Advanced Drug Delivery Platforms for the Treatment of Oral Pathogens
Abstract
:1. Introduction
2. Drug Delivery Systems for Oral Pathogens Treatment
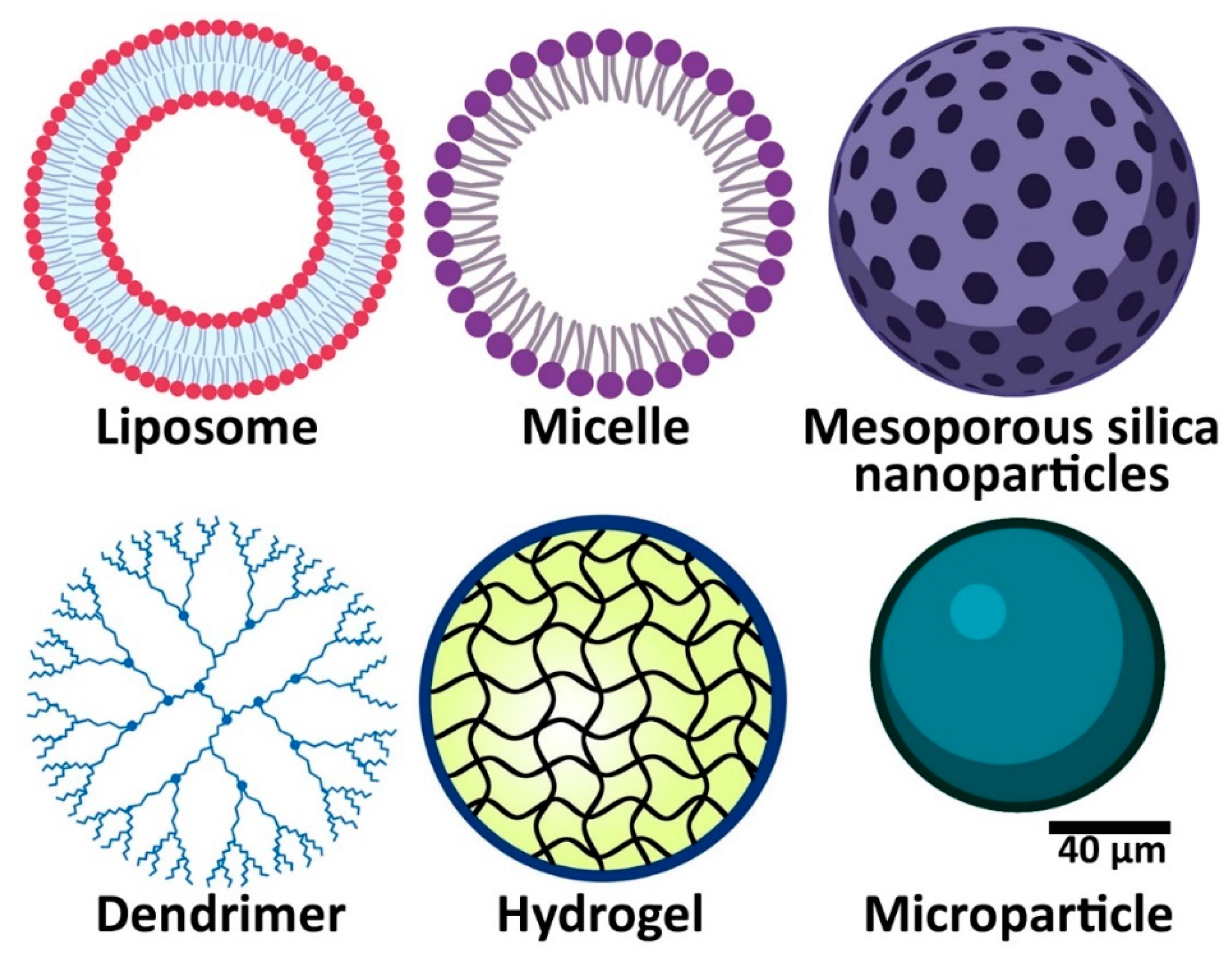
2.1. Nanoparticles
2.2. Oral Stimuli-Responsive Drug Delivery Systems
2.3. Hydrogels
2.4. Microparticles
2.5. Strips/Fibers
3. Drugs Used in the Treatment of Oral Pathogens
3.1. Antibiotic Delivery
3.2. Antifungal Delivery
3.3. Antiviral Delivery
3.3.1. Carbon-Based Polymers
3.3.2. Cyclodextrin-Based Delivery Systems
3.4. Ion Delivery
3.4.1. Fluoride Delivery
3.4.2. Calcium and Phosphate Delivery
4. Conclusions and Outlook
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liang, J.; Peng, X.; Zhou, X.; Zou, J.; Cheng, L. Emerging Applications of Drug Delivery Systems in Oral Infectious Diseases Prevention and Treatment. Molecules 2020, 25, 516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kilian, M.; Chapple, I.; Hannig, M.; Marsh, P.; Meuric, V.; Pedersen, A.; Tonetti, M.; Wade, W.; Zaura, E. The oral microbiome–an update for oral healthcare professionals. Br. Dent. J. 2016, 221, 657–666. [Google Scholar] [CrossRef] [PubMed]
- Duran-Pinedo, A.E. Metatranscriptomic analyses of the oral microbiome. Periodontology 2000 2021, 85, 28–45. [Google Scholar] [CrossRef] [PubMed]
- Rajeshwari, H.; Dhamecha, D.; Jagwani, S.; Rao, M.; Jadhav, K.; Shaikh, S.; Puzhankara, L.; Jalalpure, S. Local drug delivery systems in the management of periodontitis: A scientific review. J. Control. Release 2019, 307, 393–409. [Google Scholar]
- Ghaferi, M.; Raza, A.; Koohi, M.; Zahra, W.; Akbarzadeh, A.; Shahmabadi, H.E.; Alavi, S.E. Impact of PEGylated Liposomal Doxorubicin and Carboplatin Combination on Glioblastoma. Pharmaceutics 2022, 14, 2183. [Google Scholar] [CrossRef]
- Hamidi, M.; Azadi, A.; Rafiei, P. Hydrogel nanoparticles in drug delivery. Adv. Drug Deliv. Rev. 2008, 60, 1638–1649. [Google Scholar] [CrossRef] [PubMed]
- Álvarez, A.L.; Espinar, F.O.; Méndez, J.B. The application of microencapsulation techniques in the treatment of endodontic and periodontal diseases. Pharmaceutics 2011, 3, 538–571. [Google Scholar] [CrossRef]
- Kmiec, M.; Pighinelli, L.; Tedesco, M.; Silva, M.; Reis, V. Chitosan-properties and applications in dentistry. Adv. Tissue Eng. Regen. Med. Open Access 2017, 2, 00035. [Google Scholar] [CrossRef] [Green Version]
- Innocenzi, P.; Stagi, L. Carbon-based antiviral nanomaterials: Graphene, C-dots, and fullerenes. A perspective. Chem. Sci. 2020, 11, 6606–6622. [Google Scholar] [CrossRef] [PubMed]
- Maus, A.; Strait, L.; Zhu, D. Nanoparticles as delivery vehicles for antiviral therapeutic drugs. Eng. Regen. 2021, 2, 31–46. [Google Scholar] [CrossRef]
- Makvandi, P.; Josic, U.; Delfi, M.; Pinelli, F.; Jahed, V.; Kaya, E.; Ashrafizadeh, M.; Zarepour, A.; Rossi, F.; Zarrabi, A. Drug delivery (nano) platforms for oral and dental applications: Tissue regeneration, infection control, and cancer management. Adv. Sci. 2021, 8, 2004014. [Google Scholar] [CrossRef] [PubMed]
- Alavi, S.E.; Cabot, P.J.; Yap, G.Y.; Moyle, P.M. Optimized methods for the production and bioconjugation of site-specific, alkyne-modified glucagon-like peptide-1 (GLP-1) analogs to azide-modified delivery platforms using copper-catalyzed alkyne–azide cycloaddition. Bioconjugate Chem. 2020, 31, 1820–1834. [Google Scholar] [CrossRef] [PubMed]
- Alavi, S.E.; Ebrahimi Shahmabadi, H. Anthelmintics for drug repurposing: Opportunities and challenges. Saudi Pharm. J. SPJ 2021, 29, 434. [Google Scholar] [CrossRef]
- Alavi, S.E.; Cabot, P.J.; Moyle, P.M. Glucagon-like peptide-1 receptor agonists and strategies to improve their efficiency. Mol. Pharm. 2019, 16, 2278–2295. [Google Scholar] [CrossRef]
- Alavi, S.E.; Shahmabadi, H.E. GLP-1 peptide analogs for targeting pancreatic beta cells. Drug Discov. Today 2021, 26, 1936–1943. [Google Scholar] [CrossRef]
