3.1. Solubility Results
Experimental data for the solubility of caffeine in 0.1 mol∙kg
−1 SS aqueous solutions and the solubility of caffeine in pure water were obtained experimentally and presented in
Table 1. As can be seen, the solubility of caffeine increased with temperature in both cases. In 0.1 mol∙kg
−1 SS aqueous solutions, caffeine had a 2–2.7 times higher solubility than caffeine in pure water, depending on the temperature.
Based on the modified Van ’t Hoff equation, the apparent standard dissolution enthalpy (Δ
solH°), entropy (Δ
solS°), and Gibbs energy (Δ
solG°) change of solutes in water and 0.1 mol·kg
−1 SS aqueous solutions were calculated from the results of experimental solubility data [
26,
27]:
where
x1 is the mole fraction of caffeine in solutions,
T is the absolute temperature (K), and
R is the universal gas constant with a value of 8.314 J·K
−1·mol
−1.
Thm represents the harmonic temperature, defined as:
where
n equals the number of temperature points,
Tj is the experimental temperature, and the obtained value is 302.99 K. Δ
solH° is defined as the apparent standard mole dissolution enthalpy change of solute dissolved in water and 0.1 mol∙kg
−1 SS aqueous solution, obtained from the slope of the fitted line curves, ln
x1 versus (1/
T − 1/
Thm) (
Figure 3).
From the intercept obtained from the modified Van ’t Hoff plot, the Δ
solG° and Δ
solS° can be calculated by the following equations:
Determination of the relative contribution of enthalpy (
ζH) and the relative contribution of entropy (
ζTS) were calculated using the following expressions [
26,
27]:
The obtained apparent thermodynamic properties’ values of Δ
solG°, Δ
solH°, and Δ
solS°, as well as of ζ
H and
ζTS for caffeine in water and caffeine in SS aqueous solution, are presented in
Table 2.
Table 2 shows that Δ
solG° and Δ
solH° values were positive for both examined solutions, caffeine in aqueous solution and caffeine in SS aqueous solution. The dissolution processes are endothermic, which indicates that dissolving caffeine in water and aqueous sodium salicylate solution proceeds with absorption of heat. A positive value of the enthalpy of dissolution indicates that the heat energy released due to the hydration of compounds is less than the energy required to break down the interactions between caffeine molecules. Based on these results, we can assume that in the presence of sodium salicylate, caffeine self-aggregates are formed in an aqueous solution, similar to that in pure water. Lower enthalpy values in sodium salicylate solution may be due to the hydration of sodium and salicylate ions. The negative Δ
solS° value for both examined solutions denotes that dissolution of caffeine in water proceeds with an increase in the order of the system. The formation of caffeine self-aggregates is undoubtedly one of the explanations for the negative values of dissolution entropy.
On the other hand, the influence of caffeine molecules on the structural organization of water molecules, i.e., its structure-making/breaking properties, will be examined based on volumetric and viscometric measurements. The calculated values of the relative contribution of enthalpy and entropy imply that the main contributor to ΔsolG°, in caffeine dissolution in aqueous solutions and SS aqueous solutions, is enthalpy. Moreover, the endothermic enthalpy of the dissolution process is in accordance with the obtained solubility increase with rising temperature.
3.2. Volumetric Properties
For the determination of caffeine volumetric properties in 0.1 mol·kg
−1 SS aqueous solution, data obtained from experimental measurements of solution densities were collected and provided in
Table S2. Densities of solutions increased with a molality of caffeine at all temperatures (
Figure S1).
The obtained solution densities were used to calculate the apparent molar volume (
) of caffeine, from the equation presented in the
Supplementary Materials. The calculated values are shown in
Table S2. As shown in
Figure 4, the caffeine values in 0.1 mol∙kg
−1 SS aqueous solutions increased with caffeine molality at all temperatures.
The
values were fitted with the Masson’s equation modified for non-electrolytes [
28]:
and the plots of
versus m presented in
Figure 3. The
value represents the apparent molar volume of solute at infinite dilution, and S
v is the experimental slope, which provides information about solute–solute interactions. The obtained values of
and S
v are shown in
Table 3 with the standard deviation (
σ) and regression coefficient (
R2).
