Blood–Brain Barrier Transport of Transferrin Receptor-Targeted Nanoparticles
Abstract
1. Introduction
2. Passaging of Large Molecules through the Blood–Brain and Blood–CSF Barriers
3. The Transferrin Receptor as Target for Drug Delivery
4. Specific Proteins Expressed by Brain Endothelial Cells Enhance the Binding and Uptake of Antibodies from the Circulation
5. Specific Proteins Expressed by the Brain Endothelium also Facilitate the Binding and Uptake of Targeted Nanoparticles
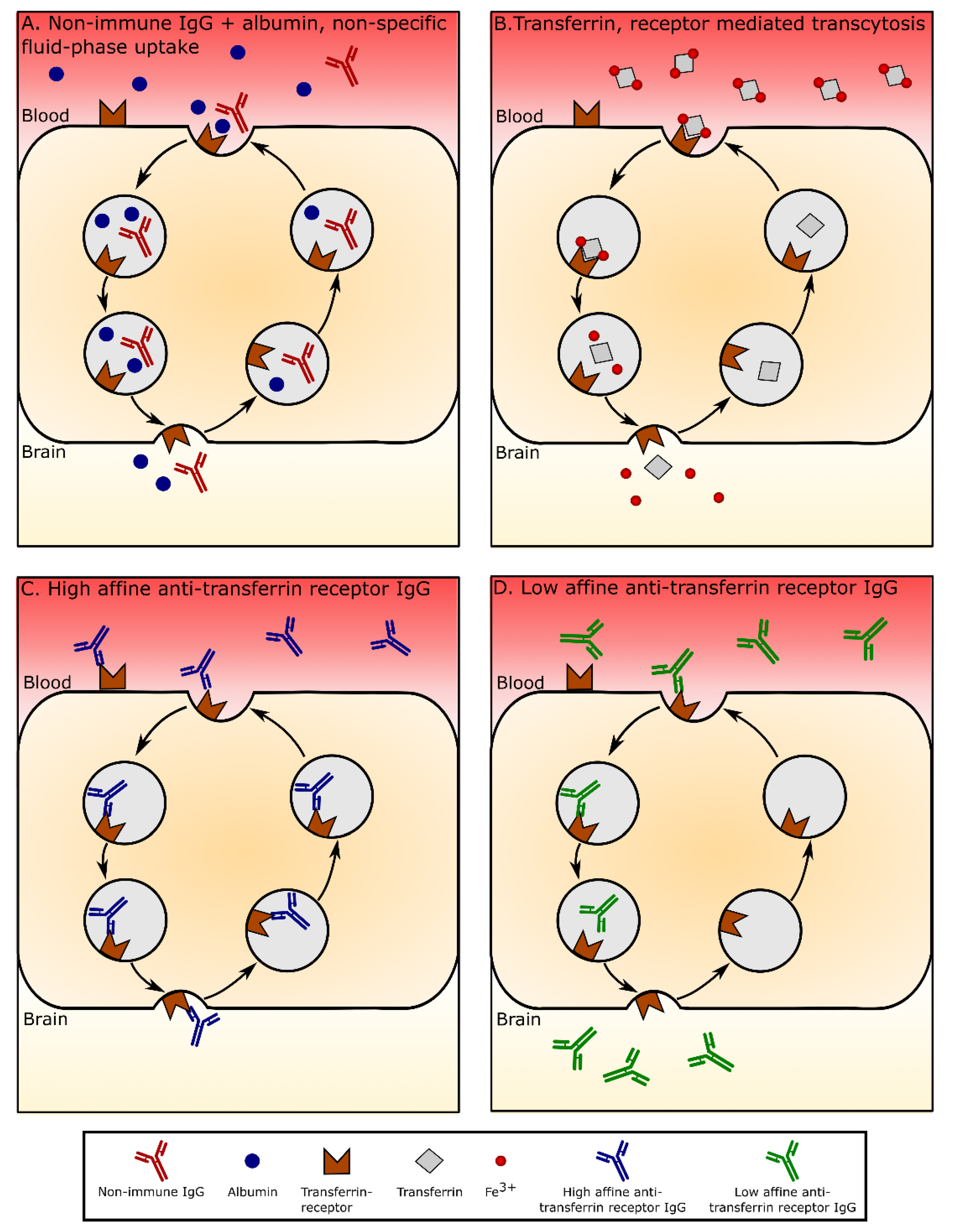
6. Anti-Transferrin Receptor Antibodies Weakened in Affinity or Lowered in Avidity Facilitate Nanoparticle Transport through BECs
7. A Mechanistic Approach to an Understanding of Trafficking of Transferrin Receptor-Targeting Liposomes Based on Studies of Iron-Transferrin and Unconjugated Anti-Transferrin Receptor Antibody Trafficking in BECs
7.1. Blood to Endothelium Transport
7.2. Endothelium to Brain Transport
8. Post-Capillary Venules Denote an Alternate Route for Transport
9. Targeting Nanoparticles to the Brain Endothelium in Pathological Conditions
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Abbott, N.J.; Patabendige, A.A.K.; Dolman, D.E.M.; Yusof, S.R.; Begley, D.J. Structure and Function of the Blood-Brain Barrier. Neurobiol. Dis. 2010, 37, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Pardridge, W.M. CSF, Blood-Brain Barrier, and Brain Drug Delivery. Expert Opin. Drug Deliv. 2016, 13, 963–975. [Google Scholar] [CrossRef] [PubMed]
- Pardridge, W.M. A Historical Review of Brain Drug Delivery. Pharmaceutics 2022, 14, 1283. [Google Scholar] [CrossRef] [PubMed]
- Profaci, C.P.; Munji, R.N.; Pulido, R.S.; Daneman, R. The Blood–Brain Barrier in Health and Disease: Important Unanswered Questions. J. Exp. Med. 2020, 217, e20190062. [Google Scholar] [CrossRef]
- Lichota, J.; Skjorringe, T.; Thomsen, L.B.; Moos, T. Macromolecular Drug Transport into the Brain Using Targeted Therapy. J. Neurochem. 2010, 113, 1–13. [Google Scholar] [CrossRef]
- Lajoie, J.M.; Shusta, E.V. Targeting Receptor-Mediated Transport for Delivery of Biologics across the Blood-Brain Barrier. Annu. Rev. Pharmacol. Toxicol. 2015, 55, 613–631. [Google Scholar] [CrossRef]
- Boado, R.J. IgG Fusion Proteins for Brain Delivery of Biologics via Blood-Brain Barrier Receptor-Mediated Transport. Pharmaceutics 2022, 14, 1476. [Google Scholar] [CrossRef]
- Futtrup, J.; Margolinsky, R.; Benros, M.E.; Moos, T.; Routhe, L.J.; Rungby, J.; Krogh, J. Blood-Brain Barrier Pathology in Patients with Severe Mental Disorders: A Systematic Review and Meta-Analysis of Biomarkers in Case-Control Studies. Brain Behav. Immun.-Health 2020, 6, 100102. [Google Scholar] [CrossRef]
- Johnsen, K.B.; Burkhart, A.; Thomsen, L.B.; Andresen, T.L.; Moos, T. Targeting the Transferrin Receptor for Brain Drug Delivery. Prog. Neurobiol. 2019, 181, 101665. [Google Scholar] [CrossRef]