- Esfahani, M.K.M.; Alavi, S.E.; Cabot, P.J.; Islam, N.; Izake, E.L. PEGylated Mesoporous Silica Nanoparticles (MCM-41): A Promising Carrier for the Targeted Delivery of Fenbendazole into Prostrate Cancer Cells. Pharmaceutics 2021, 13, 1605. [Google Scholar] [CrossRef] [PubMed]
- Movahedi, F.; Shahmabadi, H.E.; Alavi, S.E.; Koohi Moftakhari Esfahani, M. Release modeling and comparison of nanoarchaeosomal, nanoliposomal and pegylated nanoliposomal carriers for paclitaxel. Tumor Biol. 2014, 35, 8665–8672. [Google Scholar] [CrossRef]
- Alavi, S.E.; Esfahani, M.K.M.; Raza, A.; Adelnia, H.; Ebrahimi Shahmabadi, H. PEG-grafted liposomes for enhanced antibacterial and antibiotic activities: An in vivo study. NanoImpact 2022, 25, 100384. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, C.; Zhang, X.; He, C.; Zhao, P.; Li, M.; Fan, T.; Yan, R.; Lu, Y.; Lee, R.J. Platinum complexes of curcumin delivered by dual-responsive polymeric nanoparticles improve chemotherapeutic efficacy based on the enhanced anti-metastasis activity and reduce side effects. Acta Pharm. Sin. B 2020, 10, 1106–1121. [Google Scholar] [CrossRef] [PubMed]
- Luo, K.; Guo, W.; Yu, Y.; Xu, S.; Zhou, M.; Xiang, K.; Niu, K.; Zhu, X.; Zhu, G.; An, Z.; et al. Reduction-sensitive platinum (IV)-prodrug nano-sensitizer with an ultra-high drug loading for efficient chemo-radiotherapy of Pt-resistant cervical cancer in vivo. J. Control. Release 2020, 326, 25–37. [Google Scholar] [CrossRef]
- Darge, H.F.; Hanurry, E.Y.; Birhan, Y.S.; Mekonnen, T.W.; Andrgie, A.T.; Chou, H.-Y.; Lai, J.-Y.; Tsai, H.-C. Multifunctional drug-loaded micelles encapsulated in thermo-sensitive hydrogel for in vivo local cancer treatment: Synergistic effects of anti-vascular and immuno-chemotherapy. Chem. Eng. J. 2021, 406, 126879. [Google Scholar] [CrossRef]
- Ghaferi, M.; Esfahani, M.K.M.; Raza, A.; Al Harthi, S.; Shahmabadi, H.E.; Alavi, S.E. Mesoporous silica nanoparticles: Synthesis methods and their therapeutic use-recent advances. J. Drug Target. 2021, 29, 131–154. [Google Scholar] [CrossRef] [PubMed]
- Alavi, S.E.; Cabot, P.J.; Raza, A.; Moyle, P.M. Developing GLP-1 Conjugated Self-Assembling Nanofibers Using Copper-Catalyzed Alkyne–Azide Cycloaddition and Evaluation of Their Biological Activity. Bioconjug. Chem. 2021, 32, 810–820. [Google Scholar] [CrossRef] [PubMed]
- Esfahani, M.K.M.; Islam, N.; Cabot, P.J.; Izake, E.L. Development of Thiabendazole-Loaded Mesoporous Silica Nanoparticles for Cancer Therapy. ACS Biomater. Sci. Eng. 2022, 8, 4153–4162. [Google Scholar] [CrossRef] [PubMed]
- Shahmabadi, H.E.; Movahedi, F.; Esfahani, M.K.M.; Alavi, S.E.; Eslamifar, A.; Mohammadi Anaraki, G.; Akbarzadeh, A. Efficacy of Cisplatin-loaded polybutyl cyanoacrylate nanoparticles on the glioblastoma. Tumor Biol. 2014, 35, 4799–4806. [Google Scholar] [CrossRef]
- Ghaferi, M.; Zahra, W.; Akbarzadeh, A.; Shahmabadi, H.E.; Alavi, S.E. Enhancing the efficacy of albendazole for liver cancer treatment using mesoporous silica nanoparticles: An in vitro study. EXCLI J. 2022, 21, 236–249. [Google Scholar] [CrossRef]
- Esfahani, M.K.M.; Alavi, S.E.; Cabot, P.J.; Islam, N.; Izake, E.L. Application of Mesoporous Silica Nanoparticles in Cancer Therapy and Delivery of Repurposed Anthelmintics for Cancer Therapy. Pharmaceutics 2022, 14, 1579. [Google Scholar] [CrossRef]
- Bernegossi, J.; Calixto, G.M.F.; Sanches, P.R.d.S.; Fontana, C.R.; Cilli, E.M.; Garrido, S.S.; Chorilli, M. Peptide KSL-W-loaded mucoadhesive liquid crystalline vehicle as an alternative treatment for multispecies oral biofilm. Molecules 2016, 21, 37. [Google Scholar] [CrossRef]
- Niaz, T.; Shabbir, S.; Noor, T.; Abbasi, R.; Imran, M. Alginate-caseinate based pH-responsive nano-coacervates to combat resistant bacterial biofilms in oral cavity. Int. J. Biol. Macromol. 2020, 156, 1366–1380. [Google Scholar] [CrossRef]
