheng 2020 [94] | Cas9 protein + sgRNA (RNP) | Lungs/PTEN | IV | 1 | 1.5 mg/kg sgRNA | 5A2-SC8 | 11.9 | 40:1 | 5.3% gene editing |
| DOPE | 11.9 | ||||||||
| Cholesterol | 23.8 | ||||||||
| Cas9 mRNA/sgRNA | 2.5 mg/kg total RNA | DMG-PEG | 2.4 | 15.1% gene editing | |||||
| DOTAP | 50 | ||||||||
| Wei 2020 [66] | RNP | Lungs PTEN | IV | 1 | 1.5 mg/kg sgRNA | 5A2-SC8 | 11.9 | 40:1 | 13% indel frequency |
| P53; PTEN; EMl4; ALK; RB1 | 1 | 0.33 mg/kg each sgRNA | DOPE | 11.9 | Gene editing: 1.1% (P53) 3.4% (PTEN); 7.7% (EMl4); 1.1% (ALK); 7.5% (RB1) | ||||
| Cholesterol | 23.8 | ||||||||
| Eml4/Alk | 1 | 2 mg/kg sgRNA | DMG-PEG | 2.4 | 16.3% (EMl4); 4.5% (ALK) | ||||
| Eml4/Alk | 2 | 1.5 mg/kg sgRNA | DOTAP | 50 | 13.1% (EMl4); 3.5% (ALK) | ||||
| Robinson 2018 [95] | cmCFTR mRNA | Lungs/CFTR | IN | 0.6 mg cmRNA/kg | DLin-MC3-DMA | 50 | Polarization in response to CFTR | ||
| DSPC | 10 | ||||||||
| cmFLuc mRNA | Cholesterol | 38.5 | |||||||
| DMG-PEG | 1.5 | ||||||||
| Zhang 2020 [96] | FLuc mRNA | Heart, Spleen, Lung | IV | 0.5 mg/kg | FTT7 lipids | 22.04 | |||
| DOPE | 33.06 | ||||||||
| Cholesterol | 44.08 | ||||||||
| DMG-PEG | 0.82 | ||||||||
| Paunovska 2018 [97] | Lung EC | IV | 7C1 | 62 | |||||
| Cholesteryl Stearate | 30 | ||||||||
| DMG-PEG | 8 | ||||||||
| Lung Macs | 7C1 | 50 | |||||||
| DOPE | 8 | ||||||||
| 7B-OH Cholesterol | 40 | ||||||||
| DMG-PEG | 2 | ||||||||
| Paunovska 2018 [98] | b-DNA | Heart ECs, Lung ECs | IV | 0.75 mg/kg | 104-PEI600 | 62 | |||
| Lipid = C12Epoxy | 5 | ||||||||
| PEG350-C18 | 33 | ||||||||
| Lungs Macs | 7C1-PEI600 | 62 | |||||||
| Lipid = C15Epox | 5 | ||||||||
| PEG350-C14 | 33 | ||||||||
| Lungs Ecs | 7C1-PEI600 | 62 | |||||||
| Lipid = C15Epoxy | 6 | ||||||||
| DMG-PEG | 32 | ||||||||
| Lung Macs | 104-PEI600 | 62 | |||||||
| Lipid = C12Epoxy | 21 | ||||||||
| DMG-PEG-C18 | 17 | ||||||||
| Heart Macs and Lungs Macs | 104-PEI600 | 62 | |||||||
| Lipid=C12Epoxy | 18 | ||||||||
| DMG-PEG-C18 | 20 | ||||||||
| Sago 2018 [99] | two sgRNAs targeting ICAM2 (sgICAM2ab | Lung, Spleen, Kidney | IV | 3 | 1.5 mg/kg | 7C1 | 50 | Good for small RNAs but not for mRNAs | |
| 18:1Lyso PC | 20 | ||||||||
| Cholesterol | 23.5 | ||||||||
| DMG-PEG | 6.5 | ||||||||
| Qiu 2022 [100] | Cas9 mRNA + sgRNA | Lungs/LoxP | IV | 1.67 mg/kg | 306-N16B | 50 | 10:11 | ||
| DOPE (or DSPC) | 10 | ||||||||
| Cholesterol | 38.5 | ||||||||
| DMG-PEG | 1.5 | ||||||||
| Liu 2021 [101] | Cre mRNA | Lungs | IV | 9A1P9 | 46 | Transfection of ~34% of all endothelial cells, ~20% of all epithelial cells, and ~13% of immune cells | |||
| DDAB | 23 | ||||||||
| Cas9 mRNA + Tom1 sgRNA | Lungs | 0.75 mg/kg | Cholesterol | 30.7 | Specific gene editing in the lungs | ||||
| Cas9 mRNA + sgRNA | PTEN | 0.75 mg/kg | DMG-PEG | 0.3 | Efficient target gene editing | ||||
| Hagino 2021 [102] | pDNA + PEI | Lungs | IV | 40 μg de pDNA | The inner coat (half of the total lipid): DOPE STR-R8 | 640 nmol of lipid for 30 μg de pDNA | High gene Expression in the lungs | ||
| 9.55 | |||||||||
| 0.45 | |||||||||
| The outer coat: DOTMA YSK05 Cholesterol DMG-PEG Chol-GALA | |||||||||
| 4 | |||||||||
| 4 | |||||||||