- Saraiva, C.; Praça, C.; Ferreira, R.; Santos, T.; Ferreira, L.; Bernardino, L. Nanoparticle-Mediated Brain Drug Delivery: Overcoming Blood-Brain Barrier to Treat Neurodegenerative Diseases. J. Control. Release 2016, 235, 34–47. [Google Scholar] [CrossRef]
- Shi, N.; Pardridge, W.M. Noninvasive Gene Targeting to the Brain. Proc. Natl. Acad. Sci. USA 2000, 97, 7567–7572. [Google Scholar] [CrossRef]
- Shi, N.; Zhang, Y.; Zhu, C.; Boado, R.J.; Pardridge, W.M. Brain-Specific Expression of an Exogenous Gene after i.v. Administration. Proc. Natl. Acad. Sci. USA 2001, 98, 12754–12759. [Google Scholar] [CrossRef]
- Zhang, Y.; Schlachetzki, F.; Pardridge, W.M. Global Non-Viral Gene Transfer to the Primate Brain Following Intravenous Administration. Mol. Ther. 2003, 7, 11–18. [Google Scholar] [CrossRef]
- Kucharz, K.; Kristensen, K.; Johnsen, K.B.; Lund, M.A.; Lønstrup, M.; Moos, T.; Andresen, T.L.; Lauritzen, M.J. Post-Capillary Venules Are the Key Locus for Transcytosis-Mediated Brain Delivery of Therapeutic Nanoparticles. Nat. Commun. 2021, 12, 4121. [Google Scholar] [CrossRef]
- Routhe, L.J.; Thomsen, M.S.; Moos, T. The significance of the choroid plexus for cerebral iron homeostasis. In Role of the Choroid Plexus in Health and Disease; Springer: New York, NY, USA, 2020; pp. 125–148. [Google Scholar] [CrossRef]
- Moos, T.; Morgan, E.H. Restricted Transport of Anti-Transferrin Receptor Antibody (OX26) through the Blood-Brain Barrier in the Rat. J. Neurochem. 2001, 79, 119–129. [Google Scholar] [CrossRef]
- Angelova-Gateva, P. Iron Transferrin Receptors in Rat and Human Cerebrum. Agressologie 1980, 1, 27–30. [Google Scholar]
- Jefferies, W.A.; Brandon, M.R.; Hunt, S.V.; Williams, A.F.; Gatter, K.C.; Mason, D.Y. Transferrin Receptor on Endothelium of Brain Capillaries. Nature 1984, 312, 162–163. [Google Scholar] [CrossRef]
- Zuchero, Y.J.Y.; Chen, X.; Bien-Ly, N.; Bumbaca, D.; Tong, R.K.; Gao, X.; Zhang, S.; Hoyte, K.; Luk, W.; Huntley, M.A.; et al. Discovery of Novel Blood-Brain Barrier Targets to Enhance Brain Uptake of Therapeutic Antibodies. Neuron 2016, 89, 70–82. [Google Scholar] [CrossRef]
- Hoyes, K.P.; Morris, I.D.; Hendry, J.H.; Sharma, H.L. Transferrin-Mediated Uptake of Radionuclides by the Testis. J. Nucl. Med. 1996, 37, 336–340. [Google Scholar]
- Pardridge, W.M.; Buciak, J.L.; Friden, P.M. Selective Transport of an Anti-Transferrin Receptor Antibody through the Blood-Brain Barrier in Vivo. J. Pharmacol. Exp. Ther. 1991, 259, 66–70. [Google Scholar]
- Vanlandewijck, M.; He, L.; Mäe, M.A.; Andrae, J.; Ando, K.; Del Gaudio, F.; Nahar, K.; Lebouvier, T.; Laviña, B.; Gouveia, L.; et al. A Molecular Atlas of Cell Types and Zonation in the Brain Vasculature. Nature 2018, 554, 475–480. [Google Scholar] [CrossRef]