- Onnainty, R.; Onida, B.; Páez, P.; Longhi, M.; Barresi, A.; Granero, G. Targeted chitosan-based bionanocomposites for controlled oral mucosal delivery of chlorhexidine. Int. J. Pharm. 2016, 509, 408–418. [Google Scholar] [CrossRef]
- Horev, B.; Klein, M.I.; Hwang, G.; Li, Y.; Kim, D.; Koo, H.; Benoit, D.S. pH-activated nanoparticles for controlled topical delivery of farnesol to disrupt oral biofilm virulence. ACS Nano 2015, 9, 2390–2404. [Google Scholar] [CrossRef]
- Bowen, W.H. The Stephan curve revisited. Odontology 2013, 101, 2–8. [Google Scholar] [CrossRef]
- Akram, Z.; Daood, U.; Aati, S.; Ngo, H.; Fawzy, A.S. Formulation of pH-sensitive chlorhexidine-loaded/mesoporous silica nanoparticles modified experimental dentin adhesive. Mater. Sci. Eng. C 2021, 122, 111894. [Google Scholar] [CrossRef]
- Zhang, M.; Yu, Z.; Lo, E. A new pH-responsive nano micelle for enhancing the effect of a hydrophobic bactericidal agent on mature Streptococcus mutans biofilm. Front. Microbiol. 2021, 12, 761583. [Google Scholar] [CrossRef]
- Ibrahim, M.A.; Meera Priyadarshini, B.; Neo, J.; Fawzy, A.S. Characterization of chitosan/TiO2 nano-powder modified glass-ionomer cement for restorative dental applications. J. Esthet. Restor. Dent. 2017, 29, 146–156. [Google Scholar] [CrossRef]
- Seneviratne, C.J.; Leung, K.C.-F.; Wong, C.-H.; Lee, S.-F.; Li, X.; Leung, P.C.; Lau, C.B.S.; Wat, E.; Jin, L. Nanoparticle-encapsulated chlorhexidine against oral bacterial biofilms. PLoS ONE 2014, 9, e103234. [Google Scholar] [CrossRef] [Green Version]
- Chen, F.; Rice, K.C.; Liu, X.-M.; Reinhardt, R.A.; Bayles, K.W.; Wang, D. Triclosan-loaded tooth-binding micelles for prevention and treatment of dental biofilm. Pharm. Res. 2010, 27, 2356–2364. [Google Scholar] [CrossRef]
- Zambom, C.R.; da Fonseca, F.H.; Crusca Jr, E.; da Silva, P.B.; Pavan, F.R.; Chorilli, M.; Garrido, S.S. A novel antifungal system with potential for prolonged delivery of histatin 5 to limit growth of Candida albicans. Front. Microbiol. 2019, 10, 1667. [Google Scholar] [CrossRef] [Green Version]
- Sebelemetja, M.; Moeno, S.; Patel, M. Anti-acidogenic, anti-biofilm and slow release properties of Dodonaea viscosa var. angustifolia flavone stabilized polymeric nanoparticles. Arch. Oral Biol. 2020, 109, 104586. [Google Scholar] [CrossRef]
- Tian, Y.; Shen, Y.; Jv, M. Synthesis, characterization and evaluation of tinidazole-loaded mPEG–PDLLA (10/90) in situ gel forming system for periodontitis treatment. Drug Deliv. 2016, 23, 2726–2735. [Google Scholar] [CrossRef] [Green Version]
- Kong, E.F.; Tsui, C.; Boyce, H.; Ibrahim, A.; Hoag, S.W.; Karlsson, A.J.; Meiller, T.F.; Jabra-Rizk, M.A. Development and in vivo evaluation of a novel histatin-5 bioadhesive hydrogel formulation against oral candidiasis. Antimicrob. Agents Chemother. 2016, 60, 881–889. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Li, X.; Xu, X.; Li, J.; Ding, C.; Zhao, C.; Li, J. One-step phosphorylated poly (amide-amine) dendrimer loaded with apigenin for simultaneous remineralization and antibacterial of dentine. Colloids Surf. B Biointerfaces 2018, 172, 760–768. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Na, R.; Wang, X.; Liu, H.; Zhao, L.; Sun, X.; Ma, G.; Cui, F. Fabrication of antimicrobial peptide-loaded PLGA/chitosan composite microspheres for long-acting bacterial resistance. Molecules 2017, 22, 1637. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jhaveri, J.; Raichura, Z.; Khan, T. Chitosan Nanoparticles-Insight into Properties, Functionalization and Applications in Drug Delivery and Theranostics. Molecules 2021, 26, 272. [Google Scholar] [CrossRef]
- Samaranayake, L.; Matsubara, V.H. Normal oral flora and the oral ecosystem. Dent. Clin. 2017, 61, 199–215. [Google Scholar] [CrossRef]
- Baliga, S.; Muglikar, S.; Kale, R. Salivary pH: A diagnostic biomarker. J. Indian Soc. Periodontol. 2013, 17, 461. [Google Scholar] [CrossRef]
