Development of Multilayer Nanoparticles for the Delivery of Peptide-Based Subunit Vaccine against Group A Streptococcus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Instruments
2.3. Peptide Synthesis & Purification
2.4. Oxidation
2.5. Formulation and Optimization of LCP-1/Alginate
2.6. Optimization of LCP-1/Alginate/Cross-Linked Poly-Cationic Peptide
2.7. Transmission Electron Microscopy
2.8. Encapsulation Study
2.9. Animal Studies
2.10. Determination of Antibody Titres & Statistical Analyses
2.11. Opsonization Assay of Antibodies against Group A Streptococcus
3. Results and Discussion
3.1. Design of the Vaccine Delivery System
3.2. Formation and Characterization of PECs
3.3. Immunogenicity of PECs
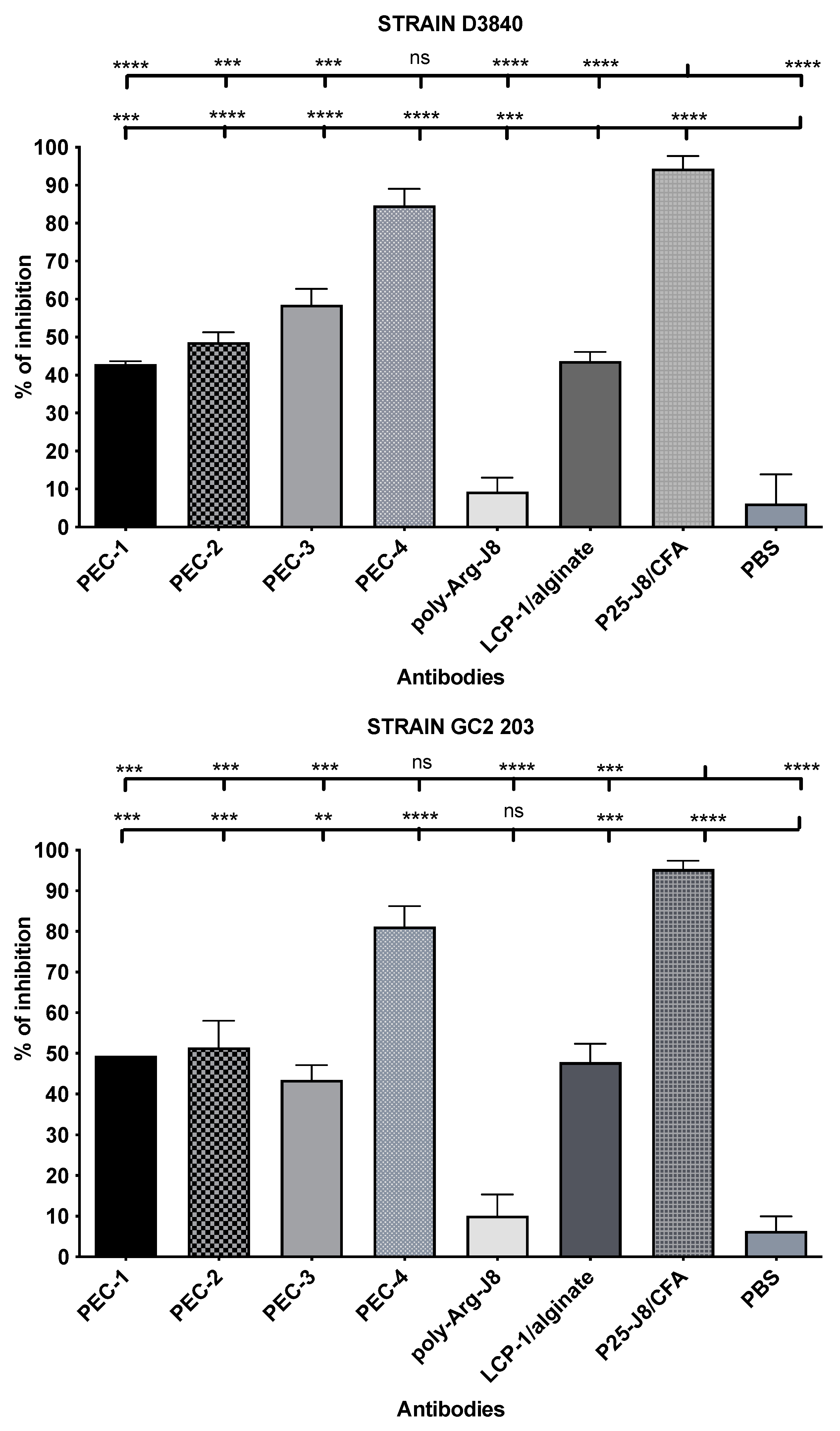
3.4. Antibody Opsonic Capability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tacket, C.O.; Mason, H.S.; Losonsky, G.; Clements, J.D.; Levine, M.M.; Arntzen, C.J. Immunogenicity in humans of a recombinant bacterial antigen delivered in a transgenic potato. Nat. Med. 1998, 4, 607–609. [Google Scholar] [CrossRef]
- Rendi-Wagner, P.; Kollaritsch, H. Chapter 9-Principles of Immunization. In Travel Medicine, 2nd ed.; Keystone, J.S., Kozarsky, P.E., Freedman, D.O., Nothdurft, H.D., Connor, B.A., Eds.; Mosby: Edinburgh, Scotland, 2008; pp. 75–84. [Google Scholar]
- Skwarczynski, M.; Toth, I. Peptide-based synthetic vaccines. Chem. Sci. 2016, 7, 842–854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmed, S.S.; Ellis, R.W.; Rappuoli, R. 66-Technologies for Making New Vaccines. In Plotkin’s Vaccines, 7th ed.; Plotkin, S.A., Orenstein, W.A., Offit, P.A., Edwards, K.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 1283–1304.e1287. [Google Scholar]
- Vogel, F.R. Improving vaccine performance with adjuvants. Clin. Infect. Dis. 2000, 30, S266–S270. [Google Scholar] [CrossRef] [PubMed]
- Firdaus, F.Z.; Skwarczynski, M.; Toth, I. Developments in Vaccine Adjuvants. In Vaccine Design: Methods and Protocols, Volume 3. Resources for Vaccine Development; Thomas, S., Ed.; Springer: New York, NY, USA, 2022; pp. 145–178. [Google Scholar]
- Bartlett, S.; Skwarczynski, M.; Toth, I. Lipids as Activators of Innate Immunity in Peptide Vaccine Delivery. Curr. Med. Chem. 2020, 27, 2887–2901. [Google Scholar] [CrossRef] [PubMed]
- Shalash, A.O.; Becker, L.; Yang, J.; Giacomin, P.; Pearson, M.; Hussein, W.M.; Loukas, A.; Skwarczynski, M.; Toth, I. Oral Peptide Vaccine against Hookworm Infection: Correlation of Antibody Titers with Protective Efficacy. Vaccines 2021, 9, 1034. [Google Scholar] [CrossRef]
