Targeting Emerging Pathogenic Mechanisms by Natural Molecules as Potential Therapeutics for Neurodegenerative Diseases
Abstract
:1. Introduction
2. Emerging Pathogenic Mechanisms Targeted by Natural Molecules
2.1. Ferroptosis
2.2. Energy Metabolism Disorders
2.3. Autophagy-Lysosomal Dysfunction
2.4. Endoplasmatic Reticulum Stress
2.5. Gut Dysbiosis
3. Intracerebral Administration Strategies for Natural Molecules
4. Clinical Trails of Natural Molecules for NDD Treatment
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fu, H.; Hardy, J.; Duff, K.E. Selective vulnerability in neurodegenerative diseases. Nat. Neurosci. 2018, 21, 1350–1358. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Xia, X.; Basnet, D.; Zheng, J.C.; Huang, J.; Liu, J. Mechanisms of ferroptosis and emerging links to the pathology of neurodegenerative diseases. Front. Aging Neurosci. 2022, 14, 904152. [Google Scholar] [CrossRef] [PubMed]
- Cunnane, S.C.; Trushina, E.; Morland, C.; Prigione, A.; Casadesus, G.; Andrews, Z.B.; Beal, M.F.; Bergersen, L.H.; Brinton, R.D.; de la Monte, S.; et al. Brain energy rescue: An emerging therapeutic concept for neurodegenerative disorders of ageing. Nat. Rev. Drug Discov. 2020, 19, 609–633. [Google Scholar] [CrossRef] [PubMed]
- Bastien, J.; Menon, S.; Messa, M.; Nyfeler, B. Molecular targets and approaches to restore autophagy and lysosomal capacity in neurodegenerative disorders. Mol. Asp. Med. 2021, 82, 101018. [Google Scholar] [CrossRef] [PubMed]
- Esmaeili, Y.; Yarjanli, Z.; Pakniya, F.; Bidram, E.; Los, M.J.; Eshraghi, M.; Klionsky, D.J.; Ghavami, S.; Zarrabi, A. Targeting autophagy, oxidative stress, and ER stress for neurodegenerative disease treatment. J. Control. Release 2022, 345, 147–175. [Google Scholar] [CrossRef] [PubMed]
- Chidambaram, S.B.; Essa, M.M.; Rathipriya, A.G.; Bishir, M.; Ray, B.; Mahalakshmi, A.M.; Tousif, A.H.; Sakharkar, M.K.; Kashyap, R.S.; Friedland, R.P.; et al. Gut dysbiosis, defective autophagy and altered immune responses in neurodegenerative diseases: Tales of a vicious cycle. Pharmacol. Ther. 2022, 231, 107988. [Google Scholar] [CrossRef] [PubMed]
- Rao, T.; Tan, Z.; Peng, J.; Guo, Y.; Chen, Y.; Zhou, H.; Ouyang, D. The pharmacogenetics of natural products: A pharmacokinetic and pharmacodynamic perspective. Pharmacol. Res. 2019, 146, 104283. [Google Scholar] [CrossRef]
- Su, X.; Zhou, M.; Li, Y.; Zhang, J.; An, N.; Yang, F.; Zhang, G.; Yuan, C.; Chen, H.; Wu, H.; et al. Protective effects of natural products against myocardial ischemia/reperfusion: Mitochondria-targeted therapeutics. Biomed. Pharmacother. 2022, 149, 112893. [Google Scholar] [CrossRef]
- Dong, S.; Guo, X.; Han, F.; He, Z.; Wang, Y. Emerging role of natural products in cancer immunotherapy. Acta Pharm. Sin. B 2022, 12, 1163–1185. [Google Scholar] [CrossRef]
- Rahman, M.H.; Bajgai, J.; Fadriquela, A.; Sharma, S.; Trinh, T.T.; Akter, R.; Jeong, Y.J.; Goh, S.H.; Kim, C.S.; Lee, K.J. Therapeutic potential of natural products in treating neurodegenerative disorders and their future prospects and challenges. Molecules 2021, 26, 5327. [Google Scholar] [CrossRef]
- Ciccone, L.; Vandooren, J.; Nencetti, S.; Orlandini, E. Natural marine and terrestrial compounds as modulators of matrix metalloproteinases-2 (MMP-2) and MMP-9 in Alzheimer’s disease. Pharmaceuticals 2021, 14, 86. [Google Scholar] [CrossRef] [PubMed]
- Malar, D.S.; Prasanth, M.I.; Brimson, J.M.; Sharika, R.; Sivamaruthi, B.S.; Chaiyasut, C.; Tencomnao, T. Neuroprotective properties of green tea (Camellia sinensis) in Parkinson’s disease: A review. Molecules 2020, 25, 3926. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, M.; Lankatillake, C.; Dias, D.A.; Docea, A.O.; Mahomoodally, M.F.; Lobine, D.; Chazot, P.L.; Kurt, B.; Tumer, T.B.; Moreira, A.C.; et al. Impact of natural compounds on neurodegenerative disorders: From preclinical to pharmacotherapeutics. J. Clin. Med. 2020, 9, 1061. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, C.; Liang, Y.; Chen, Y.; Xiong, Y.; She, Y.; Zhong, X.; Chen, H.; Huang, M. Berberine improves cognitive impairment by simultaneously impacting cerebral blood flow and beta-amyloid accumulation in an APP/tau/PS1 mouse model of Alzheimer’s disease. Cells 2021, 10, 1161. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Nehru, B. Curcumin affords neuroprotection and inhibits alpha-synuclein aggregation in lipopolysaccharide-induced Parkinson’s disease model. Inflammopharmacology 2018, 26, 349–360. [Google Scholar] [CrossRef]
