Novel Salinomycin-Based Paramagnetic Complexes—First Evaluation of Their Potential Theranostic Properties
Abstract
:1. Introduction
2. Methods
2.1. Chemicals
2.2. Synthesis of Paramagnetic Complexes of Sal
2.2.1. Synthesis of Gd(III) Salinomycinate
2.2.2. Synthesis of Mn(II) Salinomycinate
2.2.3. Loading of Gd(III)and Mn(II) Salinomycinates into Empty Bacterial Ghosts Cells (BGs)
Quantification of Sal-Gd(III)/Sal-Mn(II) Extracted from BGs
2.3. Physical Measurements
2.3.1. Infrared Spectral Analysis (IR)
2.3.2. Electroparamagnetic Resonance Analysis (EPR)
2.3.3. Electrospray Ionization Mass Spectrometry (ESI-MS)
2.4. Thermogravimetric Analysis (TGA)
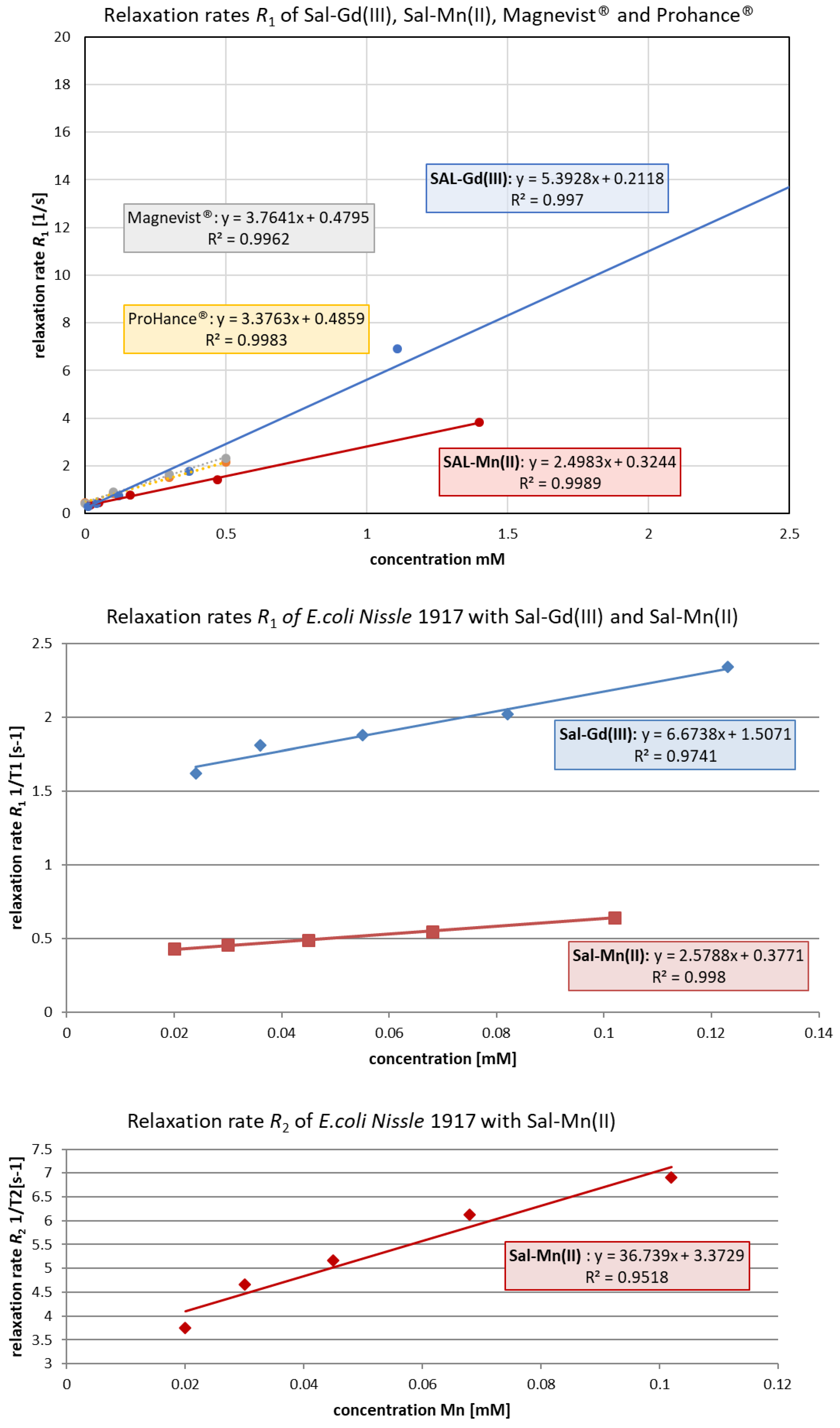
2.5. In Vitro Relaxivity Measurements
2.6. Cytotoxicity Tests
2.6.1. Cell Culture
2.6.2. MTT Assay
2.6.3. Cell Cycle Studies—Impact of Free Sal-H, Sal-Gd(III), and Sal-Mn(II) on the Cell Cycle
3. Results
3.1. Synthesis and Characterization
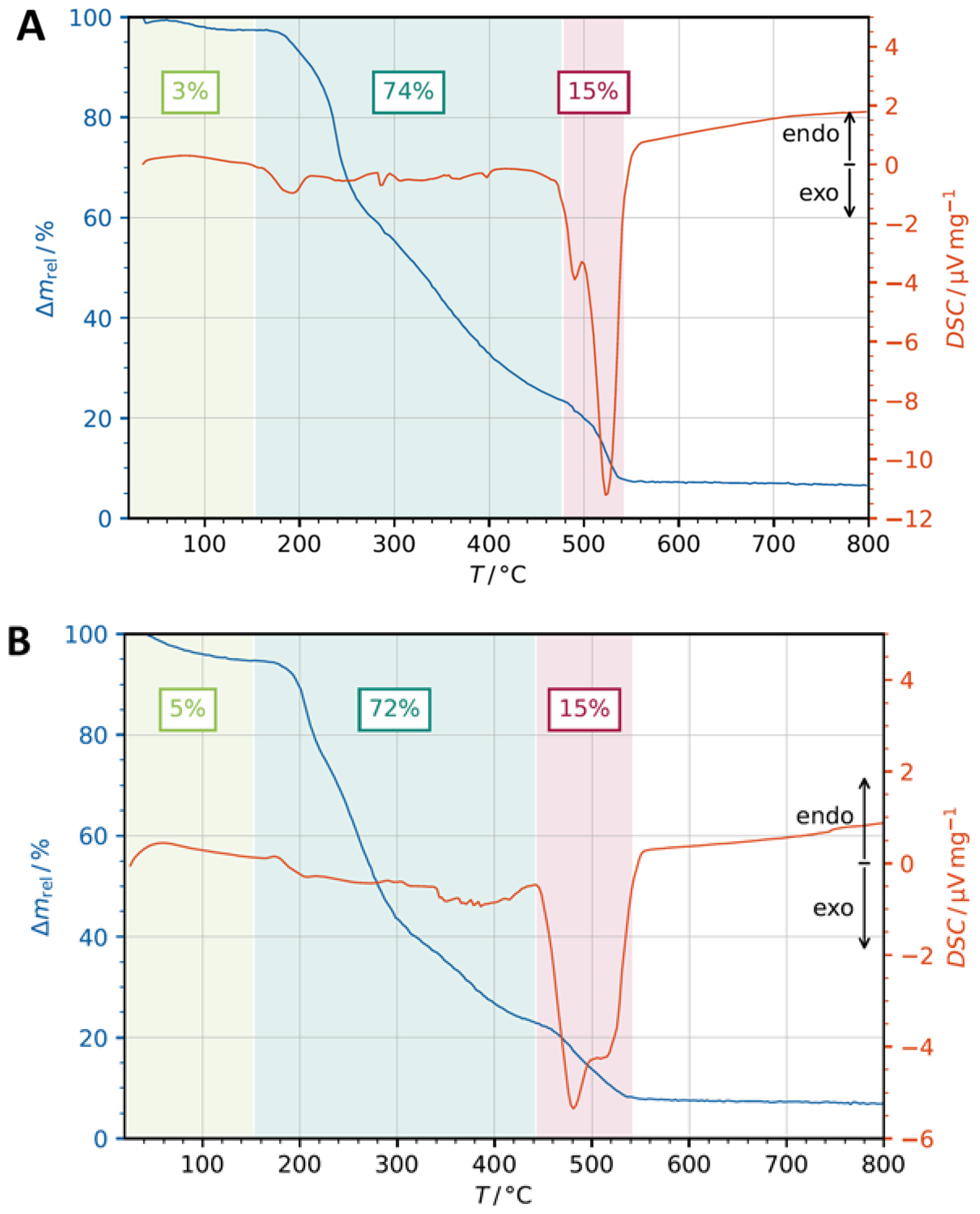
3.2. Thermogravimetric Analysis (TGA)
3.3. In Vitro MRI
4. Cell Culture Studies
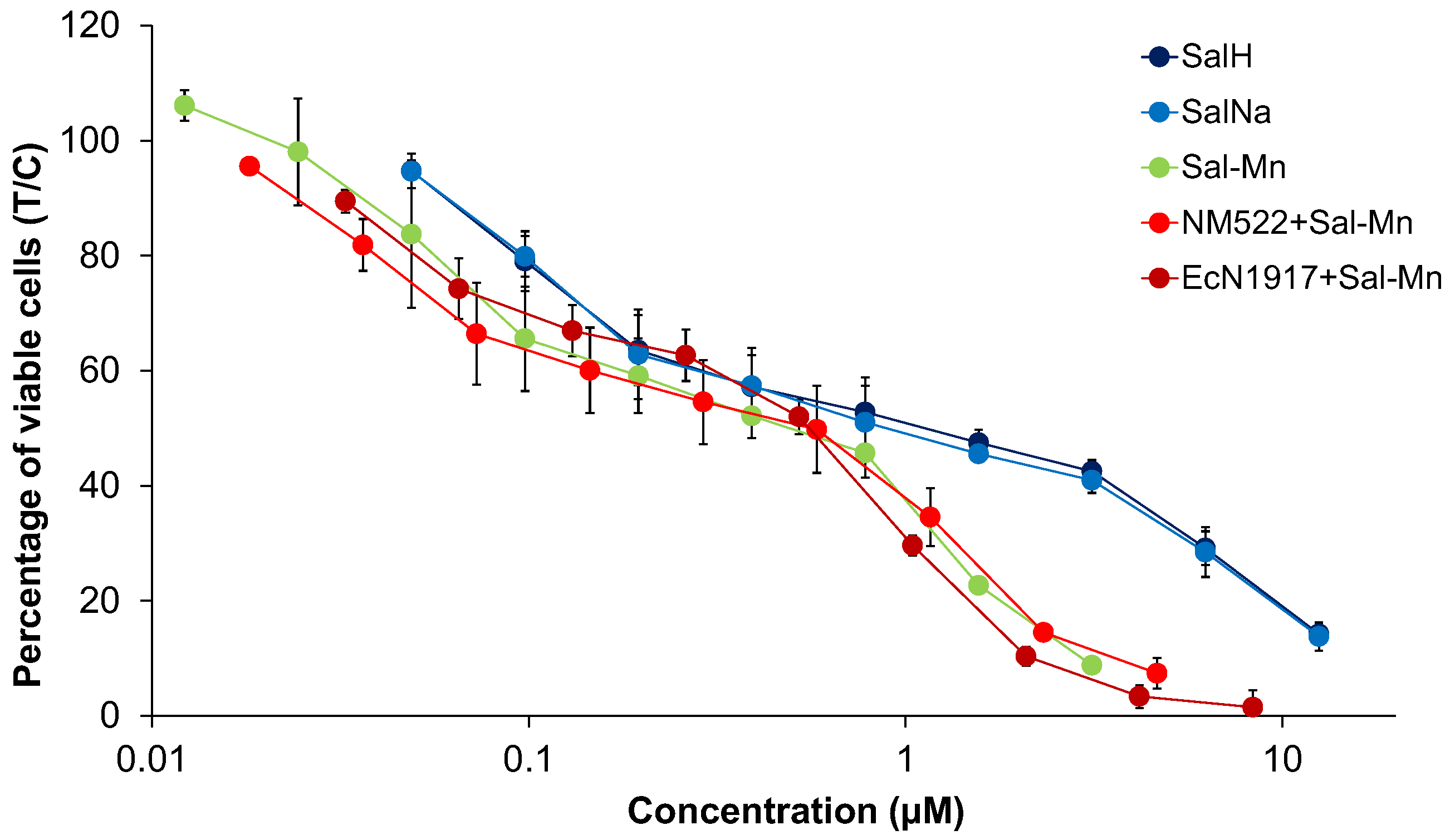
4.1. Cytotoxicity Studies
4.2. Cell Cycle Studies
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jeyamogan, S.; Khan, N.A.; Siddiqui, R. Application and Importance of Theranostics in the Diagnosis and Treatment of Cancer. Arch. Med. Res. 2021, 52, 131–142. [Google Scholar] [CrossRef]
- Sahraei, Z.; Mirabzadeh, M.; Fadaei-Fouladi, D.; Eslami, N.; Eshraghi, A. Magnetic Resonance Imaging Contrast Agents: A Review of Literature. J. Pharm. Care 2015, 2, 177–182. [Google Scholar]
- Kojima, R.; Aubel, D.; Fussenegger, M. Novel theranostic agents for next-generation personalized medicine: Small molecules, nanoparticles, and engineered mammalian cells. Curr. Opin. Chem. Biol. 2015, 28, 29–38. [Google Scholar] [CrossRef]
- Keam, S.J. Lutetium Lu 177 Vipivotide Tetraxetan: First Approval. Mol. Diagn. Ther. 2022, 26, 467–475. [Google Scholar] [CrossRef]
- Lohrke, J.; Frenzel, T.; Endrikat, J.; Alves, F.C.; Grist, T.M.; Law, M.; Lee, J.M.; Leiner, T.; Li, K.-C.; Nikolaou, K.; et al. 25 Years of Contrast-Enhanced MRI: Developments, Current Challenges and Future Perspectives. Adv. Ther. 2016, 33, 1–28. [Google Scholar] [CrossRef] [Green Version]
- Thomsen, H.S. NSF: Still relevant. J. Magn. Reson. Imaging 2014, 40, 11–12. [Google Scholar] [CrossRef]