| 2 | |||||||||
| 0.3 | |||||||||
| 0.4 |
| Reference | Delivered Cargo | Targeted Tissue/Gene | Route | No. of Doses | Dose | LNP Formulation | Lipids Molar Ratios | Lipid: RNA (or DNA) Ratio (w:w) | Results |
|---|---|---|---|---|---|---|---|---|---|
| Cheng 2020 [94] | Cas9 mRNA/sgRNA | Liver/PTEN | IV | 1 | 2.5 mg/kg total RNA | 5A2-SC8 | 19.05 | 40:1 | 2.7% gene editing |
| Cas9 protein + sgRNA (RNP) | 1 | 1.5 mg/kg sgRNA | DOPE | 19.05 | 11.6%–13.9% gene editing | ||||
| Cholesterol | 38.1 | ||||||||
| Cas9 mRNA/sgRNA | Liver/PCSK9 | 3 | 2.5 mg/kg total RNA | DMG-PEG | 3.8 | ∼60% gene editing 100% reduction in liver and serum Pcsk9 | |||
| DOTAP | 20 | ||||||||
| Wei 2020 [66] | RNP | Liver/P53, PTEN, RB1 | IV | 3 | 2.5 mg/kg sgRNA | 5A2-SC8 | 22.6 | 40:1 | 8.6% (P53); 7.9% (PTEN); 13.3% (RB1) gene editing |
| DOPE | 22.6 | ||||||||
| Cholesterol | 45.2 | ||||||||
| Liver/PCSK9 | DMG-PEG | 4.5 | 5.7% gene editing reduction of PCSK9 in serum and liver tissue | ||||||
| DOTAP | 5 | ||||||||
| Zhang 2020 [96] | hFVIII mRNA | Liver | IV | 2 mg/kg | FTT5 lipids | 22.04 | Restores the hFVIII level up to 90% of normal activity. | ||
| DOPE | 33.06 | ||||||||
| mRNA encoding ABE + sgRNA | PCSK9 | 1 mg/kg of total RNA dose | Cholesterol | 44.08 | 60% of gene editing | ||||
| DMG-PEG | 0.82 | ||||||||
| Paunovska 2018 [97] | Liver EC | IV | 7C1 | 50 | |||||
| 18:1Lyso PC | 10 | ||||||||
| 4B-OH-Cholesterol | 29 | ||||||||
| DMG-PEG | 11 | ||||||||
| Liver hepatocyte | 7C1 | 50 | |||||||
| DOPE | 8 | ||||||||
| 7B-OH Cholesterol | 40 | ||||||||
| DMG-PEG | 2 | ||||||||
| Cui 2022 [111,112] | FLuc mRNA | Liver; iWAT, gWAT | IV | 0.25 mg/kg | MC3 | 50 | 20:1 | ||
| DSPC | 10 | ||||||||
| Cholesterol | 38.5 | ||||||||
| DMG-PEG | 1.5 | ||||||||
| Rothgangl 2021 [113] | ABE + gRNA | Liver/PCSK9 | IV | 1 | 1.0, 1.5, or 3.0 mg/kg total RNA | Patent: US 2016/0376224 A1 | In mice: 4%, 12%, or 51% base editing | ||
| 2 | 1.5 or 3.0 mg/kg total RNA | In mice: 40% or 67% base editing | |||||||
| 1 | 1.5 mg/kg total RNA | In cynomolgus monkeys: 28% base editing 26% reduction in serum PCSK9 | |||||||
| 2 | 1.5 mg/kg total RNA | In cynomolgus monkeys: 24% base editing 39% reduction in serum PCSK9 | |||||||
| Liu 2019 [22] | Cas9 mRNA + gRNA | Liver, Kidney/PCSK9 | IV | 1 | 0.6 mg/kg Cas9 mRNA | BAMEA-16B | 16 (w) | 15:1 | Reduction of serum PCSK9 by 80% |
| DOPE | 4 (w) | ||||||||
| Cholesterol | 8 (w) | ||||||||
| DSPE-PEG | 1 (w) | ||||||||
| Prakash 2013 [114] | ASO | Liver/PTEN | IV | 1 | 4.5 mg/kg | DLin-KC2-DMA | 57.5 | 10:1 | In mice: around 85% of reduction of PTEN mRNA |
| DSPC | 7.5 | ||||||||
| ss-siRNA | Cholesterol | 31.5 | In mice: around 70% of re-duction of PTEN mRNA | ||||||
| siRNA | DMG-PEG | 3.5 | In mice: around 75% of re-duction of PTEN mRNA |
| Reference | Delivered Cargo | Targeted Tissue/Gene | Route | No. of Doses | Dose | LNP Formulation | Lipids Molar Ratios | Ionizable Lipid: RNA (or DNA) Ratio (w:w) | Results |
|---|---|---|---|---|---|---|---|---|---|
| Scalzo 2022 [115] | pDNA | Cardiac cells | IV | 0.2 µg of pDNA | C12-200 | 35 | 10:1 | Transfection efficiency | |
