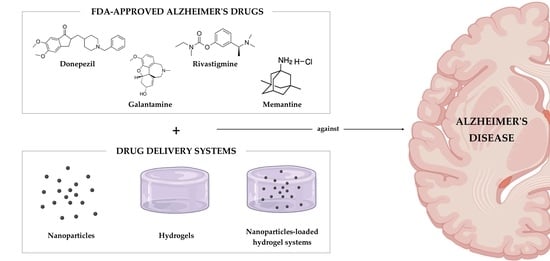
Drug Delivery Systems as a Strategy to Improve the Efficacy of FDA-Approved Alzheimer’s Drugs
Abstract
:1. Introduction
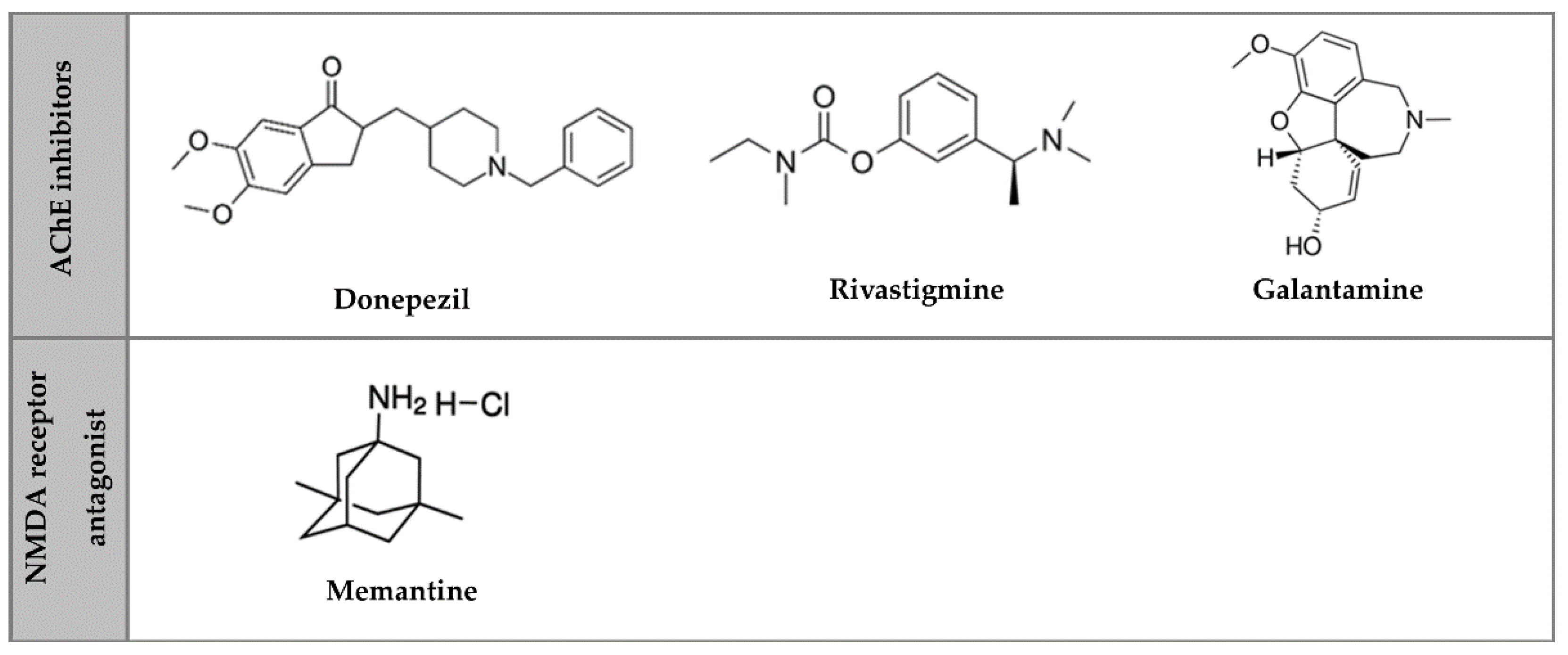
2. Pharmacological Therapeutic Strategies for AD
3. Shortcomings of AD Pharmacological Therapies
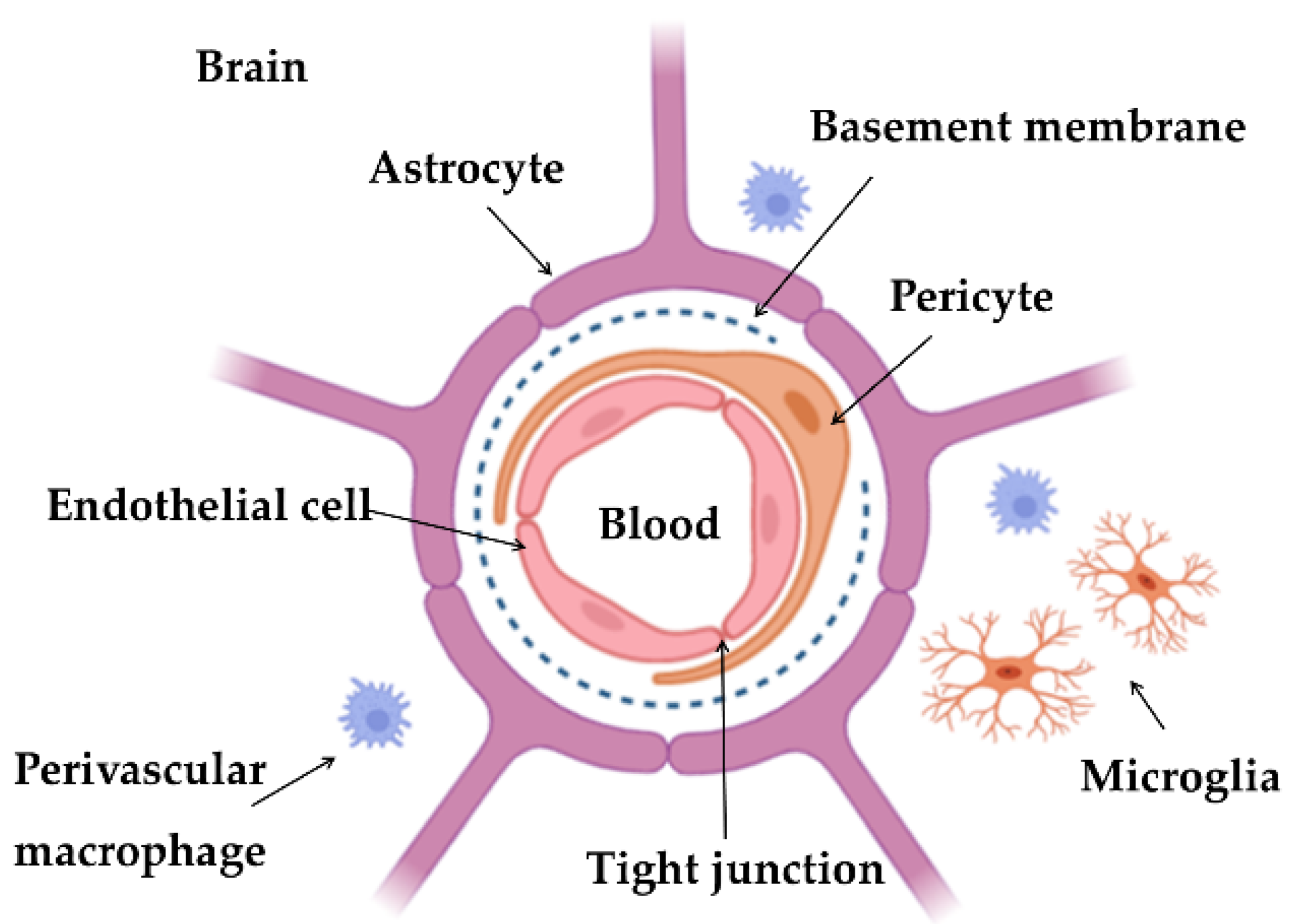
Possible Strategies to Overcome the Blood–Brain Barrier
4. Drug Delivery Systems against AD
4.1. Nanoparticles
4.1.1. Donepezil
4.1.2. Galantamine
4.1.3. Rivastigmine
4.1.4. Memantine
4.2. Hydrogels
4.3. Microformulations
4.4. Nanoparticle-Loaded Hydrogel Systems
5. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Anand, R.; Gill, K.D.; Mahdi, A.A. Therapeutics of Alzheimer’s disease: Past, present and future. Neuropharmacology 2014, 76, 27–50. [Google Scholar] [CrossRef]
- World Health Organization. Global Action Plan on the Public Health Response to Dementia 2017–2025; Licence: CC BY-NC-SA 3.0 IGO; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- IOS Press. Analysis Reveals Economic Cost of Alzheimer’s Disease and Dementia Are ‘Tip of the Iceberg; ScienceDaily, Ed.; IOS Press: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Ramirez-Bermudez, J. Alzheimer’s Disease: Critical Notes on the History of a Medical Concept. Arch. Med. Res. 2012, 43, 595–599. [Google Scholar] [CrossRef] [PubMed]
- Mecocci, P.; Boccardi, V.; Cecchetti, R.; Bastiani, P.; Scamosci, M.; Ruggiero, C.; Baroni, M. A Long Journey into Aging, Brain Aging, and Alzheimer’s Disease Following the Oxidative Stress Tracks. J. Alzheimer’s Dis. JAD 2018, 62, 1319–1335. [Google Scholar] [CrossRef] [Green Version]
- Brookmeyer, R.; Corrada, M.; Curriero, F.C.; Kawas, C. Survival Following a Diagnosis of Alzheimer Disease. Arch. Neurol. 2002, 59, 1764–1767. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.; Sun, J.; Cheng, Q.; Yang, Y.; Cordato, D.; Gao, J. The Development of Pharmacological Therapies for Alzheimer’s Disease. Neurol. Ther. 2021, 10, 609–626. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Krishnan, N.; Heo, J.; Fang, R.H.; Zhang, L. Nanoparticle-hydrogel superstructures for biomedical applications. J. Control. Release 2020, 324, 505–521. [Google Scholar] [CrossRef] [PubMed]
- Lengyel, M.; Kállai-Szabó, N.; Antal, V.; Laki, A.J.; Antal, I. Microparticles, Microspheres, and Microcapsules for Advanced Drug Delivery. Sci. Pharm. 2019, 87, 20. [Google Scholar] [CrossRef] [Green Version]
- Chang, D.; Park, K.; Famili, A. Hydrogels for sustained delivery of biologics to the back of the eye. Drug Discov. Today 2019, 24, 1470–1482. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Hao, Y.; Wang, Y.; Chen, J.; Mao, L.; Deng, Y.; Chen, J.; Yuan, S.; Zhang, T.; Ren, J.; et al. Functional Hydrogels and Their Application in Drug Delivery, Biosensors, and Tissue Engineering. Int. J. Polym. Sci. 2019, 2019, 3160732. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Li, Q.; Zhou, J.E.; Tan, J.; Li, M.; Xu, N.; Qu, F.; Chen, J.; Li, J.; Wang, J.; et al. A Photopolymerized Semi-Interpenetrating Polymer Networks-Based Hydrogel Incorporated with Nanoparticle for Local Chemotherapy of Tumors. Pharm. Res. 2021, 38, 669–680. [Google Scholar] [CrossRef] [PubMed]
- Madduma-Bandarage, U.S.K.; Madihally, S.V. Synthetic hydrogels: Synthesis, novel trends, and applications. J. Appl. Polym. Sci. 2021, 138, 50376. [Google Scholar] [CrossRef]
- Sun, Z.; Wang, X.; Liu, J.; Wang, Z.; Wang, W.; Kong, D.; Leng, X. ICG/l-Arginine Encapsulated PLGA Nanoparticle-Thermosensitive Hydrogel Hybrid Delivery System for Cascade Cancer Photodynamic-NO Therapy with Promoted Collagen Depletion in Tumor Tissues. Mol. Pharm. 2021, 18, 928–939. [Google Scholar] [CrossRef] [PubMed]
- Fonseca-Santos, B.; Chorilli, M.; Gremião, M.P.D. Nanotechnology-based drug delivery systems for the treatment of Alzheimer’s disease. Int. J. Nanomed. 2015, 10, 4981–5003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karthivashan, G.; Ganesan, P.; Park, S.-Y.; Kim, J.-S.; Choi, D.-K. Therapeutic strategies and nano-drug delivery applications in management of ageing Alzheimer’s disease. Drug Deliv. 2018, 25, 307–320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Altinoglu, G.; Adali, T. Alzheimer’s Disease Targeted Nano-Based Drug Delivery Systems. Curr. Drug Targets 2020, 21, 628–646. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.; Nguyen TT, D.; Nguyen TK, O.; Vo, T.K. Advances in developing therapeutic strategies for Alzheimer’s disease. Biomed. Pharmacother. 2021, 139, 111623. [Google Scholar] [CrossRef]
- Khan, N.H.; Mir, M.; Ngowi, E.E.; Zafar, U.; Khakwani, M.M.A.K.; Khattak, S.; Zhai, Y.-K.; Jiang, E.-S.; Zheng, M.; Duan, S.-F.; et al. Nanomedicine: A Promising Way to Manage Alzheimer’s Disease. Front. Bioeng. Biotechnol. 2021, 9, 630055. [Google Scholar] [CrossRef]
- Poudel, P.; Park, S. Recent Advances in the Treatment of Alzheimer’s Disease Using Nanoparticle-Based Drug Delivery Systems. Pharmaceutics 2022, 14, 835. [Google Scholar] [CrossRef]
- Yiannopoulou, K.G.; Papageorgiou, S.G. Current and Future Treatments in Alzheimer Disease: An Update. J. Cent. Nerv. Syst. Dis. 2020, 12, 1179573520907397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colovic, M.B.; Krstic, D.Z.; Lazarevic-Pasti, T.D.; Bondzic, A.M.; Vasic, V.M. Acetylcholinesterase inhibitors: Pharmacology and toxicology. Curr. Neuropharmacol. 2013, 11, 315–335. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chang, L.; Song, Y.; Li, H.; Wu, Y. The Role of NMDA Receptors in Alzheimer’s Disease. Front. Neurosci. 2019, 13, 43. [Google Scholar] [CrossRef] [Green Version]
- Larkin, H.D. First Donepezil Transdermal Patch Approved for Alzheimer Disease. JAMA 2022, 327, 1642. [Google Scholar] [CrossRef] [PubMed]