- Lamont, R.J.; Koo, H.; Hajishengallis, G. The oral microbiota: Dynamic communities and host interactions. Nat. Rev. Microbiol. 2018, 16, 745–759. [Google Scholar] [CrossRef]
- Dong, Y.; Ye, H.; Liu, Y.; Xu, L.; Wu, Z.; Hu, X.; Ma, J.; Pathak, J.L.; Liu, J.; Wu, G. pH dependent silver nanoparticles releasing titanium implant: A novel therapeutic approach to control peri-implant infection. Colloids Surf. B Biointerfaces 2017, 158, 127–136. [Google Scholar] [CrossRef]
- Zhou, J.; Horev, B.; Hwang, G.; Klein, M.I.; Koo, H.; Benoit, D.S. Characterization and optimization of pH-responsive polymer nanoparticles for drug delivery to oral biofilms. J. Mater. Chem. B 2016, 4, 3075–3085. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Deng, X.; Ding, J.; Zhou, W.; Zheng, X.; Tang, G. Mechanisms of drug release in pH-sensitive micelles for tumour targeted drug delivery system: A review. Int. J. Pharm. 2018, 535, 253–260. [Google Scholar] [CrossRef]
- Chang, P.C.; Chao, Y.C.; Hsiao, M.H.; Chou, H.S.; Jheng, Y.H.; Yu, X.H.; Lee, N.; Yang, C.; Liu, D.M. Inhibition of Periodontitis Induction Using a Stimuli-Responsive Hydrogel Carrying Naringin. J. Periodontol. 2017, 88, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Hoare, T.R.; Kohane, D.S. Hydrogels in drug delivery: Progress and challenges. Polymer 2008, 49, 1993–2007. [Google Scholar] [CrossRef] [Green Version]
- Peppas, N.A.; Sahlin, J.J. Hydrogels as mucoadhesive and bioadhesive materials: A review. Biomaterials 1996, 17, 1553–1561. [Google Scholar] [CrossRef]
- Tarawneh, O.; Hamadneh, I.; Huwaitat, R.; Al-Assi, A.R.; El Madani, A. Characterization of chlorhexidine-impregnated cellulose-based hydrogel films intended for the treatment of periodontitis. BioMed Res. Int. 2021, 2021, 9853977. [Google Scholar] [CrossRef]
- Alkhalidi, H.M.; Hosny, K.M.; Rizg, W.Y. Oral gel loaded by fluconazole–sesame oil nanotransfersomes: Development, optimization, and assessment of antifungal activity. Pharmaceutics 2020, 13, 27. [Google Scholar] [CrossRef]
- Garala, K.; Joshi, P.; Shah, M.; Ramkishan, A.; Patel, J. Formulation and evaluation of periodontal in situ gel. Int. J. Pharm. Investig. 2013, 3, 29. [Google Scholar] [CrossRef] [Green Version]
- Juvekar, S.; Kathpalia, H. Solvent removal precipitation based in situ forming implant for controlled drug delivery in periodontitis. J. Control. Release Off. J. Control. Release Soc. 2017, 251, 75–81. [Google Scholar] [CrossRef]
- Şenel, S.; Özdoğan, A.I.; Akca, G. Current status and future of delivery systems for prevention and treatment of infections in the oral cavity. Drug Deliv. Transl. Res. 2021, 11, 1703–1734. [Google Scholar] [CrossRef]
- Kawakita, E.R.; Ré, A.C.S.; Peixoto, M.P.G.; Ferreira, M.P.; Ricomini-Filho, A.P.; Freitas, O.; Aires, C.P. Effect of chitosan dispersion and microparticles on older Streptococcus mutans biofilms. Molecules 2019, 24, 1808. [Google Scholar] [CrossRef] [Green Version]
- Moura, L.A.; Ribeiro, F.V.; Aiello, T.B.; Duek, E.A.D.R.; Sallum, E.A.; Nociti Junior, F.H.; Casati, M.Z.; Sallum, A.W. Characterization of the release profile of doxycycline by PLGA microspheres adjunct to non-surgical periodontal therapy. J. Biomater. Sci. Polym. Ed. 2015, 26, 573–584. [Google Scholar] [CrossRef]
- Joshi, D.; Garg, T.; Goyal, A.K.; Rath, G. Advanced drug delivery approaches against periodontitis. Drug Deliv. 2016, 23, 363–377. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, D.; Friedman, M.; Soskolne, A.; Sela, M. A new degradable controlled release device for treatment of periodontal disease: In vitro release study. J. Periodontol. 1990, 61, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Goodson, J.; Cugini, M.; Kent, R.; Armitage, G.; Cobb, C.; Fine, D.; Fritz, M.; Green, E.; Imoberdorf, M.; Killoy, W. Multicenter evaluation of tetracycline fiber therapy: II. Clinical response. J. Periodontal Res. 1991, 26, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, J.; Chen, M.; Gong, H.; Thamphiwatana, S.; Eckmann, L.; Gao, W.; Zhang, L. A bioadhesive nanoparticle–hydrogel hybrid system for localized antimicrobial drug delivery. ACS Appl. Mater. Interfaces 2016, 8, 18367–18374. [Google Scholar] [CrossRef] [Green Version]