- DI Gregorio, E.; Ferrauto, G.; Furlan, C.; Lanzardo, S.; Nuzzi, R.; Gianolio, E.; Aime, S. The Issue of Gadolinium Retained in Tissues: Insights on the Role of Metal Complex Stability by Comparing Metal Uptake in Murine Tissues Upon the Concomitant Administration of Lanthanum- and Gadolinium-Diethylentriamminopentaacetate. Investig. Radiol. 2018, 53, 167–172. [Google Scholar] [CrossRef]
- Sato, T.; Ito, K.; Tamada, T.; Kanki, A.; Watanabe, S.; Nishimura, H.; Tanimoto, D.; Higashi, H.; Yamamoto, A. Tissue gadolinium deposition in renally impaired rats exposed to different gadolinium-based MRI contrast agents: Evaluation with inductively coupled plasma mass spectrometry (ICP-MS). Magn. Reson. Imaging 2013, 31, 1412–1417. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food and Drug Administration. FDA Drug Safety Communication: FDA Warns that Gadolinium Based Contrast Agents (GBCAs) are Retained in the Body; Requires New Class Warnings; U.S. Food and Drug Administration: Washington, DC, USA, 2017.
- EMA’s Final Opinion Confirms Restrictions on Use of Linear Gadolinium Agents in Body Scans. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/Referrals_document/gadolinium_contrast_agents_31/Opinion_provided_by_Committee_for_Medicinal_Products_for_Human_Use/WC500231824.pdf (accessed on 21 July 2017).
- Zhen, Z.; Xie, J. Development of Manganese-Based Nanoparticles as Contrast Probes for Magnetic Resonance Imaging. Theranostics 2012, 2, 45–54. [Google Scholar] [CrossRef]
- Naujokat, C.; Fuchs, D.; Opelz, G. Salinomycin in cancer: A new mission for an old agent. Mol. Med. Rep. 2010, 3, 555–559. [Google Scholar] [CrossRef]
- Gupta, P.B.; Onder, T.T.; Jiang, G.; Tao, K.; Kuperwasser, C.; Weinberg, R.A.; Lander, E.S. Identification of Selective Inhibitors of Cancer Stem Cells by High-Throughput Screening. Cell 2009, 138, 645–659. [Google Scholar] [CrossRef] [PubMed]
- Schaffhausen, J. Remaining hurdles to effective cancer therapy. Trends Pharmacol. Sci. 2015, 36. [Google Scholar] [CrossRef]