- Oliveira, A.M.; Cardoso, S.M.; Ribeiro, M.; Seixas, R.S.; Silva, A.M.; Rego, A.C. Protective effects of 3-alkyl luteolin derivatives are mediated by Nrf2 transcriptional activity and decreased oxidative stress in Huntington’s disease mouse striatal cells. Neurochem. Int. 2015, 91, 1–12. [Google Scholar] [CrossRef]
- Cai, M.; Yang, E.J. Ginsenoside Re attenuates neuroinflammation in a symptomatic ALS animal model. Am. J. Chin. Med. 2016, 44, 401–413. [Google Scholar]
- Moren, C.; deSouza, R.M.; Giraldo, D.M.; Uff, C. Antioxidant therapeutic strategies in neurodegenerative diseases. Int. J. Mol. Sci. 2022, 23, 9328. [Google Scholar] [CrossRef]
- Du, X.X.; Xu, H.M.; Jiang, H.; Song, N.; Wang, J.; Xie, J.X. Curcumin protects nigral dopaminergic neurons by iron-chelation in the 6-hydroxydopamine rat model of Parkinson’s disease. Neurosci. Bull. 2012, 28, 253–258. [Google Scholar] [CrossRef] [Green Version]
- Shao, L.; Dong, C.; Geng, D.; He, Q.; Shi, Y. Ginkgolide B protects against cognitive impairment in senescence-accelerated P8 mice by mitigating oxidative stress, inflammation and ferroptosis. Biochem. Biophys. Res. Commun. 2021, 572, 7–14. [Google Scholar] [CrossRef]
- Yang, S.; Xie, Z.; Pei, T.; Zeng, Y.; Xiong, Q.; Wei, H.; Wang, Y.; Cheng, W. Salidroside attenuates neuronal ferroptosis by activating the Nrf2/HO1 signaling pathway in Abeta1-42-induced Alzheimer’s disease mice and glutamate-injured HT22 cells. Chin. Med. 2022, 17, 82. [Google Scholar] [CrossRef]
- Gunesch, S.; Hoffmann, M.; Kiermeier, C.; Fischer, W.; Pinto, A.F.M.; Maurice, T.; Maher, P.; Decker, M. 7-O-Esters of taxifolin with pronounced and overadditive effects in neuroprotection, anti-neuroinflammation, and amelioration of short-term memory impairment in vivo. Redox. Biol. 2020, 29, 101378. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Zhong, Y.; Gao, C.; Li, J. Myricetin ameliorates scopolamine-induced memory impairment in mice via inhibiting acetylcholinesterase and down-regulating brain iron. Biochem. Biophys. Res. Commun. 2017, 490, 336–342. [Google Scholar] [CrossRef]
- Camandola, S.; Mattson, M.P. Brain metabolism in health, aging, and neurodegeneration. EMBO J. 2017, 36, 1474–1492. [Google Scholar] [CrossRef]
- Olzmann, J.A.; Carvalho, P. Dynamics and functions of lipid droplets. Nat. Rev. Mol. Cell Biol. 2019, 20, 137–155. [Google Scholar] [CrossRef]
- Castellanos, D.B.; Martin-Jimenez, C.A.; Rojas-Rodriguez, F.; Barreto, G.E.; Gonzalez, J. Brain lipidomics as a rising field in neurodegenerative contexts: Perspectives with Machine Learning approaches. Front. Neuroendocrinol. 2021, 61, 100899. [Google Scholar] [CrossRef]
- Yin, F. Lipid metabolism and Alzheimer’s disease: Clinical evidence, mechanistic link and therapeutic promise. FEBS J. 2022, 1–34, online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Su, C.; Feng, H.; Chen, X.; Dong, Y.; Rao, Y.; Ren, Y.; Yang, J.; Shi, J.; Tian, J.; et al. Curcumin regulates insulin pathways and glucose metabolism in the brains of APPswe/PS1dE9 mice. Int. J. Immunopathol. Pharmacol. 2017, 30, 25–43. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Jia, J.; Wang, W.; Hou, T.; Tian, Y.; Wu, Q.; Xu, L.; Wei, Y.; Wang, X. Honokiol alleviates cognitive deficits of Alzheimer’s disease (PS1V97L) transgenic mice by activating mitochondrial SIRT3. J. Alzheimers. Dis. 2018, 64, 291–302. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, S.; Shi, X.; Feng, X. Polydatin alleviates parkinsonism in MPTP-model mice by enhancing glycolysis in dopaminergic neurons. Neurochem. Int. 2020, 139, 104815. