An Immunconjugate Vaccine Alters Distribution and Reduces the Antinociceptive, Behavioral and Physiological Effects of Fentanyl in Male and Female Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Drugs
2.3. Antigens and Adjuvants
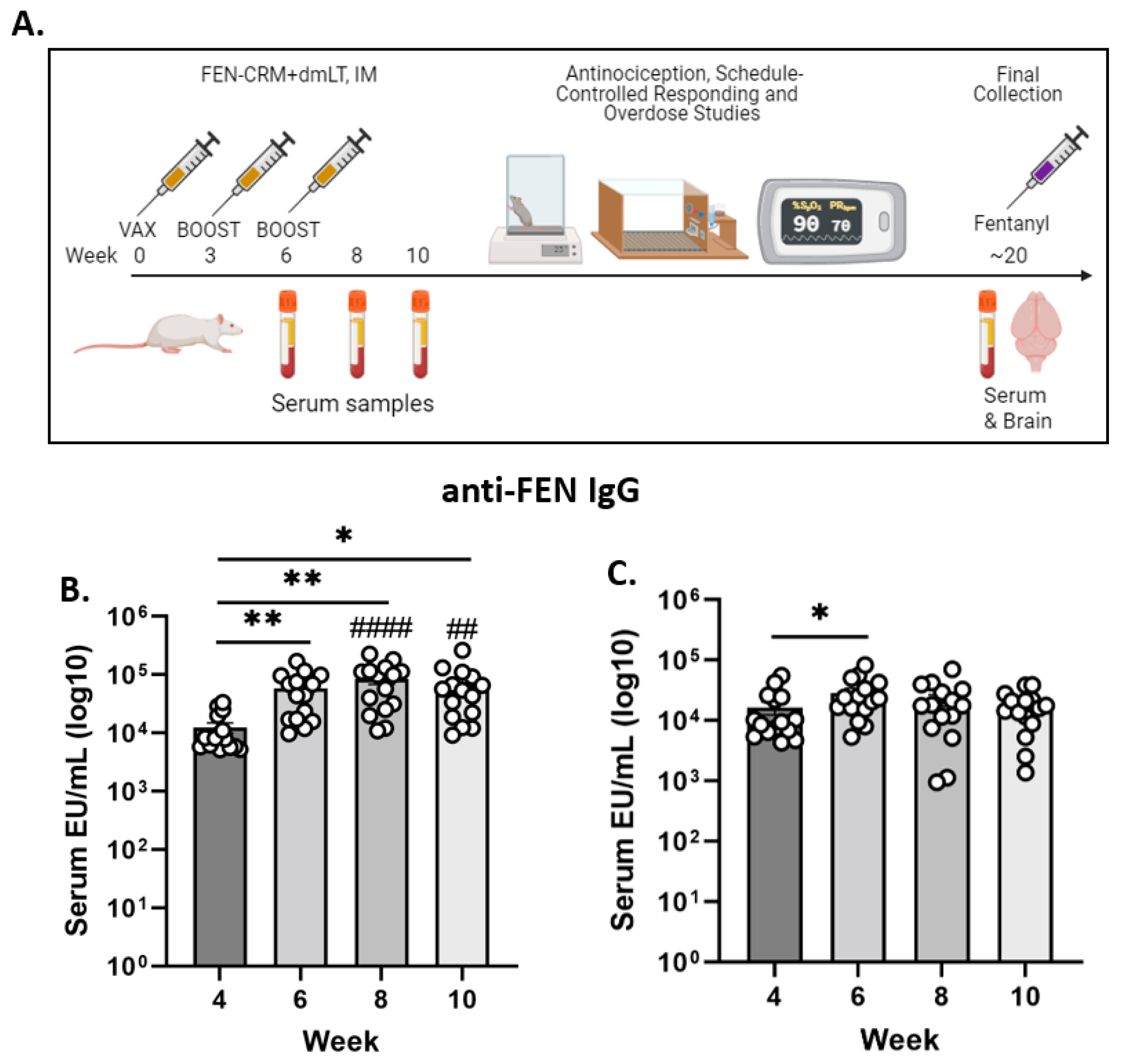
2.4. Immunization and Sample Collection
2.5. Antibody Levels, Cross-Reactivity and Fentanyl Quantification