The positive
Sv values indicate the existence of strong caffeine–caffeine interactions. These values are significantly higher than pure water and 0.1 mol∙kg
−1 ATP aqueous solution (see
Table S3). As caffeine self-aggregation occurs in pure water and 0.1 mol∙kg
−1 ATP water solution [
12], higher values of the
Sv coefficient indicate that the process of self-aggregation in the presence of SS is even more pronounced. At first glance, this is unusual. The self-aggregation of molecules usually reduces their solubility in water, while our results showed an increase in solubility of caffeine in the presence of sodium salicylate.
Furthermore, the obtained
values of caffeine in SS aqueous solutions were positive and increased with rising temperature (
Figure S2), but they were significantly lower than in pure water or in the presence of ATP molecules (see
Table S3). This means that adding caffeine to a SS aqueous solution will lead to a significantly smaller increase in volume than in pure water or in the presence of ATP. Lower values of apparent molar volumes may also be a consequence of enhanced self-aggregation of caffeine molecules in the presence of sodium salicylate and/or strong interactions between caffeine molecules and salicylate anion. It is not possible to draw a conclusion based on volumetric measurements alone. That is why we need viscosity measurements and computer simulations.
The values of
were fitted as a function of the temperature using the equation of the second order:
Calculated coefficients
a0,
a1, and
a2 of caffeine in 0.1 mol∙kg
−1 SS aqueous solutions together with regression coefficients (
R2) are presented in
Table S4. Based on the calculated coefficients, the limiting apparent molar expansibility,
, of caffeine in SS aqueous solutions was calculated and presented in
Table 4, together with values in pure water:
The values of
were positive in the whole temperature range and significantly higher at lower temperatures than in pure water (
Table 4 and
Figure S3). Higher
values indicate a faster release of water molecules from the hydration sphere. However,
values decreased much faster with increasing temperature in the presence of SS compared to pure water. The Hepler’s coefficient defines the rate of this change and can be calculated with the following equation [
29]:
The obtained negative values for Hepler’s coefficient, presented in
Table 4, suggest caffeine structure-breaking properties in sodium salicylate aqueous solutions. However, the efficiency of Hepler’s coefficient to estimate the structure-making/breaking properties in water systems where significant self-aggregation of molecules occurs is quite questionable.
From the available data for
of caffeine in water and SS aqueous solutions, the limiting apparent transfer molar volumes (
) can be calculated with the following equation [
12]:
Values of
for the aqueous solution of caffeine are presented in
Table S3. The obtained values are presented in
Table 5. The negative values of
indicate a more pronounced self-aggregation of caffeine in the presence of SS or a strong interaction between caffeine and SS, which was discussed earlier in this paper.
According to Birch et al. [
30], an organization of water molecules around the solute regulates transport in the taste epithelium, binding to the receptor and finally inducing a taste response. In this study, to define the taste quality of studied solutes, the apparent specific volume at infinite dilution,
(results in
Table 5), was calculated by dividing caffeine
values with caffeine molar mass (
M = 194.19 g·mol
−1). It was found that the apparent specific volume at infinite dilution is a parameter of taste quality in the following order: salty (0.1–0.3 cm
3·g
−1) < sour (0.3–0.5 cm
3·g
−1) < sweet (0.5–0.7 cm
3·g
−1) < bitter (0.7–0.9 cm
3·g
−1). From
Table 5, it can be seen that the calculated values of
0.69 cm
3·g
−1 are on the border between the sweet and bitter taste of caffeine in SS aqueous solutions at all examined temperatures. On the other hand, Vraneš et al. calculated values for caffeine in pure water, 0.73–0.75 cm
3·g
−1, pointing out the bitter taste of caffeine at all examined temperatures (283.15–313.15 K) [
12]. Thus, the presence of SS in water reduces the bitter taste of caffeine. These data are essential due to the joint presence of caffeine and benzoic acid derivatives in food products.
3.3. Viscosimetric Properties
The viscosity measurements of caffeine in 0.1 mol∙kg
−1 SS aqueous solutions were measured in the temperature range from
T = 293.15 to 313.15 K up to molality of caffeine
m = 0.1058 mol·kg
−1 at the atmospheric pressure (p = 1 × 10
5 Pa). Data obtained from experimental caffeine measurements in 0.1 mol∙kg
−1 SS aqueous solution viscosities are presented in
Table S5, together with the values for aqueous solutions of caffeine [
12].