- He, L.; Vanlandewijck, M.; Mäe, M.A.; Andrae, J.; Ando, K.; Del Gaudio, F.; Nahar, K.; Lebouvier, T.; Laviña, B.; Gouveia, L.; et al. Single-Cell RNA Sequencing of Mouse Brain and Lung Vascular and Vessel-Associated Cell Types. Sci. Data 2018, 5, 180160. [Google Scholar] [CrossRef]
- Moos, T.; Morgan, E.H. Transferrin and Transferrin Receptor Function in Brain Barrier Systems. Cell. Mol. Neurobiol. 2000, 20, 77–95. [Google Scholar] [CrossRef]
- Morris, C.M.; Keith, A.B.; Edwardson, J.A.; Pullen, R.G.L. Uptake and Distribution of Iron and Transferrin in the Adult Rat Brain. J. Neurochem. 1992, 59, 300–306. [Google Scholar] [CrossRef]
- Hu, Y.B.; Dammer, E.B.; Ren, R.J.; Wang, G. The Endosomal-Lysosomal System: From Acidification and Cargo Sorting to Neurodegeneration. Transl. Neurodegener. 2015, 4, 18. [Google Scholar] [CrossRef]
- Burkhart, A.; Skjorringe, T.; Johnsen, K.B.; Siupka, P.; Thomsen, L.B.; Nielsen, M.S.; Thomsen, L.L.; Moos, T. Expression of Iron-Related Proteins at the Neurovascular Unit Supports Reduction and Reoxidation of Iron for Transport Through the Blood-Brain Barrier. Mol Neurobiol 2015, 53, 7237–7253. [Google Scholar] [CrossRef]
- Fishman, J.B.; Rubin, J.B.; Handrahan, J.V.; Connor, J.R.; Fine, R.E. Receptor-Mediated Transcytosis of Transferrin across the Blood-Brain Barrier. J. Neurosci. Res. 1987, 18, 299–304. [Google Scholar] [CrossRef]
- Skarlatos, S.; Yoshikawa, T.; Pardridge, W.M. Transport of [125I]Transferrin through the Rat Blood-Brain Barrier. Brain Res. 1995, 683, 164–171. [Google Scholar] [CrossRef]
- Taylor, E.M.; Morgan, E.H. Developmental Changes in Transferrin and Iron Uptake by the Brain in the Rat. Brain Res. Dev. Brain Res. 1990, 55, 35–42. [Google Scholar] [CrossRef]
- Morgan, E.H.; Moos, T. Mechanism and Developmental Changes in Iron Transport across the Blood-Brain Barrier. Dev. Neurosci. 2002, 24, 106–113. [Google Scholar] [CrossRef]
- Moos, T.; Morgan, E.H. A Morphological Study of the Developmentally Regulated Transport of Iron into the Brain. Dev. Neurosci. 2002, 24, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Kniesel, U.; Risau, W.; Wolburg, H. Development of Blood-Brain Barrier Tight Junctions in the Rat Cortex. Brain Res. Dev. Brain Res. 1996, 96, 229–240. [Google Scholar] [CrossRef]
- Banks, W.A.; Broadwell, R.D. Blood to Brain and Brain to Blood Passage of Native Horseradish Peroxidase, Wheat Germ Agglutinin, and Albumin: Pharmacokinetic and Morphological Assessments. J. Neurochem. 1994, 62, 2404–2419. [Google Scholar] [CrossRef]
- Balin, B.J.; Broadwell, R.D. Transcytosis of Protein through the Mammalian Cerebral Epithelium and Endothelium. I. Choroid Plexus and the Blood-Cerebrospinal Fluid Barrier. J. Neurocytol. 1988, 17, 809–826. [Google Scholar] [CrossRef] [PubMed]