2.6. Nociception Assays
2.7. Schedule-Controlled Responding
2.8. Physiological Effects
2.9. Statistical Analysis
3. Results
3.1. Anti-FEN Antibody Levels
3.2. Antinociception Tests: Tail Flick and Hot Plate
3.3. Fentanyl Brain Levels
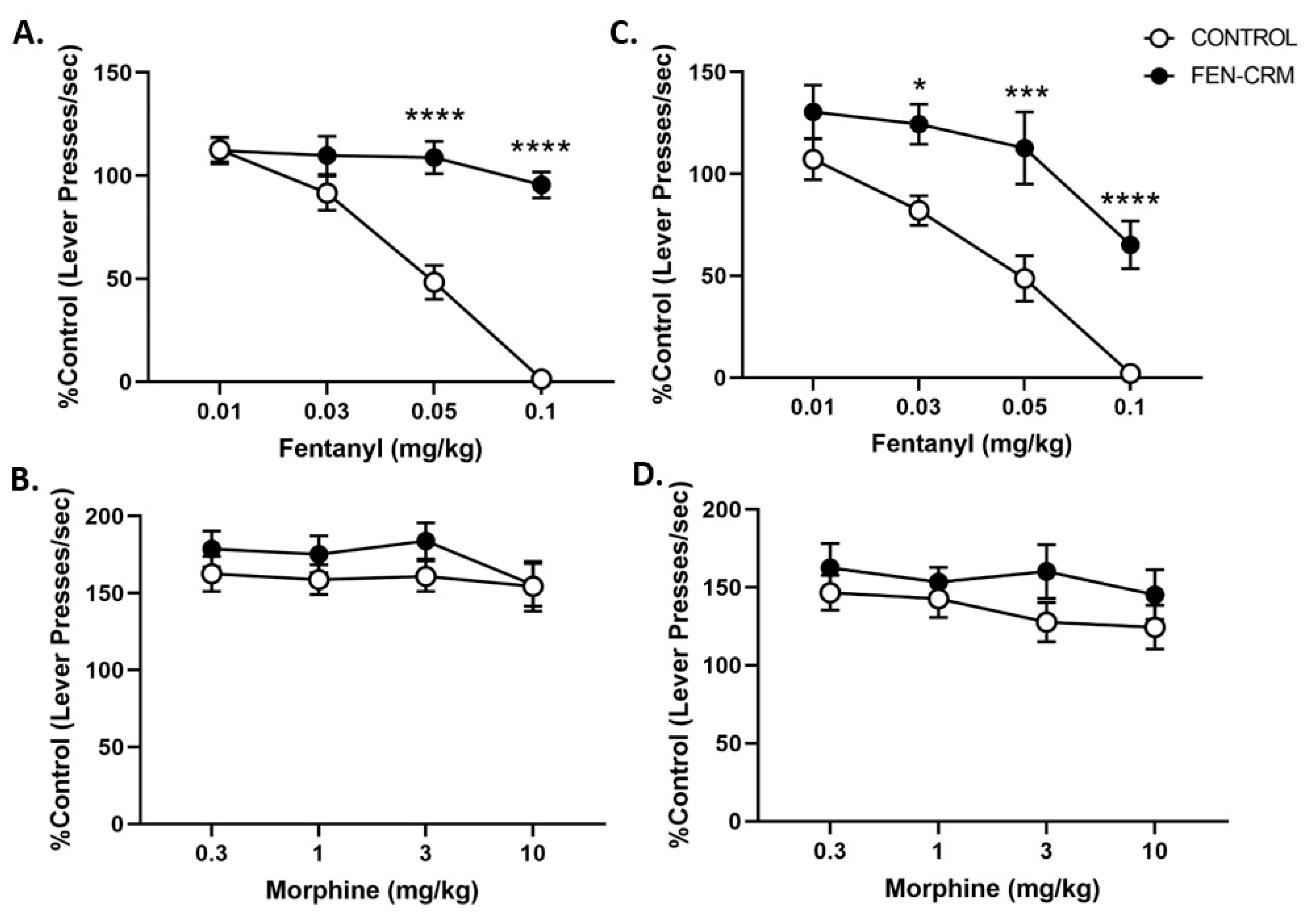
3.4. Schedule-Controlled Responding
3.5. Physiology Study
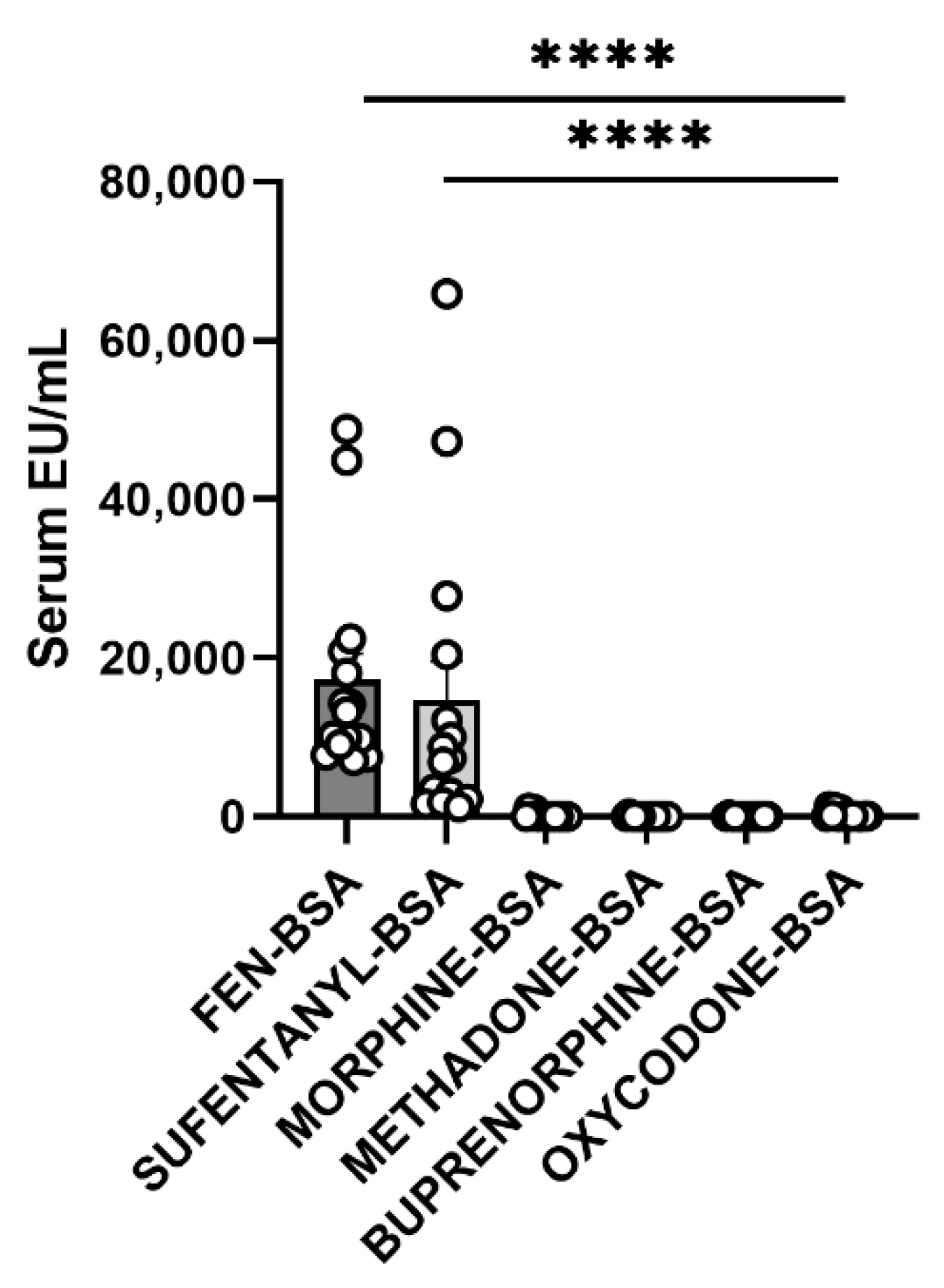
3.6. Cross-Reactivity Assay
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- CDC. Overdose Deaths Accelerating During COVID-19. Available online: https://www.naccho.org/blog/articles/overdose-deaths-accelerating-during-covid-19 (accessed on 8 March 2022).
- NCDAS. Drug Overdose Death Rates. Available online: https://drugabusestatistics.org/drug-overdose-deaths/ (accessed on 8 March 2022).
