Structural Optimization of Platinum Drugs to Improve the Drug-Loading and Antitumor Efficacy of PLGA Nanoparticles
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. All-Atom Molecular Dynamics Simulations of Polymer Carriers
2.2.1. Molecular Docking of the Investigated Molecules on the Virtually Constructed Carriers
2.2.2. Calculating the Main Descriptors of the Investigated Drugs
2.3. PASS-Based In Silico Assessment of Compounds’ Activity
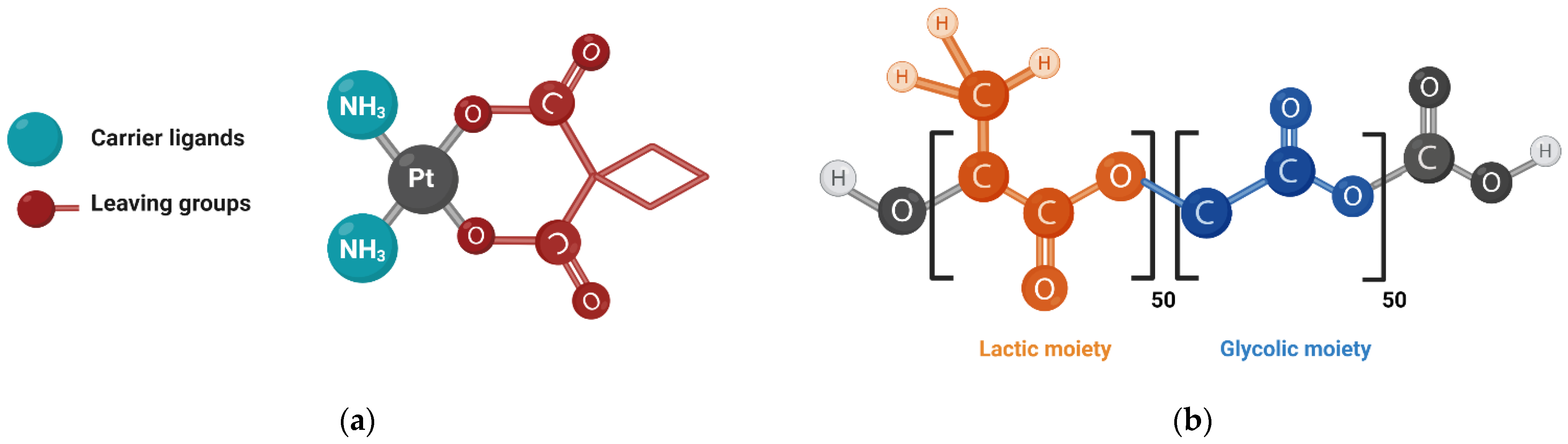
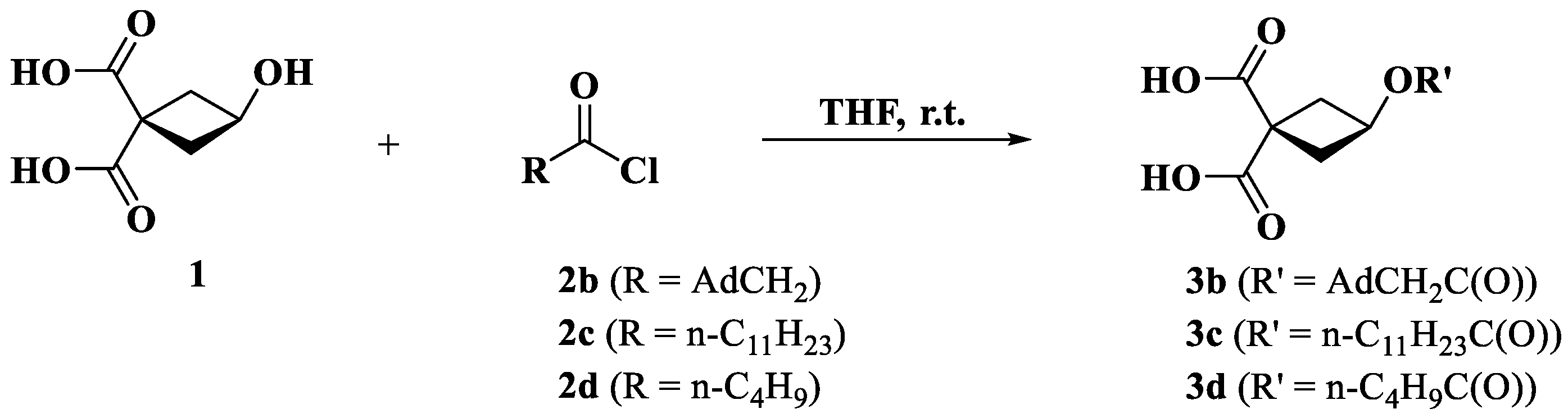
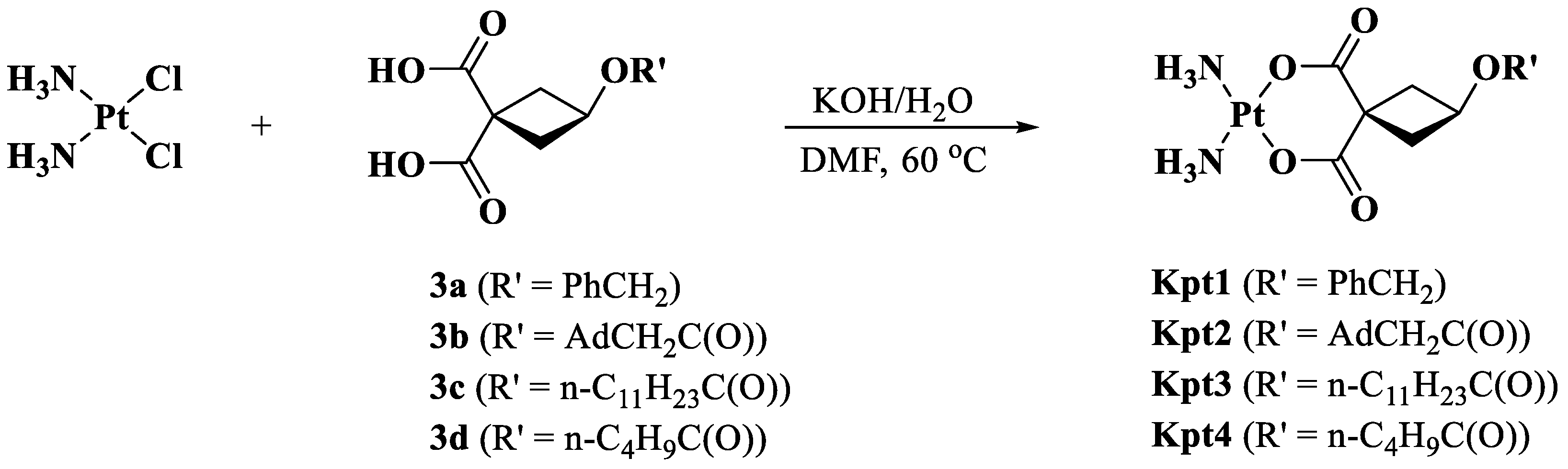
2.4. Synthesis of Carboplatin Derivatives
2.4.1. Synthesis of Ligands 3 (b–d)
2.4.2. Synthesis of Complexes Kpt1–4
