The Improved Brain-Targeted Drug Delivery of Edaravone Temperature-Sensitive Gels by Ultrasound for γ-ray Radiation-Induced Brain Injury
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Animals
2.3. Ultrasound Processing
2.4. Prescription Optimization of EDA TSGs
2.5. Characterization of EDA TSGs
2.5.1. Determination of Gelation Temperature and Gelation Time
2.5.2. Rheological Properties Investigation
2.5.3. Syringe Ability of EDA TSGs
2.5.4. The Micromorphology of EDA TSGs
2.6. Model Establishment of RBI and Administration Scheme
2.7. Behavior Evaluation
2.7.1. Spontaneous Behavior of Mice Evaluated by the Open Field Test
2.7.2. Learning and Memory of Mice Evaluated by the Novel Object Recognition Test
2.7.3. Anxiety of Mice Evaluated by the Elevated plus Maze Test
2.7.4. Reflex to Conditional Memory of Mice Assessed with the Fear Conditioning Test
2.8. H.E. Staining of Mice Brain
2.9. Expression of MDA and IL-6 in the Hippocampus of Brain
2.10. Data Analysis
3. Results and Discussion
3.1. Optimal Prescription of EDA TSGs
3.2. Rheological Properties of EDA TSGs
3.2.1. The Viscoelastic Modulus and the Compound Viscosity Varied with Temperature
3.2.2. The Viscoelastic Modulus and Compound Viscosity Varied with Frequency
3.3. Good Syringe Ability of EDA TSGs
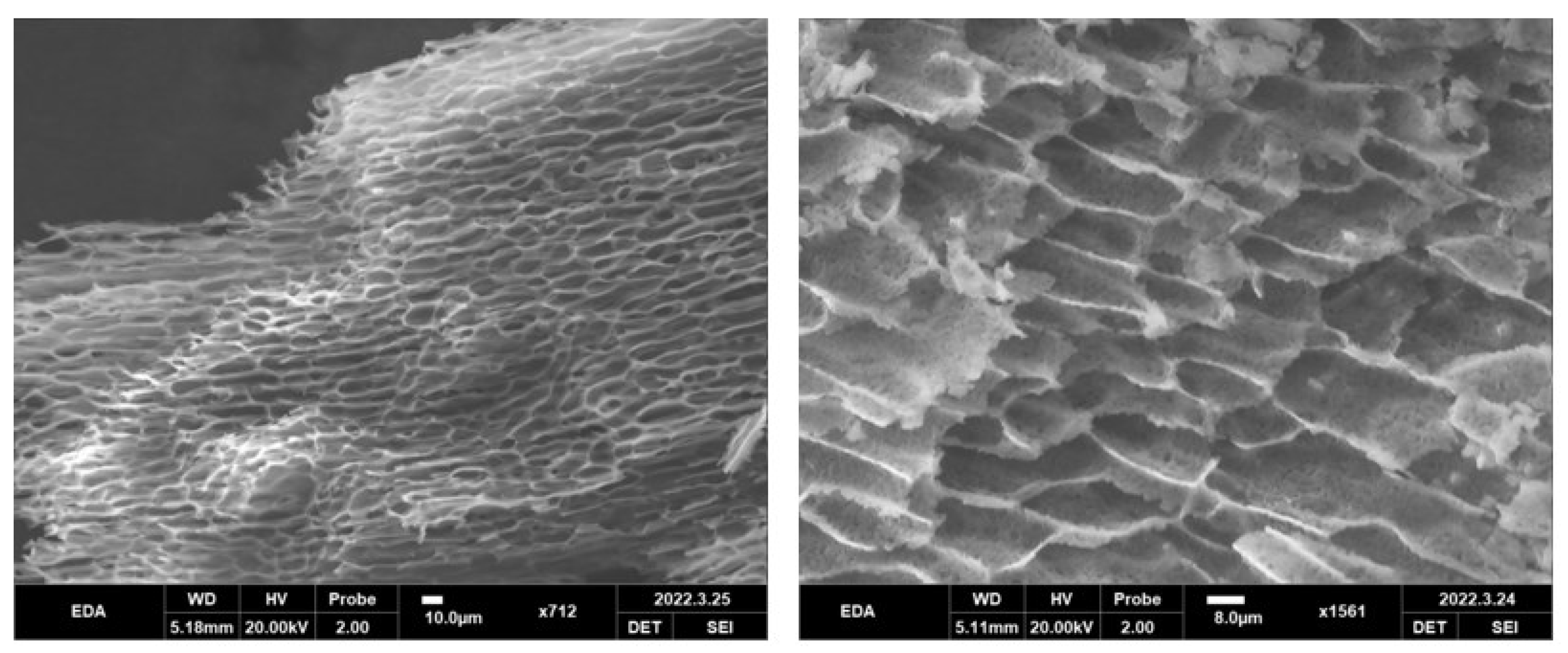
3.4. Micromorphology of EDA TSGs
3.5. In Vivo Safety of Blank TSGs
3.6. Pharmacodynamics Evaluation
3.6.1. The Open Field Test to Detect the Spontaneous Behavior
3.6.2. The Novel Object Recognition Test to Detect Learning and Memory
3.6.3. The Elevated plus Maze Test to Detect Anxiety
3.6.4. The Fear Conditioning Test to Detect the Reflex to Conditional Memory of Mice
3.6.5. Histopathological Evaluation of Hippocampus in Brain
3.6.6. Decreased Expression of MDA and IL-6
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Katalin, L.; Tünde, S.; Géza, S. Ionizing Radiation-Induced Immune and Inflammatory Reactions in the Brain. Front. Immunol. 2017, 8, 517. [Google Scholar]
- Smart, D. Radiation Toxicity in the Central Nervous System: Mechanisms and Strategies for Injury Reduction. Semin. Radiat. Oncol. 2017, 27, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Motallebzadeh, E.; Tameh, A.A.; Zavareh, S.A.T.; Farhood, B.; Aliasgharzedeh, A.; Mohseni, M. Neuroprotective effect of melatonin on radiation-induced oxidative stress and apoptosis in the brainstem of rats. J. Cell. Physiol. 2020, 235, 8791–8798. [Google Scholar] [CrossRef] [PubMed]
- Na, A.; Haghigi, N.; Drummond, K.J. Cerebral radiation necrosis. Asia-Pac. J. Clin. Oncol. 2014, 10, 11–21. [Google Scholar] [CrossRef]
- Yang, L.; Yang, J.; Li, G.; Li, Y.; Wu, R.; Cheng, J.; Tang, Y. Pathophysiological Responses in Rat and Mouse Models of Radiation-Induced Brain Injury. Mol. Neurobiol. 2017, 54, 1022–1032. [Google Scholar] [CrossRef] [PubMed]