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.T.; Sun, S.Q.; Li, Y.; Xu, S.Y.; Gan, S.W.; Xu, J.; Qiu, G.P.; Zhuo, F.; Huang, S.Q.; Jiang, X.L.; et al. Curcumin ameliorates memory deficits by enhancing lactate content and MCT2 expression in APP/PS1 transgenic mouse model of Alzheimer’s disease. Anat. Rec. 2019, 302, 332–338. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Cui, Y.; Yu, Z.; Wang, W.; Cheng, X.; Ji, W.; Guo, S.; Zhou, Q.; Wu, N.; Chen, Y.; et al. Brain endothelial cells maintain lactate homeostasis and control adult hippocampal neurogenesis. Cell Stem Cell 2019, 25, 754–767. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Gao, L.; Zhang, Y.; Su, Y.; Kong, Z.; Wang, D.; Yan, M. Acteoside-improved streptozotocin-induced learning and memory impairment by upregulating hippocampal insulin, glucose transport, and energy metabolism. Phytother. Res. 2021, 35, 392–403. [Google Scholar] [CrossRef]
- Cisternas, P.; Oliva, C.A.; Torres, V.I.; Barrera, D.P.; Inestrosa, N.C. Presymptomatic treatment with andrographolide improves brain metabolic markers and cognitive behavior in a model of early-onset Alzheimer’s disease. Front. Cell Neurosci. 2019, 13, 295. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yang, X.; Wang, S.; Song, S. Ginsenoside Rg3 prevents cognitive impairment by improving mitochondrial dysfunction in the rat model of Alzheimer’s disease. J. Agric. Food Chem. 2019, 67, 10048–10058. [Google Scholar] [CrossRef]
- Zhang, W.; Xu, C.; Sun, J.; Shen, H.M.; Wang, J.; Yang, C. Impairment of the autophagy-lysosomal pathway in Alzheimer’s diseases: Pathogenic mechanisms and therapeutic potential. Acta Pharm. Sin. B 2022, 12, 1019–1040. [Google Scholar] [CrossRef]
- Bonam, S.R.; Tranchant, C.; Muller, S. Autophagy-lysosomal pathway as potential therapeutic target in Parkinson’s disease. Cells 2021, 10, 3547. [Google Scholar] [CrossRef]
- Croce, K.R.; Yamamoto, A. A role for autophagy in Huntington’s disease. Neurobiol. Dis. 2019, 122, 16–22. [Google Scholar] [CrossRef]
- Amin, A.; Perera, N.D.; Beart, P.M.; Turner, B.J.; Shabanpoor, F. Amyotrophic lateral sclerosis and autophagy: Dysfunction and therapeutic targeting. Cells 2020, 9, 2413. [Google Scholar] [CrossRef]
- Jiang, W.; Wei, W.; Gaertig, M.A.; Li, S.; Li, X.J. Therapeutic effect of berberine on Huntington’s disease transgenic mouse model. PLoS ONE 2015, 10, e0134142. [Google Scholar] [CrossRef]
- Huang, M.; Jiang, X.; Liang, Y.; Liu, Q.; Chen, S.; Guo, Y. Berberine improves cognitive impairment by promoting autophagic clearance and inhibiting production of beta-amyloid in APP/tau/PS1 mouse model of Alzheimer’s disease. Exp. Gerontol. 2017, 91, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Chen, Y.; Liang, Y.; Chen, H.; Ji, X.; Huang, M. Berberine mitigates cognitive decline in an Alzheimer’s disease mouse model by targeting both tau hyperphosphorylation and autophagic clearance. Biomed. Pharmacother. 2020, 121, 109670. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Ma, Z. Protective effects of berberine against MPTP-induced dopaminergic neuron injury through promoting autophagy in mice. Food Funct. 2021, 12, 8366–8375. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, R.; del Valle, J.; Modol, L.; Martinez, A.; Granado-Serrano, A.B.; Ramirez-Nunez, O.; Pallas, M.; Portero-Otin, M.; Osta, R.; Navarro, X. Resveratrol improves motoneuron function and extends survival in SOD1(G93A) ALS mice. Neurotherapeutics 2014, 11, 419–432. [Google Scholar]
- Zhang, L.; Park, J.Y.; Zhao, D.; Kwon, H.C.; Yang, H.O. Neuroprotective effect of Astersaponin I against Parkinson’s disease through autophagy induction. Biomol. Ther. 2021, 29, 615–629. [Google Scholar] [CrossRef]
- Gao, L.; Li, X.; Meng, S.; Ma, T.; Wan, L.; Xu, S. Chlorogenic acid alleviates Abeta25-35-induced autophagy and cognitive impairment via the mTOR/TFEB signaling pathway. Drug Des. Devel. Ther. 2020, 14, 1705–1716. [Google Scholar] [CrossRef]