- Hall, O.T.; Trimble, C.; Garcia, S.; Entrup, P.; Deaner, M.; Teater, J. Unintentional Drug Overdose Mortality in Years of Life Lost Among Adolescents and Young People in the US From 2015 to 2019. JAMA Pediatr. 2022, 176, 415–417. [Google Scholar] [CrossRef] [PubMed]
- Comer, S.D.; Cahill, C.M. Fentanyl: Receptor pharmacology, abuse potential, and implications for treatment. Neurosci. Biobehav. Rev. 2019, 106, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, Y.; Koide, S.; Hirose, N.; Takada, K.; Tomiyama, K.; Koshikawa, N.; Cools, A.R. Fentanyl increases dopamine release in rat nucleus accumbens: Involvement of mesolimbic mu- and delta-2-opioid receptors. Neuroscience 1999, 92, 1357–1365. [Google Scholar] [CrossRef]
- Zacny, J.P.; Lichtor, J.L.; Zaragoza, J.G.; de Wit, H. Subjective and behavioral responses to intravenous fentanyl in healthy volunteers. Psychopharmacology 1992, 107, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.D.; Flynn, G.L. Solubility and related physicochemical properties of narcotic analgesics. Pharm. Res. 1988, 5, 580–586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Armenian, P.; Vo, K.T.; Barr-Walker, J.; Lynch, K.L. Fentanyl, fentanyl analogs and novel synthetic opioids: A comprehensive review. Neuropharmacology 2018, 134, 121–132. [Google Scholar] [CrossRef] [Green Version]
- Jones, C.M.; Einstein, E.B.; Compton, W.M. Changes in Synthetic Opioid Involvement in Drug Overdose Deaths in the United States, 2010–2016. JAMA 2018, 319, 1819–1821. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DEA. DEA Issues Public Safety Alert on Sharp Increase in Fake Prescription Pills Containing Fentanyl and Meth. Available online: https://www.dea.gov/press-releases/2021/09/27/dea-issues-public-safety-alert (accessed on 9 March 2022).
- Wang, Q.Q.; Kaelber, D.C.; Xu, R.; Volkow, N.D. COVID-19 risk and outcomes in patients with substance use disorders: Analyses from electronic health records in the United States. Mol. Psychiatry 2021, 26, 30–39. [Google Scholar] [CrossRef]
- Kosten, T.R.; Petrakis, I.L. The Hidden Epidemic of Opioid Overdoses During the Coronavirus Disease 2019 Pandemic. JAMA Psychiatry 2021, 78, 585–586. [Google Scholar] [CrossRef] [PubMed]
- Krieter, P.A.; Chiang, C.N.; Gyaw, S.; McCann, D.J. Comparison of the Pharmacokinetic Properties of Naloxone Following the Use of FDA-Approved Intranasal and Intramuscular Devices Versus a Common Improvised Nasal Naloxone Device. J. Clin. Pharmacol. 2019, 59, 1078–1084. [Google Scholar] [CrossRef]
- Moss, R.B.; Carlo, D.J. Higher doses of naloxone are needed in the synthetic opiod era. Subst. Abus. Treat. Prev. Policy 2019, 14, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torralva, R.; Janowsky, A. Noradrenergic Mechanisms in Fentanyl-Mediated Rapid Death Explain Failure of Naloxone in the Opioid Crisis. J. Pharmacol. Exp. Ther. 2019, 371, 453–475. [Google Scholar] [CrossRef]
- Pravetoni, M.; Comer, S.D. Development of vaccines to treat opioid use disorders and reduce incidence of overdose. Neuropharmacology 2019, 158, 107662. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, D.H.; Jaffe, J.H.; McNary, S.; Kavanagh, G.J.; Hayes, M.; Currens, M. Short-term outcomes after brief ambulatory opioid detoxification with buprenorphine in young heroin users. Addiction 2003, 98, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Smyth, B.P.; Barry, J.; Keenan, E.; Ducray, K. Lapse and relapse following inpatient treatment of opiate dependence. Ir. Med. J. 2010, 103, 176–179. [Google Scholar]