- Turnquist, C.; Harris, B.T.; Harris, C.C. Radiation-induced brain injury: Current concepts and therapeutic strategies targeting neuroinflammation. Neuro. Oncol. Adv. 2020, 2, vdaa057. [Google Scholar] [CrossRef]
- Lee, X.-R.; Xiang, G.-L. Effects of edaravone, the free radical scavenger, on outcomes in acute cerebral infarction patients treated with ultra-early thrombolysis of recombinant tissue plasminogen activator. Clin. Neurol. Neurosurg. 2018, 167, 157–161. [Google Scholar] [CrossRef]
- Fidalgo, M.; Pires, J.R.; Viseu, I.; Magalhães, P.; Gregório, H.; Afreixo, V.; Gregório, T. Edaravone for acute ischemic stroke–Systematic review with meta-analysis. Clin. Neurol. Neurosurg. 2022, 219, 107299. [Google Scholar] [CrossRef]
- Yoshino, H.; Kimura, A. Investigation of the therapeutic effects of edaravone, a free radical scavenger, on amyotrophic lateral sclerosis (Phase II study). Amyotroph. Lateral Scler. 2006, 7, 247–251. [Google Scholar] [CrossRef]
- Dang, R.; Wang, M.; Li, X.; Wang, H.; Liu, L.; Wu, Q.; Zhao, J.; Ji, P.; Zhong, L.; Licinio, J.; et al. Edaravone ameliorates depressive and anxiety-like behaviors via Sirt1/Nrf2/HO-1/Gpx4 pathway. J. Neuroinflamm. 2022, 19, 41. [Google Scholar] [CrossRef]
- Romeiro, T.H.; Da Silva, S.C.; Beggiora, P.D.S.; Sampaio, G.B.; Brandão, R.A.; Santos, M.V.; Machado, H.R.; Lopes, L.D.S. The association of Edaravone with shunt surgery improves behavioral performance, reduces astrocyte reaction and apoptosis, and promotes neuroprotection in young hydrocephalic rats. J. Chem. Neuroanat. 2021, 119, 102059. [Google Scholar] [CrossRef] [PubMed]
- Uppuluri, C.T.; Ravi, P.R.; Dalvi, A.; Shaikh, S.S.; Kale, S.R. Piribedil loaded thermo-responsive nasal in situ gelling system for enhanced delivery to the brain: Formulation optimization, physical characterization, and in vitro and in vivo evaluation. Drug Deliv. Transl. Res. 2020, 11, 909–926. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.; Wang, R.; Xing, Y.; Gao, Y.; Zhang, Q.; Xing, B.; Zhang, Y.; Yu, C.; Cai, X.; Shang, Q. Development and evaluation of Panax notoginseng saponins contained in an in situ pH-triggered gelling system for sustained ocular posterior segment drug delivery. Acupunct. Herb. Med. 2021, 1, 107–121. [Google Scholar] [CrossRef]
- Lin, L.; Gan, Y.; Zhang, X.X.; Wang, C.G.; Liu, Q.; Pan, W.S. Preparation and in vitro evaluation of thermo-and ion-sensitive in situ gels of vibavirin via intranasal administration. J. Shenyang Pharm. Univ. 2009, 77, 599–622. [Google Scholar]
- Kong, X.; Xu, W.; Zhang, C.; Kong, W. Chitosan temperature-sensitive gel loaded with drug microspheres has excellent effectiveness, biocompatibility and safety as an ophthalmic drug delivery system. Exp. Ther. Med. 2018, 15, 1442–1448. [Google Scholar] [CrossRef]