- Schelterns, P.; Feldman, H. Treatment of Alzheimer’s disease; current status and new perspectives. Lancet Neurol. 2003, 2, 539–547. [Google Scholar]
- Epperly, T.; Dunay, M.A.; Boice, J.L. Alzheimer Disease: Pharmacologic and Nonpharmacologic Therapies for Cognitive and Functional Symptoms. Am. Fam. Physician 2017, 95, 771–778. [Google Scholar] [PubMed]
- Cunha, S.; Forbes, B.; Lobo, J.M.S.; Silva, A.C. Improving Drug Delivery for Alzheimer’s Disease Through Nose-to-Brain Delivery Using Nanoemulsions, Nanostructured Lipid Carriers (NLC) and in situ Hydrogels. Int. J. Nanomed. 2021, 16, 4373–4390. [Google Scholar] [CrossRef] [PubMed]
- Cummings, J.; Aisen, P.; Lemere, C.; Atri, A.; Sabbagh, M.; Salloway, S. Aducanumab produced a clinically meaningful benefit in association with amyloid lowering. Alzheimer’s Res. Ther. 2021, 13, 98. [Google Scholar] [CrossRef]
- Homayun, B.; Lin, X.; Choi, H.-J. Challenges and Recent Progress in Oral Drug Delivery Systems for Biopharmaceuticals. Pharmaceutics 2019, 11, 129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Mooney, D.J. Designing hydrogels for controlled drug delivery. Nat. Rev. Mater. 2016, 1, 16071. [Google Scholar] [CrossRef]
- Hernando, S.; Gartziandia, O.; Herran, E.; Pedraz, J.L.; Igartua, M.; Hernandez, R.M. Advances in nanomedicine for the treatment of Alzheimer’s and Parkinson’s diseases. Nanomedicine 2016, 11, 1267–1285. [Google Scholar] [CrossRef] [PubMed]
- Tonda-Turo, C.; Origlia, N.; Mattu, C.; Accorroni, A.; Chiono, V. Current Limitations in the Treatment of Parkinson’s and Alzheimer’s Diseases: State-of-the-Art and Future Perspective of Polymeric Carriers. Curr. Med. Chem. 2018, 25, 5755–5771. [Google Scholar] [CrossRef] [PubMed]
- Wen, M.M.; El-Salamouni, N.S.; El-Refaie, W.M.; Hazzah, H.A.; Ali, M.M.; Tosi, G.; Farid, R.M.; Blanco-Prieto, M.J.; Billa, N.; Hanafy, A.S. Nanotechnology-based drug delivery systems for Alzheimer’s disease management: Technical, industrial, and clinical challenges. J. Control. Release 2017, 245, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Bellotti, E.; Schilling, A.L.; Little, S.R.; Decuzzi, P. Injectable thermoresponsive hydrogels as drug delivery system for the treatment of central nervous system disorders: A review. J. Control. Release 2021, 329, 16–35. [Google Scholar] [CrossRef] [PubMed]
- Hogan, D.B. Progress update: Pharmacological treatment of Alzheimer’s disease. Neuropsychiatr. Dis. Treat. 2007, 3, 569–578. [Google Scholar] [PubMed]
- Hyde, C.; Peters, J.; Bond, M.; Rogers, G.; Hoyle, M.; Anderson, R.; Jeffreys, M.; Davis, S.; Thokala, P.; Moxham, T. Evolution of the evidence on the effectiveness and cost-effectiveness of acetylcholinesterase inhibitors and memantine for Alzheimer’s disease: Systematic review and economic model. Age Ageing 2013, 42, 14–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Casey, D.A.; Antimisiaris, D.; O’Brien, J. Drugs for Alzheimer’s disease: Are they effective? Pharm. Ther. Peer-Rev. J. Formul. Manag. 2010, 35, 208–211. [Google Scholar]
- van Marum, R.J. Update on the use of memantine in Alzheimer’s disease. Neuropsychiatr. Dis. Treat. 2009, 5, 237–247. [Google Scholar] [CrossRef] [Green Version]
- Alam, M.I.; Beg, S.; Samad, A.; Baboota, S.; Kohli, K.; Ali, J.; Ahuja, A.; Akbar, M. Strategy for effective brain drug delivery. Eur. J. Pharm. Sci. 2010, 40, 385–403. [Google Scholar] [CrossRef]
- Lalatsa, A.; Butt, A.M. Chapter 3—Physiology of the Blood–Brain Barrier and Mechanisms of Transport Across the BBB. In Nanotechnology-Based Targeted Drug Delivery Systems for Brain Tumors; Kesharwani, P., Gupta, U., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 49–74. [Google Scholar]
- Wahl, M.; Unterberg, A.; Baethmann, A.; Schilling, L. Mediators of blood-brain barrier dysfunction and formation of vasogenic brain edema. J. Cereb. Blood Flow Metab. 1988, 8, 621–634. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Liu, L. Modern methods for delivery of drugs across the blood-brain barrier. Adv. Drug Deliv. Rev. 2012, 64, 640–665. [Google Scholar] [CrossRef]
- Ballabh, P.; Braun, A.; Nedergaard, M. The blood-brain barrier: An overview: Structure, regulation, and clinical implications. Neurobiol. Dis. 2004, 16, 1–13. [Google Scholar] [CrossRef]
- Sharif, Y.; Jumah, F.; Coplan, L.; Krosser, A.; Sharif, K.; Tubbs, R.S. Blood brain barrier: A review of its anatomy and physiology in health and disease. Clin. Anat. 2018, 31, 812–823. [Google Scholar] [CrossRef] [PubMed]
- Wong, A.D.; Ye, M.; Levy, A.F.; Rothstein, J.D.; Bergles, D.E.; Searson, P.C. The blood-brain barrier: An engineering perspective. Front. Neuroeng. 2013, 6, 7. [Google Scholar] [CrossRef] [PubMed]
- Barar, J.; Rafi, M.A.; Pourseif, M.M.; Omidi, Y. Blood-brain barrier transport machineries and targeted therapy of brain diseases. BioImpacts BI 2016, 6, 225–248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kargozar, S.; Mozafari, M. Nanotechnology and Nanomedicine: Start small, think big. Mater. Today Proc. 2018, 5 Pt 3, 15492–15500. [Google Scholar] [CrossRef]
- El-Sayed, A.; Kamel, M. Advances in nanomedical applications: Diagnostic, therapeutic, immunization, and vaccine production. Environ. Sci. Pollut. Res. 2020, 27, 19200–19213. [Google Scholar] [CrossRef]
- Nobile, L.; Nobile, S. Recent advances of nanotechnology in medicine and engineering. AIP Conf. Proc. 2016, 1736, 020058. [Google Scholar]
- Srivastava, N.; Saxena, S.K. Current Advances in Nanotechnology and Medicine. In NanoBioMedicine; Saxena, S.K., Khurana, S.M.P., Eds.; Springer: Singapore, 2020; pp. 3–16. [Google Scholar]