- Banks, M.L.; Olson, M.E.; Janda, K.D. Immunopharmacotherapies for Treating Opioid Use Disorder. Trends Pharmacol. Sci. 2018, 39, 908–911. [Google Scholar] [CrossRef]
- Heekin, R.D.; Shorter, D.; Kosten, T.R. Current status and future prospects for the development of substance abuse vaccines. Expert Rev. Vaccines 2017, 16, 1067–1077. [Google Scholar] [CrossRef]
- Ohia-Nwoko, O.; Kosten, T.A.; Haile, C.N. Animal Models and the Development of Vaccines to Treat Substance Use Disorders. Int. Rev. Neurobiol. 2016, 126, 263–291. [Google Scholar] [CrossRef]
- Kosten, T.A.; Shen, X.Y.; O’Malley, P.W.; Kinsey, B.M.; Lykissa, E.D.; Orson, F.M.; Kosten, T.R. A morphine conjugate vaccine attenuates the behavioral effects of morphine in rats. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2013, 45, 223–229. [Google Scholar] [CrossRef] [Green Version]
- Robinson, C.; Gradinati, V.; Hamid, F.; Baehr, C.; Crouse, B.; Averick, S.; Kovaliov, M.; Harris, D.; Runyon, S.; Baruffaldi, F.; et al. Therapeutic and Prophylactic Vaccines to Counteract Fentanyl Use Disorders and Toxicity. J. Med. Chem. 2020, 63, 14647–14667. [Google Scholar] [CrossRef] [PubMed]
- Barrientos, R.C.; Bow, E.W.; Whalen, C.; Torres, O.B.; Sulima, A.; Beck, Z.; Jacobson, A.E.; Rice, K.C.; Matyas, G.R. Novel Vaccine That Blunts Fentanyl Effects and Sequesters Ultrapotent Fentanyl Analogues. Mol. Pharm. 2020, 17, 3447–3460. [Google Scholar] [CrossRef] [PubMed]
- Raleigh, M.D.; Baruffaldi, F.; Peterson, S.J.; Le Naour, M.; Harmon, T.M.; Vigliaturo, J.R.; Pentel, P.R.; Pravetoni, M. A Fentanyl Vaccine Alters Fentanyl Distribution and Protects against Fentanyl-Induced Effects in Mice and Rats. J. Pharmacol. Exp. Ther. 2019, 368, 282–291. [Google Scholar] [CrossRef] [PubMed]
- Stone, A.E.; Scheuermann, S.E.; Haile, C.N.; Cuny, G.D.; Velasquez, M.L.; Linhuber, J.P.; Duddupudi, A.L.; Vigliaturo, J.R.; Pravetoni, M.; Kosten, T.A.; et al. Fentanyl conjugate vaccine by injected or mucosal delivery with dmLT or LTA1 adjuvants implicates IgA in protection from drug challenge. NPJ Vaccines 2021, 6, 69. [Google Scholar] [CrossRef] [PubMed]
- El-Kamary, S.S.; Cohen, M.B.; Bourgeois, A.L.; Van De Verg, L.; Bauers, N.; Reymann, M.; Pasetti, M.F.; Chen, W.H. Safety and immunogenicity of a single oral dose of recombinant double mutant heat-labile toxin derived from enterotoxigenic Escherichia coli. Clin. Vaccine Immunol. 2013, 20, 1764–1770. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lundgren, A.; Bourgeois, L.; Carlin, N.; Clements, J.; Gustafsson, B.; Hartford, M.; Holmgren, J.; Petzold, M.; Walker, R.; Svennerholm, A.M. Safety and immunogenicity of an improved oral inactivated multivalent enterotoxigenic Escherichia coli (ETEC) vaccine administered alone and together with dmLT adjuvant in a double-blind, randomized, placebo-controlled Phase I study. Vaccine 2014, 32, 7077–7084. [Google Scholar] [CrossRef] [Green Version]
- Harro, C.; Louis Bourgeois, A.; Sack, D.; Walker, R.; DeNearing, B.; Brubaker, J.; Maier, N.; Fix, A.; Dally, L.; Chakraborty, S.; et al. Live attenuated enterotoxigenic Escherichia coli (ETEC) vaccine with dmLT adjuvant protects human volunteers against virulent experimental ETEC challenge. Vaccine 2019, 37, 1978–1986. [Google Scholar] [CrossRef]
- Bagley, S.M.; Gai, M.J.; Earlywine, J.J.; Schoenberger, S.F.; Hadland, S.E.; Barocas, J.A. Incidence and Characteristics of Nonfatal Opioid Overdose Among Youths Aged 11 to 24 Years by Sex. JAMA Netw. Open 2020, 3, e2030201. [Google Scholar] [CrossRef]