- Amer, Z.; Mahdi, H.Z.; Alhamadany, A.T. Formulation and Evaluation of ocular in-Situ gelling system containing ciprofloxacin and Naproxen Sodium. Res. J. Pharm. Technol. 2021, 14, 91–95. [Google Scholar]
- Pang, L.; Zhu, S.; Ma, J.; Zhu, L.; Liu, Y.; Ou, G.; Li, R.; Wang, Y.; Liang, Y.; Jin, X.; et al. Intranasal temperature-sensitive hydrogels of cannabidiol inclusion complex for the treatment of post-traumatic stress disorder. Acta Pharm. Sin. B 2021, 11, 2031–2047. [Google Scholar] [CrossRef]
- Zhang, D.Y.; Dmello, C.; Chen, L.; Arrieta, V.A.; Gonzalez-Buendia, E.; Kane, J.R.; Magnusson, L.P.; Baran, A.; James, C.D.; Horbinski, C.; et al. Ultrasound-mediated Delivery of Paclitaxel for Glioma: A Comparative Study of Distribution, Toxicity, and Efficacy of Albumin-bound Versus Cremophor Formulations. Clin. Cancer Res. 2020, 26, 477–486. [Google Scholar] [CrossRef]
- Endo-Takahashi, Y.; Kurokawa, R.; Sato, K.; Takizawa, N.; Katagiri, F.; Hamano, N.; Suzuki, R.; Maruyama, K.; Nomizu, M.; Takagi, N.; et al. Ternary Complexes of pDNA, Neuron-Binding Peptide, and PEGylated Polyethyleneimine for Brain Delivery with Nano-Bubbles and Ultrasound. Pharmaceutics 2021, 13, 1003. [Google Scholar] [CrossRef]
- Gu, F.; Fan, H.; Cong, Z.; Li, S.; Wang, Y.; Wu, C. Preparation, characterization, and in vivo pharmacokinetics of thermosensitive in situ nasal gel of donepezil hydrochloride. Acta Pharm. 2020, 70, 411–422. [Google Scholar] [CrossRef]
- Hao, J.; Zhao, J.; Zhang, S.; Tong, T.; Zhuang, Q.; Jin, K.; Chen, W.; Tang, H. Fabrication of an ionic-sensitive in situ gel loaded with resveratrol nanosuspensions intended for direct nose-to-brain delivery. Colloids Surf. B Biointerfaces 2016, 147, 376–386. [Google Scholar] [CrossRef] [PubMed]
- Tobin, K.; Fiegel, J.; Brogden, N. Thermosensitive Gels Used to Improve Microneedle-Assisted Transdermal Delivery of Naltrexone. Polymers 2021, 13, 933. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Wang, C.; Sun, Y.; Pang, L.; Zhu, S.; Liu, Y.; Zhu, L.; Zhang, S.; Wang, L.; Du, L. Comparative study of oral and intranasal puerarin for prevention of brain injury induced by acute high-altitude hypoxia. Int. J. Pharm. 2020, 591, 120002. [Google Scholar] [CrossRef] [PubMed]
- Hyungtaek, K.; Dahee, S.; Ngo, H.V.; Gang, J.; Chulhun, P.; Jun-Bom, P.; Beom-Jin, L. Modulation of the clinically accessible gelation time using glucono-d-lactone and pyridoxal 5′-phosphate for long-acting alginate in situ forming gel injectable. Carbohydr. Polym. 2021, 272, 118453. [Google Scholar]
- Zhuang, B.; Chen, T.; Huang, Y.; Xiao, Z.; Jin, Y. Chemo-photothermal immunotherapy for eradication of orthotopic tumors and inhibition of metastasis by intratumoral injection of polydopamine versatile hydrogels. Acta Pharm. Sin. B 2022, 2, 1447–1459. [Google Scholar] [CrossRef]