- Pehlivan, S.B. Nanotechnology-based drug delivery systems for targeting, imaging and diagnosis of neurodegenerative diseases. Pharm. Res. 2013, 30, 2499–2511. [Google Scholar] [CrossRef]
- Domínguez, A.; Suárez-Merino, B.; Goñi-De-Cerio, F. Nanoparticles and Blood-Brain Barrier: The Key to Central Nervous System Diseases. J. Nanosci. Nanotechnol. 2014, 14, 766–779. [Google Scholar] [CrossRef]
- Vieira, D.B.; Gamarra, L.F. Getting into the brain: Liposome-based strategies for effective drug delivery across the blood-brain barrier. Int. J. Nanomed. 2016, 11, 5381–5414. [Google Scholar] [CrossRef] [Green Version]
- Ramalho, M.J.; Coelho, M.A.; Pereira, M.C. Chapter 18—Nanocarriers for the delivery of temozolomide in the treatment of glioblastoma: A review. In Design and Development of New Nanocarriers; Grumezescu, A.M., Ed.; William Andrew Publishing: Norwich, NY, USA, 2018; pp. 687–722. [Google Scholar]
- Shankar, R.; Joshi, M.; Pathak, K. Lipid Nanoparticles: A Novel Approach for Brain Targeting. Pharm. Nanotechnol. 2018, 6, 81–93. [Google Scholar] [CrossRef]
- Ribovski, L.; Hamelmann, N.M.; Paulusse, J.M.J. Polymeric Nanoparticles Properties and Brain Delivery. Pharmaceutics 2021, 13, 2045. [Google Scholar] [CrossRef]
- Park, K. Drug delivery of the future: Chasing the invisible gorilla. J. Control. Release 2016, 240, 2–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saraiva, C.; Praça, C.; Ferreira, R.; Santos, T.; Ferreira, L.; Bernardino, L. Nanoparticle-mediated brain drug delivery: Overcoming blood-brain barrier to treat neurodegenerative diseases. J. Control. Release 2016, 235, 34–47. [Google Scholar] [CrossRef] [PubMed]
- Wais, U.; Jackson, A.W.; He, T.; Zhang, H. Nanoformulation and encapsulation approaches for poorly water-soluble drug nanoparticles. Nanoscale 2016, 8, 1746–1769. [Google Scholar] [CrossRef]
- Al Asmari, A.K.; Ullah, Z.; Tariq, M.; Fatani, A. Preparation, characterization, and in vivo evaluation of intranasally administered liposomal formulation of donepezil. Drug Des. Dev. Ther. 2016, 10, 205–215. [Google Scholar]
- Bhavna; Shadab; Ali, M.; Ali, R.; Bhatnagar, A.; Baboota, S.; Ali, J. Donepezil nanosuspension intended for nose to brain targeting: In vitro and in vivo safety evaluation. Int. J. Biol. Macromol. 2014, 67, 418–425. [Google Scholar] [CrossRef] [PubMed]
- Bhavna, M.S.; Ali, M.; Baboota, S.; Sahni, J.K.; Bhatnagar, A. Preparation, characterization, in vivo biodistribution and pharmacokinetic studies of donepezil-loaded PLGA nanoparticles for brain targeting. Drug Dev. Ind. Pharm. 2014, 40, 278–287. [Google Scholar]
- Baysal, I.; Ucar, G.; Gultekinoglu, M.; Ulubayram, K.; Yabanoglu-Ciftci, S. Donepezil loaded PLGA-b-PEG nanoparticles: Their ability to induce destabilization of amyloid fibrils and to cross blood brain barrier in vitro. J. Neural Transm. 2017, 124, 33–45. [Google Scholar] [CrossRef]
- Topal, G.R.; Mészáros, M.; Porkoláb, G.; Szecskó, A.; Polgár, T.F.; Siklós, L.; Deli, M.A.; Veszelka, S.; Bozkir, A. ApoE-Targeting Increases the Transfer of Solid Lipid Nanoparticles with Donepezil Cargo across a Culture Model of the Blood–Brain Barrier. Pharmaceutics 2021, 13, 38. [Google Scholar] [CrossRef]
- Li, W.; Zhou, Y.; Zhao, N.; Hao, B.; Wang, X.; Kong, P. Pharmacokinetic behavior and efficiency of acetylcholinesterase inhibition in rat brain after intranasal administration of galanthamine hydrobromide loaded flexible liposomes. Environ. Toxicol. Pharmacol. 2012, 34, 272–279. [Google Scholar] [CrossRef]
- Fornaguera, C.; Feiner-Gracia, N.; Calderó, G.; García-Celma, M.J.; Solans, C. Galantamine-loaded PLGA nanoparticles, from nano-emulsion templating, as novel advanced drug delivery systems to treat neurodegenerative diseases. Nanoscale 2015, 7, 12076–12084. [Google Scholar] [CrossRef] [PubMed]
- Misra, S.; Chopra, K.; Sinha, V.R.; Medhi, B. Galantamine-loaded solid–lipid nanoparticles for enhanced brain delivery: Preparation, characterization, in vitro and in vivo evaluations. Drug Deliv. 2016, 23, 1434–1443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sunena; Singh, S.K.; Mishra, D.N.; Jha, S. Nose to Brain Delivery of Galantamine Loaded Nanoparticles: In-vivo Pharmacodynamic and Biochemical Study in Mice. Curr. Drug Deliv. 2019, 16, 51–58. [Google Scholar]
- Mohamadpour, H.; Azadi, A.; Rostamizadeh, K.; Andalib, S.; Saghatchi Zanjani, M.R.; Hamidi, M. Preparation, Optimization, and Evaluation of Methoxy Poly(ethylene glycol)-co-Poly(ε-caprolactone) Nanoparticles Loaded by Rivastigmine for Brain Delivery. ACS Chem. Neurosci. 2020, 11, 783–795. [Google Scholar] [CrossRef]
- Fazil, M.M.S.; Haque, S.; Kumar, M.; Baboota, S.; kaur Sahni, J.; Ali, J. Development and evaluation of rivastigmine loaded chitosan nanoparticles for brain targeting. Eur. J. Pharm. Sci. 2012, 47, 6–15. [Google Scholar] [CrossRef]
- Rompicherla, S.K.L.; Arumugam, K.; Bojja, S.L.; Kumar, N.; Rao, C.M. Pharmacokinetic and pharmacodynamic evaluation of nasal liposome and nanoparticle based rivastigmine formulations in acute and chronic models of Alzheimer’s disease. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2021, 394, 1737–1755. [Google Scholar] [CrossRef]
- El-Helaly, S.N.; Elbary, A.A.; Kassem, M.A.; El-Nabarawi, M.A. Electrosteric stealth Rivastigmine loaded liposomes for brain targeting: Preparation, characterization, ex vivo, bio-distribution and in vivo pharmacokinetic studies. Drug Deliv. 2017, 24, 692–700. [Google Scholar] [CrossRef] [Green Version]
- Ismail, M.F.; ElMeshad, A.N.; Salem, N.A.H. Potential therapeutic effect of nanobased formulation of rivastigmine on rat model of Alzheimer’s disease. Int. J. Nanomed. 2013, 8, 393–406. [Google Scholar] [CrossRef]