| DOPE | 56.5 | Day 2 = 60% | |||||||
| Cholesterol | 6 | Day 4 = 80% | |||||||
| DMG-PEG | 2.5 | A twofold increase in GFP expression in the heart tissue compared to the control group | |||||||
| Zhang 2020 [96] | FLuc mRNA | Heart, Spleen, Lung | IV | 0.5 mg/kg | FTT7 lipids | 22.04 | |||
| DOPE | 33.06 | ||||||||
| Cholesterol | 44.08 | ||||||||
| DMG-PEG | 0.82 | ||||||||
| Paunovska 2018 [98] | b-DNA | Heart ECs, Lung ECs | IV | 0.75 mg/kg | 104-PEI600 | 62 | |||
| Lipid=C12Epoxy | 5 | ||||||||
| DMG-PEG350-C18 | 33 | ||||||||
| Heart ECs and Macs | 102-Spermidine | 35 | |||||||
| Lipid=C12Epoxy | 35 | ||||||||
| DMG-PEG | 30 | ||||||||
| Heart Ecs | 104-PEI600 | 62 | |||||||
| DMG-PEG-C18 | 38 | ||||||||
| Heart Macs and Lungs Macs | 104-PEI600 | 62 | |||||||
| Lipid=C12Epoxy | 18 | ||||||||
| DMG-PEG-C18 | 20 |
| Reference | Delivered Cargo | Targeted Tissue/Gene | Route | No. of Doses | Dose | LNP Formulation | Lipids Molar Ratios | Ionizable Lipid: RNA (or DNA) Ratio (w:w) | Results |
|---|---|---|---|---|---|---|---|---|---|
| Cheng 2020 [94] | Cas9 mRNA/sgRNA | Spleen/PTEN | IV | 1 | 4.0 mg/kg | 5A2-SC8 | 16.7 | 40:1 (total lipid: mRNA) | |
| DOPE | 16.7 | ||||||||
| Cholesterol | 33.3 | ||||||||
| DMG-PEG | 3.3 | ||||||||
| 18PA | 30 | ||||||||
| Zhang 2020 [96] | FLuc mRNA | Spleen | IV | 0.5 mg/kg | FTT3 lipids | 22.04 | |||
| DOPE | 33.06 | ||||||||
| Cholesterol | 44.08 | ||||||||
| DMG-PEG | 0.82 | ||||||||
| Paunovska 2018 [97] | Spleen EC | IV | 7C1 | 50 | |||||
| DSPC | 8 | ||||||||
| Cholesteryl Stearate | 40 | ||||||||
| DMG-PEG | 2 | ||||||||
| Spleen Macs | 7C1 | 50 | |||||||
| DOPE | 8 | ||||||||
| 7B-OH Cholesterol | 40 | ||||||||
| DMG-PEG | 2 | ||||||||
| Sago 2018 [99] | SpCas9 mRNA and sgICAM2ab | Spleen ECs | IV | 2 | 2 mg/kg | 7C1 | 60 | The best formulations for mRNA | |
| DOPE | 5 | ||||||||
| Cholesterol | 10 | ||||||||
| SpCas9 mRNA + e-sgICAM2 | DMG-PEG | 25 | |||||||
| two sgRNAs targeting ICAM2 (sgICAM2ab) | Lung, Spleen, Kidney | 3 | 1.5 mg/kg | 7C1 | 50 | Good for small RNAs but not for mRNA | |||
| 18:1Lyso PC | 20 | ||||||||
| Cholesterol | 23.5 | ||||||||
| DMG-PEG | 6.5 | ||||||||
| Maugeri 2019 [116] | hEPO mRNA | Spleen | IV | 1.5 µg per mouse | DLin-MC3-DMA | 50 | 3:1 | ||
| DSPC | 10 | ||||||||
| Cholesterol | 38.5 | ||||||||
| DMPE-PEG | 1.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Godbout, K.; Tremblay, J.P. Delivery of RNAs to Specific Organs by Lipid Nanoparticles for Gene Therapy. Pharmaceutics 2022, 14, 2129. https://doi.org/10.3390/pharmaceutics14102129
Godbout K, Tremblay JP. Delivery of RNAs to Specific Organs by Lipid Nanoparticles for Gene Therapy. Pharmaceutics. 2022; 14(10):2129. https://doi.org/10.3390/pharmaceutics14102129
Chicago/Turabian StyleGodbout, Kelly, and Jacques P. Tremblay. 2022. "Delivery of RNAs to Specific Organs by Lipid Nanoparticles for Gene Therapy" Pharmaceutics 14, no. 10: 2129. https://doi.org/10.3390/pharmaceutics14102129
APA StyleGodbout, K., & Tremblay, J. P. (2022). Delivery of RNAs to Specific Organs by Lipid Nanoparticles for Gene Therapy. Pharmaceutics, 14(10), 2129. https://doi.org/10.3390/pharmaceutics14102129