- Chandra, S.; Roy, A.; Jana, M.; Pahan, K. Cinnamic acid activates PPARalpha to stimulate lysosomal biogenesis and lower Amyloid plaque pathology in an Alzheimer’s disease mouse model. Neurobiol. Dis. 2019, 124, 379–395. [Google Scholar] [CrossRef]
- Song, J.X.; Malampati, S.; Zeng, Y.; Durairajan, S.S.K.; Yang, C.B.; Tong, B.C.; Iyaswamy, A.; Shang, W.B.; Sreenivasmurthy, S.G.; Zhu, Z.; et al. A small molecule transcription factor EB activator ameliorates beta-amyloid precursor protein and Tau pathology in Alzheimer’s disease models. Aging Cell 2020, 19, e13069. [Google Scholar] [CrossRef] [Green Version]
- Long, T.; Wu, Q.; Wei, J.; Tang, Y.; He, Y.N.; He, C.L.; Chen, X.; Yu, L.; Yu, C.L.; Law, B.Y.; et al. Ferulic acid exerts neuroprotective effects via autophagy induction in C. elegans and cellular models of Parkinson’s disease. Oxid. Med. Cell. Longev. 2022, 2022, 3723567. [Google Scholar] [CrossRef]
- Choy, K.W.; Murugan, D.; Mustafa, M.R. Natural products targeting ER stress pathway for the treatment of cardiovascular diseases. Pharmacol. Res. 2018, 132, 119–129. [Google Scholar] [CrossRef]
- Li, J.; Yu, H.; Yang, C.; Ma, T.; Dai, Y. Therapeutic potential and molecular mechanisms of echinacoside in neurodegenerative diseases. Front. Pharmacol. 2022, 13, 841110. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Long, H.; Zhou, F.; Zhu, W.; Ruan, J.; Zhao, Y.; Lu, Y. Echinacoside’s nigrostriatal dopaminergic protection against 6-OHDA-Induced endoplasmic reticulum stress through reducing the accumulation of Seipin. J. Cell Mol. Med. 2017, 21, 3761–3775. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Han, G.; Xu, S.; Yuan, Y.; Zhao, C.; Ma, T. Echinacoside suppresses amyloidogenesis and modulates F-actin remodeling by targeting the ER stress sensor PERK in a mouse model of Alzheimer’s disease. Front. Cell Dev. Biol. 2020, 8, 593659. [Google Scholar] [CrossRef] [PubMed]
- Ge, B.; Li, S.L.; Li, F.R. Astragaloside-IV regulates endoplasmic reticulum stress-mediated neuronal apoptosis in a murine model of Parkinson’s disease via the lincRNA-p21/CHOP pathway. Exp. Mol. Pathol. 2020, 115, 104478. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Ye, C.; Chen, Y.; Chen, Y.; Diao, S.; Huang, M. Berberine improves behavioral and cognitive deficits in a mouse model of Alzheimer’s disease via regulation of beta-amyloid production and endoplasmic reticulum stress. ACS Chem. Neurosci. 2021, 12, 1894–1904. [Google Scholar] [CrossRef]
- Wu, Y.; Chen, Q.; Wen, B.; Wu, N.; He, B.; Chen, J. Berberine Reduces Abeta42 Deposition and Tau Hyperphosphorylation via Ameliorating Endoplasmic Reticulum Stress. Front. Pharmacol. 2021, 12, 640758. [Google Scholar] [CrossRef] [PubMed]
- Kou, J.; Wang, M.; Shi, J.; Zhang, H.; Pu, X.; Song, S.; Yang, C.; Yan, Y.; Doring, Y.; Xie, X.; et al. Curcumin reduces cognitive deficits by inhibiting neuroinflammation through the endoplasmic reticulum stress pathway in apolipoprotein E4 transgenic mice. ACS Omega 2021, 6, 6654–6662. [Google Scholar] [CrossRef]
- Du, K.; Liu, M.; Zhong, X.; Yao, W.; Xiao, Q.; Wen, Q.; Yang, B.; Wei, M. Epigallocatechin gallate reduces amyloid beta-induced neurotoxicity via inhibiting endoplasmic reticulum stress-mediated apoptosis. Mol. Nutr. Food Res. 2018, 62, e1700890. [Google Scholar] [CrossRef]
- Tana; Nakagawa, T. Luteolin ameliorates depression-like behaviors by suppressing ER stress in a mouse model of Alzheimer’s disease. Biochem. Biophys. Res. Commun. 2022, 588, 168–174. [Google Scholar] [CrossRef]
- Chen, J.; Deng, X.; Liu, N.; Li, M.; Liu, B.; Fu, Q.; Qu, R.; Ma, S. Quercetin attenuates tau hyperphosphorylation and improves cognitive disorder via suppression of ER stress in a manner dependent on AMPK pathway. J. Funct. Foods 2016, 22, 463–476. [Google Scholar] [CrossRef]
- Gaballah, H.H.; Zakaria, S.S.; Elbatsh, M.M.; Tahoon, N.M. Modulatory effects of resveratrol on endoplasmic reticulum stress-associated apoptosis and oxido-inflammatory markers in a rat model of rotenone-induced Parkinson’s disease. Chem. Biol. Interact. 2016, 251, 10–16. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Ruganzu, J.B.; Lin, C.; Ding, B.; Zheng, Q.; Wu, X.; Ma, R.; Liu, Q.; Wang, Y.; Jin, H.; et al. Tanshinone IIA ameliorates cognitive deficits by inhibiting endoplasmic reticulum stress-induced apoptosis in APP/PS1 transgenic mice. Neurochem. Int. 2020, 133, 104610. [Google Scholar] [CrossRef] [PubMed]