- Liu, G.; Nie, Y.; Huang, C.; Zhu, G.; Zhang, X.; Hu, C.; Li, Z.; Gao, Y.; Ma, Z. Ferulic acid produces neuroprotection against radiation-induced neuroinflammation by affecting NLRP3 inflammasome activation. Int. J. Radiat. Biol. 2022, 98, 1442–1451. [Google Scholar] [CrossRef]
- Khadrawy, Y.A.; Khoder, N.M.; Sawie, H.G.; Sharada, H.M.; Hosny, E.N.; Abdulla, M.S. The neuroprotective effect of alpha lipoic acid and/or metformin against the behavioral and neurochemical changes induced by hypothyroidism in rat. Neuroendocrinology 2022, 112, 1129–1142. [Google Scholar] [CrossRef]
- Lakshmi, R.; Bill, M.; Mei, H.; Yoshihiro, O.; Herbert, Y.M. The novel object recognition test in rodents in relation to cognitive impairment in schizophrenia. Curr. Pharm. Des. 2014, 20, 5104–5114. [Google Scholar]
- Jihye, L.; Huiyoung, K.; Eunbi, C.; Jieun, J.; InKyu, L.; WanSeob, C.; Jin, P.S.; Seungheon, L.; Hyun, K.D.; Wook, J.J. Hydrangea macrophylla and Thunberginol C Attenuate Stress-Induced Anxiety in Mice. Antioxidants 2022, 11, 234. [Google Scholar]
- Norrholm, S.D.; Jovanovic, T. Fear Processing, Psychophysiology, and PTSD. Harv. Rev. Psychiatry 2018, 26, 129–141. [Google Scholar] [CrossRef]
- Mehla, J.; Lacoursiere, S.; Stuart, E.; McDonald, R.J.; Mohajerani, M.H. Gradual Cerebral Hypoperfusion Impairs Fear Conditioning and Object Recognition Learning and Memory in Mice: Potential Roles of Neurodegeneration and Cholinergic Dysfunction. J. Alzheimer’s Dis. 2017, 61, 283–293. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Zhang, S.; Yu, X.; Zhu, S.; Ou, G.; Li, Q.; Zhang, Y.; Wang, L.; Zhuang, X.; Du, L.; et al. Application of armodafinil-loaded microneedle patches against the negative influence induced by sleep deprivation. Eur. J. Pharm. Biopharm. 2021, 169, 178–188. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Diao, P.; Shu, X.; Li, L.; Xiong, L. Quercetin and Quercitrin Attenuates the Inflammatory Response and Oxidative Stress in LPS-Induced RAW264.7 Cells: In Vitro Assessment and a Theoretical Model. Biomed. Res. Int. 2019, 2019, 7039802. [Google Scholar] [CrossRef] [PubMed]
- Dumortier, G.; Grossiord, J.L.; Agnely, F.; Chaumeil, J.C. A Review of Poloxamer 407 Pharmaceutical and Pharmacological Characteristics. Pharm. Res. 2006, 23, 2709–2728. [Google Scholar] [CrossRef]
- Aurea, P.; Ningrui, L.; Lechtenber, K.J.; Rosenberg, J.; Airan, R.D.; James, M.L.; Bouley, M.D.; Pauly, B.K. Histologic evaluation of activation of acute inflammatory response in a mouse model following ultrasound-mediated blood-brain barrier using different acoustic pressures and microbubble doses. Nanotheranostics 2020, 4, 210–223. [Google Scholar]