- Salimi, A.; Ghobadian, H.; Sharif Makhmalzadeh, B. Dermal pharmacokinetics of rivastigmine-loaded liposomes: An ex vivo–in vivo correlation study. J. Liposome Res. 2021, 31, 246–254. [Google Scholar] [CrossRef]
- Shah, B.; Khunt, D.; Bhatt, H.; Misra, M.; Padh, H. Application of quality by design approach for intranasal delivery of rivastigmine loaded solid lipid nanoparticles: Effect on formulation and characterization parameters. Eur. J. Pharm. Sci. 2015, 78, 54–66. [Google Scholar] [CrossRef]
- Arora, D.; Bhatt, S.; Kumar, M.; Verma, R.; Taneja, Y.; Kaushal, N.; Tiwari, A.; Tiwari, V.; Alexiou, A.; Albogami, S.; et al. QbD-based rivastigmine tartrate-loaded solid lipid nanoparticles for enhanced intranasal delivery to the brain for Alzheimer’s therapeutics. Front. Aging Neurosci. 2022, 14, 869. [Google Scholar] [CrossRef]
- Basharzad, S.F.; Hamidi, M.; Maleki, A.; Karami, Z.; Mohamadpour, H.; Zanjani, M.R.S. Polysorbate-coated mesoporous silica nanoparticles as an efficient carrier for improved rivastigmine brain delivery. Brain Res. 2022, 1781, 147786. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-López, E.; Ettcheto, M.; Egea, M.A.; Espina, M.; Cano, A.; Calpena, A.C.; Camins, A.; Carmona-Ule, N.; Silva, A.M.; Souto, E.B.; et al. Memantine loaded PLGA PEGylated nanoparticles for Alzheimer’s disease: In vitro and in vivo characterization. J. Nanobiotechnol. 2018, 16, 32. [Google Scholar] [CrossRef] [PubMed]
- Gothwal, A.; Kumar, H.; Nakhate, K.; Uddin, A.; Dutta, A.; Borah, A.; Gupta, U. Lactoferrin Coupled Lower Generation PAMAM Dendrimers for Brain Targeted Delivery of Memantine in Aluminum-Chloride-Induced Alzheimer’s Disease in Mice. Bioconjug. Chem. 2019, 30, 2573–2583. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, A.S. Hydrogels for biomedical applications. Adv. Drug Deliv. Rev. 2012, 64, 18–23. [Google Scholar] [CrossRef]
- Huang, H.; Qi, X.; Chen, Y.; Wu, Z. Thermo-sensitive hydrogels for delivering biotherapeutic molecules: A review. Saudi Pharm. J. 2019, 27, 990–999. [Google Scholar] [CrossRef]
- Rajkumar, M.; Sakthivel, M.; Senthilkumar, K.; Thangaraj, R.; Kannan, S. Galantamine tethered hydrogel as a novel therapeutic target for streptozotocin-induced Alzheimer’s disease in Wistar rats. Curr. Res. Pharmacol. Drug Discov. 2022, 3, 100100. [Google Scholar] [CrossRef] [PubMed]
- Bashyal, S.; Shin, C.Y.; Hyun, S.M.; Jang, S.W.; Lee, S. Preparation, Characterization, and In Vivo Pharmacokinetic Evaluation of Polyvinyl Alcohol and Polyvinyl Pyrrolidone Blended Hydrogels for Transdermal Delivery of Donepezil HCl. Pharmaceutics 2020, 12, 270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gu, F.; Fan, H.; Cong, Z.; Li, S.; Wang, Y.; Wu, C. Preparation, characterization, and pharmacokinetics of thermosensitive nasal gel of donepezil hydrochloride. Acta Pharm. 2020, 70, 411–422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
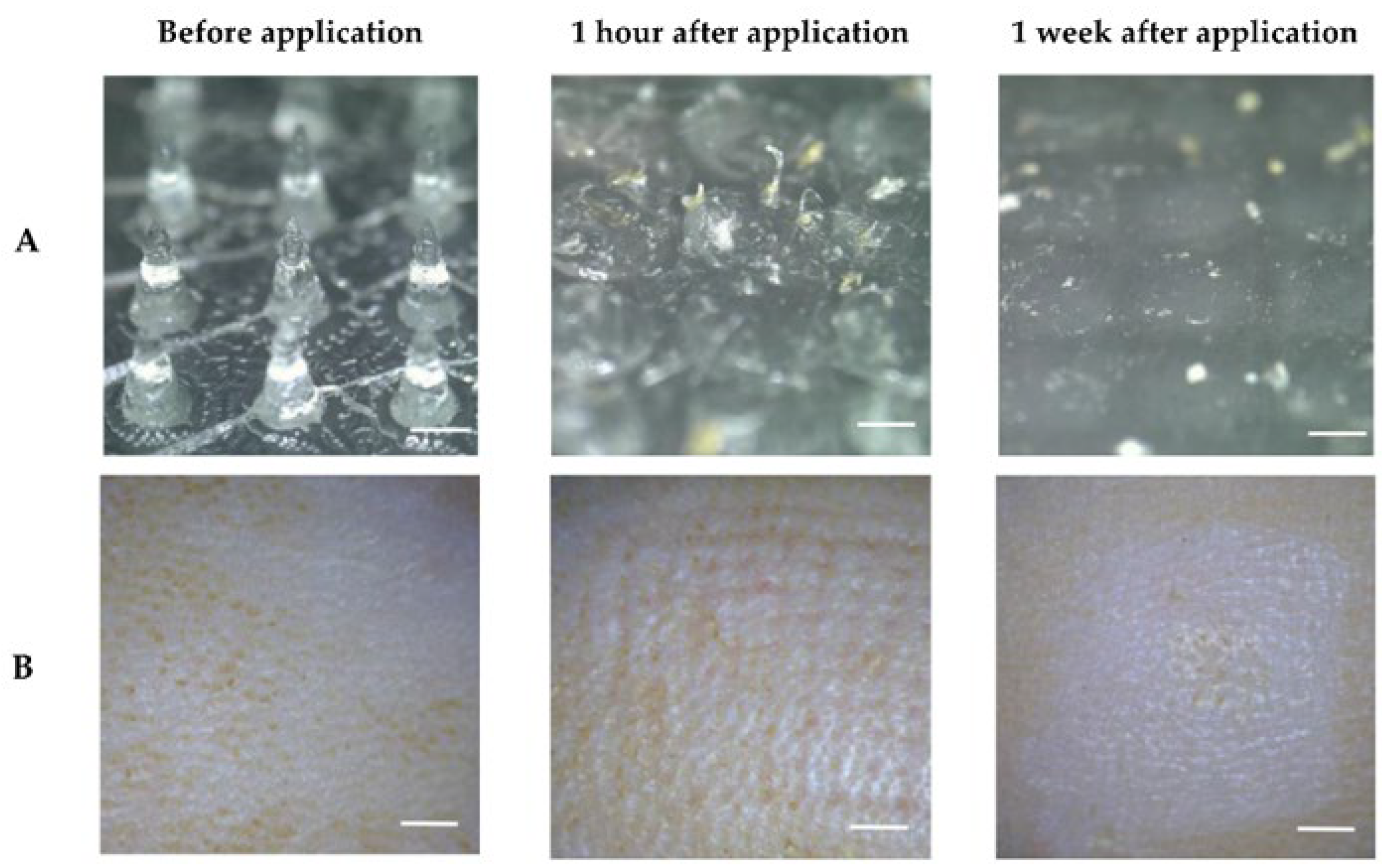
- Waghule, T.; Singhvi, G.; Dubey, S.K.; Pandey, M.M.; Gupta, G.; Singh, M.; Dua, K. Microneedles: A smart approach and increasing potential for transdermal drug delivery system. Biomed. Pharmacother. 2019, 109, 1249–1258. [Google Scholar] [CrossRef] [PubMed]
- Aldawood, F.K.; Andar, A.; Desai, S. A Comprehensive Review of Microneedles: Types, Materials, Processes, Characterizations and Applications. Polymers 2021, 13, 2815. [Google Scholar] [CrossRef]
- Kim, J.-Y.; Han, M.-R.; Kim, Y.-H.; Shin, S.-W.; Nam, S.-Y.; Park, J.-H. Tip-loaded dissolving microneedles for transdermal delivery of donepezil hydrochloride for treatment of Alzheimer’s disease. Eur. J. Pharm. Biopharm. 2016, 105, 148–155. [Google Scholar] [CrossRef]
- Kearney, M.-C.; Caffarel-Salvador, E.; Fallows, S.J.; McCarthy, H.O.; Donnelly, R.F. Microneedle-mediated delivery of donepezil: Potential for improved treatment options in Alzheimer’s disease. Eur. J. Pharm. Biopharm. 2016, 103, 43–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rehman, N.U.; Song, C.; Kim, J.; Noh, I.; Rhee, Y.-S.; Chung, H.J. Pharmacokinetic Evaluation of a Novel Donepezil-Loaded Dissolving Microneedle Patch in Rats. Pharmaceutics 2022, 14, 5. [Google Scholar] [CrossRef]
- Vos, P.J.; Kuijt, N.; Kaya, M.; Rol, S.; Van Der Maaden, K. Nanoporous microneedle arrays seamlessly connected to a drug reservoir for tunable transdermal delivery of memantine. Eur. J. Pharm. Sci. 2020, 150, 105331. [Google Scholar] [CrossRef] [PubMed]
- Cummings, J.L.; Farlow, M.R.; Meng, X.; Tekin, S.; Olin, J.T. Rivastigmine Transdermal Patch Skin Tolerability. Clin. Drug Investig. 2010, 30, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, T.M.T.; Moniz, T.; Nunes, C.; Zaharieva, M.M.; Kaleva, M.; Yoncheva, K.; Najdenski, H.; Lima, S.A.C.; Reis, S. Polymeric Microneedles for Transdermal Delivery of Rivastigmine: Design and Application in Skin Mimetic Model. Pharmaceutics 2022, 14, 752. [Google Scholar] [CrossRef] [PubMed]
- Boyuklieva, R.; Pilicheva, B. Micro- and Nanosized Carriers for Nose-to-Brain Drug Delivery in Neurodegenerative Disorders. Biomedicines 2022, 10, 1706. [Google Scholar] [CrossRef]
- Simon, A.; Amaro, M.I.; Cabral, L.M.; Healy, A.M.; de Sousa, V.P. Development of a novel dry powder inhalation formulation for the delivery of rivastigmine hydrogen tartrate. Int. J. Pharm. 2016, 501, 124–138. [Google Scholar] [CrossRef] [PubMed]
- Haider, M.; Elsayed, I.; Ahmed, I.; Fares, A. In Situ-Forming Microparticles for Controlled Release of Rivastigmine: In Vitro Optimization and In Vivo Evaluation. Pharmaceuticals 2021, 14, 66. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Almalki, W.H.; Afzal, O.; Panda, S.K.; Kazmi, I.; Alrobaian, M.; Katouah, H.A.; Altamimi, A.S.A.; Al-Abbasi, F.A.; Alshehri, S.; et al. Systematic development of lectin conjugated microspheres for nose-to-brain delivery of rivastigmine for the treatment of Alzheimer’s disease. Biomed. Pharmacother. 2021, 141, 111829. [Google Scholar] [CrossRef]