- Sivamaruthi, B.S.; Suganthy, N.; Kesika, P.; Chaiyasut, C. The role of microbiome, dietary supplements, and probiotics in Autism Spectrum Disorder. Int. J. Environ. Res. Public Health 2020, 17, 2647. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Luo, Y.; Ray Chaudhuri, K.; Reynolds, R.; Tan, E.K.; Pettersson, S. The role of gut dysbiosis in Parkinson’s disease: Mechanistic insights and therapeutic options. Brain 2021, 144, 2571–2593. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Liu, L.; Li, X.Y.; Ji, H.F. Regulation of gut microbiota in Alzheimer’s disease mice by silibinin and silymarin and their pharmacological implications. Appl. Microbiol. Biotechnol. 2019, 103, 7141–7149. [Google Scholar] [CrossRef]
- Xie, Z.; Lu, H.; Yang, S.; Zeng, Y.; Li, W.; Wang, L.; Luo, G.; Fang, F.; Zeng, T.; Cheng, W. Salidroside attenuates cognitive dysfunction in senescence-accelerated mouse prone 8 (SAMP8) mice and modulates inflammation of the gut-brain axis. Front. Pharmacol. 2020, 11, 568423. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; Pan, J.; Sun, M.; Fang, Y. Comparative multiomics study of the effects of Ellagic acid on the gut environment in young and adult mice. Food Res. Int. 2022, 161, 111819. [Google Scholar] [CrossRef]
- Garcia-Villalba, R.; Gimenez-Bastida, J.A.; Cortes-Martin, A.; Avila-Galvez, M.A.; Tomas-Barberan, F.A.; Selma, M.V.; Espin, J.C.; Gonzalez-Sarrias, A. Urolithins: A comprehensive update on their metabolism, bioactivity, and associated gut microbiota. Mol. Nutr. Food Res. 2022, e2101019, online ahead of print. [Google Scholar] [CrossRef]
- Li, J.; Meng, P.; Zhang, J.; He, M. Effect of berberine hydrochloride on the diversity of intestinal flora in Parkinson’s disease patients. Contrast Media Mol. Imaging 2022, 2022, 8381870. [Google Scholar] [CrossRef]
- Sun, Z.Z.; Li, X.Y.; Wang, S.; Shen, L.; Ji, H.F. Bidirectional interactions between curcumin and gut microbiota in transgenic mice with Alzheimer’s disease. Appl. Microbiol. Biotechnol. 2020, 104, 3507–3515. [Google Scholar] [CrossRef]
- Fasina, O.B.; Wang, J.; Mo, J.; Osada, H.; Ohno, H.; Pan, W.; Xiang, L.; Qi, J. Gastrodin from gastrodia elata enhances cognitive function and neuroprotection of AD mice via the regulation of gut microbiota composition and inhibition of neuron inflammation. Front. Pharmacol. 2022, 13, 814271. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Ye, T.; Zhang, Y.; Zhang, R.; Kong, Y.; Zhang, Y.; Sun, J. Protective effect of Ginkgolide B against cognitive impairment in mice via regulation of gut microbiota. J. Agric. Food Chem. 2021, 69, 12230–12240. [Google Scholar] [CrossRef]
- Wang, L.; Lu, J.; Zeng, Y.; Guo, Y.; Wu, C.; Zhao, H.; Zheng, H.; Jiao, J. Improving Alzheimer’s disease by altering gut microbiota in tree shrews with ginsenoside Rg1. FEMS Microbiol. Lett. 2020, 367, 4. [Google Scholar] [CrossRef] [PubMed]
- Qu, C.; Li, Q.P.; Su, Z.R.; Ip, S.P.; Yuan, Q.J.; Xie, Y.L.; Xu, Q.Q.; Yang, W.; Huang, Y.F.; Xian, Y.F.; et al. Nano-Honokiol ameliorates the cognitive deficits in TgCRND8 mice of Alzheimer’s disease via inhibiting neuropathology and modulating gut microbiota. J. Adv. Res. 2022, 35, 231–243. [Google Scholar] [CrossRef]
- Li, C.; Wang, N.; Zheng, G.; Yang, L. Oral administration of resveratrol-selenium-peptide nanocomposites alleviates Alzheimer’s disease-like pathogenesis by inhibiting Abeta aggregation and regulating gut microbiota. ACS Appl. Mater. Interfaces 2021, 13, 46406–46420. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Gong, Y.; Xie, W.; Huang, A.; Yuan, X.; Zhou, H.; Zhu, X.; Chen, X.; Liu, J.; Liu, J.; et al. Microbubbles in combination with focused ultrasound for the delivery of quercetin-modified sulfur nanoparticles through the blood brain barrier into the brain parenchyma and relief of endoplasmic reticulum stress to treat Alzheimer’s disease. Nanoscale 2020, 12, 6498–6511. [Google Scholar] [CrossRef] [PubMed]