- Millsop, J.W.; Fazel, N. Oral candidiasis. Clin. Dermatol. 2016, 34, 487–494. [Google Scholar] [CrossRef]
- Di Cosola, M.; Cazzolla, A.P.; Charitos, I.A.; Ballini, A.; Inchingolo, F.; Santacroce, L. Candida albicans and oral carcinogenesis. A brief review. J. Fungi 2021, 7, 476. [Google Scholar] [CrossRef] [PubMed]
- Yehia, S.A.; El-Gazayerly, O.N.; Basalious, E.B. Design and in vitro/in vivo evaluation of novel mucoadhesive buccal discs of an antifungal drug: Relationship between swelling, erosion, and drug release. AAPS PharmSciTech 2008, 9, 1207–1217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abd El Hady, W.E.; Mohamed, E.A.; Soliman, O.A.E.-A.; El-Sabbagh, H.M. In vitro–in vivo evaluation of chitosan-PLGA nanoparticles for potentiated gastric retention and anti-ulcer activity of diosmin. Int. J. Nanomed. 2019, 14, 7191. [Google Scholar] [CrossRef] [Green Version]
- Mendes, A.; Silva, A.C.; Catita, J.A.M.; Cerqueira, F.; Gabriel, C.; Lopes, C.M. Miconazole-loaded nanostructured lipid carriers (NLC) for local delivery to the oral mucosa: Improving antifungal activity. Colloids Surf. B Biointerfaces 2013, 111, 755–763. [Google Scholar] [CrossRef]
- Martin, M.J.; Calpena, A.C.; Fernandez, F.; Mallandrich, M.; Gálvez, P.; Clares, B. Development of alginate microspheres as nystatin carriers for oral mucosa drug delivery. Carbohydr. Polym. 2015, 117, 140–149. [Google Scholar] [CrossRef]
- Tonglairoum, P.; Ngawhirunpat, T.; Rojanarata, T.; Kaomongkolgit, R.; Opanasopit, P. Fabrication of a novel scaffold of clotrimazole-microemulsion-containing nanofibers using an electrospinning process for oral candidiasis applications. Colloids Surf. B: Biointerfaces 2015, 126, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Cartagena, A.F.; Esmerino, L.A.; Polak-Junior, R.; Parreiras, S.O.; Michél, M.D.; Farago, P.V.; Campanha, N.H. New denture adhesive containing miconazole nitrate polymeric microparticles: Antifungal, adhesive force and toxicity properties. Dent. Mater. 2017, 33, e53–e61. [Google Scholar] [CrossRef] [PubMed]
- Felipe Cartagena, A.; Martinez Lyra, A.; Cristina Kapuchczinski, A.; Migliorini Urban, A.; Antonio Esmerino, L.; Klein, T.; Mendes Nadal, J.; Vitor Farago, P.; Hellen Campanha, N. Miconazole nitrate-loaded microparticles for buccal use: Immediate drug release and antifungal effect. Curr. Drug Deliv. 2017, 14, 1144–1153. [Google Scholar] [CrossRef] [PubMed]
- Tejada, G.; Lamas, M.C.; Sortino, M.; Alvarez, V.A.; Leonardi, D. Composite microparticles based on natural mucoadhesive polymers with promising structural properties to protect and improve the antifungal activity of miconazole nitrate. AAPS PharmSciTech 2018, 19, 3712–3722. [Google Scholar] [CrossRef] [PubMed]
- SK, S.R.; Anuba, P.; Swetha, B.; Aishwarya, P.; Sabarathinam, S. Drug interaction risk between cardioprotective drugs and drugs used in treatment of COVID-19: A evidence-based review from six databases. Diabetes Metab. Syndr. Clin. Res. Rev. 2022, 16, 102451. [Google Scholar]
- Prashantha, C.; Gouthami, K.; Lavanya, L.; Bhavanam, S.; Jakhar, A.; Shakthiraju, R.; Suraj, V.; Sahana, K.; Sujana, H.; Guruprasad, N. Molecular screening of antimalarial, antiviral, anti-inflammatory and HIV protease inhibitors against spike glycoprotein of coronavirus. J. Mol. Graph. Model. 2021, 102, 107769. [Google Scholar] [CrossRef]
