Topographical and Compositional Gradient Tubular Scaffold for Bone to Tendon Interface Regeneration
Abstract
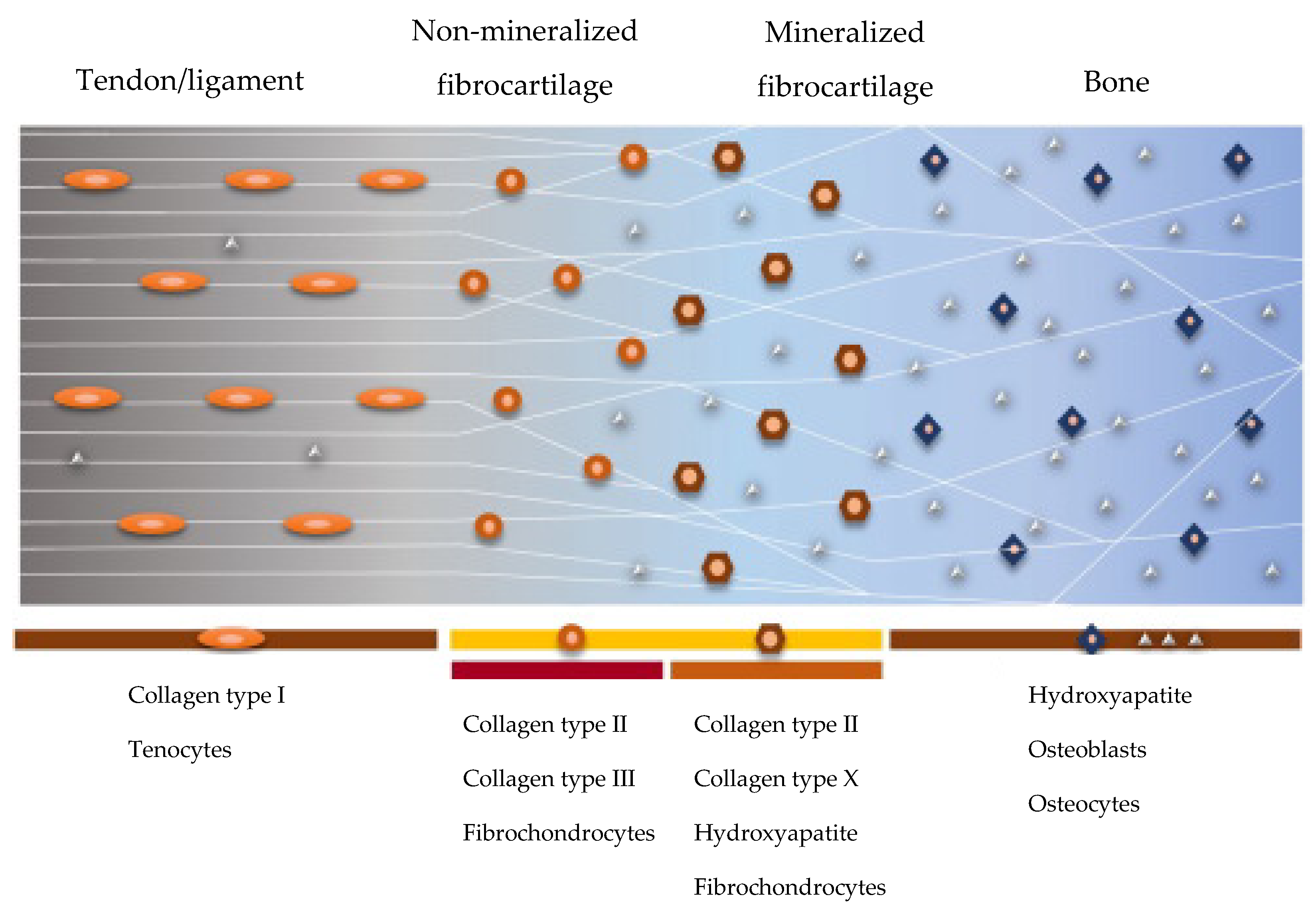
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Methods
2.2.1. Preparation of the Polymeric Blends
2.2.2. Preparation of the Tubular Scaffold
2.2.3. Structural Characterization
2.2.4. Biocompatibility
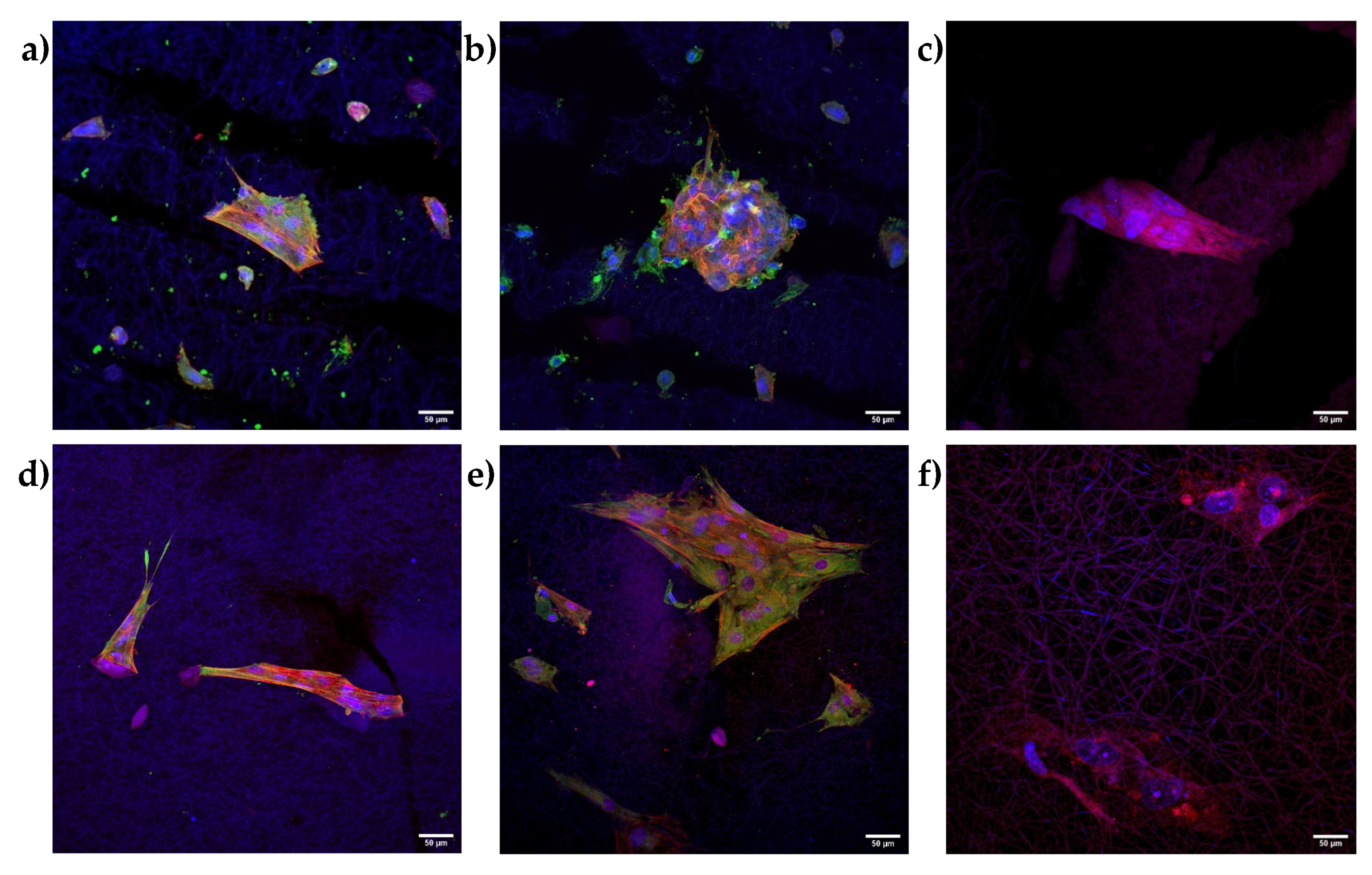
2.2.5. Immunofluorescence Analysis
2.2.6. Statistical Analysis
3. Results and Discussion
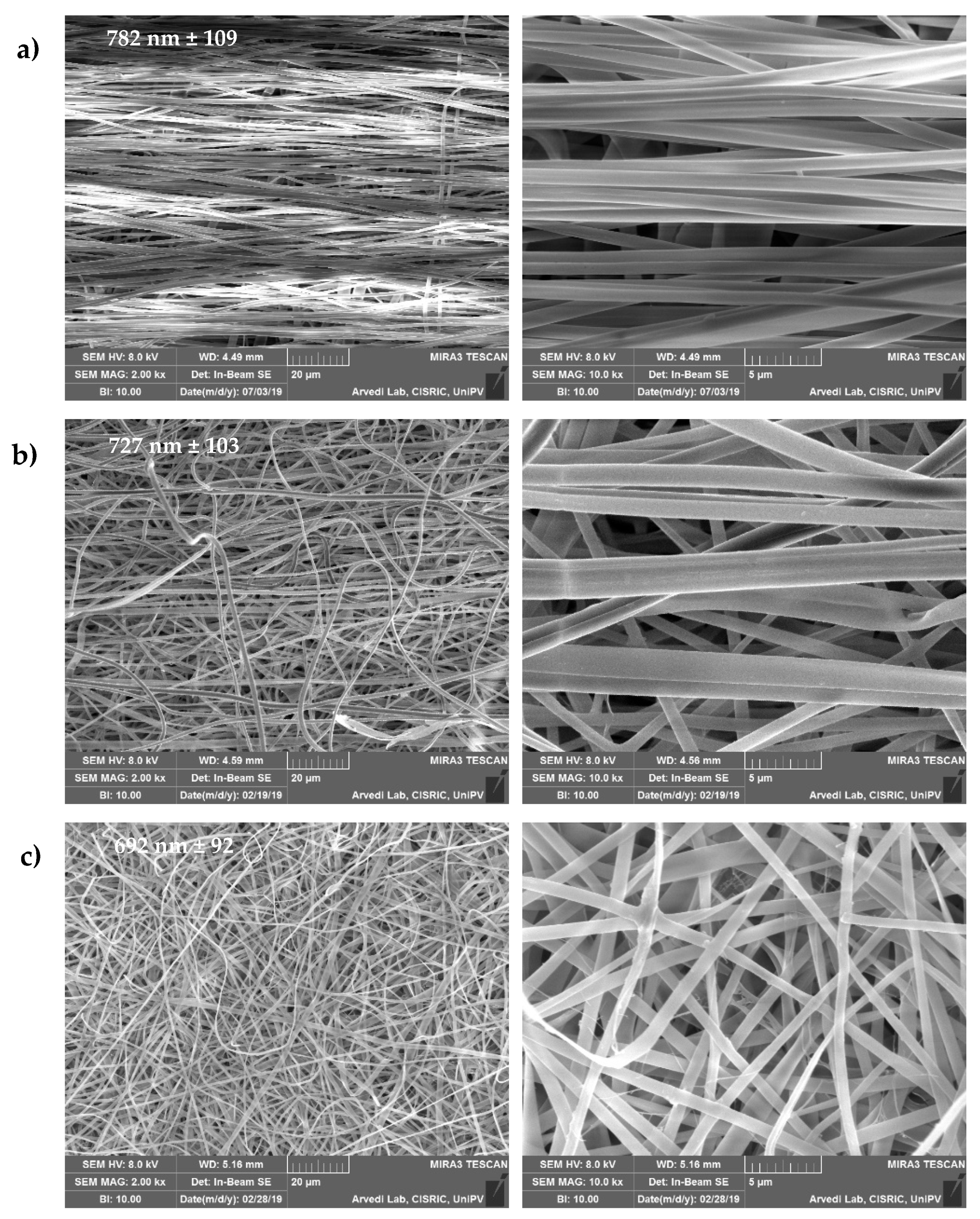
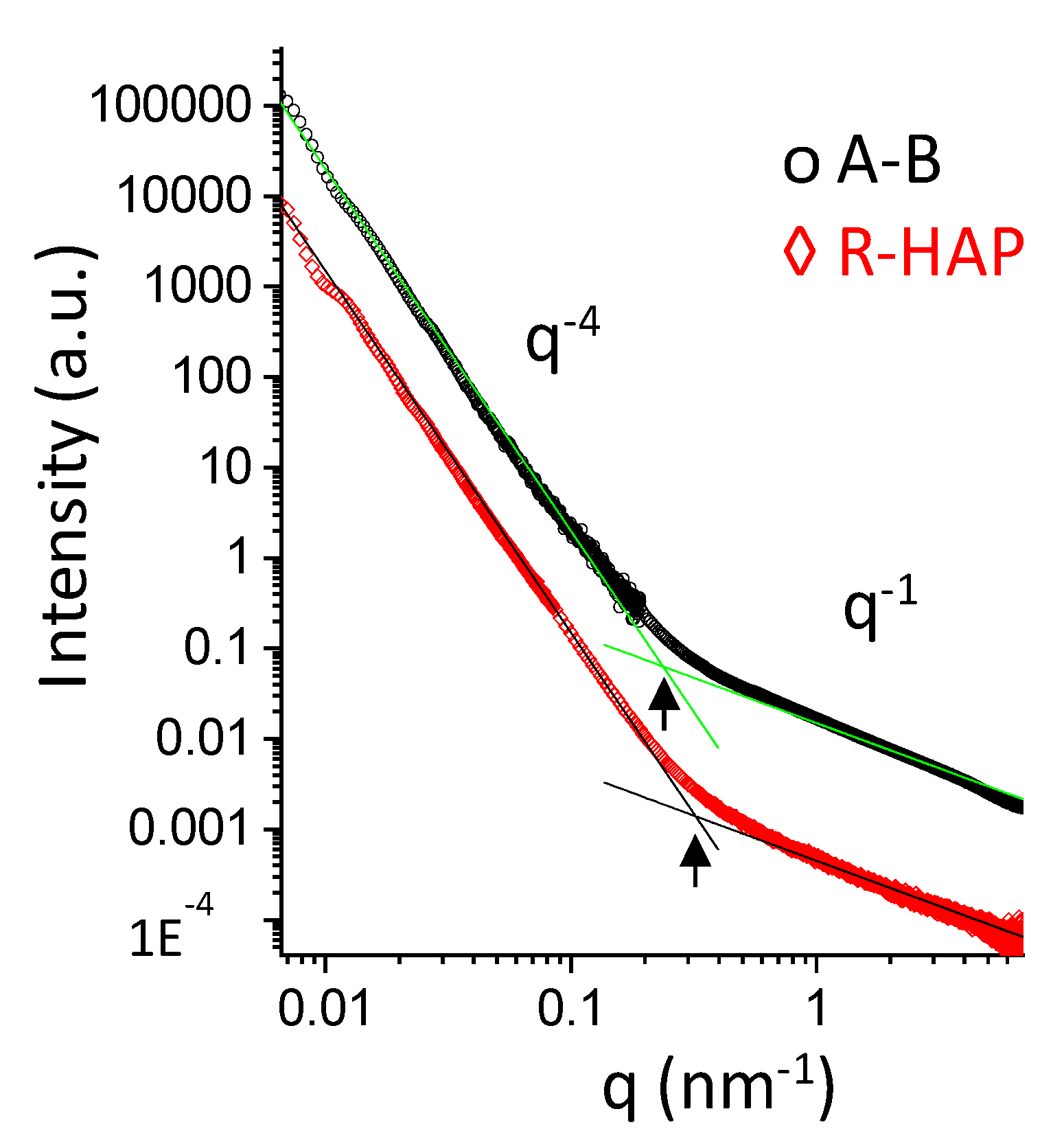
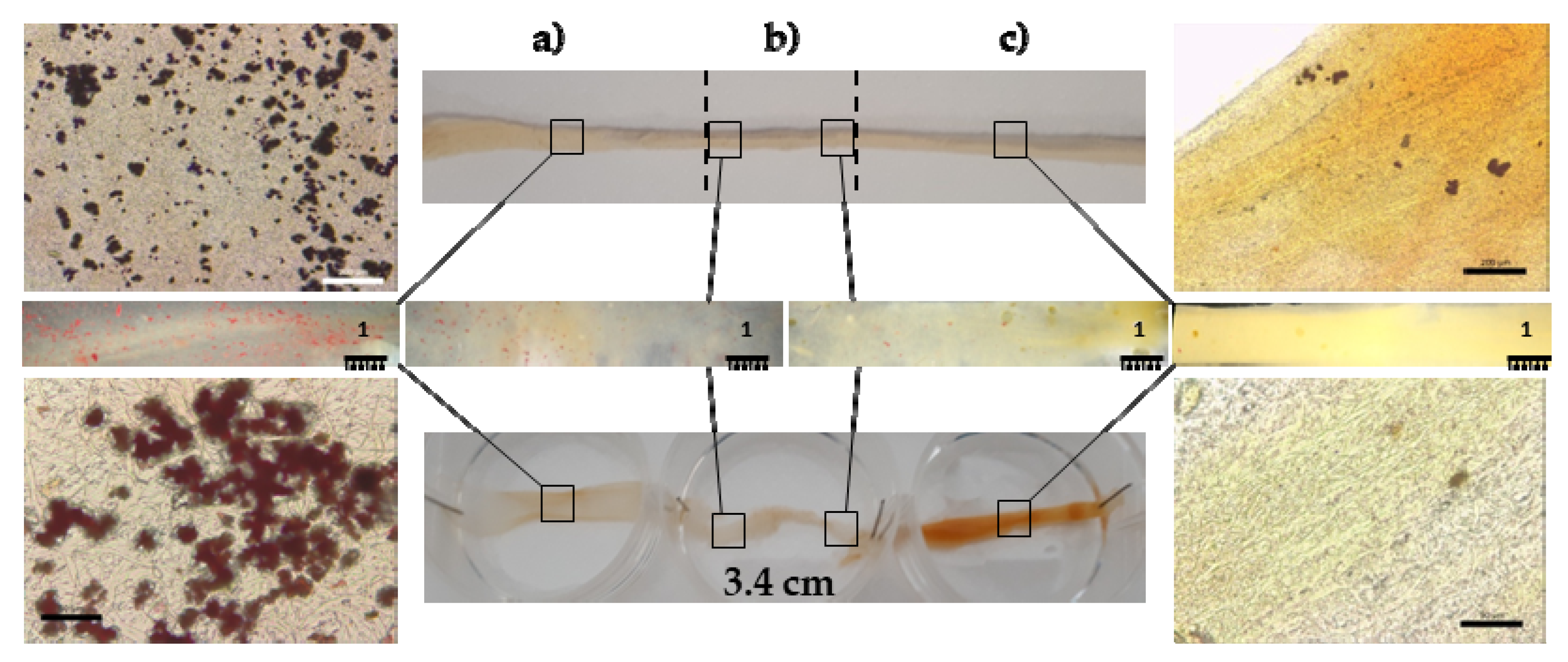
3.1. Structural Characterizations
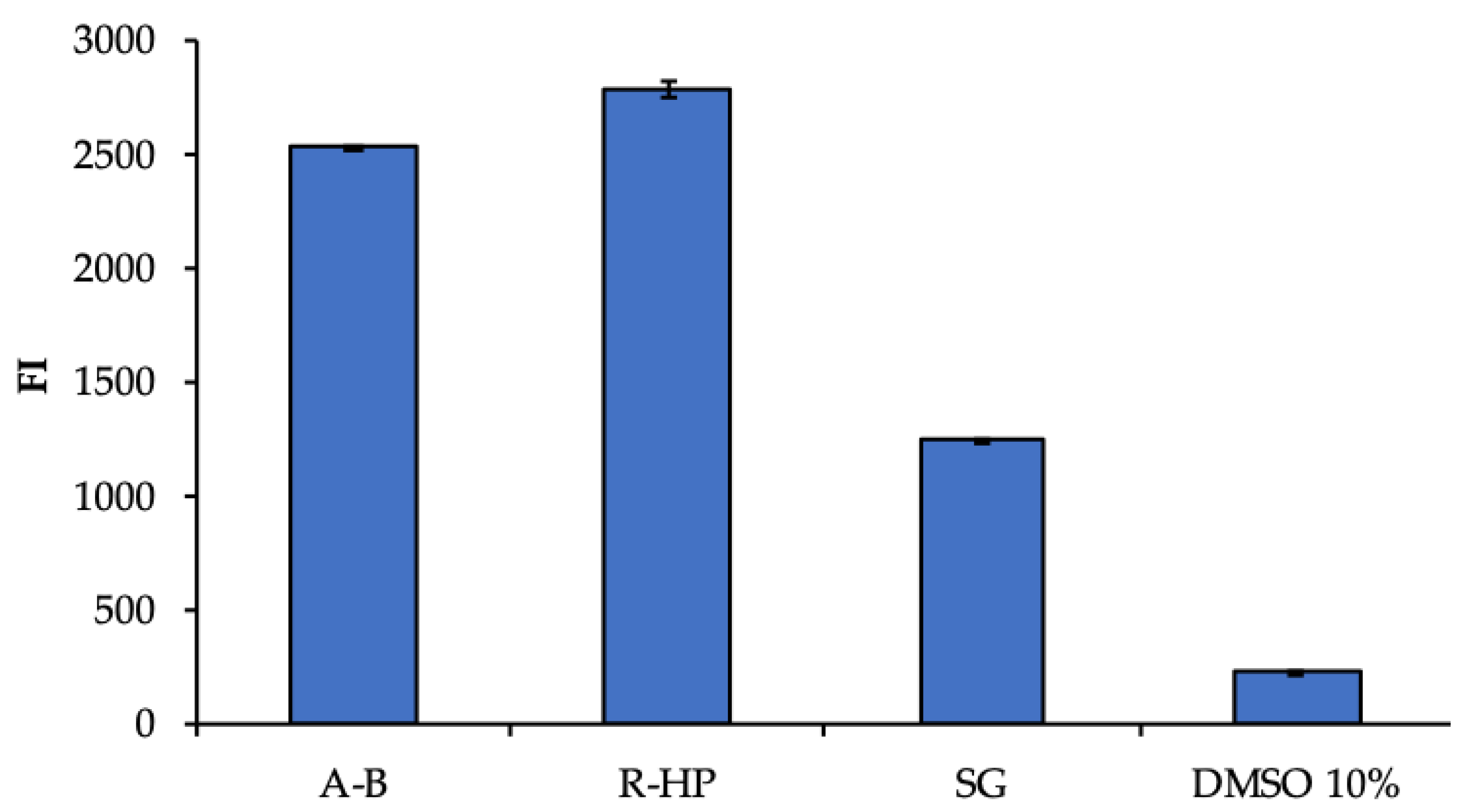
3.2. Biopharmaceutical Characterization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bianchi, E.; Ruggeri, M.; Rossi, S.; Vigani, B.; Miele, D.; Bonferoni, M.C.; Sandri, G.; Ferrari, F. Innovative