- Crowe, T.P.; Hsu, W.H. Evaluation of recent intranasal drug delivery systems to the central nervous system. Pharmaceutics 2022, 14, 629. [Google Scholar] [CrossRef]
- Lofts, A.; Abu-Hijleh, F.; Rigg, N.; Mishra, R.K.; Hoare, T. Using the intranasal route to administer drugs to treat neurological and psychiatric illnesses: Rationale, successes, and future needs. CNS Drugs 2022, 36, 739–770. [Google Scholar] [CrossRef]
- Dou, Y.; Zhao, D.; Yang, F.; Tang, Y.; Chang, J. Natural phyto-antioxidant albumin nanoagents to treat advanced Alzheimer’s disease. ACS Appl. Mater. Interfaces 2021, 13, 30373–30382. [Google Scholar] [CrossRef]
- Liu, J.; Liu, C.; Zhang, J.; Zhang, Y.; Liu, K.; Song, J.X.; Sreenivasmurthy, S.G.; Wang, Z.; Shi, Y.; Chu, C.; et al. A self-assembled alpha-synuclein nanoscavenger for Parkinson’s disease. ACS Nano 2020, 14, 1533–1549. [Google Scholar] [CrossRef]
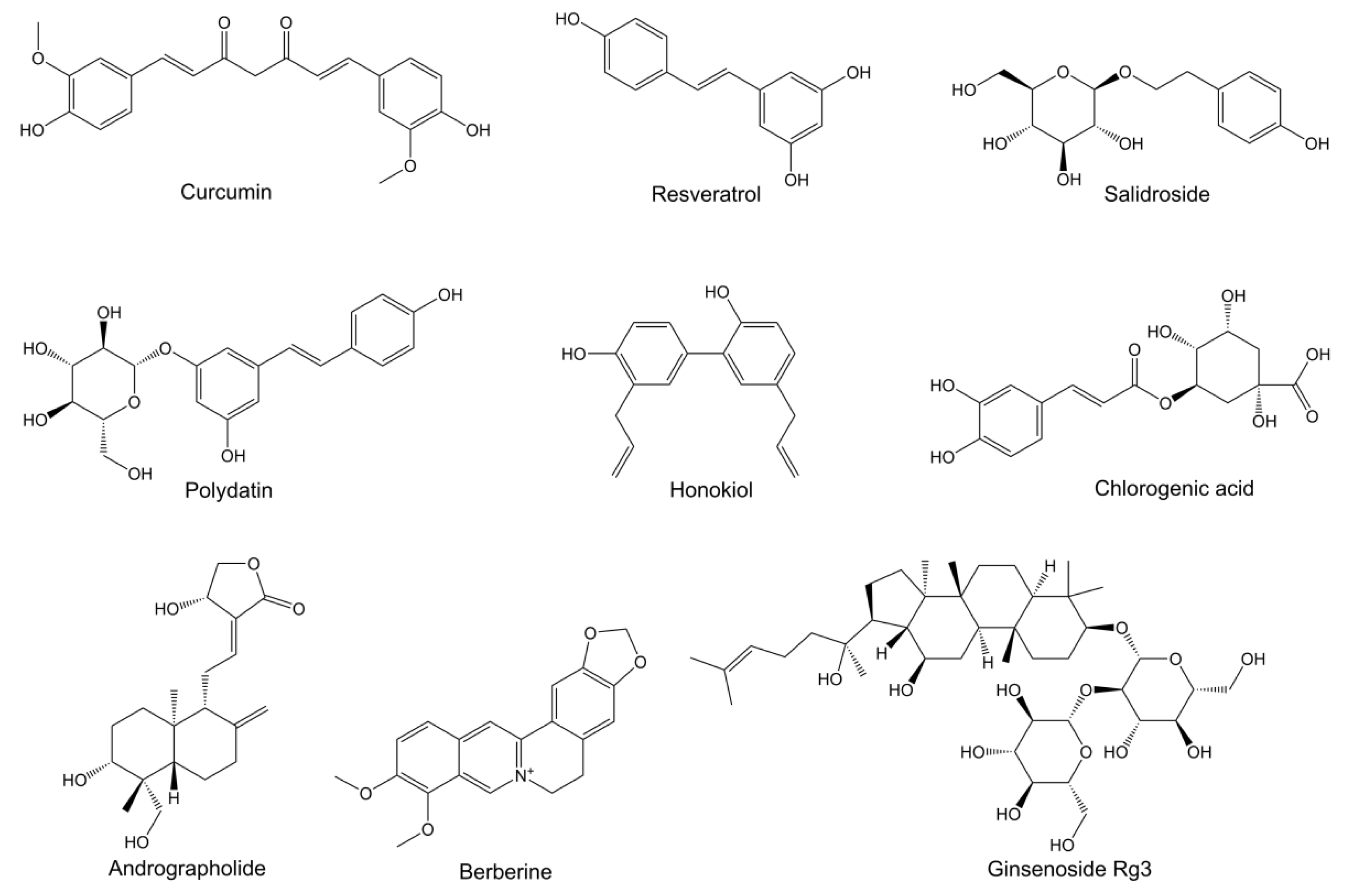
| Compounds | Models | Mechanisms | Administration | Ref. |
|---|---|---|---|---|
| Curcumin | 6-OHDA induced PD rats | Suppressing the iron-induced degeneration of nigral dopaminergic neurons by iron-chelating activity | i.g. | [19] |
| 7-O-Esters of taxifolin | Glutamate-induced HT22 cells; Aβ25-35-induced AD mice | Resisting oxytosis, ferroptosis and ATP depletion | i.p. | [22] |
| Ginkgolide B | SAMP8 mice as AD model | Mitigating ferroptosis by reducing iron content, decreasing TFR1 and NCOA4 expressions, increasing FTH1 expression, and activating the Nrf2/GPX4 signaling pathway | i.g. | [20] |
| Myricetin | Fe2+-induced SH-SY5Y cells; scopolamine-induced AD mice | Downregulating acetylcholinesterase and brain iron content; inhibiting TFR1 expression; increasing antioxidant enzyme activity | i.g. | [23] |
| Salidroside | Glutamate-induced HT22 cells; Aβ1-42-induced AD mice | Reducing lipid peroxidation and ROS levels; increasing GPX4 and SLC7A11 protein expressions; improving mitochondrial ultrastructure; attenuating neuronal ferroptosis by activating the Nrf2/HO1 signaling pathway | i.g. | [21] |
| Compounds | Models | Mechanisms | Administration | Ref. |
|---|---|---|---|---|
| Acteoside | Streptozotocin- induced AD mice | Increasing the protein expression of glucose transporters and glucose levels; enhancing ATP production; reducing the ROS level | i.g. | [33] |
| Andrographolide | J20 Tg mice as AD model | Increasing the uptake and utilization of glucose; enhancing ATP production | i.p. | [34] |
| Curcumin | APP/PS1 mice as AD model | Improving the glucose uptake in cerebrum; increasing the protein expression of GLUT1 and GLUT3; enhancing lactate content | i.g./i.p. | [28,31] |
| Ginsenoside Rg3 | D-galactose-induced AD rats | Resisting the oxidative stress; regulating the energy metabolism-related biomarkers; recovering mitochondrial ETC perturbations | i.g. | [35] |
| Honokiol | Aβ1-42-induced primary hippocampal neurons; PS1V97L Tg mice | Increasing mitochondrial SIRT3 expression; enhancing ATP production; weakening mitochondrial ROS production | i.p. | [29] |
| Polydatin | MPTP-induced PD mice | Inhibiting glycolytic metabolism; increasing pyruvate content; restoring ATP production level | i.g. | [30] |
| Compounds | Models | Mechanisms | Administration | Ref. |
|---|---|---|---|---|
| Astersaponin I | MPP+-induced SH-SY5Y cells; MPTP-induced PD mice | Upregulating autophagy by activating the ERK/mTOR and AMPK/mTOR pathways | i.g. | [45] |
| Berberine | GFP-exon1 HTT containing transfected HEK293 cells; transgenic N171-82Q mice as HD model | Promoting the degradation of mHTT by enhancing autophagic function | i.g. | [40] |
| 3 × Tg AD mice | Reducing Aβ production; facilitating Aβ clearance in an autophagy-dependent manner | oral | [41] | |
| 3 × Tg AD mice | Reducing the accumulation of total tau and hyperphosporylated tau via the Akt/GSK3β pathway; promoting tau clearance by modulating the class III PI3K/Beclin-1 autophagic pathway | oral | [42] | |