- Jiang, J.; Li, H.; Qaed, E.; Zhang, J.; Song, Y.; Wu, R.; Bu, X.; Wang, Q.; Tang, Z. Salinomycin, as an autophagy modulator-- a new avenue to anticancer: A review. J. Exp. Clin. Cancer Res. 2018, 37, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Q.; Liu, F.; Wang, L.; Xie, C.; Wu, P.; Du, S.; Zhou, S.; Sun, Z.; Liu, Q.; Yu, L.; et al. Enhanced and Prolonged Antitumor Effect of Salinomycin-Loaded Gelatinase-Responsive Nanoparticles via Targeted Drug Delivery and Inhibition of Cervical Cancer Stem Cells. Int. J. Nanomed. 2020, 15, 1283–1295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antoszczak, M.; Huczyński, A. Salinomycin and its derivatives—A new class of multiple-targeted “magic bullets”. Eur. J. Med. Chem. 2019, 176, 208–227. [Google Scholar] [CrossRef]
- Dewangan, J.; Srivastava, S.; Rath, S.K. Salinomycin: A new paradigm in cancer therapy. Tumor Biol. 2017, 39, 1010428317695035. [Google Scholar] [CrossRef] [Green Version]
- Huczynski, A. Salinomycin—A New Cancer Drug Candidate. Chem. Biol. Drug Des. 2012, 79, 235–238. [Google Scholar] [CrossRef]
- Momekova, D.; Momekov, G.; Ivanova, J.; Pantcheva, I.; Drakalska, E.; Stoyanov, N.; Guenova, M.; Michova, A.; Balashev, K.; Arpadjan, S.; et al. Sterically stabilized liposomes as a platform for salinomycin metal coordination compounds: Physicochemical characterization and in vitro evaluation. J. Drug Deliv. Sci. Technol. 2013, 23, 215–223. [Google Scholar] [CrossRef]
- Ivanova, J.; Pantcheva, I.N.; Zhorova, R.; Momekov, G.; Simova, S.; Stoyanova, R.; Zhecheva, E.; Ivanova, S.; Mitewa, M. Synthesis, spectral properties, antibacterial and antitumor activity of salinomycin complexes with Co(II), Ni(II), Cu(II) and Zn(II) transition metal ions. J. Chem Chem Eng. 2012, 6, 551–562. [Google Scholar]
- Koller, V.J.; Dirsch, V.M.; Beres, H.; Donath, O.; Reznicek, G.; Lubitz, W.; Kudela, P. Modulation of bacterial ghosts—induced nitric oxide production in macrophages by bacterial ghost-delivered resveratrol. FEBS J. 2013, 280, 1214–1225. [Google Scholar] [CrossRef]
- Langemann, T.; Koller, V.J.; Muhammad, A.; Kudela, P.; Mayr, U.B.; Lubitz, W. The bacterial ghost platform system: Production and applications. Bioeng. Bugs 2010, 1, 326–336. [Google Scholar] [CrossRef] [Green Version]
- Tabrizi, C.A.; Walcher, P.; Mayr, U.B.; Stiedl, T.; Binder, M.; McGrath, J.; Lubitz, W. Bacterial ghosts—biological particles as delivery systems for antigens, nucleic acids and drugs. Curr. Opin. Biotechnol. 2004, 15, 530–537. [Google Scholar] [CrossRef]
- Mazur, M.; Poprac, P.; Valko, M.; Rhodes, C.J. ‘U-spectrum’ type of Gd(III) EPR spectra recorded at various stages of TEOS-based sol–gel process. J. Sol-Gel Sci. Technol. 2016, 79, 220–227. [Google Scholar] [CrossRef]