- Wong, S.N.; Weng, J.; Ip, I.; Chen, R.; Lakerveld, R.; Telford, R.; Blagden, N.; Scowen, I.J.; Chow, S.F. Rational Development of a Carrier-Free Dry Powder Inhalation Formulation for Respiratory Viral Infections via Quality by Design: A Drug-Drug Cocrystal of Favipiravir and Theophylline. Pharmaceutics 2022, 14, 300. [Google Scholar] [CrossRef]
- Lotufo, M.A.; Horliana, A.C.R.T.; Santana, T.; de Queiroz, A.C.; Gomes, A.O.; Motta, L.J.; Ferrari, R.A.M.; dos Santos Fernandes, K.P.; Bussadori, S.K. Efficacy of photodynamic therapy on the treatment of herpes labialis: A systematic review. Photodiagnosis Photodyn. Ther. 2020, 29, 101536. [Google Scholar] [CrossRef]
- Clarkson, E.; Mashkoor, F.; Abdulateef, S. Oral viral infections: Diagnosis and management. Dent. Clin. 2017, 61, 351–363. [Google Scholar]
- Chen, L.; Liang, J. An overview of functional nanoparticles as novel emerging antiviral therapeutic agents. Mater. Sci. Eng. C 2020, 112, 110924. [Google Scholar] [CrossRef]
- Hu, X.; Mu, L.; Wen, J.; Zhou, Q. Covalently synthesized graphene oxide-aptamer nanosheets for efficient visible-light photocatalysis of nucleic acids and proteins of viruses. Carbon 2012, 50, 2772–2781. [Google Scholar] [CrossRef]
- Tong, T.; Hu, H.; Zhou, J.; Deng, S.; Zhang, X.; Tang, W.; Fang, L.; Xiao, S.; Liang, J. Glycyrrhizic-acid-based carbon dots with high antiviral activity by multisite inhibition mechanisms. Small 2020, 16, 1906206. [Google Scholar] [CrossRef] [Green Version]
- Du, T.; Lu, J.; Liu, L.; Dong, N.; Fang, L.; Xiao, S.; Han, H. Antiviral activity of graphene oxide–silver nanocomposites by preventing viral entry and activation of the antiviral innate immune response. ACS Appl. Bio Mater. 2018, 1, 1286–1293. [Google Scholar] [CrossRef]
- Barras, A.; Pagneux, Q.; Sane, F.; Wang, Q.; Boukherroub, R.; Hober, D.; Szunerits, S. High efficiency of functional carbon nanodots as entry inhibitors of herpes simplex virus type 1. ACS Appl. Mater. Interfaces 2016, 8, 9004–9013. [Google Scholar] [CrossRef]
- Du, T.; Liang, J.; Dong, N.; Liu, L.; Fang, L.; Xiao, S.; Han, H. Carbon dots as inhibitors of virus by activation of type I interferon response. Carbon 2016, 110, 278–285. [Google Scholar] [CrossRef]
- Fahmi, M.; Sukmayani, W.; Khairunisa, S.Q.; Witaningrum, A.; Indriati, D.; Matondang, M.; Chang, J.-Y.; Kotaki, T.; Kameoka, M. Design of boronic acid-attributed carbon dots on inhibits HIV-1 entry. RSC Adv. 2016, 6, 92996–93002. [Google Scholar] [CrossRef]
- Troshina, O.A.; Troshin, P.A.; Peregudov, A.S.; Kozlovskiy, V.I.; Balzarini, J.; Lyubovskaya, R.N. Chlorofullerene C 60 Cl 6: A precursor for straightforward preparation of highly water-soluble polycarboxylic fullerene derivatives active against HIV. Org. Biomol. Chem. 2007, 5, 2783–2791. [Google Scholar] [CrossRef]
- Hirayama, F.; Uekama, K. Cyclodextrin-based controlled drug release system. Adv. Drug Deliv. Rev. 1999, 36, 125–141. [Google Scholar] [CrossRef]
- Donalisio, M.; Argenziano, M.; Rittà, M.; Bastiancich, C.; Civra, A.; Lembo, D.; Cavalli, R. Acyclovir-loaded sulfobutyl ether-β-cyclodextrin decorated chitosan nanodroplets for the local treatment of HSV-2 infections. Int. J. Pharm. 2020, 587, 119676. [Google Scholar] [CrossRef]
- Delbem, A.C.; Pessan, J.P. Fluoride agents and dental caries. In Pediatric Restorative Dentistry; Springer: Berlin/Heidelberg, Germany, 2019; pp. 57–73. [Google Scholar]
- Ahmadian, E.; Shahi, S.; Yazdani, J.; Dizaj, S.M.; Sharifi, S. Local treatment of the dental caries using nanomaterials. Biomed. Pharmacother. 2018, 108, 443–447. [Google Scholar] [CrossRef]
- Ullah, R.; Zafar, M.S.; Shahani, N. Potential fluoride toxicity from oral medicaments: A review. Iran. J. Basic Med. Sci. 2017, 20, 841. [Google Scholar] [PubMed]