Strategies in Tendon Tissue Engineering. Pharmaceutics 2021, 13, 89. [Google Scholar] [CrossRef]
- Bianchi, E.; Vigani, B.; Viseras, C.; Ferrari, F.; Rossi, S.; Sandri, G. Inorganic Nanomaterials in Tissue Engineering. Pharmaceutics 2022, 14, 1127. [Google Scholar] [CrossRef] [PubMed]
- Font Tellado, S.; Balmayor, E.R.; Van Griensven, M. Strategies to engineer tendon/ligament-to-bone interface: Biomaterials, cells and growth factors. Adv. Drug Deliv. Rev. 2015, 94, 126–140. [Google Scholar] [CrossRef] [PubMed]
- Morais, D.S.; Torres, J.; Guedes, R.M.; Lopes, M.A. Current Approaches and Future Trends to Promote Tendon Repair. Ann. Biomed. Eng. 2015, 43, 2025–2035. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, C.; Li, C.; Qin, Y.; Wang, Z.; Yang, F.; Li, Z.; Wang, J. A functional chitosan-based hydrogel as a wound dressing and drug delivery system in the treatment of wound healing. RSC Adv. 2018, 8, 7533–7549. [Google Scholar] [CrossRef] [Green Version]
- Sandri, G.; Rossi, S.; Bonferoni, M.C.; Miele, D.; Faccendini, A.; Del Favero, E.; Di Cola, E.; Icaro Cornaglia, A.; Boselli, C.; Luxbacher, T.; et al. Chitosan/glycosaminoglycan scaffolds for skin reparation. Carbohydr. Polym. 2019, 15, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.S.; Kaur, N.; Rana, V.; Kennedy, J.F. Pullulan: A novel molecule for biomedical applications. Carbohydr. Polym. 2017, 171, 102–121. [Google Scholar] [CrossRef] [PubMed]
- Calejo, I.; Costa-Almeida, R.; Reis, R.L.; Gomes, M.E. A textile platform using continuous aligned and textured composite microfibers to engineer tendon-to-bone interface gradient scaffolds. Adv. Health Mater. 