- Yang, J.; Firdaus, F.; Azuar, A.; Khalil, Z.G.; Marasini, N.; Capon, R.J.; Hussein, W.M.; Toth, I.; Skwarczynski, M. Cell-Penetrating Peptides-Based Liposomal Delivery System Enhanced Immunogenicity of Peptide-Based Vaccine against Group A Streptococcus. Vaccines 2021, 9, 499. [Google Scholar] [CrossRef]
- Hamley, I.W. Lipopeptides for Vaccine Development. Bioconjug. Chem. 2021, 32, 1472–1490. [Google Scholar] [CrossRef]
- Avire, N.J.; Whiley, H.; Ross, K. A Review of Streptococcus pyogenes: Public Health Risk Factors, Prevention and Control. Pathogens 2021, 10, 248. [Google Scholar] [CrossRef] [PubMed]
- Stevens, D.L. Streptococcal toxic shock syndrome associated with necrotizing fasciitis. Annu. Rev. Med. 2000, 51, 271–288. [Google Scholar] [CrossRef]
- Passàli, D.; Lauriello, M.; Passàli, G.C.; Passàli, F.M.; Bellussi, L. Group A streptococcus and its antibiotic resistance. Acta Otorhinolaryngol. Ital. 2007, 27, 27–32. [Google Scholar] [PubMed]
- Watkins, D.A.; Johnson, C.O.; Colquhoun, S.M.; Karthikeyan, G.; Beaton, A.; Bukhman, G.; Forouzanfar, M.H.; Longenecker, C.T.; Mayosi, B.M.; Mensah, G.A.; et al. Global, Regional, and National Burden of Rheumatic Heart Disease, 1990–2015. N. Engl. J. Med. 2017, 377, 713–722. [Google Scholar] [CrossRef] [PubMed]
- Henningham, A.; Barnett, T.C.; Maamary, P.G.; Walker, M.J. Pathogenesis of group A streptococcal infections. Discov. Med. 2012, 13, 329–342. [Google Scholar] [PubMed]
- Massell, B.F.; Honikman, L.H.; Amezcua, J. Rheumatic fever following streptococcal vaccination. Report of three cases. JAMA 1969, 207, 1115–1119. [Google Scholar] [CrossRef]
- Cunningham, M.W.; McCormack, J.M.; Fenderson, P.G.; Ho, M.K.; Beachey, E.H.; Dale, J.B. Human and murine antibodies cross-reactive with streptococcal M protein and myosin recognize the sequence GLN-LYS-SER-LYS-GLN in M protein. J. Immunol. 1989, 143, 2677–2683. [Google Scholar] [PubMed]
- Dale, J.B.; Beachey, E.H. Multiple, heart-cross-reactive epitopes of streptococcal M proteins. J. Exp. Med. 1985, 161, 113–122. [Google Scholar] [CrossRef] [Green Version]
- Dale, J.B.; Beachey, E.H. Sequence of myosin-crossreactive epitopes of streptococcal M protein. J. Exp. Med. 1986, 164, 1785–1790. [Google Scholar] [CrossRef] [Green Version]
- Azuar, A.; Jin, W.; Mukaida, S.; Hussein, W.M.; Toth, I.; Skwarczynski, M. Recent advances in the development of peptide vaccines and their delivery systems against group A streptococcus. Vaccines 2019, 7, 58. [Google Scholar] [CrossRef] [Green Version]