| MPP+-induced SH-SY5Y cells; MPTP-induced PD mice | Enhancing autophagy and AMPK phosphorylation | i.g. | [43] | |
| Chlorogenic Acid | Aβ25-35-induced SH-SY5Y cells; APP/PS1 mice as AD model | Enhancing autophagosome–lysosome fusion by decreasing autophagosome production and increasing autolysosome content; enhancing lysosomal activity via the mTOR/TFEB pathway | i.g. | [46] |
| Cinnamic acid | 5xFAD mice | Activating the nuclear hormone receptor PPARα to transcriptionally upregulate TFEB; stimulating lysosomal biogenesis | i.g. | [47] |
| Curcumin analog C1 | 5xFAD mice, P301S mice, and 3xTg-AD mice | Activating TFEB; enhancing autophagy and lysosomal activity; reducing APP, APP CTF-β/α, Aβ peptide and tau aggregate content | oral | [48] |
| Ferulic Acid | Stable GFP-RFP-LC3 U87 cells and PC-12 cells; α-synuclein- and 6-OHDA-induced C. elegans as PD model | Inducing autophagy by inhibiting SQST-1/p62 protein expression and increasing mRNA levels of 3 key autophagy-related genes including lgg-1, vps-34, and unc-51 | medium | [49] |
| Resveratrol | SOD1G93A ALS mice | Increasing expression and activation of SIRT1 and AMPK to promote normalization of the autophagic flux and increase mitochondrial biogenesis | oral | [44] |
| Compounds | Models | Mechanisms | Administration | Ref. |
|---|---|---|---|---|
| Astragaloside-IV | MPP+-induced MN9D cells; MPTP-induced PD mice | Lessening the expression of CHOP protein by restraining the expression of lincRNA-p21 | i.p. | [54] |
| Berberine | 3 × Tg AD mice or APP/PS1 mice | Reducing Aβ production and ER stress by inhibiting PERK/eIF2α-mediated BACE1 translation | oral | [55,56] |
| Curcumin | ApoE4 transgenic mice as AD model | Inhibiting ER stress by reducing GRP78 and IRE1α expression | i.p. | [57] |
| Echinacoside | 6-OHDA-induced PC12 cells and rats as PD model | Relieving ER stress by promoting seipin degradation and GRP94/BIP-ATF4-CHOP pathway; decreasing ROS accumulation | i.p. | [52] |
| Aβ1-42-induced SH-SY5Y cells; APP/PS1 mice as AD model | Reducing Aβ production and deposition by repressing BACE1 activity; inhibiting ER stress via the PERK/eIF2α pathway | i.g. | [53] | |
| Epigallocatechin Gallate | Aβ1-42-induced SH-SY5Y cells; APP/PS1 mice as AD model | Mitigating ER abnormal ultrastructural swelling; downregulating GRP78, CHOP, cleaved-caspase-12 and -3 expressions | oral | [58] |
| Luteolin | APP23 mice as AD model | Preventing ER stress to suppress microglial activation by suppressing CHOP and IL-1β mRNA levels | i.p. | [59] |
| Quercetin | Okadaic acid-induced SH-SY5Y cells; high fat diet-induced AD mice | Suppressing ER stress by decreasing phosphorylation of IRE1α and PERK; inhibiting TXNIP and NLRP3 inflammasome activation; attenuating tau phosphorylation | oral | [60] |
| Resveratrol | Rotenone-induced PD rats | Ameliorating ER stress by downregulating CHOP and GRP78 expressions and hampering caspase-3 activity; restoring redox balance by suppressing xanthine oxidase activity and protein carbonyls formation and activating glutathione peroxidase and Nrf2 signaling pathway | i.g. | [61] |
| Tanshinone IIA | APP/PS1 mice as AD model | Preventing abnormal expression of GRP78, eIF2α, IRE1α, ATF6; suppressing the activation of CHOP and JNK pathways | i.p. | [62] |
| Compounds | Models | Mechanisms | Administration | Ref. |
|---|---|---|---|---|
| Berberine | PD patients | Improving the disorder of intestinal flora and suppressing the expression of inflammatory factors | oral | [69] |
| Curcumin | APP/PS1 mice as AD model | Altering the relative abundances of bacterial taxa | i.g. | [70] |
| Gastrodin | D-galactose-induced AD mice | Changing the gut microbiome composition | i.g. | [71] |
| Ginkgolide B | D-galactose and aluminum chloride-induced AD mice | Reconstructing gut microbiota by reversing the decreased abundance of Lactobacillus and the increased abundance of Bacteroidales, Muribaculaceae, and Alloprevotella | i.g. | [72] |