2.5. NMR Spectra Analysis
2.6. High Resolution Mass Spectra Analysis
2.7. Nanoparticles Formulation
2.8. Measurement of Size, Zeta Potential, and Polydispersity Index (PDI) Measurement
2.9. Nanoparticles’ Morphology
2.10. Drug Loading and Encapsulation Efficiency Measurement
2.11. Animals
2.12. Hemolytic Activity Study
2.13. In Vitro Release Study
2.14. Cell Culture
2.15. Cytotoxicity Assay
2.16. Apoptosis Assay
2.17. Statistical Analysis
3. Results and Discussion
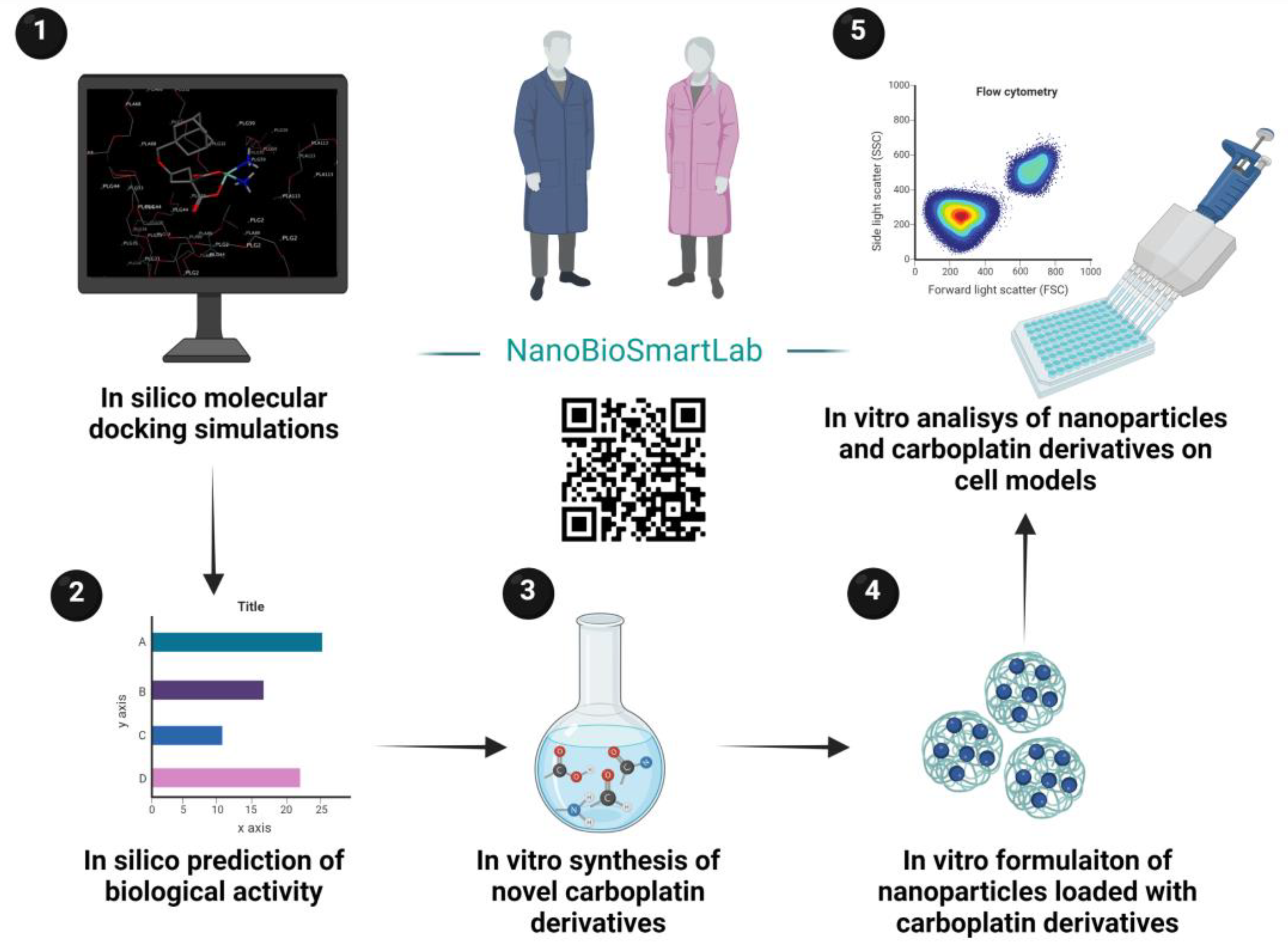
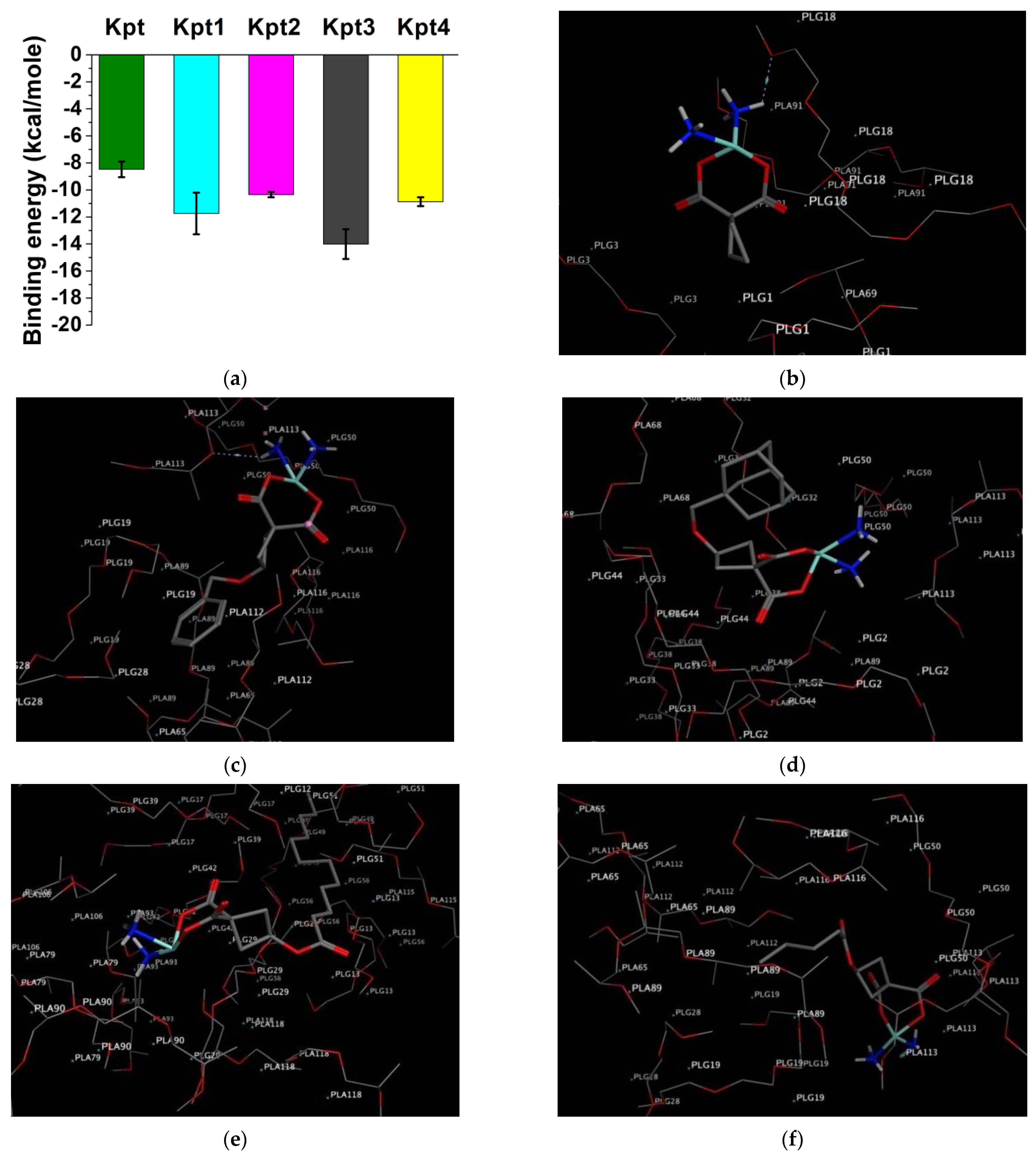
3.1. In Silico Molecular Docking on PLGA Matrix
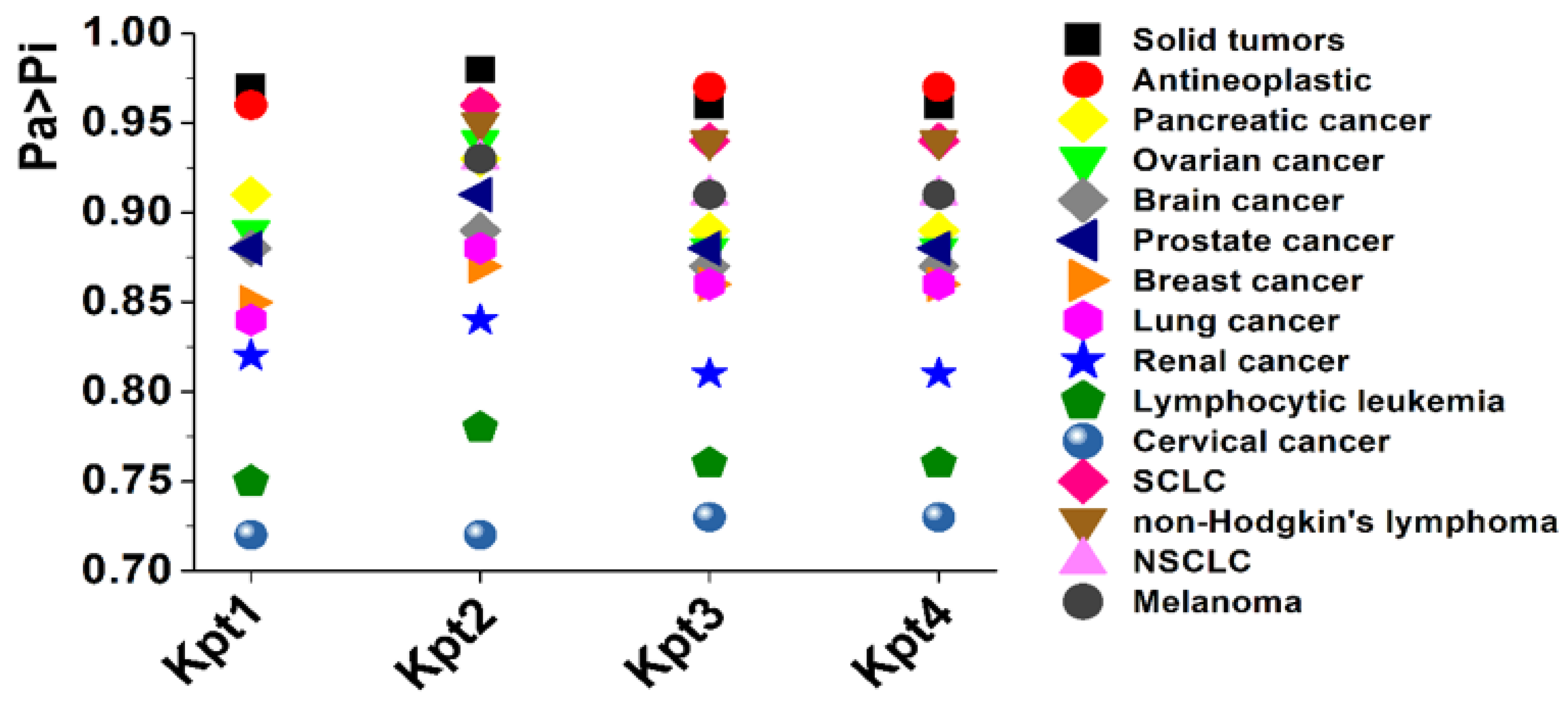
3.2. PASS-Based In Silico Assessment of Compounds’ Activity