- Friden, P.M.; Walus, L.R.; Musso, G.F.; Taylor, M.A.; Malfroy, B.; Starzyk, R.M. Anti-Transferrin Receptor Antibody and Antibody-Drug Conjugates Cross the Blood-Brain Barrier. Proc. Natl. Acad. Sci. USA 1991, 88, 4771–4775. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.U.; Friden, P.; Moran, M.; Olson, T.; Kang, Y.S.; Pardridge, W.M.; Morrison, S.L. Transferrin-Antibody Fusion Proteins Are Effective in Brain Targeting. Proc. Natl. Acad. Sci. USA 1995, 92, 2820–2824. [Google Scholar] [CrossRef]
- Frank, H.J.L.; Pardridge, W.M. A Direct in Vitro Demonstration of Insulin Binding to Isolated Brain Microvessels. Diabetes 1981, 30, 757–761. [Google Scholar] [CrossRef]
- Paris-Robidas, S.; Emond, V.; Tremblay, C.; Soulet, D.; Calon, F. In Vivo Labeling of Brain Capillary Endothelial Cells after Intravenous Injection of Monoclonal Antibodies Targeting the Transferrin Receptor. Mol. Pharmacol. 2011, 80, 32–39. [Google Scholar] [CrossRef]
- Yu, Y.J.; Zhang, Y.; Kenrick, M.; Hoyte, K.; Luk, W.; Lu, Y.; Atwal, J.; Elliott, J.M.; Prabhu, S.; Watts, R.J.; et al. Boosting Brain Uptake of a Therapeutic Antibody by Reducing Its Affinity for a Transcytosis Target. Sci. Transl. Med. 2011, 3, 84ra44. [Google Scholar] [CrossRef]
- Niewoehner, J.; Bohrmann, B.; Collin, L.; Urich, E.; Sade, H.; Maier, P.; Rueger, P.; Stracke, J.O.; Lau, W.; Tissot, A.C.; et al. Increased Brain Penetration and Potency of a Therapeutic Antibody Using a Monovalent Molecular Shuttle. Neuron 2014, 81, 49–60. [Google Scholar] [CrossRef]
- Kariolis, M.S.; Wells, R.C.; Getz, J.A.; Kwan, W.; Mahon, C.S.; Tong, R.; Kim, D.J.; Srivastava, A.; Bedard, C.; Henne, K.R.; et al. Brain Delivery of Therapeutic Proteins Using an Fc Fragment Blood-Brain Barrier Transport Vehicle in Mice and Monkeys. Sci. Transl. Med. 2020, 12, eaay1359. [Google Scholar] [CrossRef]
- Sonoda, H.; Morimoto, H.; Yoden, E.; Koshimura, Y.; Kinoshita, M.; Golovina, G.; Takagi, H.; Yamamoto, R.; Minami, K.; Mizoguchi, A.; et al. A Blood-Brain-Barrier-Penetrating Anti-Human Transferrin Receptor Antibody Fusion Protein for Neuronopathic Mucopolysaccharidosis II. Mol. Ther. 2018, 26, 1366–1374. [Google Scholar] [CrossRef]
- Sehlin, D.; Fang, X.T.; Cato, L.; Antoni, G.; Lannfelt, L.; Syvänen, S. Antibody-Based PET Imaging of Amyloid Beta in Mouse Models of Alzheimer’s Disease. Nat. Commun. 2016, 7, 10759. [Google Scholar] [CrossRef]
- Hultqvist, G.; Syvänen, S.; Fang, X.T.; Lannfelt, L.; Sehlin, D. Bivalent Brain Shuttle Increases Antibody Uptake by Monovalent Binding to the Transferrin Receptor. Theranostics 2017, 7, 308–318. [Google Scholar] [CrossRef]
- Stocki, P.; Szary, J.; Rasmussen, C.L.M.; Demydchuk, M.; Northall, L.; Logan, D.B.; Gauhar, A.; Thei, L.; Moos, T.; Walsh, F.S.; et al. Blood-Brain Barrier Transport Using a High Affinity, Brain-Selective VNAR Antibody Targeting Transferrin Receptor 1. FASEB J. 2021, 35, e21172. [Google Scholar] [CrossRef]