- Kiran, N.; Kummara, V.K.; Ravi, N.; Lenine, D. Structure and EPR investigations on Gd3+ ions in magnesium-lead-borophosphate glasses. J. Mol. Struct. 2020, 1208, 127877. [Google Scholar] [CrossRef]
- Gagnon, D.M.; Hadley, R.C.; Ozarowski, A.; Nolan, E.M.; Britt, R.D. High-Field EPR Spectroscopic Characterization of Mn(II) Bound to the Bacterial Solute-Binding Proteins MntC and PsaA. J. Phys. Chem. B 2019, 123, 4929–4934. [Google Scholar] [CrossRef] [PubMed]
- Thurston, J.H.; Trahan, D.; Ould-Ely, T.; Whitmire, K.H. Toward a General Strategy for the Synthesis of Heterobimetallic Coordination Complexes for Use as Precursors to Metal Oxide Materials: Synthesis, Characterization, and Thermal Decomposition of Bi2(Hsal)6·M(Acac)3 (M = Al, Co, V.; Fe, Cr). Inorg. Chem. 2004, 43, 3299–3305. [Google Scholar] [CrossRef] [PubMed]
- Moreira, J.M.; Campos, G.F.; Pinto, L.M.D.C.; Martins, G.R.; Tirloni, B.; Schwalm, C.S.; de Carvalho, C.T. Copper (II) complexes with novel Schiff-based ligands: Synthesis, crystal structure, thermal (TGA–DSC/FT-IR), spectroscopic (FT-IR, UV-Vis) and theoretical studies. J. Therm. Anal. Calorim. 2022, 147, 4087–4098. [Google Scholar] [CrossRef]
- Varbanov, H.P.; Göschl, S.; Heffeter, P.; Theiner, S.; Roller, A.; Jensen, F.; Jakupec, M.A.; Berger, W.; Galanski, M.S.; Keppler, B.K. A Novel Class of Bis- and Tris-Chelate Diam(m)inebis(dicarboxylato)platinum(IV) Complexes as Potential Anticancer Prodrugs. J. Med. Chem. 2014, 57, 6751–6764. [Google Scholar] [CrossRef] [Green Version]
- Banfić, J.; Legin, A.A.; Jakupec, M.A.; Galanski, M.; Keppler, B.K. Platinum(IV) complexes featuring one or two axial ferrocene bearing ligands—synthesis, characterization, and cytotoxicity. Eur J. Inorg Chem. 2014, 2014, 484–492. [Google Scholar] [CrossRef]
- Naujokat, C.; Steinhart, R. Salinomycin as a Drug for Targeting Human Cancer Stem Cells. J. Biomed. Biotechnol. 2012, 2012, 950658. [Google Scholar] [CrossRef]
- Atanasov, V.; Stoykova, S.; Goranova, Y.; Nedzhib, A.; Tancheva, L.; Ivanova, J.; Pantcheva, I. Preliminary study on in vivo toxicity of monensin, salinomycin and their metal complexes. Bul. Chem.Commun. 2014, 46, 233–273. [Google Scholar]