- Pruksakorn, S.; Galbraith, A.; Houghten, R.A.; Good, M.F. Conserved T and B cell epitopes on the M protein of group A streptococci. Induction of bactericidal antibodies. J. Immunol. 1992, 149, 2729–2735. [Google Scholar]
- Sekuloski, S.; Batzloff, M.R.; Griffin, P.; Parsonage, W.; Elliott, S.; Hartas, J.; O’Rourke, P.; Marquart, L.; Pandey, M.; Rubin, F.A.; et al. Evaluation of safety and immunogenicity of a group A streptococcus vaccine candidate (MJ8VAX) in a randomized clinical trial. PLoS ONE 2018, 13, e0198658. [Google Scholar] [CrossRef] [Green Version]
- Zhao, L.; Skwarczynski, M.; Toth, I. Polyelectrolyte-Based Platforms for the Delivery of Peptides and Proteins. ACS Biomater. Sci. Eng. 2019, 5, 4937–4950. [Google Scholar] [CrossRef]
- Liu, Z.; Jiao, Y.; Wang, Y.; Zhou, C.; Zhang, Z. Polysaccharides-based nanoparticles as drug delivery systems. Adv. Drug Deliv. Rev. 2008, 60, 1650–1662. [Google Scholar] [CrossRef]
- Nevagi, R.J.; Dai, W.; Khalil, Z.G.; Hussein, W.M.; Capon, R.J.; Skwarczynski, M.; Toth, I. Self-assembly of trimethyl chitosan and poly(anionic amino acid)-peptide antigen conjugate to produce a potent self-adjuvanting nanovaccine delivery system. Bioorg. Med. Chem. 2019, 27, 3082–3088. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.; Li, J.; Zhang, Y.; Li, Y.; Shen, G.; Zhu, J.; Tao, J. Polyethylenimine-based micro/nanoparticles as vaccine adjuvants. Int. J. Nanomed. 2017, 12, 5443–5460. [Google Scholar] [CrossRef] [Green Version]
- Gu, P.; Wusiman, A.; Wang, S.; Zhang, Y.; Liu, Z.; Hu, Y.; Liu, J.; Wang, D. Polyethylenimine-coated PLGA nanoparticles-encapsulated Angelica sinensis polysaccharide as an adjuvant to enhance immune responses. Carbohydr. Polym. 2019, 223, 115128. [Google Scholar] [CrossRef] [PubMed]
- Borges, O.; Borchard, G.; Verhoef, J.C.; de Sousa, A.; Junginger, H.E. Preparation of coated nanoparticles for a new mucosal vaccine delivery system. Int. J. Pharm. 2005, 299, 155–166. [Google Scholar] [CrossRef] [Green Version]
- Razmshoar, P.; Shakoorjavan, S.; Akbari, S. Chapter 12-Surface-engineered dendrimers in targeting and delivery of drugs. In Dendrimer-Based Nanotherapeutics; Kesharwani, P., Ed.; Academic Press: Cambridge, MA, USA, 2021; pp. 203–223. [Google Scholar]
- Zhao, L.; Jin, W.; Cruz, J.G.; Marasini, N.; Khalil, Z.G.; Capon, R.J.; Hussein, W.M.; Skwarczynski, M.; Toth, I. Development of polyelectrolyte complexes for the delivery of peptide-based subunit vaccines against group a streptococcus. Nanomaterials 2020, 10, 823. [Google Scholar] [CrossRef]