- Arguello, A.; Mahon, C.S.; Calvert, M.E.K.; Chan, D.; Dugas, J.C.; Pizzo, M.E.; Thomsen, E.R.; Chau, R.; Damo, L.A.; Duque, J.; et al. Molecular Architecture Determines Brain Delivery of a Transferrin Receptor-Targeted Lysosomal Enzyme. J. Exp. Med. 2022, 219, e20211057. [Google Scholar] [CrossRef]
- Boado, R.J.; Li, J.Y.; Nagaya, M.; Zhang, C.; Pardridge, W.M. Selective Expression of the Large Neutral Amino Acid Transporter at the Blood-Brain Barrier. Proc. Natl. Acad. Sci. USA 1999, 96, 12079–12084. [Google Scholar] [CrossRef]
- Pardridges, W.M.; Boado, R.J.; Farrell, C.R. Brain-Type Glucose Transporter (GLUT-1) Is Selectively Localized to the Blood-Brain Barrier. Studies with Quantitative Western Blotting and in Situ Hybridization. J. Biol. Chem. 1990, 265, 18035–18040. [Google Scholar] [CrossRef]
- Pardridge, W.M. The Isolated Brain Microvessel: A Versatile Experimental Model of the Blood-Brain Barrier. Front. Physiol. 2020, 11, 398. [Google Scholar] [CrossRef]
- Gosk, S.; Vermehren, C.; Storm, G.; Moos, T. Targeting Anti-Transferrin Receptor Antibody (OX26) and OX26-Conjugated Liposomes to Brain Capillary Endothelial Cells Using in Situ Perfusion. J. Cereb. Blood Flow Metab. 2004, 24, 1193–1204. [Google Scholar] [CrossRef]
- Van Rooy, I.; Mastrobattista, E.; Storm, G.; Hennink, W.E.; Schiffelers, R.M. Comparison of Five Different Targeting Ligands to Enhance Accumulation of Liposomes into the Brain. J. Control. Release 2011, 150, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Johnsen, K.B.; Burkhart, A.; Melander, F.; Kempen, P.J.; Vejlebo, J.B.; Siupka, P.; Nielsen, M.S.; Andresen, T.L.; Moos, T. Targeting Transferrin Receptors at the Blood-Brain Barrier Improves the Uptake of Immunoliposomes and Subsequent Cargo Transport into the Brain Parenchyma. Sci. Rep. 2017, 7, 10396. [Google Scholar] [CrossRef] [PubMed]
- Johnsen, K.B.; Bak, M.; Kempen, P.J.; Melander, F.; Burkhart, A.; Thomsen, M.S.; Nielsen, M.S.; Moos, T.; Andresen, T.L. Antibody Affinity and Valency Impact Brain Uptake of Transferrin Receptor-Targeted Gold Nanoparticles. Theranostics 2018, 8, 3416–3436. [Google Scholar] [CrossRef] [PubMed]
- Paris-Robidas, S.; Brouard, D.; Emond, V.; Parent, M.; Calon, F. Internalization of Targeted Quantum Dots by Brain Capillary Endothelial Cells in Vivo. J. Cereb. Blood Flow Metab. 2016, 36, 731–742. [Google Scholar] [CrossRef]
- Michaelis, K.; Hoffmann, M.M.; Dreis, S.; Herbert, E.; Alyautdin, R.N.; Michaelis, M.; Kreuter, J.; Langer, K. Covalent Linkage of Apolipoprotein e to Albumin Nanoparticles Strongly Enhances Drug Transport into the Brain. J. Pharmacol. Exp. Ther. 2006, 317, 1246–1253. [Google Scholar] [CrossRef]