- Ivanova, J.; Pantcheva, I.N.; Mitewa, M.; Simova, S.; Tanabe, M.; Osakada, K. Cd(II) and Pb(II) complexes of the polyether ionophorous antibiotic salinomycin. Chem. Cent. J. 2011, 5, 52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azzoni, C.B.; Martino, D.D. EPR study of Gd3+ doped lead oxide based glasses. J. Mater. Sci. 1999, 34, 3931–3935. [Google Scholar] [CrossRef]
- Malchukova, E.; Boizot, B.; Ghaleb, D. Guillaume Petite β-Irradiation Effects in Gd-Doped Borosilicate Glasses Studied by EPR and Raman Spectroscopies. J. Non Cryst Solids 2006, 352, 297–303. [Google Scholar] [CrossRef]
- Diehl, S.J.; Lehmann, K.J.; Gaa, J.; McGill, S.; Hoffmann, V.; Georgi, M. MR Imaging of Pancreatic Lesions. Comparison of manganese-DPDP and gadolinium chelate. Investig. Radiol. 1999, 34, 589–595. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, H.; Ramsay, I.A.; Erstad, D.J.; Fuchs, B.C.; Tanabe, K.K.; Caravan, P.; Gale, E.M. Manganese-Based Contrast Agents for Magnetic Resonance Imaging of Liver Tumors: Structure–Activity Relationships and Lead Candidate Evaluation. J. Med. Chem. 2018, 61, 8811–8824. [Google Scholar] [CrossRef] [PubMed]
- Pan, D.; Caruthers, S.D.; Senpan, A.; Schmieder, A.H.; Wickline, S.A.; Lanza, G.M. Revisiting an old friend: Manganese-based MRI contrast agents. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnology 2011, 3, 162–173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gale, E.M.; Wey, H.-Y.; Ramsay, I.; Yen, Y.-F.; Sosnovik, D.E.; Caravan, P. A Manganese-based Alternative to Gadolinium: Contrast-enhanced MR Angiography, Excretion, Pharmacokinetics, and Metabolism. Radiology 2018, 286, 865–872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Islam, K.M.; Kim, S.; Kim, H.-K.; Park, S.; Lee, G.-H.; Kang, H.J.; Jung, J.-C.; Park, J.-S.; Kim, T.-J.; Chang, Y. Manganese Complex of Ethylenediaminetetraacetic Acid (EDTA)–Benzothiazole Aniline (BTA) Conjugate as a Potential Liver-Targeting MRI Contrast Agent. J. Med. Chem. 2017, 60, 2993–3001. [Google Scholar] [CrossRef]
- Gale, E.M.; Mukherjee, S.; Liu, C.; Loving, G.S.; Caravan, P. Structure–Redox–Relaxivity Relationships for Redox Responsive Manganese-Based Magnetic Resonance Imaging Probes. Inorg. Chem. 2014, 53, 10748–10761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borodin, O.Y.; Sannikov, M.Y.; Belyanin, M.L.; Filimonov, V.D.; Usov, V.Y.; Rybakov, Y.L.; Gukasov, V.M.; Shimanovskii, N.L. Relaxivity of Paramagnetic Complexes of Manganese and Gadolinium. Pharm. Chem. J. 2019, 53, 635–637. [Google Scholar] [CrossRef]