- Froelich, A.; Osmałek, T.; Jadach, B.; Puri, V.; Michniak-Kohn, B. Microemulsion-Based Media in Nose-to-Brain Drug Delivery. Pharmaceutics 2021, 13, 201. [Google Scholar] [CrossRef]
- Shah, B.; Khunt, D.; Misra, M.; Padh, H. Formulation and In-vivo Pharmacokinetic Consideration of Intranasal Microemulsion and Mucoadhesive Microemulsion of Rivastigmine for Brain Targeting. Pharm. Res. 2018, 35, 8. [Google Scholar] [CrossRef]
- Espinoza, L.C.; Vacacela, M.; Clares, B.; García, M.L.; Fabrega, M.J.; Calpena, A.C. Development of a Nasal Donepezil-loaded Microemulsion for the Treatment of Alzheimer’s Disease: In vitro and ex vivo Characterization. CNS Neurol. Disord.-Drug Targets 2018, 17, 43–53. [Google Scholar] [CrossRef]
- Biondi, M.; Borzacchiello, A.; Mayol, L.; Ambrosio, L. Nanoparticle-Integrated Hydrogels as Multifunctional Composite Materials for Biomedical Applications. Gels 2015, 1, 162–178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thoniyot, P.; Tan, M.J.; Karim, A.A.; Young, D.J.; Loh, X.J. Nanoparticle–Hydrogel Composites: Concept, Design, and Applications of These Promising, Multi-Functional Materials. Adv. Sci. 2015, 2, 1400010. [Google Scholar] [CrossRef]
- Desfrançois, C.; Auzély, R.; Texier, I. Lipid Nanoparticles and Their Hydrogel Composites for Drug Delivery: A Review. Pharmaceuticals 2018, 11, 118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brachi, G.; Ruiz-Ramírez, J.; Dogra, P.; Wang, Z.; Cristini, V.; Ciardelli, G.; Rostomily, R.C.; Ferrari, M.; Mikheev, A.M.; Blanco, E.; et al. Intratumoral injection of hydrogel-embedded nanoparticles enhances retention in glioblastoma. Nanoscale 2020, 12, 23838–23850. [Google Scholar] [CrossRef] [PubMed]
- Rafael, D.; Melendres, M.M.R.; Andrade, F.; Montero, S.; Martinez-Trucharte, F.; Vilar-Hernandez, M.; Durán-Lara, E.F.; Schwartz, S., Jr.; Abasolo, I. Thermo-responsive hydrogels for cancer local therapy: Challenges and state-of-art. Int. J. Pharm. 2021, 606, 120954. [Google Scholar] [CrossRef] [PubMed]
- Nunes, D.; Andrade, S.; Ramalho, M.J.; Loureiro, J.A.; Pereira, M.C. Polymeric Nanoparticles-Loaded Hydrogels for Biomedical Applications: A Systematic Review on In Vivo Findings. Polymers 2022, 14, 1010. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.-H.; Lee, S.Y.; Kim, S.; Yang, M.; Jeong, D.I.; Hwang, C.; Kim, M.-H.; Kim, H.-J.; Lee, J.; Lee, K.; et al. Monopotassium phosphate-reinforced in situ forming injectable hyaluronic acid hydrogels for subcutaneous injection. Int. J. Biol. Macromol. 2020, 163, 2134–2144. [Google Scholar] [CrossRef]
- Kang, N.-W.; Yoon, S.-Y.; Kim, S.; Yu, N.-Y.; Park, J.-H.; Lee, J.-Y.; Cho, H.-J.; Kim, D.-D. Subcutaneously Injectable Hyaluronic Acid Hydrogel for Sustained Release of Donepezil with Reduced Initial Burst Release: Effect of Hybridization of Microstructured Lipid Carriers and Albumin. Pharmaceutics 2021, 13, 864. [Google Scholar] [CrossRef]
- Mendes, I.; Ruela, A.; Carvalho, F.; Freitas, J.; Bonfilio, R.; Pereira, G. Development and characterization of nanostructured lipid carrier-based gels for the transdermal delivery of donepezil. Colloids Surf. B Biointerfaces 2019, 177, 274–281. [Google Scholar] [CrossRef]
- Al Harthi, S.; Alavi, S.E.; Radwan, M.A.; El Khatib, M.M.; Alsarra, I.A. Nasal delivery of donepezil HCl-loaded hydrogels for the treatment of Alzheimer’s disease. Sci. Rep. 2019, 9, 9563. [Google Scholar] [CrossRef] [Green Version]
- Rajput, A.; Butani, S. Donepezil HCl Liposomes: Development, Characterization, Cytotoxicity, and Pharmacokinetic Study. AAPS PharmSciTech 2022, 23, 74. [Google Scholar] [CrossRef]
- Chauhan, M.K.; Sharma, P.K. Optimization and characterization of rivastigmine nanolipid carrier loaded transdermal patches for the treatment of dementia. Chem. Phys. Lipids 2019, 224, 104794. [Google Scholar] [CrossRef]
- Cunha, S.; Swedrowska, M.; Bellahnid, Y.; Xu, Z.; Lobo, J.S.; Forbes, B.; Silva, A. Thermosensitive in situ hydrogels of rivastigmine-loaded lipid-based nanosystems for nose-to-brain delivery: Characterisation, biocompatibility, and drug deposition studies. Int. J. Pharm. 2022, 620, 121720. [Google Scholar] [CrossRef]
- Salatin, S.; Barar, J.; Barzegar-Jalali, M.; Adibkia, K.; Jelvehgari, M. Thermosensitive in situ nanocomposite of rivastigmine hydrogen tartrate as an intranasal delivery system: Development, characterization, ex vivo permeation and cellular studies. Colloids Surf. B Biointerfaces 2017, 159, 629–638. [Google Scholar] [CrossRef]
- Salatin, S.; Barar, J.; Barzegar-Jalali, M.; Adibkia, K.; Alami-Milani, M.; Jelvehgari, M. Formulation and Evaluation of Eudragit RL-100 Nanoparticles Loaded In-Situ Forming Gel for Intranasal Delivery of Rivastigmine. Adv. Pharm. Bull. 2020, 10, 20–29. [Google Scholar] [CrossRef] [Green Version]
- Yuan, H.; Wang, L.-L.; Du, Y.-Z.; You, J.; Hu, F.-Q.; Zeng, S. Preparation and characteristics of nanostructured lipid carriers for control-releasing progesterone by melt-emulsification. Colloids Surf. B Biointerfaces 2007, 60, 174–179. [Google Scholar] [CrossRef]
- Shashi, K.; Satinder, K.; Parashar, B. A complete review on: Liposomes. Int. Res. J. Pharm. 2012, 3, 10–16. [Google Scholar]
- Andrade, S.; Ramalho, M.J.; Loureiro, J.A.; Pereira, M.C. Liposomes as biomembrane models: Biophysical techniques for drug-membrane interaction studies. J. Mol. Liq. 2021, 334, 116141. [Google Scholar] [CrossRef]
- Andrade, S.; Ramalho, M.J.; Loureiro, J.A.; Pereira, M.C. The biophysical interaction of ferulic acid with liposomes as biological membrane model: The effect of the lipid bilayer composition. J. Mol. Liq. 2021, 324, 114689. [Google Scholar] [CrossRef]