Table S5 shows viscosity values for 0.1 mol∙kg
−1 SS in water. Viscosity values of 0.1 mol∙kg
−1 SS in water were higher than viscosities of pure water.
The viscosity values for caffeine in 0.1 mol∙kg
−1 SS aqueous solution were higher than the values in pure water. In both systems, viscosity decreased with the temperature increase. One of the most common criteria for the determination of water organization around solute is the viscosity
B-coefficient obtained from the Jones–Dole’s equation [
31]:
where
ηo is the viscosity of the pure solvent,
η is the viscosity of the caffeine in SS aqueous solutions, and
c is concentration. Following the Jones–Dole’s equation, the reduced viscosity (
η/
ηo) dependence on the concentration was linear (
Figure 5). Calculated
B-coefficients are presented in
Table 6.
The relation between the
B-coefficient from the Jones–Dole’s equation and the change in the structural order of water molecules after adding a solute is well-known. If values of the
B-coefficient are positive, that indicates the structure-making properties of the solute, pointing out an increase in the local order of the water molecules. Negative
B-coefficient values indicate structure-breaking properties, weakening hydrogen bonds between water molecules near the solute [
32]. From the calculated results given in
Table 6,
B-coefficients for caffeine in SS aqueous solutions had positive values and decreased with the rising temperature. Thus, both conditions (
B > 0 and d
B/d
T < 0) were satisfied, and the caffeine molecule in 0.1 mol∙kg
−1 sodium salicylate water solution can be declared a structure-maker.
Table 6 also includes the
B-coefficients of caffeine in aqueous solutions tested by Vraneš et al. [
12]. From
Table 6, it can be seen that
B-coefficients in pure water were positive and increased with the rising temperature, suggesting that caffeine is an atypical structure-maker. The values of the
B-coefficient in the presence of sodium salicylate were higher compared to pure water, especially at lower temperatures. At higher temperatures (313.15 K), these values were similar in both systems. The obtained trends indicate that the influence of caffeine on the local arrangement of water molecules at higher temperatures is similar and that the presence of SS makes a significant difference only at lower temperatures. After analyzing the results of volumetric and viscometric measurements, we can conclude that the presence of SS promotes the self-aggregation of caffeine in water. The formation of self-aggregates contributes to the structural arrangement of water. However, it remains unclear what interactions occur between caffeine molecules and sodium salicylate and whether salicylate anion is incorporated into aggregates.
3.4. Thermodynamic Activation Parameters for Viscous Flow
Feakins et al. [
33] suggested calculation of the transition-state treatment of relative viscosity using viscosity obtained data:
where
is free energy of activation of viscous flow per mole of the pure water,
is Avogadro’s number,
is partial molar volumes of water at infinite dilution,
h is the Planck constant,
ηo is the viscosity of the solvent,
R is the universal gas constant, and
T is the absolute temperature. The following equation calculates the free energy of activation of viscous flow per mole of solute:
where
B is the viscosity coefficient, and
is the partial molar volume at infinite dilution of solute. The calculated values of
,
,
, and
are presented in
Table 7.
The values of
were positive and larger than
at all investigated temperatures, indicating that the interactions between caffeine and solvent molecules (water and SS) are more pronounced in the ground state than in the transition state. The transition state formation occurs with the breaking and deformation of the intermolecular bonds between solvent molecules [
34,
35]. The positive values of the term (
were described by Glasstone et al. as “loss of the structure-breaking contribution special to liquid water” [
36]. In other words, the positive values of a mentioned term are typical for structure-making compounds. These data agree well with the analyzed results of the Jones-Dole’s equation’s
B-coefficient.
The equation for determination of the entropy of activation for the viscous flow (
) of the caffeine in 0.1 mol∙kg
−1 SS aqueous solutions is:
while the activation enthalpy (
) can be calculated with the equation:
The values of
and
were 239.66 J·mol
−1·K
−1 and 162.33 kJ·mol
−1 for caffeine in SS aqueous solutions. These results support that formation of the transition state of caffeine in SS aqueous solutions is associated with bond-breaking and a decrease in order [
34,
35].