- Hoxha, A.; Gillam, D.G.; Bushby, A.J.; Agha, A.; Patel, M.P. Layered double hydroxide fluoride release in dental applications: A systematic review. Dent. J. 2019, 7, 87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schlagenhauf, U.; Kunzelmann, K.H.; Hannig, C.; May, T.W.; Hösl, H.; Gratza, M.; Viergutz, G.; Nazet, M.; Schamberger, S.; Proff, P. Impact of a non-fluoridated microcrystalline hydroxyapatite dentifrice on enamel caries progression in highly caries-susceptible orthodontic patients: A randomized, controlled 6-month trial. J. Investig. Clin. Dent. 2019, 10, e12399. [Google Scholar] [CrossRef]
- Hu, M.-L.; Zheng, G.; Zhang, Y.-D.; Yan, X.; Li, X.-C.; Lin, H. Effect of desensitizing toothpastes on dentine hypersensitivity: A systematic review and meta-analysis. J. Dent. 2018, 75, 12–21. [Google Scholar] [CrossRef]
- Ghafar, H.; Khan, M.I.; Sarwar, H.S.; Yaqoob, S.; Hussain, S.Z.; Tariq, I.; Madni, A.U.; Shahnaz, G.; Sohail, M.F. Development and characterization of bioadhesive film embedded with lignocaine and calcium fluoride nanoparticles. AAPS PharmSciTech 2020, 21, 60. [Google Scholar] [CrossRef]
- Keegan, G.M.; Smart, J.D.; Ingram, M.J.; Barnes, L.-M.; Burnett, G.R.; Rees, G.D. Chitosan microparticles for the controlled delivery of fluoride. J. Dent. 2012, 40, 229–240. [Google Scholar] [CrossRef]
- De Francisco, L.M.B.; Cerquetani, J.A.; Bruschi, M.L. Development and characterization of gelatin and ethylcellulose microparticles designed as platforms to delivery fluoride. Drug Dev. Ind. Pharm. 2013, 39, 1644–1650. [Google Scholar] [CrossRef]
- Nguyen, S.; Escudero, C.; Sediqi, N.; Smistad, G.; Hiorth, M. Fluoride loaded polymeric nanoparticles for dental delivery. Eur. J. Pharm. Sci. 2017, 104, 326–334. [Google Scholar] [CrossRef] [Green Version]
- Arifa, M.K.; Ephraim, R.; Rajamani, T. Recent advances in dental hard tissue remineralization: A review of literature. Int. J. Clin. Pediatr. Dent. 2019, 12, 139. [Google Scholar] [CrossRef] [PubMed]
- Samarehfekri, H.; Ranjbar, M.; Pardakhty, A.; Amanatfard, A. Systematic study of naf nanoparticles in micelles loaded on polylactic acid nanoscaffolds: In vitro efficient delivery. J. Clust. Sci. 2020, 31, 453–461. [Google Scholar] [CrossRef]
- Zhu, B.; He, H.; Guo, D.; Zhao, M.; Hou, T. Two novel calcium delivery systems fabricated by casein phosphopeptides and chitosan oligosaccharides: Preparation, characterization, and bioactive studies. Food Hydrocoll. 2020, 102, 105567. [Google Scholar] [CrossRef]
- Liang, K.; Wang, S.; Tao, S.; Xiao, S.; Zhou, H.; Wang, P.; Cheng, L.; Zhou, X.; Weir, M.D.; Oates, T.W. Dental remineralization via poly (amido amine) and restorative materials containing calcium phosphate nanoparticles. Int. J. Oral Sci. 2019, 11, 15. [Google Scholar] [CrossRef] [Green Version]
- Cieplik, F.; Rupp, C.M.; Hirsch, S.; Muehler, D.; Enax, J.; Meyer, F.; Hiller, K.-A.; Buchalla, W. Ca2+ release and buffering effects of synthetic hydroxyapatite following bacterial acid challenge. BMC Oral Health 2020, 20, 85. [Google Scholar] [CrossRef]
- Viana, Í.E.L.; Lopes, R.M.; Silva, F.R.O.; Lima, N.B.; Aranha, A.C.C.; Feitosa, S.; Scaramucci, T. Novel fluoride and stannous-functionalized β-tricalcium phosphate nanoparticles for the management of dental erosion. J. Dent. 2020, 92, 103263. [Google Scholar] [CrossRef]
- Sudradjat, H.; Meyer, F.; Loza, K.; Epple, M.; Enax, J. In vivo effects of a hydroxyapatite-based oral care gel on the calcium and phosphorus levels of dental plaque. Eur. J. Dent. 2020, 14, 206–211. [Google Scholar] [CrossRef] [Green Version]
- Tao, S.; Fan, M.; Xu, H.H.; Li, J.; He, L.; Zhou, X.; Liang, K.; Li, J. The remineralization effectiveness of PAMAM dendrimer with different terminal groups on demineralized dentin in vitro. RSC Adv. 2017, 7, 54947–54955. [Google Scholar] [CrossRef] [Green Version]
- Luo, X.-J.; Yang, H.-Y.; Niu, L.-N.; Mao, J.; Huang, C.; Pashley, D.H.; Tay, F.R. Translation of a solution-based biomineralization concept into a carrier-based delivery system via the use of expanded-pore mesoporous silica. Acta Biomater. 2016, 31, 378–387. [Google Scholar] [CrossRef]