- Hagl, S.; Kocher, A.; Schiborr, C.; Kolesova, N.; Frank, J.; Eckert, G.P. Curcumin micelles improve mitochondrial function in neuronal PC12 cells and brains of NMRI mice—Impact on bioavailability. Neurochem. Int. 2015, 89, 234–242. [Google Scholar] [CrossRef]
- Yang, P.; Sheng, D.; Guo, Q.; Wang, P.; Xu, S.; Qian, K.; Li, Y.; Cheng, Y.; Wang, L.; Lu, W.; et al. Neuronal mitochondria-targeted micelles relieving oxidative stress for delayed progression of Alzheimer’s disease. Biomaterials 2020, 238, 119844. [Google Scholar] [CrossRef]
- Zhang, B.; Gao, Y.; Zhang, X.; Jiang, J.; Ren, J.; Wang, S.; Hu, H.; Zhao, Y.; Chen, L.; Zhao, K.; et al. Ultra-stable dextran conjugated prodrug micelles for oxidative stress and glycometabolic abnormality combination treatment of Alzheimer’s disease. Int. J. Biol. Macromol. 2022, 203, 430–444. [Google Scholar] [CrossRef]
- Agrawal, M.; Prathyusha, E.; Ahmed, H.; Dubey, S.K.; Kesharwani, P.; Singhvi, G.; Naidu, V.G.; Alexander, A. Biomaterials in treatment of Alzheimer’s disease. Neurochem. Int. 2021, 145, 105008. [Google Scholar] [CrossRef]
- Nguyen, T.-T.-L.; Maeng, H.-J. Pharmacokinetics and Pharmacodynamics of Intranasal Solid Lipid Nanoparticles and Nanostructured Lipid Carriers for Nose-to-Brain Delivery. Pharmaceutics 2022, 14, 572. [Google Scholar] [CrossRef]
- Theunis, C.; Crespo-Biel, N.; Gafner, V.; Pihlgren, M.; López-Deber, M.P.; Reis, P.; Hickman, D.T.; Adolfsson, O.; Chuard, N.; Ndao, D.M.; et al. Efficacy and Safety of A Liposome-Based Vaccine against Protein Tau, Assessed in Tau.P301L Mice That Model Tauopathy. PLoS ONE 2013, 8, e72301. [Google Scholar] [CrossRef] [Green Version]
- Mintun, M.A.; Lo, A.C.; Duggan Evans, C.; Wessels, A.M.; Ardayfio, P.A.; Andersen, S.W.; Shcherbinin, S.; Sparks, J.; Sims, J.R.; Brys, M.; et al. Donanemab in Early Alzheimer’s Disease. N. Engl. J. Med. 2021, 384, 1691–1704. [Google Scholar] [CrossRef]
- Ostrowitzki, S.; Lasser, R.A.; Dorflinger, E.; Scheltens, P.; Barkhof, F.; Nikolcheva, T.; Ashford, E.; Retout, S.; Hofmann, C.; Delmar, P.; et al. A phase III randomized trial of gantenerumab in prodromal Alzheimer’s disease. Alzheimer’s Res. Ther. 2017, 9, 95. [Google Scholar] [CrossRef] [Green Version]
- Swanson, C.J.; Zhang, Y.; Dhadda, S.; Wang, J.; Kaplow, J.; Lai, R.Y.; Lannfelt, L.; Bradley, H.; Rabe, M.; Koyama, A.; et al. A randomized, double-blind, phase 2b proof-of-concept clinical trial in early Alzheimer’s disease with lecanemab, an anti-Aβ protofibril antibody. Alzheimer’s Res. Ther. 2021, 13, 80. [Google Scholar] [CrossRef]
- Shcherbinin, S.; Kielbasa, W.; Dubois, S.; Lowe, S.L.; Phipps, K.M.; Tseng, J.; Kevin, D.B.; Natanegara, F.; Warner, S.; Dreyfus, N.; et al. Brain target occupancy of LY3372689, an inhibitor of the O-GlcNAcase (OGA) enzyme: Translation from rat to human. Alzheimer’s Dement. 2020, 16 (Suppl. S4), e040558. [Google Scholar] [CrossRef]
- Evans, E.E.; Fisher, T.L., Jr.; Leonard, J.E.; Reader, A.M.; Mishra, V.; Mallow, C.L.; Balch, L.; Howell, A.; Smith, E.S.; Feigin, A.; et al. Evidence that semaphorin 4D is upregulated in neurons in Huntington’s and Alzheimer’s diseases: Effects of a SEMA4D blocking antibody on FDG-PET in a clinical trial, and treatment rationale for its use in AD. Alzheimer’s Dement. 2020, 16 (Suppl. S9), e043971. [Google Scholar] [CrossRef]
- Burns, L.H.; Doehner, T.; Puente, J.; Beck, B.; Gonzalez-Rojas, Y.; Lopez-Brignoni, E.; Nikolov, B.; Wang, H.; Pei, Z.; Hernandez, A.; et al. Encouraging interim results at 9 months from an open-label study of simufilam in patients with Alzheimer’s disease. Alzheimer’s Dement. 2021, 17 (Suppl. S9), e054395. [Google Scholar] [CrossRef]
- Siemers, E.R.; Sundell, K.L.; Carlson, C.; Case, M.; Sethuraman, G.; Liu-Seifert, H.; Dowsett, S.A.; Pontecorvo, M.J.; Dean, R.A.; Demattos, R. Phase 3 solanezumab trials: Secondary outcomes in mild Alzheimer’s disease patients. Alzheimer’s Dement. 2016, 12, 110–120. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.Y.; Wang, P.N.; Chiu, M.J.; Finstad, C.L.; Lin, F.; Lynn, S.; Tai, Y.H.; De Fang, X.; Zhao, K.; Hung, C.H.; et al. UB-311, a novel UBITh® amyloid β peptide vaccine for mild Alzheimer’s disease. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2017, 3, 262–272. [Google Scholar] [CrossRef]




| Drug | NPs Type | NPs Composition | Route of Administration | Main Outcomes | Ref. |
|---|---|---|---|---|---|
| Donepezil | Liposomes | Carboxymethyl cellulose, DSPC, cholesterol, and PEG | Intranasal | Sustained release of donepezil and enhanced bioavailability in the plasma and brain using liposomes. | [60] |
| Polymeric | Chitosan | Intranasal | NPs improved the pharmacokinetic properties and bioavailability of the drug, increasing its concentration in the target tissue. | [61] | |
| PLGA | Intravenous | NPs significantly increased drug transport to the brain, resulting in higher drug concentration in the target tissue. | [62] | ||
| PLGA-PEG | Intravenous | Donepezil was successfully delivered across the BBB by NPs and released in a controller manner. | [63] | ||
| SLNs | Dynasan® 116 | Intravenous | NPs exhibited a sustained release of the drug, a higher uptake by cells, and increased permeability. | [64] | |
| Galantamine | Liposomes | Soya phosphatidylcholine, cholesterol, and PG | Intranasal | Liposomes could effectively deliver galantamine by the nose-to-brain route with superior pharmacokinetic behavior and enhance AChE inhibition. | [65] |
| Polymeric | PLGA | Intravenous | NPs provided a sustained release of the drug compared to galantamine solution and are predicted to boost therapeutic effects and reduce side effects. | [66] | |