3.6. Computational Study
Computational studies were used to confirm the results obtained from volumetric, viscometric, and solubility measurements. MD simulations were performed at caffeine molality m = 0.25 mol∙kg−1 for both tested cases at a temperature 298.15 K (250 molecules of caffeine, 100 molecules of SS (0.1 mol∙kg−1), and 55,555 molecules of water, while in the case of caffeine in pure water, 250 molecules of caffeine and 55,555 molecules of water were used).
It is a well-known scientific fact that caffeine molecules show a tendency towards self-aggregation in water solutions at higher concentrations [
5,
38]. As shown in
Figure 6a, caffeine molecules tend to interact with each other, forming self-aggregates at molality
m = 0.25 mol∙kg
−1. Experimental results indicate that the presence of sodium salicylates increases the self-aggregation of caffeine molecules in water. Therefore, the radial distributions of caffeine molecules around the caffeine in pure water and the presence of SS were calculated.
Figure 7 shows the obtained results from RDFs of the caffeine–caffeine center of mass. The first and second peaks of this distribution function appeared at 3.65 and 7.1 Å, indicating the existence of caffeine’s first and second coordination shells around caffeine.
The intensity of both peaks of radial function increased after the addition of SS to water, indicating increasing probabilities of self-aggregation between caffeine molecules. The large number of caffeine molecules in the coordination shells of caffeine confirmed that the presence of SS increased the self-aggregation of caffeine in water. Caffeine molecules interact through π–π interactions, as shown in
Figure 6b. In
Figure 6b, it can also be seen that salicylate molecules are not involved in caffeine self-aggregates. To confirm the absence of SS molecules from caffeine aggregates, RDF caffeine-salicylate was calculated, considering the center of masses of caffeine and salicylate. The calculated values were g(r) = 0 in the whole distance range from 0 to 10 Å, which confirms the absence of any interactions between these particles in equilibrium and at selected concentrations (
Figure 8).
To further analyze the hydration of caffeine molecules in the presence and absence of SS, calculations of the radial distribution function (RDFs) were applied.
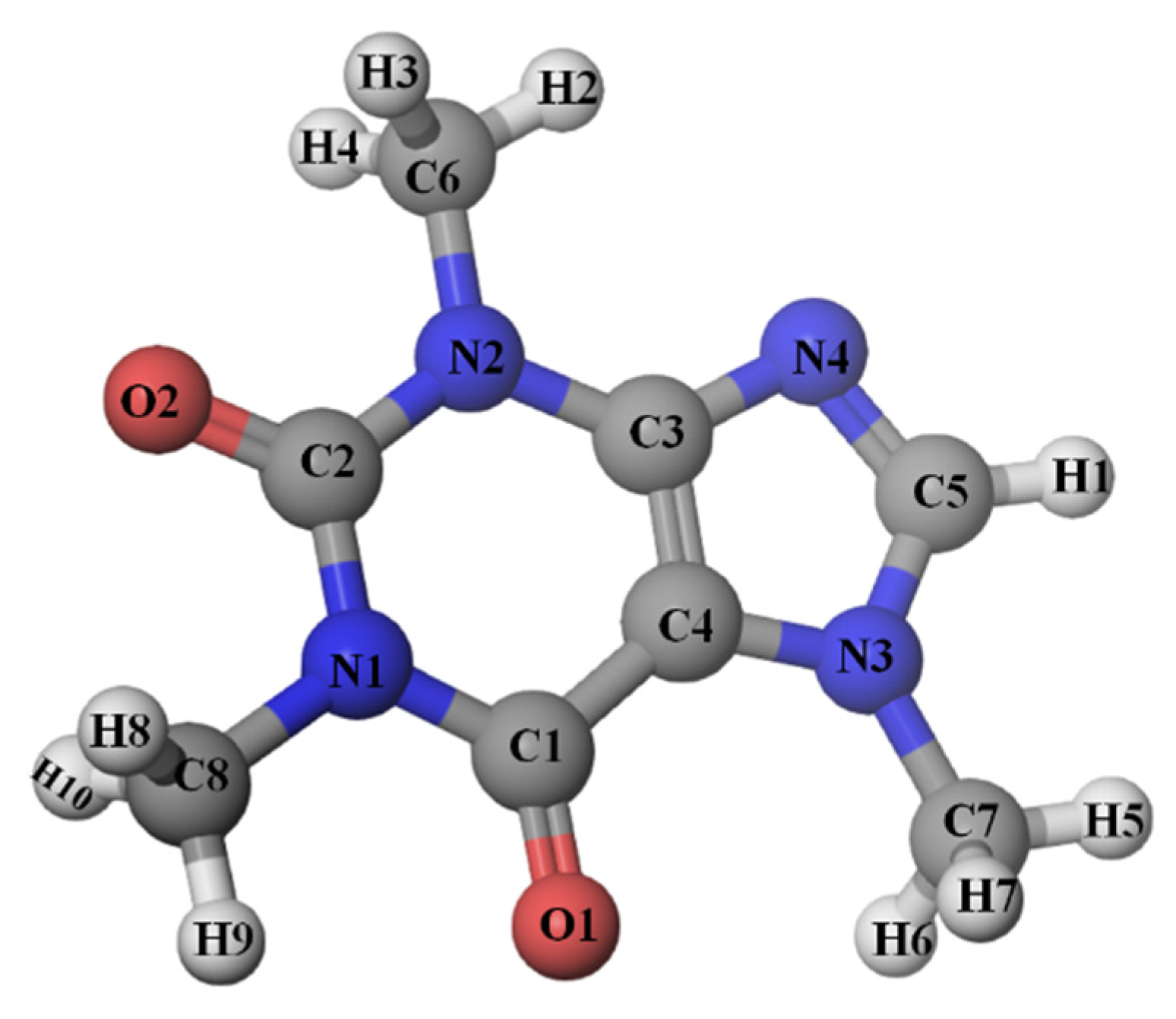