- Zubieta, J.K.; Smith, Y.R.; Bueller, J.A.; Xu, Y.; Kilbourn, M.R.; Jewett, D.M.; Meyer, C.R.; Koeppe, R.A.; Stohler, C.S. mu-opioid receptor-mediated antinociceptive responses differ in men and women. J. Neurosci. 2002, 22, 5100–5107. [Google Scholar] [CrossRef] [Green Version]
- Santoro, G.C.; Carrion, J.; Dewey, S.L. Imaging Sex Differences in Regional Brain Metabolism during Acute Opioid Withdrawal. J. Alcohol. Drug Depend. 2017, 5, 262. [Google Scholar] [CrossRef]
- Wiesenfeld-Hallin, Z. Sex differences in pain perception. Gend. Med. 2005, 2, 137–145. [Google Scholar] [CrossRef]
- Romanescu, M.; Buda, V.; Lombrea, A.; Andor, M.; Ledeti, I.; Suciu, M.; Danciu, C.; Dehelean, C.A.; Dehelean, L. Sex-Related Differences in Pharmacological Response to CNS Drugs: A Narrative Review. J. Pers. Med. 2022, 12, 907. [Google Scholar] [CrossRef]
- Kest, B.; Sarton, E.; Dahan, A. Gender differences in opioid-mediated analgesia: Animal and human studies. Anesthesiology 2000, 93, 539–547. [Google Scholar] [CrossRef] [Green Version]
- Bartok, R.E.; Craft, R.M. Sex differences in opioid antinociception. J. Pharmacol. Exp. Ther. 1997, 282, 769–778. [Google Scholar]
- Peckham, E.M.; Traynor, J.R. Comparison of the antinociceptive response to morphine and morphine-like compounds in male and female Sprague-Dawley rats. J. Pharmacol. Exp. Ther. 2006, 316, 1195–1201. [Google Scholar] [CrossRef] [Green Version]
- Towers, E.B.; Setaro, B.; Lynch, W.J. Sex- and Dose-Dependent Differences in the Development of an Addiction-Like Phenotype Following Extended-Access Fentanyl Self-Administration. Front. Pharmacol. 2022, 13, 841873. [Google Scholar] [CrossRef]
- Townsend, E.A.; Negus, S.S.; Caine, S.B.; Thomsen, M.; Banks, M.L. Sex differences in opioid reinforcement under a fentanyl vs. food choice procedure in rats. Neuropsychopharmacology 2019, 44, 2022–2029. [Google Scholar] [CrossRef] [PubMed]
- Gaulden, A.D.; Burson, N.; Sadik, N.; Ghosh, I.; Khan, S.J.; Brummelte, S.; Kallakuri, S.; Perrine, S.A. Effects of fentanyl on acute locomotor activity, behavioral sensitization, and contextual reward in female and male rats. Drug Alcohol Depend. 2021, 229, 109101. [Google Scholar] [CrossRef] [PubMed]
- Fischinger, S.; Boudreau, C.M.; Butler, A.L.; Streeck, H.; Alter, G. Sex differences in vaccine-induced humoral immunity. Semin. Immunopathol. 2019, 41, 239–249. [Google Scholar] [CrossRef] [Green Version]
- Bremer, P.T.; Kimishima, A.; Schlosburg, J.E.; Zhou, B.; Collins, K.C.; Janda, K.D. Combatting Synthetic Designer Opioids: A Conjugate Vaccine Ablates Lethal Doses of Fentanyl Class Drugs. Angew. Chem. 2016, 55, 3772–3775. [Google Scholar] [CrossRef] [Green Version]
- Guerrieri, D.; Kjellqvist, F.; Kronstrand, R.; Gréen, H. Validation and Cross-Reactivity Data for Fentanyl Analogs with the Immunalysis Fentanyl ELISA. J. Anal. Toxicol. 2019, 43, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Haile, C.N.; Kosten, T.A. Differential effects of D1- and D2-like compounds on cocaine self-administration in Lewis and Fischer 344 inbred rats. J. Pharmacol. Exp. Ther. 2001, 299, 509–518. [Google Scholar] [PubMed]
- Clements, J.D.; Norton, E.B. The Mucosal Vaccine Adjuvant LT(R192G/L211A) or dmLT. mSphere 2018, 3, e00215-18. [Google Scholar] [CrossRef] [PubMed]