| SLNs | Glyceryl Behenate | Oral | SLNs enhanced the bioavailability of the drug, modulated its time course in vivo, and provided a controlled release. | [67] | |
| Polymeric | Chitosan | Intranasal | The pharmacodynamic behavior of the drug was enhanced by NPs. The animals given the NPs had higher AChE levels and recovered significantly from induced amnesia | [68] | |
| Rivastigmine | Polymeric | MPEG-PCL | Intravenous | NPs were able to delay the drug release and increase the in vivo brain uptake clearance of rivastigmine, which translated into improved memory deficit. | [69] |
| Chitosan | Intranasal | NPs provided a controlled and sustained release of the drug, with superior brain targeting efficiency than rivastigmine solution. | [70] | ||
| Liposomes | Soya lecithin and cholesterol | Intranasal | Liposomes improved the pharmacokinetic and pharmacodynamic parameters of the drug. Drug-loaded liposomes reversed the memory deficit characteristic of AD compared to the free drug. | [71] | |
| PEG-DSPE, Lecithin, DDAB, and Tween® 80 | Intranasal | Liposomes prolonged the release of rivastigmine and improved its bioavailability. The drug levels in both plasma and brain were increased about fourfold. | [72] | ||
| Phosphatidylcholine, Dihexadecyl phosphate, cholesterol, and glycerol | Subcutaneous | Liposomes provided a sustained and controlled release of the drug. The use of nanocarriers also resulted in significantly improved cognitive impairment and increased AChE activity. | [73] | ||
| Cholesterol, Lecithin, oleic acid, Labrafil®, Labrasol®, Pluronic® F-127, PG, and PEG | Transdermal | Liposomes enhanced rivastigmine permeation through the skin and maintained plasma levels within the therapeutic window after topical application. | [74] | ||
| SLNs | Glyceryl Behenate | Intranasal | SLNs provided higher in vitro and ex vivo nasal permeation of the drug. The nasal mucosa remained intact, proving its safety for intranasal administration. | [75] | |
| Glyceryl monostearate | Intranasal | The pharmacokinetic drug profile, bioavailability, and drug concentration in plasma and the brain were improved by SLNs in vivo. | [76] | ||
| Organic NPs | Silica | Intravenous | NPs allowed for a sustained release in vitro and improved the drug pharmacokinetics parameters. | [77] | |
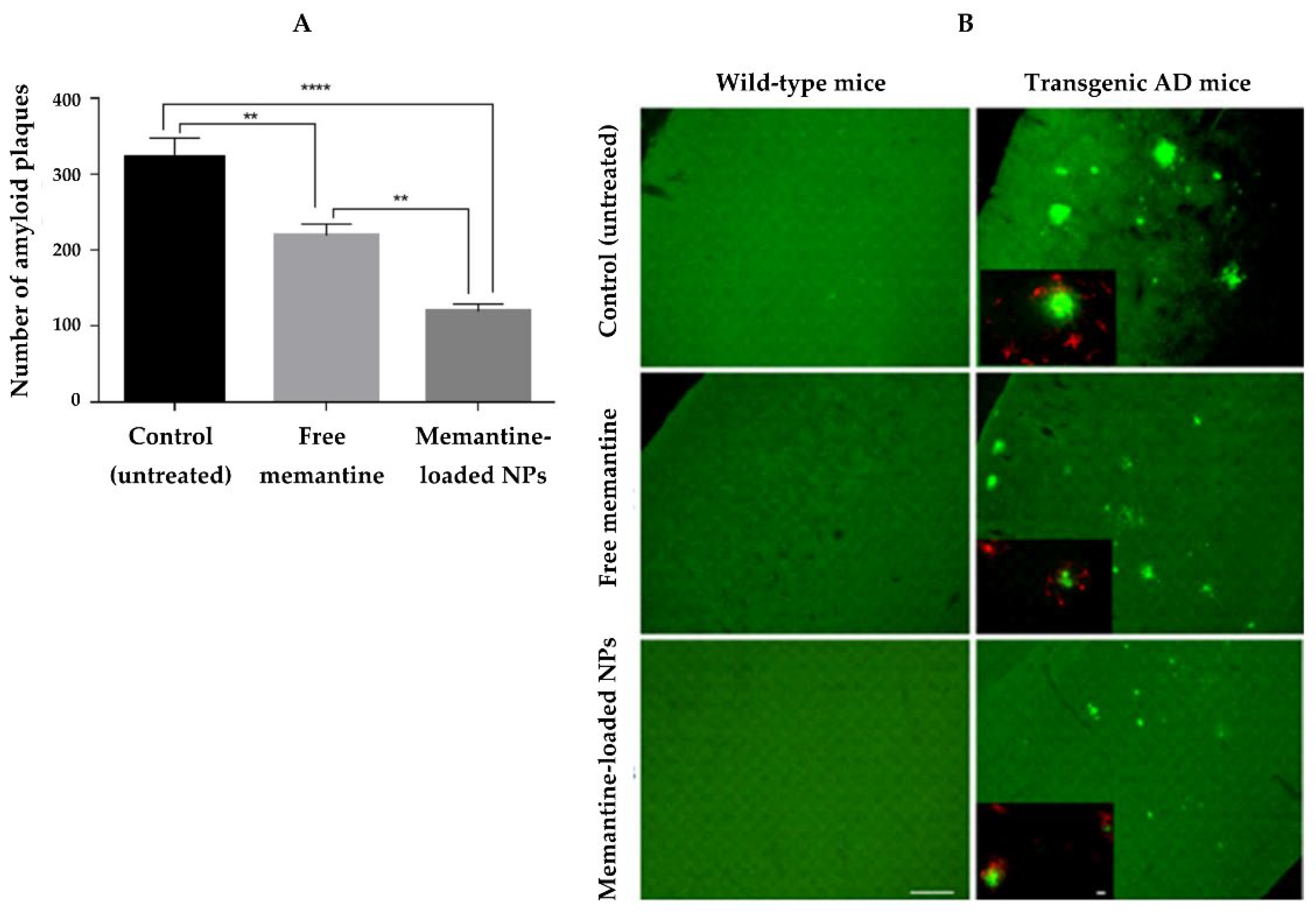
| Memantine | Polymeric | PLGA | Oral | NPs prolonged the drug release, which reduces the frequency of oral administration. In vivo, memantine-loaded NPs improved learning abilities and reduced β-amyloid brain plaques and inflammation associated with AD. | [78] |
| Dendrimers | PAMAM | Intravenous | Dendrimers improved the pharmacokinetic parameters of the drug. The DDS revealed significant improvement in behavioral responses and memory in vivo. | [79] |
| Drug | Hydrogel Composition | Route of Administration | Main Outcomes | Ref. |
|---|---|---|---|---|
| Galantamine | Methacrylated gelatin | Intracerebroventricular injection | Galantamine administration via hydrogel was found to be effective in reducing Aβ aggregation while also enhancing neuroinflammation, antioxidant activity, and neuronal development. | [82] |
| Donepezil | PVA and PVP | Transdermal | The hydrogel enhanced drug bioavailability and increased its plasma levels, allowing for long-term maintenance of drug doses. | [83] |
| Poloxamer 407 and Poloxamer 188 | Intranasal | The hydrogel improved drug bioavailability and targeting efficiency, resulting in more effective drug delivery to the brain. | [84] |
| Microformulation Type | Drug | Microformulation Composition | Route of Administration | Main Outcomes | Ref. |
|---|---|---|---|---|---|
| Microneedles | Donepezil | Hydrogel base: carboxymethyl cellulose Microneedles: HPMC | Transdermal | The microneedles efficiently transported donepezil across the skin, and the DDS increased drug bioavailability. | [87] |
| Microneedles: PMVE/MAH, PEG, sodium carbonate Drug reservoir: PVP and glycerol | Transdermal | In vitro, the DDS significantly improved drug penetration through the skin. Drug plasma concentrations in vivo were considerably higher than the control. | [88] | ||
| Hydrogel base: Polydimethylsiloxane Microneedles: PVP | Transdermal | The hydrogel improved the drug’s bioavailability and enabled its sustained release through the skin. The DDS has the potential to minimize drug delivery frequency while also improving patient compliance. | [89] | ||
| Memantine | Microneedles: PDMS and alumina Drug reservoir: PLA | Transdermal | The DDS was well tolerated by the skin and was able to deliver memantine transdermally for 3 days. | [90] | |
| Rivastigmine | Hydrogel base and microneedles: alginate and k-carrageenan | Transdermal | Compared to commercially available drug patches, the new DDS was safer and did not cause skin irritation. When the DDS was employed, the drug was released in a more efficient and controlled manner. | [92] | |
| Microparticles | Rivastigmine | Ethanol, water, and l-leucine | Inhalation | Spray-dried microparticles presented suitable physicochemical characteristics, aerodynamic properties, and aerosolization performance for rivastigmine inhalation delivery. | [94] |
| SAIB, PLGA, NMP, and Pluronic® F-68 | Intramuscular | Microparticles extended plasma levels of rivastigmine and increased its bioavailability. | [95] | ||
| Microspheres | Rivastigmine | Ethyl cellulose, chitosan, and PVA | Intranasal | The DDS significantly improved memory retention, biochemical parameters, and drug pharmacodynamics in vivo. The modification of the microsphere surface improved its mucoadhesion. | [96] |