- Shen, Y.; Goerner, F.L.; Snyder, C.; Morelli, J.N.; Hao, D.; Hu, D.; Li, X.; Runge, V.M. T1 Relaxivities of Gadolinium-Based Magnetic Resonance Contrast Agents in Human Whole Blood at 1.5, 3, and 7 T. Investig. Radiol. 2015, 50, 330–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wahsner, J.; Gale, E.M.; Rodríguez-Rodríguez, A.; Caravan, P. Chemistry of MRI Contrast Agents: Current Challenges and New Frontiers. Chem. Rev. 2019, 119, 957–1057. [Google Scholar] [CrossRef] [PubMed]
- Varbanov, H.P.; Valiahdi, S.M.; Kowol, C.R.; Jakupec, M.A.; Galanski, M.S.; Keppler, B.K. Novel tetracarboxylatoplatinum(iv) complexes as carboplatin prodrugs. Dalton Trans. 2012, 41, 14404–14415. [Google Scholar] [CrossRef] [PubMed]
- Uivarosi, V.; Munteanu, A. Flavanoid complexes as promising anticancer metallodrugs. In Flavanoids—From Biosynthesis to Human Health; IntechOpen Ltd.: London, UK, 2017; Chapter 14; pp. 305–333. [Google Scholar]
- Anđelković, K.; Milenković, M.R.; Pevec, A.; Turel, I.; Matić, I.Z.; Vujčić, M.; Sladić, D.; Radanović, D.; Brađan, G.; Belošević, S.; et al. Synthesis, characterization and crystal structures of two pentagonal-bipyramidal Fe(III) complexes with dihydrazone of 2,6-diacetylpyridine and Girard’s T reagent. Anticancer properties of various metal complexes of the same ligand. J. Inorg. Biochem. 2017, 174, 137–149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, F.-Y.; Xi, Q.-Y.; Huang, K.-B.; Tang, X.-M.; Chen, Z.-F.; Liu, Y.-C.; Liang, H. Crystal structure, cytotoxicity and action mechanism of Zn(II)/Mn(II) complexes with isoquinoline ligands. J. Inorg. Biochem. 2017, 169, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Icsel, C.; Yilmaz, V.T.; Aydinlik, Ş.; Aygun, M. New manganese(II), iron(II), cobalt(II), nickel(II) and copper(II) saccharinate complexes of 2,6-bis(2-benzimidazolyl)pyridine as potential anticancer agents. Eur. J. Med. Chem. 2020, 202, 112535. [Google Scholar] [CrossRef] [PubMed]
- Kovala-Demertzi, D.; Staninska, M.; Garcia-Santos, I.; Castineiras, A.; Demertzis, M.A. Synthesis, crystal structures and spectroscopy of meclofenamic acid and its metal complexes with manganese(II), copper(II), zinc(II) and cadmium(II). Antiproliferative and superoxide dismutase activity. J. Inorg. Biochem. 2011, 105, 1187–1195. [Google Scholar] [CrossRef]