- Gaillard, P.J.; Visser, C.C.; de Boer, A.G. Targeted Delivery across the Blood-Brain Barrier. Expert Opin. Drug Deliv. 2005, 2, 299–309. [Google Scholar] [CrossRef]
- Shao, K.; Huang, R.; Li, J.; Han, L.; Ye, L.; Lou, J.; Jiang, C. Angiopep-2 Modified PE-PEG Based Polymeric Micelles for Amphotericin B Delivery Targeted to the Brain. J. Control. Release 2010, 147, 118–126. [Google Scholar] [CrossRef]
- Johnsen, K.B.; Bak, M.; Melander, F.; Thomsen, M.S.; Burkhart, A.; Kempen, P.J.; Andresen, T.L.; Moos, T. Modulating the Antibody Density Changes the Uptake and Transport at the Blood-Brain Barrier of Both Transferrin Receptor-Targeted Gold Nanoparticles and Liposomal Cargo. J. Control. Release 2019, 295, 237–249. [Google Scholar] [CrossRef]
- Kolhar, P.; Anselmo, A.C.; Gupta, V.; Pant, K.; Prabhakarpandian, B.; Ruoslahti, E.; Mitragotri, S. Using Shape Effects to Target Antibody-Coated Nanoparticles to Lung and Brain Endothelium. Proc. Natl. Acad. Sci. USA 2013, 110, 10753–10758. [Google Scholar] [CrossRef]
- Sepand, M.R.; Ghavami, M.; Zanganeh, S.; Stacks, S.; Ghasemi, F.; Montazeri, H.; Corbo, C.; Derakhshankhah, H.; Ostad, S.N.; Ghahremani, M.H.; et al. Impact of Plasma Concentration of Transferrin on Targeting Capacity of Nanoparticles. Nanoscale 2020, 12, 4935–4944. [Google Scholar] [CrossRef]
- Kristensen, K.; Münter, R.; Kempen, P.J.; Thomsen, M.E.; Stensballe, A.; Andresen, T.L. Isolation Methods Commonly Used to Study the Liposomal Protein Corona Suffer from Contamination Issues. Acta Biomater. 2021, 130, 460–472. [Google Scholar] [CrossRef]
- Kristensen, K.; Urquhart, A.J.; Thormann, E.; Andresen, T.L. Binding of Human Serum Albumin to PEGylated Liposomes: Insights into Binding Numbers and Dynamics by Fluorescence Correlation Spectroscopy. Nanoscale 2016, 8, 19726–19736. [Google Scholar] [CrossRef]
- Kristensen, K.; Engel, T.B.; Stensballe, A.; Simonsen, J.B.; Andresen, T.L. The Hard Protein Corona of Stealth Liposomes Is Sparse. J. Control. Release 2019, 307, 1–15. [Google Scholar] [CrossRef]
- Dos Santos Rodrigues, B.; Lakkadwala, S.; Kanekiyo, T.; Singh, J. Dual-Modified Liposome for Targeted and Enhanced Gene Delivery into Mice Brain. J. Pharmacol. Exp. Ther. 2020, 374, 354–365. [Google Scholar] [CrossRef]
- Dos Santos Rodrigues, B.; Arora, S.; Kanekiyo, T.; Singh, J. Efficient Neuronal Targeting and Transfection Using RVG and Transferrin-Conjugated Liposomes. Brain Res. 2020, 1734, 146738. [Google Scholar] [CrossRef]
- Dos Santos Rodrigues, B.; Kanekiyo, T.; Singh, J. In Vitro and in Vivo Characterization of CPP and Transferrin Modified Liposomes Encapsulating PDNA. Nanomedicine 2020, 28, 102225. [Google Scholar] [CrossRef]