2019, 15, 1900200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costa-Almeida, R.; Calejo, I.; Gomes, M.E. Mesenchymal Stem Cells Empowering Tendon Regenerative Therapies. Int. J. Mol. Sci. 2019, 20, 3002. [Google Scholar] [CrossRef] [Green Version]
- Sandri, G.; Faccendini, A.; Longo, M.; Ruggeri, M.; Rossi, S.; Bonferoni, M.C.; Miele, D.; Prina-Mello, A.; Aguzzi, C.; Viseras, C.; et al. Halloysite- and Montmorillonite-Loaded Scaffolds as Enhancers of Chronic Wound Healing. Pharmaceutics 2020, 12, 179. [Google Scholar] [CrossRef] [PubMed]
- Faccendini, A.; Ruggeri, M.; Miele, D.; Rossi, S.; Bonferoni, M.C.; Aguzzi, C.; Grisoli, P.; Viseras, C.; Vigani, B.; Sandri, G.; et al. Norfloxacin-Loaded Electrospun Scaffolds: Montmorillonite Nanocomposite vs. Free Drug. Pharmaceutics 2020, 12, 325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kupiec, T.C.; Matthews, P.; Ahmad, R. Dry-heat sterilization of parenteral oil vehicles. Int. J. Pharm. Compd. 2000, 4, 223–224. [Google Scholar] [PubMed]
- Faccendini, A.; Bianchi, E.; Ruggeri, M.; Vigani, B.; Perotti, C.; Pavesi, F.C.; Caliogna, L.; Natali, F.; Del Favero, E.; Cantu’, L.; et al. Smart Device for Biologically Enhanced Functional Regeneration of Osteo–Tendon Interface. Pharmaceutics 2021, 13, 1996. [Google Scholar] [CrossRef] [PubMed]
- Zubillaga, V.; Salaberria, A.M.; Palomares, T.; Alonso-Varona, A.; Kootala, S.; Labidi, J.; Fernandes, S.C.M. Chitin Nanoforms Provide Mechanical and Topological Cues to Support Growth of Human Adipose Stem Cells in Chitosan Matrices. Biomacromolecules 2018, 7, 3000–3012. [Google Scholar] [CrossRef] [PubMed]