| Microemulsion | Rivastigmine | Glyceryl caprylate, labrasol, transcutol-P, and water | Intranasal | Mucoadhesive microspheres increased the drug’s bioavailability and brain concentration following intranasal administration. | [98] |
| Donepezil | Castor oil, labrasol, transcutol-P, PG, and water | Intranasal | The microspheres were able to release donepezil in a sustained manner. In ex vivo nasal mucosa, a high permeability was likewise attained. | [99] |
| Drug | NPs Type | NPs Composition | Hydrogel Composition | Route of Administration | Outcome | Ref. |
|---|---|---|---|---|---|---|
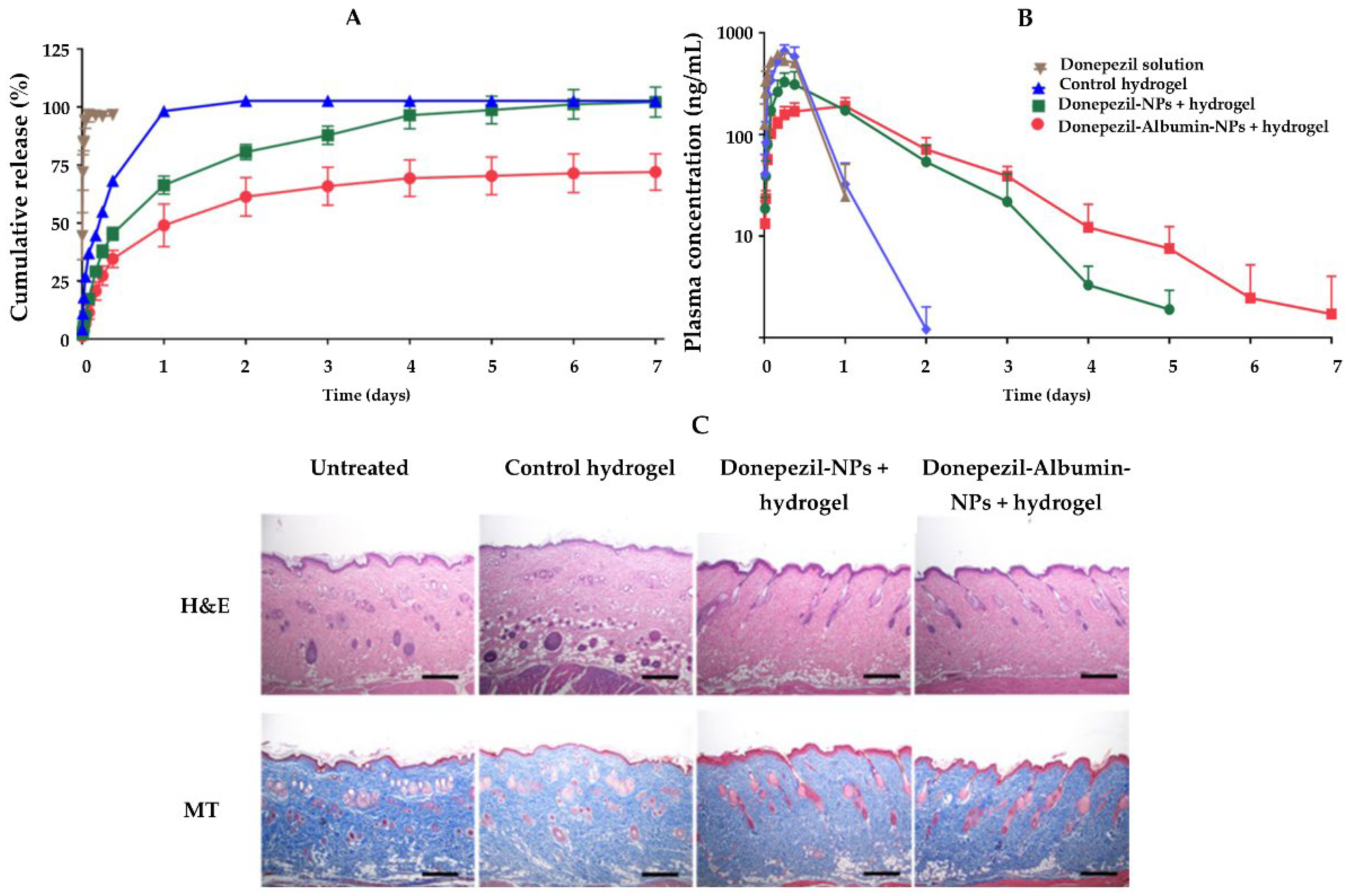
| Donepezil | Polymeric | PLGA | Hyaluronic acid | Subcutaneous | The NLH system provides a sustained release of the drug, which can minimize donepezil dose frequency and enhance patient compliance. | [106] |
| NLCs | Stearic acid and oleic acid | Hyaluronic acid | Subcutaneous | The NLH system revealed a biodegradation time of roughly 7 days and a sustained release of the drug, making it a viable option for a local depot with long-term drug release. | [107] | |
| Stearic acid, oleic acid, lecithin, and sodium taurodeoxycholate | Carbopol® 940 | Transdermal | The addition of drug-loaded NPs to the hydrogel resulted in a sustained release and a significant increase in drug penetration into the skin. | [108] | ||
| Liposomes | Cholesterol and dipalmitoylphosphocholine | Thiolated chitosan | Intranasal | Donepezil-loaded NPs combined into a hydrogel could give a long-lasting release, as well as significantly boost drug levels in the blood and brain. | [109] | |
| Hydrogenated soy phosphatidyl choline and cholesterol | Gellan gum and xanthan gum | Intranasal | Intranasal administration of the NLH increased drug levels in the target tissue due to a direct conduit from the nose to the brain, reducing systemic toxicity. The AChE activity was decreased, alleviating cognitive impairments. | [110] | ||
| Rivastigmine | NLCs | Glycerol monostearate and castor oil | Eudragit® E-100 and PBMACMM | Transdermal | Rivastigmine-loaded NLH enhanced skin permeation of the drug, achieving higher drug levels in plasma compared to conventional rivastigmine transdermal therapy. | [111] |
| Precirol® 5 ATO, and Vitamin E | Pluronic® F-127 and HPMC | Intranasal | The NLH system provided a prolonged drug release and higher nasal mucoadhesion, increasing drug retention time. The results highlight the potential of the DDS to improve nose-to-brain delivery. | [112] | ||
| Polymeric | PLGA | Pluronic® F-127 | Intranasal | A high cellular uptake of NPs by cells was obtained, which may provide an enhanced therapeutic efficacy in vivo with a decrease in side effects. | [113] | |
| Eudragit® RL-100 | Pluronic® F-127 | Intranasal | [114] |
| Drug | Target | Mechanism of Action | Route of Administration | Sponsor | FDA Status | Clinical Trial Identifier | Ref. |
|---|---|---|---|---|---|---|---|
| ACI-35 | Tau | A liposome-based vaccine to elicit an immune response targeted to pathological conformers of phosphorylated tau. | Intramuscular | AC Immune SA (Lausanne, Switzerland), Janssen (Belcey, Belgium) | Phase II | NCT04445831 | [124] |
| Donanemab | Amyloid | An antibody designed to bind to a pyroglutamate form of Aβ that is aggregated in amyloid plaques. | Intravenous | Eli Lilly & Co. (Indianapolis, IN, USA) | Phase III | NCT04437511 | [125] |
| Gantenerumab | Amyloid | An antibody designed to bind to Aβ fibrils. | Subcutaneous | Roche (Basel, Switzerland) | Phase III | NCT04374253 | [126] |
| Lecanemab | Amyloid | An antibody designed to bind to Aβ protofibrils. | Intravenous | Biogen, Eisai Co., Ltd. (Tokyo, Japan) | Phase III | NCT03887455 | [127] |
| LY3372689 | Tau | Inhibitor of the O-GlcNAcase enzyme. | Oral | Eli Lilly & Co. (Indianapolis, IN, USA) | Phase II | NCT05063539 | [128] |
| Pepinemab | Inflammation | Antibody to semaphorin 4D, a multifunctional membrane glycoprotein expressed by oligodendrocytes and astrocytes in the CNS. | Intravenous | Vaccinex, Inc. (New York, NY, USA) | Phase I/ II | NCT04381468 | [129] |
| Simufilam | Amyloid | Molecule designed to bind to filamin, a protein that stabilizes Aβ-42 and the α7 nicotinic acetylcholine receptor (reported to trigger tau phosphorylation). | Oral | Cassava Sciences (Austin, TX, USA) | Phase II | NCT04079803 | [130] |
| Solanezumab | Amyloid | An antibody directed against the mid-domain of the Aβ peptide. | Intravenous | Eli Lilly & Co. (Indianapolis, IN, USA) | Phase III | NCT00905372 | [131] |
| UB-311 | Amyloid | Synthetic peptide vaccine generated N-terminal anti-Aβ antibodies, which neutralized Aβ toxicity and promoted plaque clearance. | Intramuscular | United Neuroscience (Dublin, Ireland) | Phase III | NCT02551809 | [132] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nunes, D.; Loureiro, J.A.; Pereira, M.C. Drug Delivery Systems as a Strategy to Improve the Efficacy of FDA-Approved Alzheimer’s Drugs. Pharmaceutics 2022, 14, 2296. https://doi.org/10.3390/pharmaceutics14112296
Nunes D, Loureiro JA, Pereira MC. Drug Delivery Systems as a Strategy to Improve the Efficacy of FDA-Approved Alzheimer’s Drugs. Pharmaceutics. 2022; 14(11):2296. https://doi.org/10.3390/pharmaceutics14112296
Chicago/Turabian StyleNunes, Débora, Joana A. Loureiro, and Maria Carmo Pereira. 2022. "Drug Delivery Systems as a Strategy to Improve the Efficacy of FDA-Approved Alzheimer’s Drugs" Pharmaceutics 14, no. 11: 2296. https://doi.org/10.3390/pharmaceutics14112296
APA StyleNunes, D., Loureiro, J. A., & Pereira, M. C. (2022). Drug Delivery Systems as a Strategy to Improve the Efficacy of FDA-Approved Alzheimer’s Drugs. Pharmaceutics, 14(11), 2296. https://doi.org/10.3390/pharmaceutics14112296