- Jalah, R.; Torres, O.B.; Mayorov, A.V.; Li, F.; Antoline, J.F.; Jacobson, A.E.; Rice, K.C.; Deschamps, J.R.; Beck, Z.; Alving, C.R.; et al. Efficacy, but not antibody titer or affinity, of a heroin hapten conjugate vaccine correlates with increasing hapten densities on tetanus toxoid, but not on CRM197 carriers. Bioconjug. Chem. 2015, 26, 1041–1053. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baruffaldi, F.; Kelcher, A.H.; Laudenbach, M.; Gradinati, V.; Limkar, A.; Roslawski, M.; Birnbaum, A.; Lees, A.; Hassler, C.; Runyon, S.; et al. Preclinical Efficacy and Characterization of Candidate Vaccines for Treatment of Opioid Use Disorders Using Clinically Viable Carrier Proteins. Mol. Pharm. 2018, 15, 4947–4962. [Google Scholar] [CrossRef]
- Deuis, J.R.; Dvorakova, L.S.; Vetter, I. Methods Used to Evaluate Pain Behaviors in Rodents. Front. Mol. Neurosci. 2017, 10, 284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Terpstra, G.K.; Raaijmakers, J.A.; Kreukniet, J. Comparison of vaccination of mice and rats with Haemophilus influenzae and Bordetella pertussis as models of atopy. Clin. Exp. Pharmacol. Physiol. 1979, 6, 139–149. [Google Scholar] [CrossRef]
- Martignoni, M.; Groothuis, G.M.; de Kanter, R. Species differences between mouse, rat, dog, monkey and human CYP-mediated drug metabolism, inhibition and induction. Expert Opin. Drug Metab. Toxicol. 2006, 2, 875–894. [Google Scholar] [CrossRef]
- Sakhuja, A.; Sztajnkrycer, M.; Vallabhajosyula, S.; Cheungpasitporn, W.; Patch, R., 3rd; Jentzer, J. National trends and outcomes of cardiac arrest in opioid overdose. Resuscitation 2017, 121, 84–89. [Google Scholar] [CrossRef]
- Pattinson, K.T. Opioids and the control of respiration. Br. J. Anaesth. 2008, 100, 747–758. [Google Scholar] [CrossRef] [Green Version]
- Reagan-Shaw, S.; Nihal, M.; Ahmad, N. Dose translation from animal to human studies revisited. FASEB J. 2008, 22, 659–661. [Google Scholar] [CrossRef] [Green Version]
- Dahan, A.; Yassen, A.; Bijl, H.; Romberg, R.; Sarton, E.; Teppema, L.; Olofsen, E.; Danhof, M. Comparison of the respiratory effects of intravenous buprenorphine and fentanyl in humans and rats. Br. J. Anaesth. 2005, 94, 825–834. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lister, N.; Warrington, S.; Boyce, M.; Eriksson, C.; Tamaoka, M.; Kilborn, J. Pharmacokinetics, safety, and tolerability of ascending doses of sublingual fentanyl, with and without naltrexone, in Japanese subjects. J. Clin. Pharmacol. 2011, 51, 1195–1204. [Google Scholar] [CrossRef] [PubMed]
- Gill, J.R.; Lin, P.T.; Nelson, L. Reliability of postmortem fentanyl concentrations in determining the cause of death. J. Med. Toxicol. Off. J. Am. Coll. Med. Toxicol. 2013, 9, 34–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andresen, H.; Gullans, A.; Veselinovic, M.; Anders, S.; Schmoldt, A.; Iwersen-Bergmann, S.; Mueller, A. Fentanyl: Toxic or therapeutic? Postmortem and antemortem blood concentrations after transdermal fentanyl application. J. Anal. Toxicol. 2012, 36, 182–194. [Google Scholar] [CrossRef]
- Thompson, J.G.; Baker, A.M.; Bracey, A.H.; Seningen, J.; Kloss, J.S.; Strobl, A.Q.; Apple, F.S. Fentanyl concentrations in 23 postmortem cases from the hennepin county medical examiner’s office. J. Forensic Sci. 2007, 52, 978–981. [Google Scholar] [CrossRef]
- Flanagan, K.L.; Fink, A.L.; Plebanski, M.; Klein, S.L. Sex and Gender Differences in the Outcomes of Vaccination over the Life Course. Annu. Rev. Cell Dev. Biol. 2017, 33, 577–599. [Google Scholar] [CrossRef]
- Castellsagué, X.; Giuliano, A.R.; Goldstone, S.; Guevara, A.; Mogensen, O.; Palefsky, J.M.; Group, T.; Shields, C.; Liu, K.; Maansson, R.; et al. Immunogenicity and safety of the 9-valent HPV vaccine in men. Vaccine 2015, 33, 6892–6901. [Google Scholar] [CrossRef]
- Klein, S.L.; Flanagan, K.L. Sex differences in immune responses. Nature reviews. Immunology 2016, 16, 626–638. [Google Scholar] [CrossRef]