- Huang, Y.; Hu, L.; Zhang, T.; Zhong, H.; Zhou, J.; Liu, Z.; Wang, H.; Guo, Z.; Chen, Q. Mn3[Co(CN)6]2@SiO2 Core-shell Nanocubes: Novel bimodal contrast agents for MRI and optical imaging. Sci. Rep. 2013, 3, 2647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaur, J.; Tsvetkova, Y.; Arroub, K.; Sahnoun, S.; Kiessling, F.; Mathur, S. Synthesis, characterization, and relaxation studies of Gd-DO3A conjugate of chlorambucil as a potential theranostic agent. Chem. Biol. Drug Des. 2017, 89, 269–276. [Google Scholar] [CrossRef] [Green Version]
- Niwa, A.M.; D′epiro, G.F.R.; Marques, L.A.; Semprebon, S.C.; Sartori, D.; Ribeiro, L.R.; Mantovani, M.S. Salinomycin efficiency assessment in non-tumor (HB4a) and tumor (MCF-7) human breast cells. Naunyn-Schmiedebergs Arch. Fur Exp. Pathol. Und Pharmakol. 2016, 389, 557–571. [Google Scholar] [CrossRef] [PubMed]




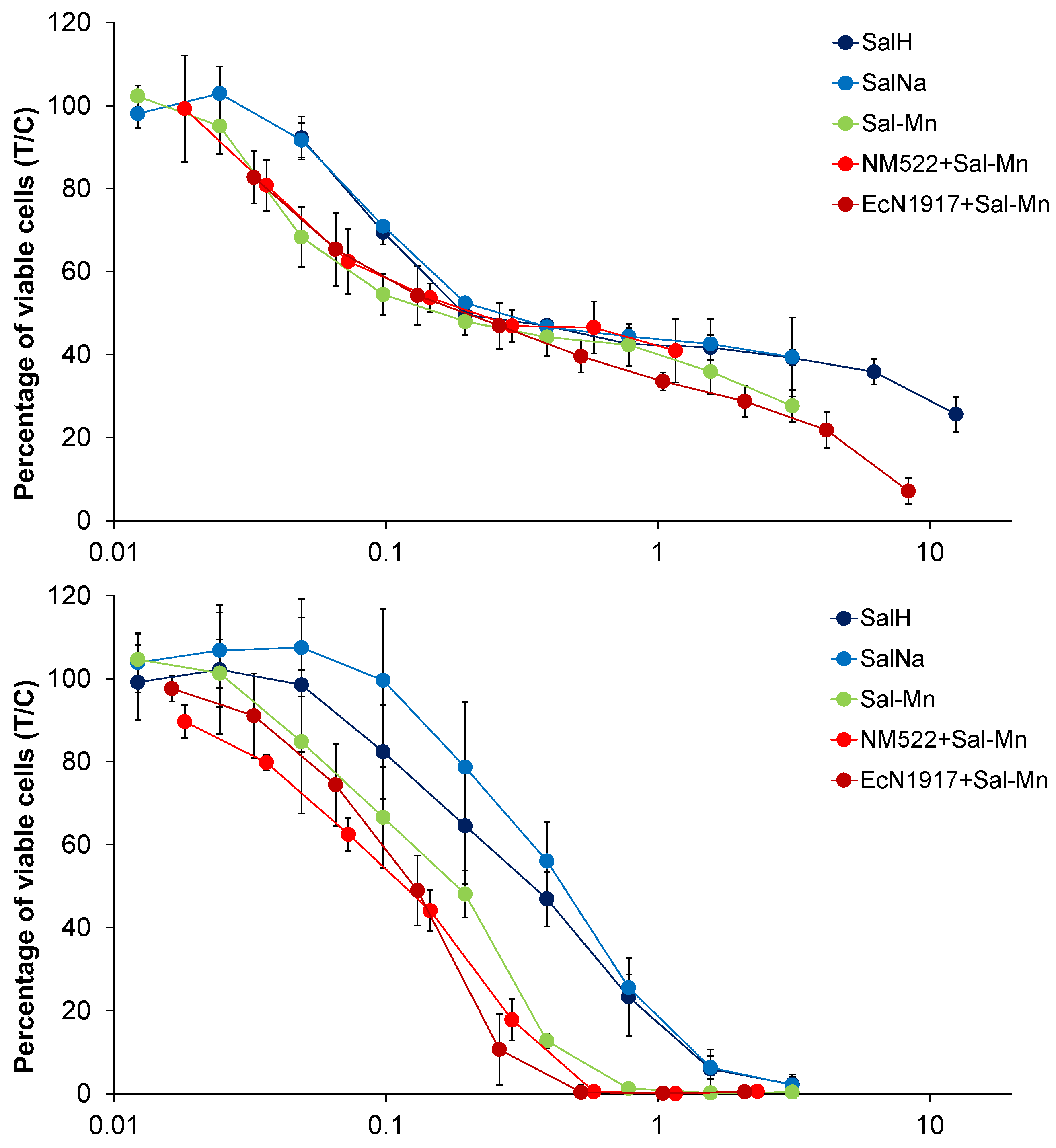
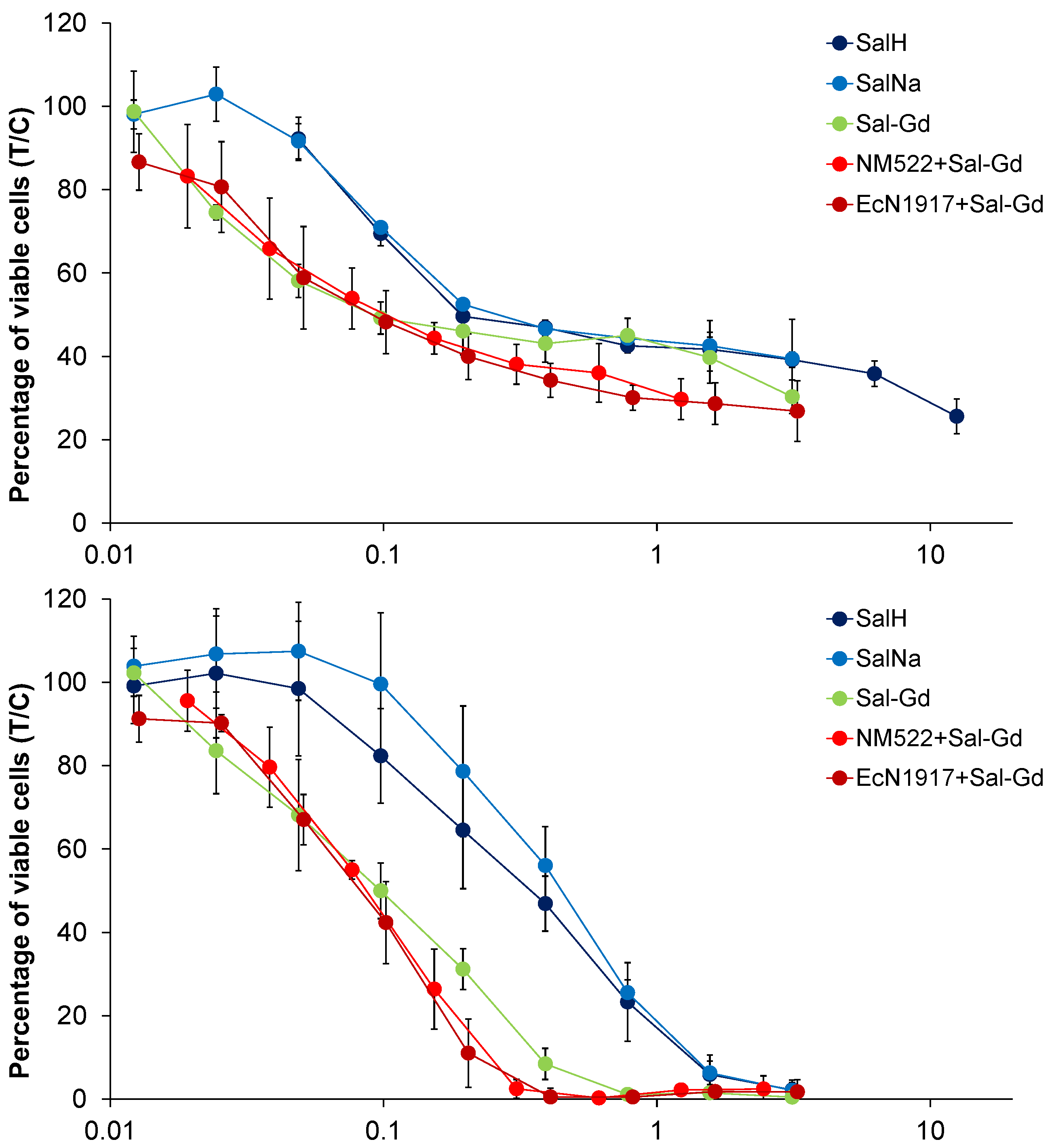
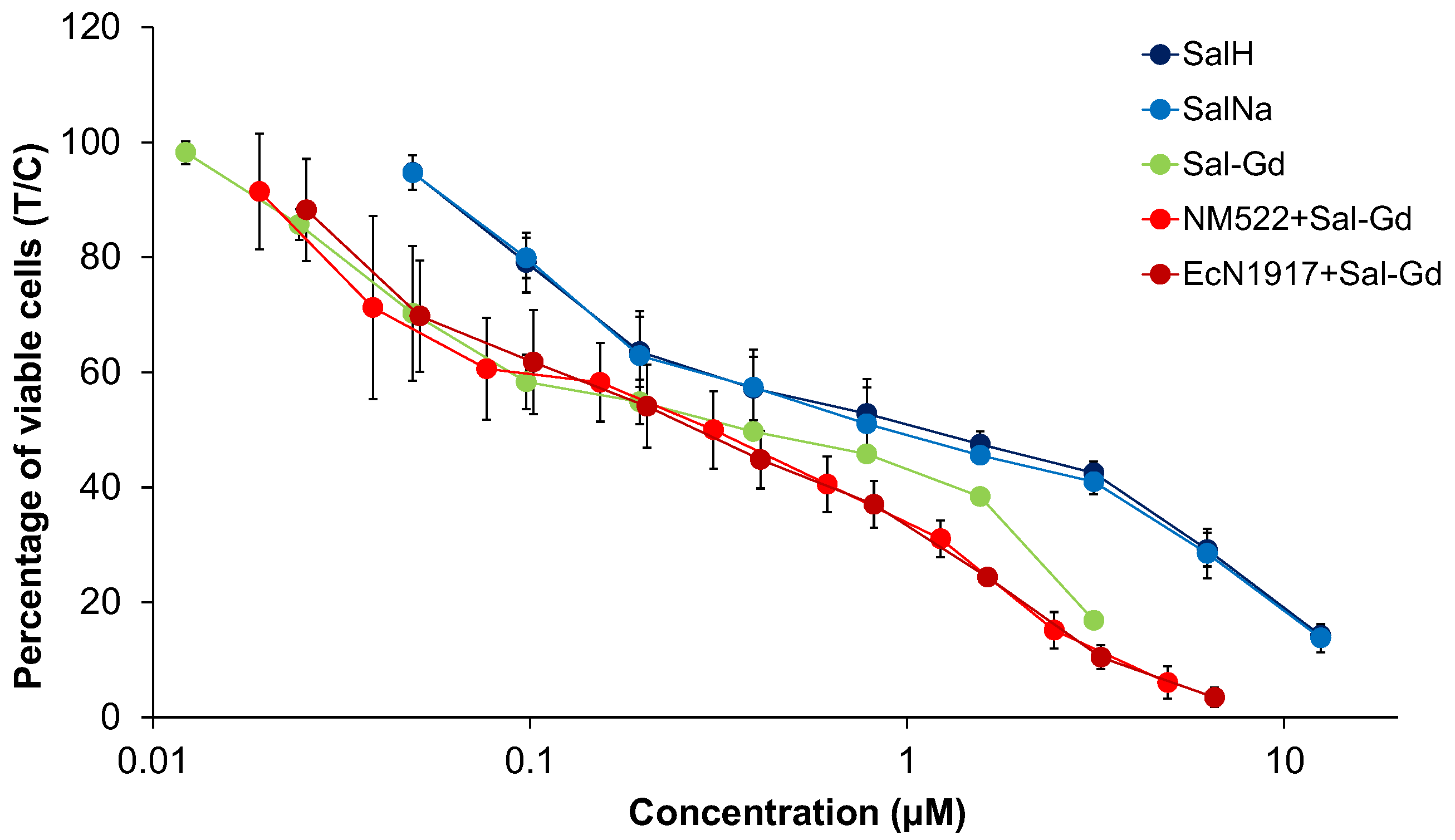
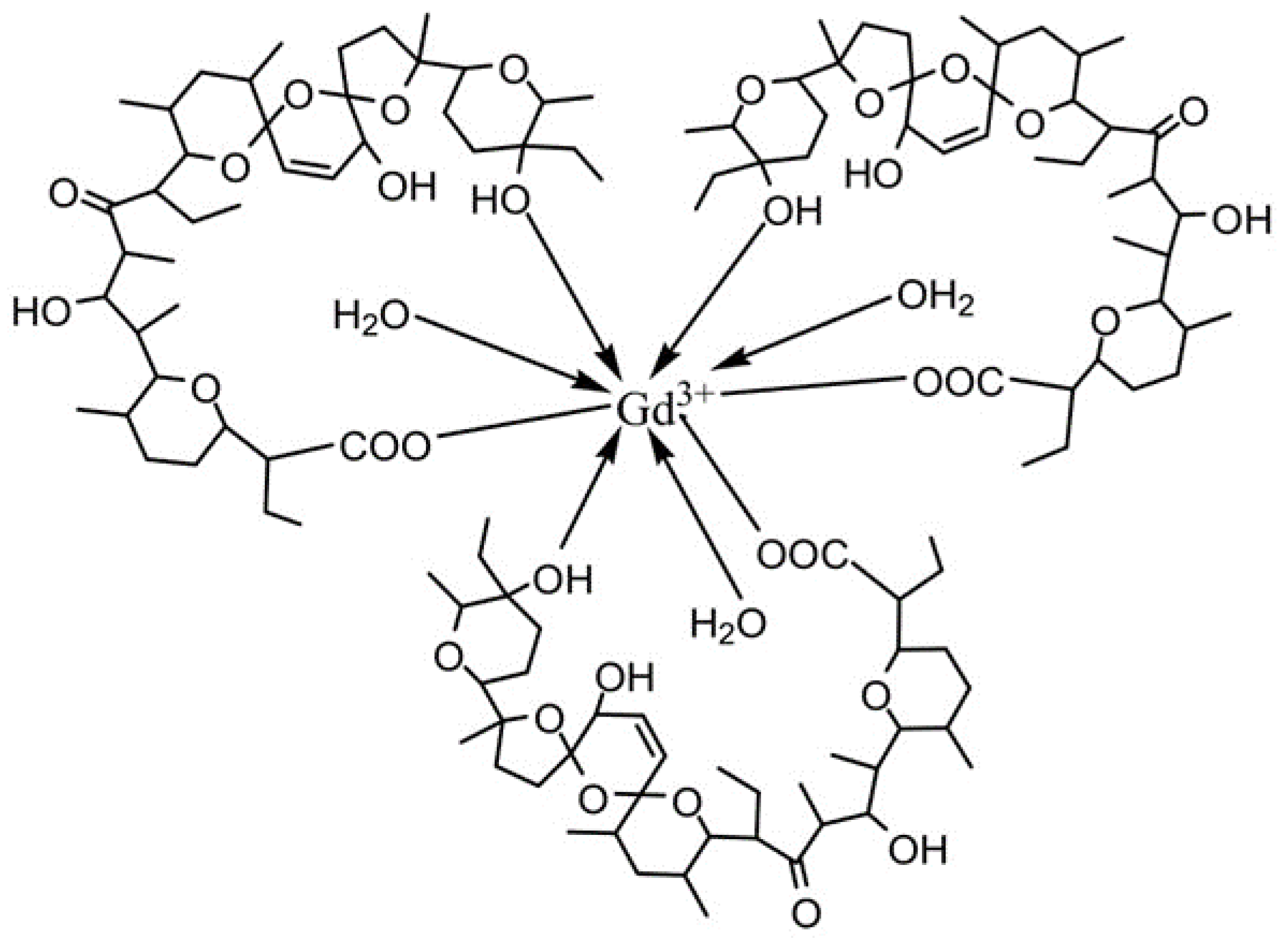

| Cell Line | A549 | SW480 | CH1/PA-1 | ||
|---|---|---|---|---|---|
| Sample | |||||
| 1. | Sal-H | 0.23 ± 0.06 | 1.1 ± 0.6 | 0.32 ± 0.12 | |
| 2. | Sal-Na | 0.27 ± 0.02 | 0.88 ± 0.44 | 0.43 ± 0.11 | |
| 3. | [Mn(Sal)2(H2O)2] = Sal-Mn(II) | 0.19 ± 0.11 * | 0.52 ± 0.22 | 0.17 ± 0.05 | |
| 4. | [Gd(Sal)3(H2O)3] = Sal-Gd(III) | 0.15 ± 0.12 * | 0.36 ± 0.12 | 0.093 ± 0.025 | |
| 5. | EcN1917 + 10 mg/mL Sal-Mn(II) | 0.22 ± 0.09 | 0.55 ± 0.06 | 0.12 ± 0.03 | |
| 6. | EcN1917 + 10 mg/mL Sal-Gd(III) | 0.093 ± 0.043 | 0.28 ± 0.14 | 0.086 ± 0.021 | |
| 7. | NM522 + 5 mg/mL Sal-Mn(II) | 0.28 ± 0.17 * | 0.54 ± 0.27 | 0.12 ± 0.02 | |
| 8. | NM522 + 5 mg/mL Sal-Gd(III) | 0.092 ± 0.041 | 0.31 ± 0.15 | 0.088 ± 0.008 | |
| Complex | Relaxivity Values r1/r2, [1/mM·s] (RT/20 °C/37 °C) | Magnetic Field Strength, [T] |
|---|---|---|
| Gd-DTPA2− (Magnevist®) | 3.76/n.d. | 9.4 |
| Gd-HPDO3A (ProHance®) | 3.37/n.d. | 9.4 |
| JTIP1/[Mn(Sal)2(H2O)2] | 2.50/n.d. | 9.4 |
| JTIP3/[Gd(Sal)3(H2O)3] | 5.40/n.d. | 9.4 |
| E. coli Nissle 1917 loaded with JTIP1/[Mn(Sal)2(H2O)2] | 2.58/36.74 | 9.4 |
| E. coli Nissle 1917 loaded with JTIP3/[Gd(Sal)3(H2O)3] | 6.67/n.a. | 9.4 |