- Amblard, M.; Fehrentz, J.-A.; Martinez, J.; Subra, G. Methods and protocols of modern solid-phase peptide synthesis. Mol. Biotechnol. 2006, 33, 239–254. [Google Scholar] [CrossRef]
- Raghupathi, K.; Thayumanavan, S. Chapter Sixteen-Nano-Armoring of Enzymes: Rational Design of Polymer-Wrapped Enzymes. In Methods in Enzymology; Kumar, C.V., Ed.; Academic Press: Cambridge, MA, USA, 2017; Volume 590, pp. 381–411. [Google Scholar]
- Nevagi, R.J.; Khalil, Z.G.; Hussein, W.M.; Powell, J.; Batzloff, M.R.; Capon, R.J.; Good, M.F.; Skwarczynski, M.; Toth, I. Polyglutamic acid-trimethyl chitosan-based intranasal peptide nano-vaccine induces potent immune responses against group A streptococcus. Acta Biomater. 2018, 80, 278–287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azuar, A.; Shibu, M.A.; Adilbish, N.; Marasini, N.; Hung, H.; Yang, J.; Luo, Y.; Khalil, Z.G.; Capon, R.J.; Hussein, W.M.; et al. Poly(hydrophobic amino acid) Conjugates for the Delivery of Multiepitope Vaccine against Group A Streptococcus. Bioconjugate Chem. 2021, 32, 2307–2317. [Google Scholar] [CrossRef]
- Dai, C.C.; Yang, J.; Hussein, W.M.; Zhao, L.; Wang, X.; Khalil, Z.G.; Capon, R.J.; Toth, I.; Stephenson, R.J. Polyethylenimine: An Intranasal Adjuvant for Liposomal Peptide-Based Subunit Vaccine against Group A Streptococcus. ACS Infect. Dis. 2020, 6, 2502–2512. [Google Scholar] [CrossRef]
- Werth, S.; Urban-Klein, B.; Dai, L.; Höbel, S.; Grzelinski, M.; Bakowsky, U.; Czubayko, F.; Aigner, A. A low molecular weight fraction of polyethylenimine (PEI) displays increased transfection efficiency of DNA and siRNA in fresh or lyophilized complexes. J. Control. Release 2006, 112, 257–270. [Google Scholar] [CrossRef]
- Yang, H.-W.; Ye, L.; Guo, X.D.; Yang, C.; Compans, R.W.; Prausnitz, M.R. Ebola Vaccination Using a DNA Vaccine Coated on PLGA-PLL/γPGA Nanoparticles Administered Using a Microneedle Patch. Adv. Healthc. Mater. 2017, 6, 1600750. [Google Scholar] [CrossRef]
- Cappellano, G.; Abreu, H.; Casale, C.; Dianzani, U.; Chiocchetti, A. Nano-Microparticle Platforms in Developing Next-Generation Vaccines. Vaccines 2021, 9, 606. [Google Scholar] [CrossRef]
- Al Hasan, A.; Azam, A.T.M.Z. Chapter 9-Small RNA-mediated prevention, diagnosis and therapies of cancer. In Design of Nanostructures for Theranostics Applications; Grumezescu, A.M., Ed.; William Andrew Publishing: Norwich, NY, USA, 2018; pp. 341–436. [Google Scholar]