- Pisanu, C.; Franconi, F.; Gessa, G.L.; Mameli, S.; Pisanu, G.M.; Campesi, I.; Leggio, L.; Agabio, R. Sex differences in the response to opioids for pain relief: A systematic review and meta-analysis. Pharmacol. Res. 2019, 148, 104447. [Google Scholar] [CrossRef]
- Bodnar, R.J.; Kest, B. Sex differences in opioid analgesia, hyperalgesia, tolerance and withdrawal: Central mechanisms of action and roles of gonadal hormones. Horm. Behav. 2010, 58, 72–81. [Google Scholar] [CrossRef]
- Dews, P. Assessing the effects of drugs. In Methods in Psychobiology; Myers, R.D., Ed.; Academic Press: New York, NY, USA, 1972; Volume 2, pp. 83–124. [Google Scholar]
- Picker, M.J.; Negus, S.S.; Powell, K.R. Differential cross-tolerance to mu and kappa opioid agonists in morphine-tolerant rats responding under a schedule of food presentation. Psychopharmacology 1991, 103, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Butelman, E.R.; Negus, S.S.; Lewis, J.W.; Woods, J.H. Clocinnamox antagonism of opioid suppression of schedule-controlled responding in rhesus monkeys. Psychopharmacology 1996, 123, 320–324. [Google Scholar] [CrossRef] [PubMed]
- Siemian, J.N.; Obeng, S.; Zhang, Y.; Zhang, Y.; Li, J.X. Antinociceptive Interactions between the Imidazoline I2 Receptor Agonist 2-BFI and Opioids in Rats: Role of Efficacy at the μ-Opioid Receptor. J. Pharmacol. Exp. Ther. 2016, 357, 509–519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tenney, R.D.; Blake, S.; Bremer, P.T.; Zhou, B.; Hwang, C.S.; Poklis, J.L.; Janda, K.D.; Banks, M.L. Vaccine blunts fentanyl potency in male rhesus monkeys. Neuropharmacology 2019, 158, 107730. [Google Scholar] [CrossRef]
- Fitzpatrick, Z.; Frazer, G.; Ferro, A.; Clare, S.; Bouladoux, N.; Ferdinand, J.; Tuong, Z.K.; Negro-Demontel, M.L.; Kumar, N.; Suchanek, O.; et al. Gut-educated IgA plasma cells defend the meningeal venous sinuses. Nature 2020, 587, 472–476. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haile, C.N.; Baker, M.D.; Sanchez, S.A.; Lopez Arteaga, C.A.; Duddupudi, A.L.; Cuny, G.D.; Norton, E.B.; Kosten, T.R.; Kosten, T.A. An Immunconjugate Vaccine Alters Distribution and Reduces the Antinociceptive, Behavioral and Physiological Effects of Fentanyl in Male and Female Rats. Pharmaceutics 2022, 14, 2290. https://doi.org/10.3390/pharmaceutics14112290
Haile CN, Baker MD, Sanchez SA, Lopez Arteaga CA, Duddupudi AL, Cuny GD, Norton EB, Kosten TR, Kosten TA. An Immunconjugate Vaccine Alters Distribution and Reduces the Antinociceptive, Behavioral and Physiological Effects of Fentanyl in Male and Female Rats. Pharmaceutics. 2022; 14(11):2290. https://doi.org/10.3390/pharmaceutics14112290
Chicago/Turabian StyleHaile, Colin N., Miah D. Baker, Sergio A. Sanchez, Carlos A. Lopez Arteaga, Anantha L. Duddupudi, Gregory D. Cuny, Elizabeth B. Norton, Thomas R. Kosten, and Therese A. Kosten. 2022. "An Immunconjugate Vaccine Alters Distribution and Reduces the Antinociceptive, Behavioral and Physiological Effects of Fentanyl in Male and Female Rats" Pharmaceutics 14, no. 11: 2290. https://doi.org/10.3390/pharmaceutics14112290
APA StyleHaile, C. N., Baker, M. D., Sanchez, S. A., Lopez Arteaga, C. A., Duddupudi, A. L., Cuny, G. D., Norton, E. B., Kosten, T. R., & Kosten, T. A. (2022). An Immunconjugate Vaccine Alters Distribution and Reduces the Antinociceptive, Behavioral and Physiological Effects of Fentanyl in Male and Female Rats. Pharmaceutics, 14(11), 2290. https://doi.org/10.3390/pharmaceutics14112290