| [Mn(DPDP)]4− (Teslascan®) [39] | 2.80/3.70 | 4.7 |
| Mn-PyC3A [40] | 3.4/n.d. | 3 |
| Mn-EDTA-BTA [41] | 3.50 | 0.47 |
| Mn(HBET-NO2)]2− [42] | 2.33 | 1.4 |
| Mn-CDTA2− [43] | 2.99 | 0.47 |
| Mn-EDTA2− [43] | 2.12 | 0.47 |
| Mn-DTPA3− [43] | 1.57 | 0.47 |
| Cell Line | A549 | SW480 | CH1/PA-1 | ||
|---|---|---|---|---|---|
| Sample | |||||
| 1. | Sal-H | 0.23 ± 0.06 | 1.06 ± 0.58 | 0.32 ± 0.12 | |
| 2. | Sal-Na | 0.27 ± 0.02 | 0.88 ± 0.44 | 0.43 ± 0.11 | |
| 3. | [Mn(Sal)2(H2O)2] = Sal-Mn(II) | 0.19 ± 0.11 | 0.52 ± 0.22 | 0.17 ± 0.05 | |
| 4. | [Gd(Sal)3(H2O)3] = Sal-Gd(III) | 0.15 ± 0.12 | 0.36 ± 0.12 | 0.09 ± 0.03 | |
| 5. | EcN1917 + 10 mg/mL Sal-Mn(II) | 0.33 ± 0.14 | 0.82 ± 0.10 | 0.19 ± 0.04 | |
| 6. | EcN1917 + 10 mg/mL Sal-Gd(III) | 0.09 ± 0.04 | 0.27 ± 0.13 | 0.082 ± 0.020 | |
| 7. | NM522 + 5 mg/mL Sal-Mn(II) | 0.75 ± 0.46 | 1.44 ± 0.74 | 0.32 ± 0.054 | |
| 8. | NM522 + 5 mg/mL Sal-Gd(III) | 0.12 ± 0.05 | 0.40 ± 0.19 | 0.112 ± 0.010 | |
| Satraplatin [30] | Pt(NH3)(cha)Cl2(OAc)2 | 6.4 ± 0.4 | 1.5 ± 0.1 | 0.10 ± 0.12 | |
| Cisplatin [30] | Pt(NH3)2Cl2 | 6.2 ± 1.2 | 3.3 ± 0.2 | 0.077 ± 0.006 | |
| Carboplatin [46] | Pt(NH3)2(CBDCA) | 91 ± 102 | 37 ± 1 | 0.81 ± 0.17 | |
| Oxaliplatin [31] | Pt(DACH)(ox) | 0.98 ± 0.21 | 0.29 ± 0.05 | 0.18 ± 0.01 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pashkunova-Martic, I.; Kukeva, R.; Stoyanova, R.; Pantcheva, I.; Dorkov, P.; Friske, J.; Hejl, M.; Jakupec, M.; Hohagen, M.; Legin, A.; et al. Novel Salinomycin-Based Paramagnetic Complexes—First Evaluation of Their Potential Theranostic Properties. Pharmaceutics 2022, 14, 2319. https://doi.org/10.3390/pharmaceutics14112319
Pashkunova-Martic I, Kukeva R, Stoyanova R, Pantcheva I, Dorkov P, Friske J, Hejl M, Jakupec M, Hohagen M, Legin A, et al. Novel Salinomycin-Based Paramagnetic Complexes—First Evaluation of Their Potential Theranostic Properties. Pharmaceutics. 2022; 14(11):2319. https://doi.org/10.3390/pharmaceutics14112319
Chicago/Turabian StylePashkunova-Martic, Irena, Rositsa Kukeva, Radostina Stoyanova, Ivayla Pantcheva, Peter Dorkov, Joachim Friske, Michaela Hejl, Michael Jakupec, Mariam Hohagen, Anton Legin, and et al. 2022. "Novel Salinomycin-Based Paramagnetic Complexes—First Evaluation of Their Potential Theranostic Properties" Pharmaceutics 14, no. 11: 2319. https://doi.org/10.3390/pharmaceutics14112319