- Mendes, A.C.; Restrepo, M.; Bussaneli, D.; Zuanon, A.C. Use of casein amorphous calcium phosphate (CPP-ACP) on white-spot lesions: Randomised clinical trial. Oral Health Prev. Dent. 2018, 16, 27–31. [Google Scholar]


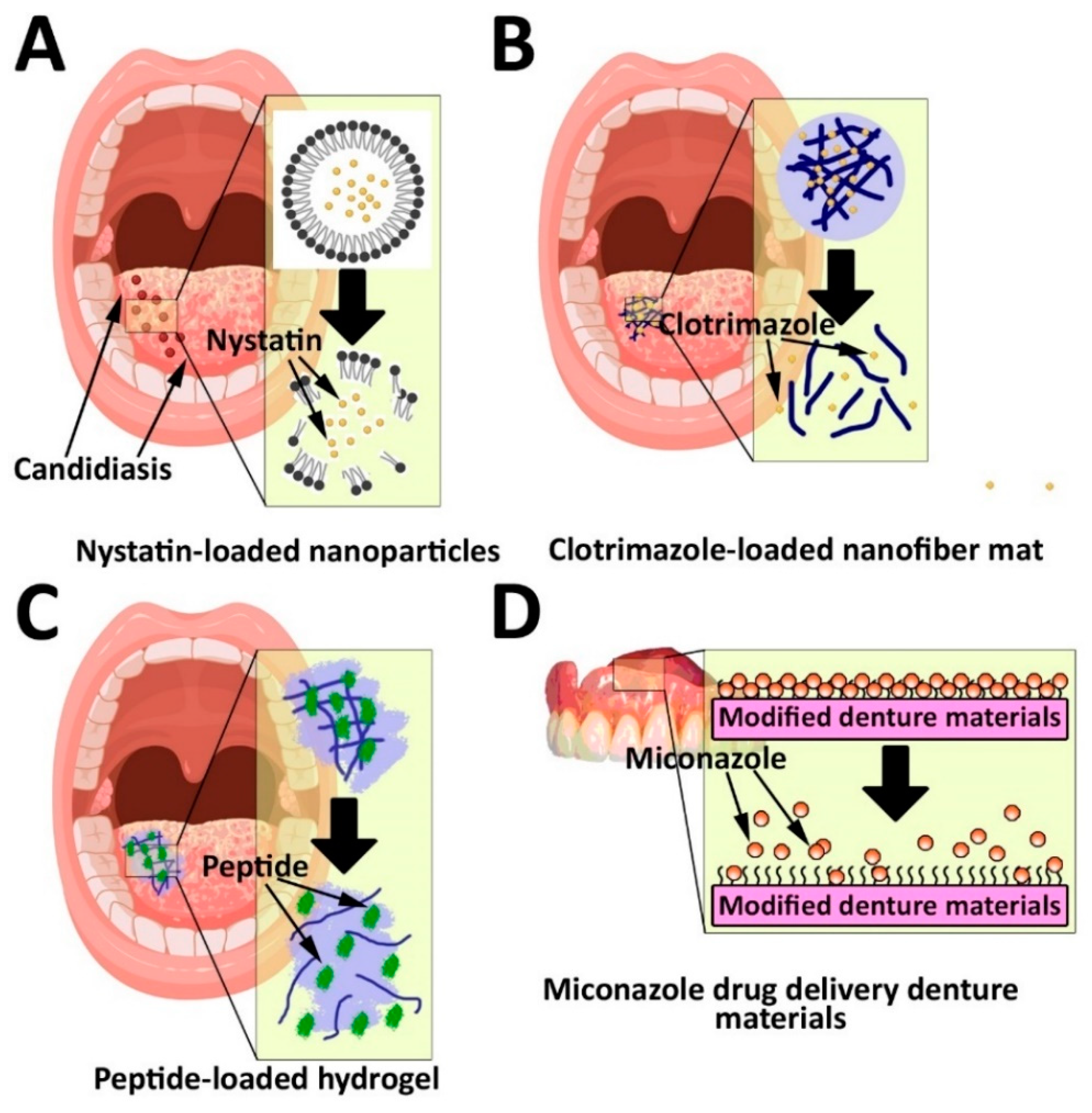
| Carrier | Therapeutic Agent | Oral Infectious Diseases | Results |
|---|---|---|---|
| Chitosan nanoparticles | Glass ionomer cement and titanium oxide nanoparticles | Dental caries | Chitosan nanoparticles incorporating glass ionomer cement and titanium oxide nanoparticles enhanced the antimicrobial (Streptococcus mutans (S. mutans)) activity by approximately 1.7-fold [35]. |
| Mesoporous silica nanoparticles (MSNs) | Chlorhexidine | Dental caries | Chlorhexidine-loaded MSNs demonstrated antibacterial activity against biofilms of S. mutans (minimum inhibitory concentration (MIC): 100 µg/mL), Streptococcus sobrinus (MIC: 200 µg/mL), Fusobacterium nucleatum (F. nucleatum) (MIC: 100 µg/mL), Aggregatibacter actinomycetemcomitans (MIC: 100 µg/mL), and Enterococcus faecalis (MIC: 200 µg/mL) [36]. |
| Micelle nanoparticles | Triclosan | Dental caries | Triclosan-loaded micelle nanoparticles inhibited the initial biofilm growth of S. mutans by 6-log colony-forming unit (CFU)/hydroxyapatite (HA) disc compared to the untreated control [37]. |
| Liposomes nanoparticles | 0WHistatin 5 peptide | Oral candidiasis | Liposomes could increase the cytotoxicity effects of 0Whistatin 5 by approximately 13-fold against Candida albicans (C. albicans) [38]. |
| PEG-PLGA nanoparticles | Dodonaea viscosa var. Angustifolia (DVA) | Dental caries | DVA-loaded PEG-PLGA nanoparticles demonstrated antibacterial activity against biofilms of S. mutans by 8-fold compared to the blank nanoparticles [39]. |
| Hydrogel | Tinidazole | Periodontitis | Tinidazole-loaded hydrogel (monomethoxy poly(ethylene glycol)-block-poly(d,l-lactide) (mPEG-PDLLA)) could increase the t1/2 (h), tmax (h), and area under the curve (AUC)0–168 (h µg/mL) of tinidazole by 16-, 8-, and 21-fold, compared to the control group, in a rabbit periodontitis model [40]. |
| Hydrogel | Histatin-5 | Oral candidiasis | Histatin-5-loaded hydrogel (hydroxypropyl methylcellulose (HPMC)) could increase the antifungal (C. albicans) effects of histatin-5 by approximately 9-fold compared to the control hydrogel [41]. |
| Dendrimer | Apigenin | Dental caries | The apigenin-loaded dendrimer demonstrated an increase in the antibacterial (S. mutans) activity by 1.6-fold compared to the control nanoparticle [42]. |
| PLGA/chitosan composite microsphere | KSL-W peptide | Periodontitis | KSL-W-loaded PLGA/chitosan composite microsphere, compared to the control group, demonstrates significant antibacterial (F. nucleatum) activity (inhibition zone of 0 and 2.26 cm in control and KSL-W-loaded PLGA/chitosan composite microsphere receiving groups, respectively) [43]. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alavi, S.E.; Raza, A.; Gholami, M.; Giles, M.; Al-Sammak, R.; Ibrahim, A.; Ebrahimi Shahmabadi, H.; Sharma, L.A. Advanced Drug Delivery Platforms for the Treatment of Oral Pathogens. Pharmaceutics 2022, 14, 2293. https://doi.org/10.3390/pharmaceutics14112293
Alavi SE, Raza A, Gholami M, Giles M, Al-Sammak R, Ibrahim A, Ebrahimi Shahmabadi H, Sharma LA. Advanced Drug Delivery Platforms for the Treatment of Oral Pathogens. Pharmaceutics. 2022; 14(11):2293. https://doi.org/10.3390/pharmaceutics14112293
Chicago/Turabian StyleAlavi, Seyed Ebrahim, Aun Raza, Max Gholami, Michael Giles, Rayan Al-Sammak, Ali Ibrahim, Hasan Ebrahimi Shahmabadi, and Lavanya A. Sharma. 2022. "Advanced Drug Delivery Platforms for the Treatment of Oral Pathogens" Pharmaceutics 14, no. 11: 2293. https://doi.org/10.3390/pharmaceutics14112293