- Mojarad-Jabali, S.; Farshbaf, M.; Hemmati, S.; Sarfraz, M.; Motasadizadeh, H.; Shahbazi Mojarrad, J.; Atyabi, F.; Zakeri-Milani, P.; Valizadeh, H. Comparison of Three Synthetic Transferrin Mimetic Small Peptides to Promote the Blood-Brain Barrier Penetration of Vincristine Liposomes for Improved Glioma Targeted Therapy. Int. J. Pharm. 2022, 613, 121395. [Google Scholar] [CrossRef]
- Bien-Ly, N.; Yu, Y.J.; Bumbaca, D.; Elstrott, J.; Boswell, C.A.; Zhang, Y.; Luk, W.; Lu, Y.; Dennis, M.S.; Weimer, R.M.; et al. Transferrin Receptor (TfR) Trafficking Determines Brain Uptake of TfR Antibody Affinity Variants. J. Exp. Med. 2014, 211, 233–244. [Google Scholar] [CrossRef]
- Toth, A.E.; Holst, M.R.; Nielsen, M.S. Vesicular Transport Machinery in Brain Endothelial Cells: What We Know and What We Do Not. Curr. Pharm. Des. 2020, 26, 1405–1416. [Google Scholar] [CrossRef]
- Haqqani, A.S.; Delaney, C.E.; Brunette, E.; Baumann, E.; Farrington, G.K.; Sisk, W.; Eldredge, J.; Ding, W.; Tremblay, T.L.; Stanimirovic, D.B. Endosomal Trafficking Regulates Receptor-Mediated Transcytosis of Antibodies across the Blood Brain Barrier. J. Cereb. Blood Flow Metab. 2018, 38, 727–740. [Google Scholar] [CrossRef]
- Haqqani, A.S.; Delaney, C.E.; Tremblay, T.L.; Sodja, C.; Sandhu, J.K.; Stanimirovic, D.B. Method for Isolation and Molecular Characterization of Extracellular Microvesicles Released from Brain Endothelial Cells. Fluids Barriers CNS 2013, 10, 4. [Google Scholar] [CrossRef]
- Moos, T.; Gudbergsson, J.M.; Johnsen, K.B. Transport of Transferrin Receptor-Targeted Antibodies through the Blood-Brain Barrier for Drug Delivery to the Brain. AAPS Adv. Pharm. Sci. Ser. 2022, 33, 527–549. [Google Scholar] [CrossRef]
- Kucharz, K.; Kutuzov, N.; Zhukov, O.; Mathiesen Janiurek, M.; Lauritzen, M. Shedding Light on the Blood-Brain Barrier Transport with Two-Photon Microscopy In Vivo. Pharm. Res. 2022, 39, 1457–1468. [Google Scholar] [CrossRef]
- Nagy, Z.; Peters, H.; Huttner, I. Fracture Faces of Cell Junctions in Cerebral Endothelium during Normal and Hyperosmotic Conditions. Lab. Investig. 1984, 50, 313–322. [Google Scholar]
- Elegbede, A.I.; Banerjee, J.; Hanson, A.J.; Tobwala, S.; Ganguli, B.; Wang, R.; Lu, X.; Srivastava, D.K.; Mallik, S. Mechanistic Studies of the Triggered Release of Liposomal Contents by Matrix Metalloproteinase-9. J. Am. Chem. Soc. 2008, 130, 10633–10642. [Google Scholar] [CrossRef]
- Zhao, B.Q.; Wang, S.; Kim, H.Y.; Storrie, H.; Rosen, B.R.; Mooney, D.J.; Wang, X.; Lo, E.H. Role of Matrix Metalloproteinases in Delayed Cortical Responses after Stroke. Nat. Med. 2006, 12, 441–445. [Google Scholar] [CrossRef]
- Bruch, G.E.; Fernandes, L.F.; Bassi, B.L.T.; Alves, M.T.R.; Pereira, I.O.; Frézard, F.; Massensini, A.R. Liposomes for Drug Delivery in Stroke. Brain Res. Bull. 2019, 152, 246–256. [Google Scholar] [CrossRef]