Figure 9 shows the RDFs of water oxygen atoms (Ow) around selected atomic sites of caffeine: O1, O2, N4, and H1. Hydrophilic centers in the caffeine molecule have been selected because they can form the strongest interactions with water molecules. As hydrogen bond acceptors, oxygen atoms from carbonyl groups, O1 and O2, as well as N4 atom from the imidazole ring were selected. The most acidic hydrogen atom (H1) was also considered the potential proton-donor for forming the H-bond with water molecules.
Figure 9a–d show the values of g(r) in pure water and in the presence of sodium salicylate for all selected caffeine atoms. The obtained results indicate that sodium salicylate reduces the number of water molecules in the hydration spheres of O1 and H1 atoms. In the hydration sphere of the imidazole nitrogen atom (N4), the number of water molecules increased in the presence of SS, while in the case of carbonyl oxygen (O2), it did not change.
According to the procedure published elsewhere [
12], the hydration number of caffeine in the presence and absence of SS was calculated to quantify the obtained results. The calculated value of the hydration number of caffeine in pure water at 298.15 K and caffeine molality
m = 0.25 mol∙kg
−1 was
hn = 5.25. In 0.1 mol∙kg
−1 sodium salicylate aqueous solutions, at the same molality, the hydration number of caffeine had a lower value,
hn = 4.24. This value of hydration number in the presence of SS is in good agreement with the experimentally obtained results (
Table 8). In our previous work [
12], the hydration number of caffeine at a molality of 0.06 mol∙kg
−1 (unfortunately, these data are missing in that paper) was calculated using an identical computer simulations procedure, and a value of
hn = 0.82 at
T = 298.15 K was obtained. At a molality of 0.06 mol∙kg
−1, self-aggregation of caffeine in water was not observed. Thus, computer simulations show that the caffeine hydration number increased significantly during self-aggregation (from 0.82 to 5.25). The main reason for the increase in hydration number is that caffeine molecules form aggregates primarily through π–π interactions (see
Figure 6b). As a result, caffeine molecules’ hydrophobic surfaces in self-aggregate are reduced [
38], while hydrophilic centers have more probability for interaction with water molecules.
The presence of SS reduces the hydration number of caffeine (from 5.25 to 4.24), which is in concordance with the results of other studies. Sodium salicylate and many other polar molecules, such as sodium chloride [
38], ATP [
12], and sucrose [
5], have a dehydrating effect on caffeine molecules. The mentioned molecules have more pronounced hydration abilities than caffeine, and by binding water molecules to themselves, they reduce the number of water molecules in the hydration shells of caffeine.