- Kosaraju, R.; Rennert, R.C.; Maan, Z.N.; Duscher, D.; Barrera, J.; Whittam, A.J.; Januszyk, M.; Rajadas, J.; Rodrigues, M.; Gurtner, G.C. Adipose-Derived Stem Cell-Seeded Hydrogels Increase Endogenous Progenitor Cell Recruitment and Neovascularization in Wounds. Tissue Eng. Part A 2016, 22, 295–305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pulyala, P.; Singh, A.; Dias-Netipanyj, M.F.; Cogo, S.C.; Santos, L.S.; Soares, P.; Gopal, V.; Suganthan, V.; Manivasagam, G.; Popat, K.C. In-vitro cell adhesion and proliferation of adipose derived stem cell on hydroxyapatite composite surfaces. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 75, 1305–1316. [Google Scholar] [CrossRef] [PubMed]
- Schweitzer, R.; Chyung, J.H.; Murtaugh, L.C.; Brent, A.E.; Rosen, V.; Olson, E.N.; Lassar, A.; Tabin, C.J. Analysis of the tendon cell fate using Scleraxis, a specific marker for tendons and ligaments. Development 2001, 19, 3855–3866. [Google Scholar] [CrossRef]
- Killian, M.L.; Thomopoulos, S. Scleraxis is required for the development of a functional tendon enthesis. FASEB J. 2016, 1, 301–311. [Google Scholar] [CrossRef] [Green Version]
- Wang, P.; Zhao, L.; Liu, J.; Weir, M.D.; Zhou, X.; Xu, H.H. Bone tissue engineering via nanostructured calcium phosphate biomaterials and stem cells. Bone Res. 2014, 30, 14017. [Google Scholar] [CrossRef] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bianchi, E.; Faccendini, A.; Del Favero, E.; Ricci, C.; Caliogna, L.; Vigani, B.; Pavesi, F.C.; Perotti, C.; Domingues, R.M.A.; Gomes, M.E.; et al. Topographical and Compositional Gradient Tubular Scaffold for Bone to Tendon Interface Regeneration. Pharmaceutics 2022, 14, 2153. https://doi.org/10.3390/pharmaceutics14102153
Bianchi E, Faccendini A, Del Favero E, Ricci C, Caliogna L, Vigani B, Pavesi FC, Perotti C, Domingues RMA, Gomes ME, et al. Topographical and Compositional Gradient Tubular Scaffold for Bone to Tendon Interface Regeneration. Pharmaceutics. 2022; 14(10):2153. https://doi.org/10.3390/pharmaceutics14102153
Chicago/Turabian StyleBianchi, Eleonora, Angela Faccendini, Elena Del Favero, Caterina Ricci, Laura Caliogna, Barbara Vigani, Francesco Claudio Pavesi, Cesare Perotti, Rui M. A. Domingues, Manuela E. Gomes, and et al. 2022. "Topographical and Compositional Gradient Tubular Scaffold for Bone to Tendon Interface Regeneration" Pharmaceutics 14, no. 10: 2153. https://doi.org/10.3390/pharmaceutics14102153