- Muralikrishna Adibhatla, R.; Hatcher, J.F.; Dempsey, R.J. Phospholipase A2, Hydroxyl Radicals, and Lipid Peroxidation in Transient Cerebral Ischemia. Antioxid. Redox Signal. 2003, 5, 647–654. [Google Scholar] [CrossRef]
- Heidari, P.; Blayney, S.; Butler, J.; Hitomi, E.; Luby, M.; Leigh, R. The Relationship Between Penumbral Tissue and Blood-Brain Barrier Disruption in Acute Stroke Patients Presenting in an Extended Time Window. Front. Neurol. 2020, 11, 582994. [Google Scholar] [CrossRef]
- Olsman, M.; Sereti, V.; Mühlenpfordt, M.; Johnsen, K.B.; Andresen, T.L.; Urquhart, A.J.; de Lange Davies, C. Focused Ultrasound and Microbubble Treatment Increases Delivery of Transferrin Receptor-Targeting Liposomes to the Brain. Ultrasound Med. Biol. 2021, 47, 1343–1355. [Google Scholar] [CrossRef]
- Hall, C.N.; Reynell, C.; Gesslein, B.; Hamilton, N.B.; Mishra, A.; Sutherland, B.A.; O’Farrell, F.M.; Buchan, A.M.; Lauritzen, M.; Attwell, D. Capillary Pericytes Regulate Cerebral Blood Flow in Health and Disease. Nature 2014, 508, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Grubb, S.; Lauritzen, M.; Aalkjær, C. Brain Capillary Pericytes and Neurovascular Coupling. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2021, 254, 110893. [Google Scholar] [CrossRef] [PubMed]
- Marcos-Contreras, O.A.; Greineder, C.F.; Kiseleva, R.Y.; Parhiz, H.; Walsh, L.R.; Zuluaga-Ramirez, V.; Myerson, J.W.; Hood, E.D.; Villa, C.H.; Tombacz, I.; et al. Selective Targeting of Nanomedicine to Inflamed Cerebral Vasculature to Enhance the Blood–Brain Barrier. Proc. Natl. Acad. Sci. USA 2020, 117, 3405–3414. [Google Scholar] [CrossRef] [PubMed]
- Bourassa, P.; Alata, W.; Tremblay, C.; Paris-Robidas, S.; Calon, F. Transferrin Receptor-Mediated Uptake at the Blood-Brain Barrier Is Not Impaired by Alzheimer’s Disease Neuropathology. Mol. Pharm. 2019, 16, 583–594. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thomsen, M.S.; Johnsen, K.B.; Kucharz, K.; Lauritzen, M.; Moos, T. Blood–Brain Barrier Transport of Transferrin Receptor-Targeted Nanoparticles. Pharmaceutics 2022, 14, 2237. https://doi.org/10.3390/pharmaceutics14102237
Thomsen MS, Johnsen KB, Kucharz K, Lauritzen M, Moos T. Blood–Brain Barrier Transport of Transferrin Receptor-Targeted Nanoparticles. Pharmaceutics. 2022; 14(10):2237. https://doi.org/10.3390/pharmaceutics14102237
Chicago/Turabian StyleThomsen, Maj Schneider, Kasper Bendix Johnsen, Krzysztof Kucharz, Martin Lauritzen, and Torben Moos. 2022. "Blood–Brain Barrier Transport of Transferrin Receptor-Targeted Nanoparticles" Pharmaceutics 14, no. 10: 2237. https://doi.org/10.3390/pharmaceutics14102237
APA StyleThomsen, M. S., Johnsen, K. B., Kucharz, K., Lauritzen, M., & Moos, T. (2022). Blood–Brain Barrier Transport of Transferrin Receptor-Targeted Nanoparticles. Pharmaceutics, 14(10), 2237. https://doi.org/10.3390/pharmaceutics14102237