However, the main question remains open: how does sodium salicylate increase the solubility of caffeine in water? If we compare the results of the molecular dynamics simulations for the system NaCl + caffeine with our results, we can conclude that they are very similar [
38]. In the presence of NaCl, caffeine self-aggregation in water increases, and RDFs peaks occur at the exact distances from the caffeine–caffeine center of mass. The addition of NaCl reduces the hydration number of water molecules around the hydrophilic centers of caffeine. These results were also obtained in this paper. Furthermore, both NaCl and SS have structure-making properties to water molecules.
The work of Ahmad et al. [
7] showed that the presence of a cosmotrope (structure-makers, such as NaCl and Na
2SO
4) increases self-aggregation and decreases caffeine solubility, which is a consequence of the salting-out effect of these salts. The salting-out effect on caffeine molecules is also shown by some neutral molecules, such as sucrose [
5]. Sucrose has structure-making properties, increasing the self-aggregation of caffeine and reducing its solubility.
However, SS has structure-maker properties but increases the solubility of caffeine.
The cosmotropic behavior of some additives does not appear to be a “driving force” to promote caffeine self-aggregation in water. Combining the Kirkwood–Buff solution theory with the isodesmic model of caffeine association, Shimizu [
39] concluded that changes in the water structure upon the addition of cosmotropic or chaotropic additives have a negligible effect on the self-aggregation of caffeine. In the same paper, Shimizu concludes that the real driving force for caffeine self-aggregation is the interaction between additives and caffeine. Self-aggregation increases if additives are removed from caffeine molecules and decreases if additives stick around caffeine. In the mentioned manuscript, the author emphasizes that a new theory has important consequences for predicting the influence of additives on the solubility of macromolecules, but he did not elaborate on that influence.
Therefore, we need to analyze what interactions SS can form with the caffeine molecule, increasing its solubility, unlike sodium chloride and sucrose. The logical answer is π–π interactions because both salicylate and caffeine are aromatic compounds. However, we have already discussed (
Figure 6b) that salicylate anions are not included in caffeine self-aggregates. Therefore, we set up a new MD simulation with a significantly lower concentration of caffeine (0.06 mol∙kg
−1). At this concentration, self-association in pure water does not occur. The concentration of SS was 0.1 mol∙kg
−1 and the number of water molecules was 5555.
Figure 10 shows a snapshot of the distribution of caffeine and SS molecules after equilibrium (30 ns). It can be seen from the figure that the presence of SS initiated the self-aggregation of caffeine, but that not all molecules are included in the aggregates. However,
Figure 10 also shows π–π interactions between caffeine and salicylate molecules. We calculated the radial distribution function concerning the center of masses to better understand the spatial relationship of salicylate and caffeine molecules (see
Figure 8). The peak maximum appeared at a distance of 3.86 Å, which is very close to the value of the first peak of the radial distribution of the caffeine–caffeine center of mass (3.65 Å). This means that a molecule of caffeine displaces salicylate during aggregation and takes its place next to another molecule of caffeine. Finally, we can assume the mechanism of salicylate increasing caffeine’s solubility.
After adding caffeine to the SS solution (
Figure 11a), a parallel stacking (π–π) aromatic complex between aromatic rings was formed (
Figure 11b). Caffeine-salicylate complexes provide better solubility of caffeine monomers and allow local caffeine supersaturation formation (
Figure 10c). After contact with two or more complexes, the salicylate anion was released, forming a π–π interaction between caffeine molecules (
Figure 11d). The driving force for this process is the good solvation of the salicylate anion by water molecules and the tendency of caffeine molecules to aggregate with each other. We calculated the hydration number for salicylate anions not bonded with caffeine (see
Figure 6b), and the average value was 5.47. Hydration of the salicylate ion is an exothermic process. Additional heat release due to hydration of the salicylate anion is a reason why the enthalpy of dissolution is lower in the presence of SS than in pure water. Releasing salicylate molecules from the complex with caffeine and its hydration after that is probably the main reason for increasing the solubility of caffeine in water.
Therefore, for an additive to increase caffeine’s solubility in water through forming caffeine self-aggregates, it needs to form π–π interactions with caffeine and have pronounced hydrating properties. This hypothesis needs to be tested in the presence of several additives. It is necessary to choose additives that can and cannot form π–π interactions with caffeine and additives with different hydrating properties, i.e., structure-makers and structure-breakers.