- Howard, J.T.; Janak, J.C.; Bukhman, V.; Robertson, C.; Frolov, I.; Nawn, C.D.; Schiller, A.M.; Convertino, V.A. The neurovascular complexity index as a potential indicator of traumatic brain injury severity: A case-series study. J. Trauma Acute Care Surg. 2017, 83 (Suppl. S1), S77–S82. [Google Scholar] [CrossRef]
- Wang, J.; Pan, H.; Lin, Z.; Xiong, C.; Wei, C.; Li, H.; Tong, F.; Dong, X. Neuroprotective Effect of Fractalkine on Radiation-induced Brain Injury Through Promoting the M2 Polarization of Microglia. Mol. Neurobiol. 2020, 58, 1074–1087. [Google Scholar] [CrossRef]
- Krausz, A.D.; Korley, F.K.; Burns, M.A. A Variable Height Microfluidic Device for Multiplexed Immunoassay Analysis of Traumatic Brain Injury Biomarkers. Biosensors 2021, 11, 320. [Google Scholar] [CrossRef]











| Number | Poloxamer (%) | Temperature (°C) | ||||||
|---|---|---|---|---|---|---|---|---|
| 407 | 188 | 25 | 30 | 32 | 34 | 36 | 37 | |
| 1 | 18 | 3 | unset, good liquidity | unset, good liquidity | unset, good liquidity | unset, good liquidity | 5 min unset | 5.17 min solidify |
| 2 | 20 | 3.3 | good liquidity | 5 min unset | 3 min solidify | 2 min solidify | 1.33 min solidify | |
| 3 | 22 | 3.7 | good liquidity | 3 min solidify | 1.67 min solidify | 1 min 15 s solidify | 50 s solidify | 50 s solidify |
| 4 | 24 | 4 | poor liquidity, unset | 1.67 min solidify | 1 min solidify | 50 s solidify | 30 s solidify | 30 s solidify |
| 5 | 30 | 5 | 2 min solidify | 50 s solidify | 50 s solidify | 40 s solidify | 30 s solidify | 20 s solidify |
| 6 | 40 | 6.7 | solidified at 16 °C | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Q.; Zhang, Y.; Hu, J.; Yuan, B.; Zhang, P.; Wang, Y.; Jin, X.; Du, L.; Jin, Y. The Improved Brain-Targeted Drug Delivery of Edaravone Temperature-Sensitive Gels by Ultrasound for γ-ray Radiation-Induced Brain Injury. Pharmaceutics 2022, 14, 2281. https://doi.org/10.3390/pharmaceutics14112281
Li Q, Zhang Y, Hu J, Yuan B, Zhang P, Wang Y, Jin X, Du L, Jin Y. The Improved Brain-Targeted Drug Delivery of Edaravone Temperature-Sensitive Gels by Ultrasound for γ-ray Radiation-Induced Brain Injury. Pharmaceutics. 2022; 14(11):2281. https://doi.org/10.3390/pharmaceutics14112281
Chicago/Turabian StyleLi, Qian, Yizhi Zhang, Jinglu Hu, Bochuan Yuan, Pengcheng Zhang, Yaxin Wang, Xu Jin, Lina Du, and Yiguang Jin. 2022. "The Improved Brain-Targeted Drug Delivery of Edaravone Temperature-Sensitive Gels by Ultrasound for γ-ray Radiation-Induced Brain Injury" Pharmaceutics 14, no. 11: 2281. https://doi.org/10.3390/pharmaceutics14112281
APA StyleLi, Q., Zhang, Y., Hu, J., Yuan, B., Zhang, P., Wang, Y., Jin, X., Du, L., & Jin, Y. (2022). The Improved Brain-Targeted Drug Delivery of Edaravone Temperature-Sensitive Gels by Ultrasound for γ-ray Radiation-Induced Brain Injury. Pharmaceutics, 14(11), 2281. https://doi.org/10.3390/pharmaceutics14112281