| Ginsenoside Rg1 | Aβ25–35-induced tree shrews as AD model | Changing the abundance of gut microbiota and increasing lactobacillaceae | i.g. | [73] |
| Salidroside | SAMP8 mice as AD model | Improving the gut barrier integrity and modifying the gut microbiota | i.g. | [66] |
| Silibinin and silymarin | APP/PS1 mice as AD model | Regulating the microbiota diversity and abundance of several key bacterial species associated with AD | i.g. | [65] |
| Natural Molecule-Based Agents | Disease | Status (CT.gov ID) | Phase | Date |
|---|---|---|---|---|
| Caffeine | AD | Recruiting (NCT04570085) | Phase 3 | 2021–2024 |
| Colchicine | ALS | Active, not recruiting (NCT03693781) | Phase 2 | 2019–2022 |
| Combination product: antioxidants | ALS | Recruiting (NCT04244630) | Phase 2 | 2022–2023 |
| Conventional medication and chinese herbal medicine | PD | Not yet recruiting (NCT05001217) | Phase 2, 3 | 2021–2023 |
| Curcumin and yoga | AD | Active, not recruiting (NCT01811381) | Phase 2 | 2014–2020 |
| Dasatinib and quercetin | AD | Active, not recruiting (NCT04063124) | Phase 1, 2 | 2020–2022 |
| Dasatinib and quercetin | AD | Enrolling by invitation (NCT04785300) | Phase 1,2 | 2022–2023 |
| Dasatinib and quercetin | AD | Recruiting (NCT05422885) | Phase 1, 2 | 2022–2023 |
| Dasatinib and quercetin | AD | Recruiting (NCT04685590) | Phase 2 | 2021–2032 |
| DHA | AD | Active, not recruiting (NCT03613844) | Phase 2 | 2018–2025 |
| Flos gossypii flavonoids tablet | AD | Recruiting (NCT05269173) | Phase 2 | 2020–2023 |
| Ganoderma | PD | Recruiting (NCT03594656) | Phase 3 | 2018–2021 |
| Huperzine A | AD | Not yet recruiting (NCT02931136) | Phase 4 | 2019–2025 |
| Icosapent ethyl (IPE) | AD | Active, not recruiting (NCT02719327) | Phase 2, 3 | 2017–2023 |
| Medical cannabis | PD | Recruiting (NCT05106504) | Unknown | 2021–2024 |
| Meganatural-Az grapeseed extract | AD | Active, not recruiting (NCT02033941) | Phase 2 | 2014–2021 |
| Memantine and sodium oligomannate (GV-971) | AD | Not yet recruiting (NCT05430867) | Phase 4 | 2022–2024 |
| Omega-3 | AD | Recruiting (NCT03691519) | Phase 3 | 2018–2023 |
| Omega 3 PUFA | AD | Active, not recruiting (NCT01953705) | Phase 2 | 2014–2021 |
| Rapamycin | AD | Recruiting (NCT04629495) | Phase 2 | 2021–2024 |
| Salsalate | AD | Active, not recruiting (NCT03277573) | Phase 1 | 2017–2021 |
| Scopolamine, atropine, edaravone and dexmedetomidine | ALS | Not yet recruiting (NCT04391361) | Phase 2 | 2020–2023 |
| SLS-005 (Trehalose injection) | AD | Not yet recruiting (NCT05332678) | Phase 2 | 2022–2024 |
| Sodium oligomannate capsules (GV-971) | AD | Recruiting (NCT05058040) | Phase 4 | 2021–2024 |
| Sodium oligomannate (GV-971) | AD | Recruiting (NCT04520412) | Phase 3 | 2020–2026 |
| Sodium oligomannate capsules (GV-971) | AD | Recruiting (NCT05181475) | Phase 4 | 2021–2025 |
| Sulforaphane | PD | Not yet recruiting (NCT05084365) | Phase 2 | 2021–2022 |
| Trehalose | AD | Recruiting (NCT04663854) | Phase 1 | 2020–2022 |
| Trehalose | PD | Not yet recruiting (NCT05355064) | Phase 4 | 2022–2023 |
| Yangxue Qingnao pills | AD | Recruiting (NCT04780399) | Phase 2 | 2021–2024 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dou, Y.; Zhao, D. Targeting Emerging Pathogenic Mechanisms by Natural Molecules as Potential Therapeutics for Neurodegenerative Diseases. Pharmaceutics 2022, 14, 2287. https://doi.org/10.3390/pharmaceutics14112287
Dou Y, Zhao D. Targeting Emerging Pathogenic Mechanisms by Natural Molecules as Potential Therapeutics for Neurodegenerative Diseases. Pharmaceutics. 2022; 14(11):2287. https://doi.org/10.3390/pharmaceutics14112287
Chicago/Turabian StyleDou, Yan, and Dongju Zhao. 2022. "Targeting Emerging Pathogenic Mechanisms by Natural Molecules as Potential Therapeutics for Neurodegenerative Diseases" Pharmaceutics 14, no. 11: 2287. https://doi.org/10.3390/pharmaceutics14112287
APA StyleDou, Y., & Zhao, D. (2022). Targeting Emerging Pathogenic Mechanisms by Natural Molecules as Potential Therapeutics for Neurodegenerative Diseases. Pharmaceutics, 14(11), 2287. https://doi.org/10.3390/pharmaceutics14112287