- Allolio, C.; Magarkar, A.; Jurkiewicz, P.; Baxová, K.; Javanainen, M.; Mason, P.E.; Šachl, R.; Cebecauer, M.; Hof, M.; Horinek, D.; et al. Arginine-rich cell-penetrating peptides induce membrane multilamellarity and subsequently enter via formation of a fusion pore. Proc. Natl. Acad. Sci. USA 2018, 115, 11923. [Google Scholar] [CrossRef] [Green Version]
- Marasini, N.; Giddam, A.K.; Batzloff, M.R.; Good, M.F.; Skwarczynski, M.; Toth, I. Poly-L-lysine-coated nanoparticles are ineffective in inducing mucosal immunity against group a streptococcus. Biochem. Compd. 2017, 5, 1. [Google Scholar] [CrossRef]
- Mattner, F.; Fleitmann, J.-K.; Lingnau, K.; Schmidt, W.; Egyed, A.; Fritz, J.; Zauner, W.; Wittmann, B.; Gorny, I.; Berger, M.; et al. Vaccination with poly-L-arginine as immunostimulant for peptide vaccines: Induction of potent and long-lasting T-cell responses against cancer antigens. Cancer Res. 2002, 62, 1477–1480. [Google Scholar]


| Formulation | Negative Polymer | Cationic Polymer | Size (nm) | PDI | Zeta Potential (mV) | Initial Mass Ratio 1 |
|---|---|---|---|---|---|---|
| LCP-1/alginate | alginate | ---- | 176 ± 12 | 0.07 ± 0.02 | −24 ± 1 | 10:4:0 |
| PEC-0 | PEI 25 kDa | 153 ± 10 | 0.04 ± 0.02 | 33 ± 1 | 10:4:2 | |
| PEC-1 | Cross-linked poly-Arg-J8 | 330 ± 56 | 0.19 ± 0.02 | 24 ± 1 | 10:4:11 | |
| PEC-2 | Cross-linked poly-Arg | 217 ± 1 | 0.10 ± 0.01 | 27 ± 1 | 10:4:7 | |
| PEC-3 | Cross-linked mannosylated poly-Arg | 286 ± 10 4300 ± 20 | 0.34 ± 0.04 | 32 ± 1 | 10:4:9 | |
| PEC-4 | Cross-linked poly-Lys | 191 ± 16 ~4000 | 0.17 ± 0.05 | 31 ± 1 | 10:4:6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kiong, J.; Nahar, U.J.; Jin, S.; Shalash, A.O.; Zhang, J.; Koirala, P.; Khalil, Z.G.; Capon, R.J.; Skwarczynski, M.; Toth, I.; et al. Development of Multilayer Nanoparticles for the Delivery of Peptide-Based Subunit Vaccine against Group A Streptococcus. Pharmaceutics 2022, 14, 2151. https://doi.org/10.3390/pharmaceutics14102151
Kiong J, Nahar UJ, Jin S, Shalash AO, Zhang J, Koirala P, Khalil ZG, Capon RJ, Skwarczynski M, Toth I, et al. Development of Multilayer Nanoparticles for the Delivery of Peptide-Based Subunit Vaccine against Group A Streptococcus. Pharmaceutics. 2022; 14(10):2151. https://doi.org/10.3390/pharmaceutics14102151
Chicago/Turabian StyleKiong, Jolynn, Ummey Jannatun Nahar, Shengbin Jin, Ahmed O. Shalash, Jiahui Zhang, Prashamsa Koirala, Zeinab G. Khalil, Robert J. Capon, Mariusz Skwarczynski, Istvan Toth, and et al. 2022. "Development of Multilayer Nanoparticles for the Delivery of Peptide-Based Subunit Vaccine against Group A Streptococcus" Pharmaceutics 14, no. 10: 2151. https://doi.org/10.3390/pharmaceutics14102151