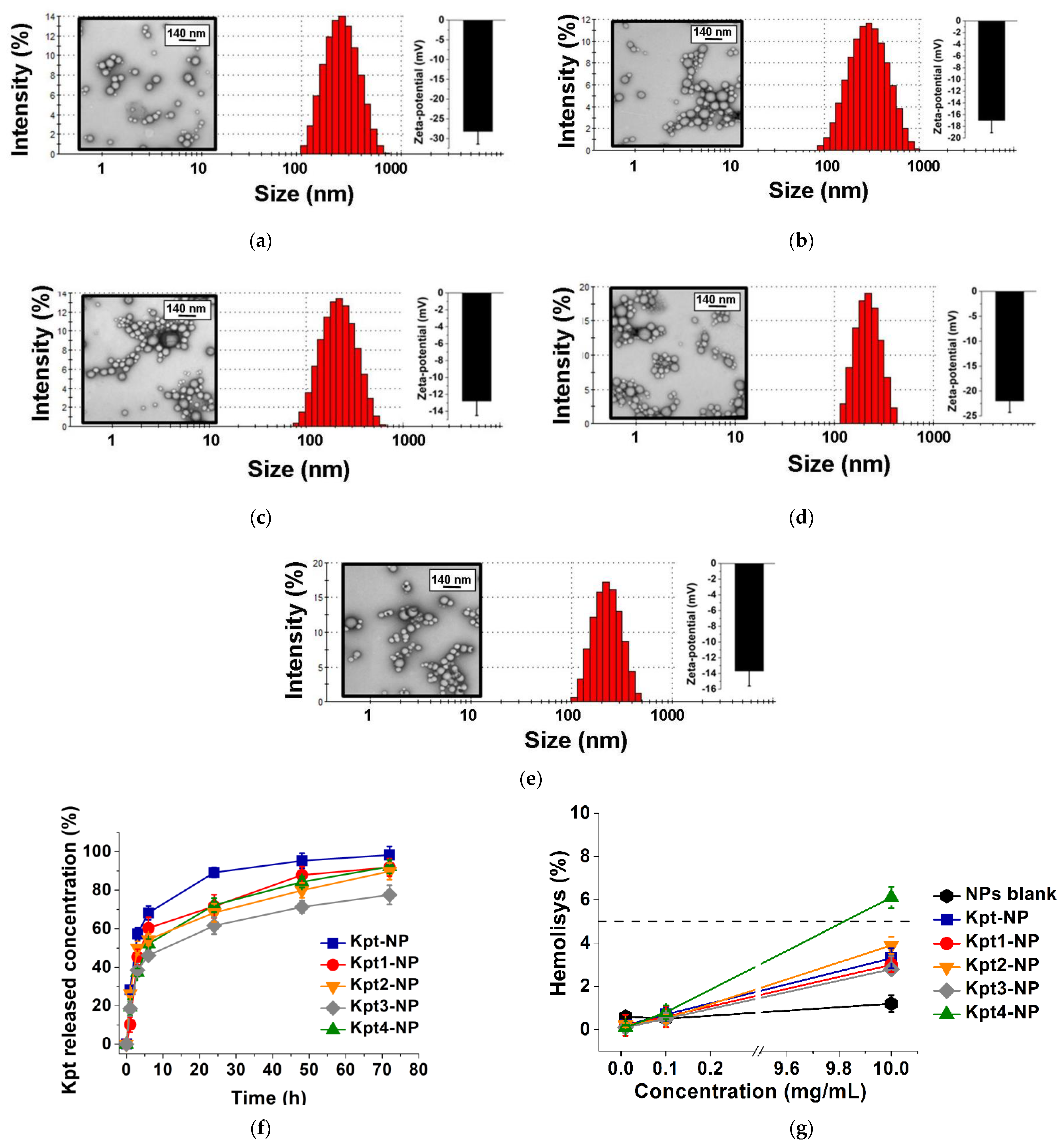
3.3. Formulation of Nanoparticles
3.4. In Vitro Kpt Release
3.5. Hemolytic Activity Study
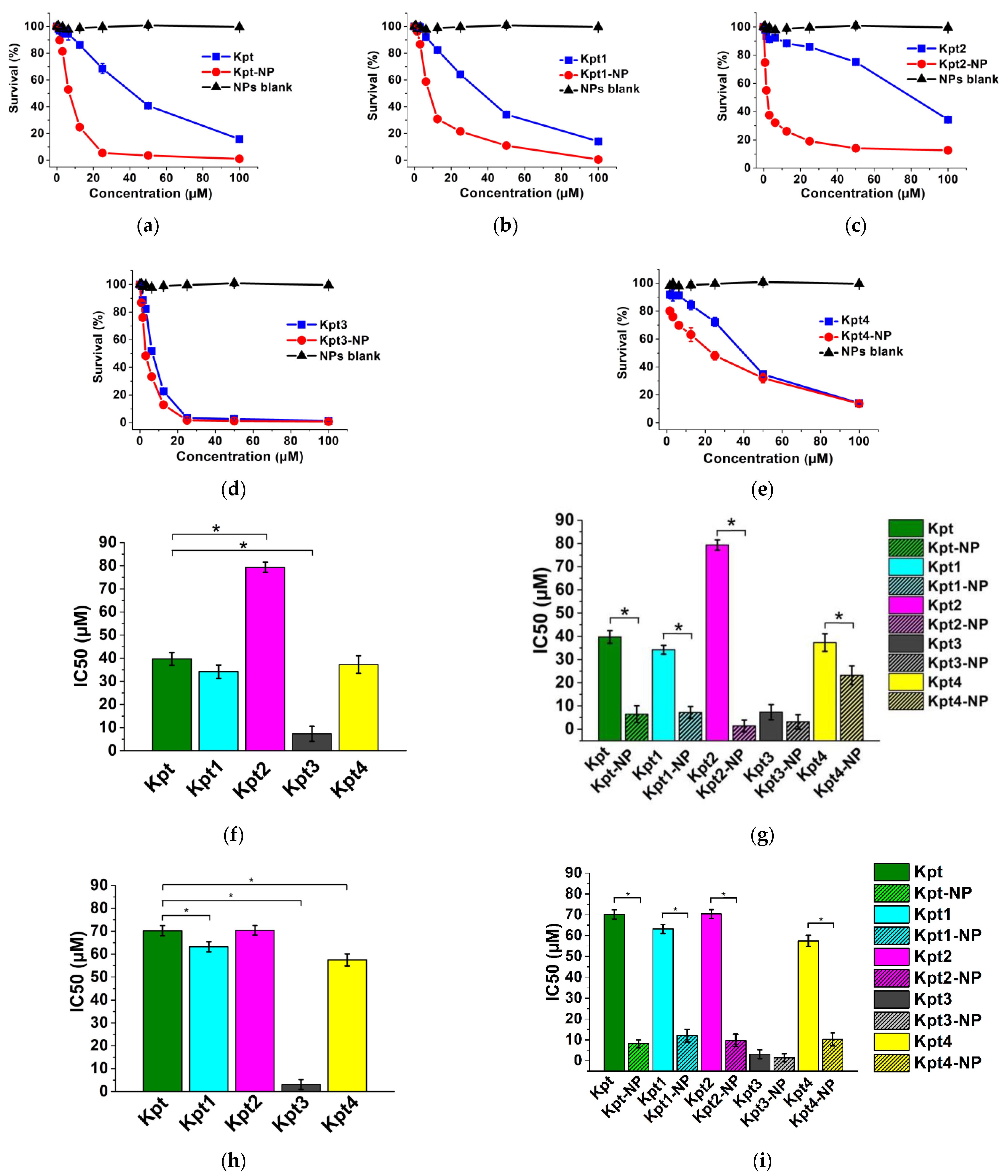
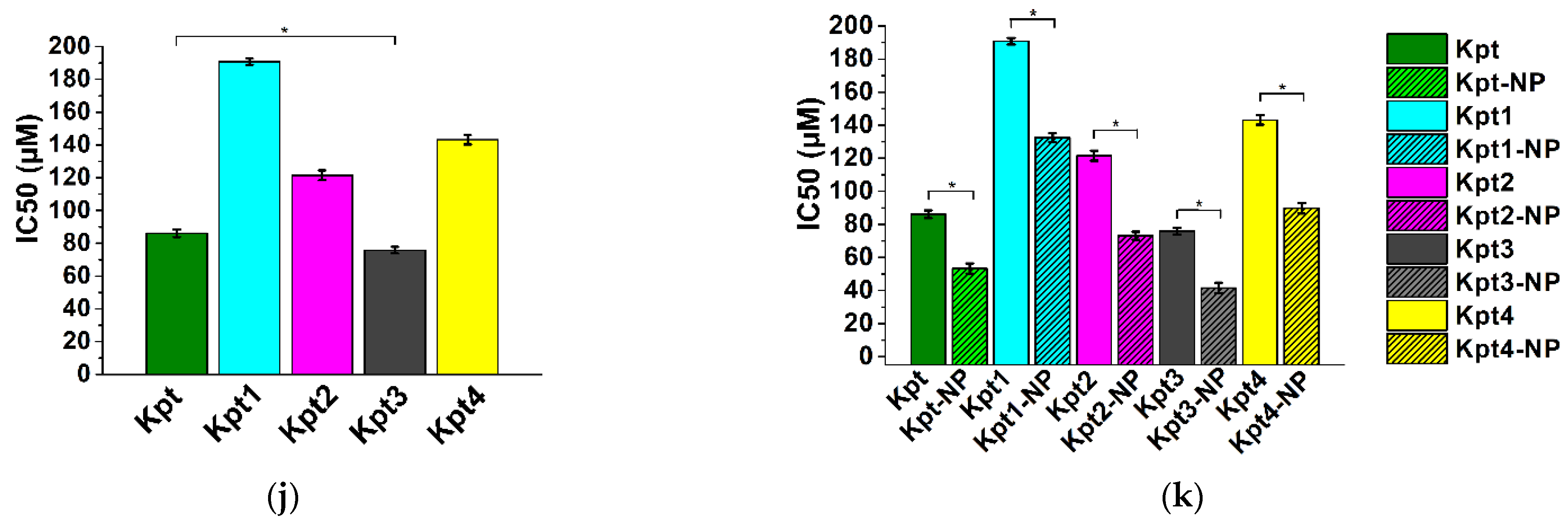
3.6. In Vitro Cytotoxicity Study
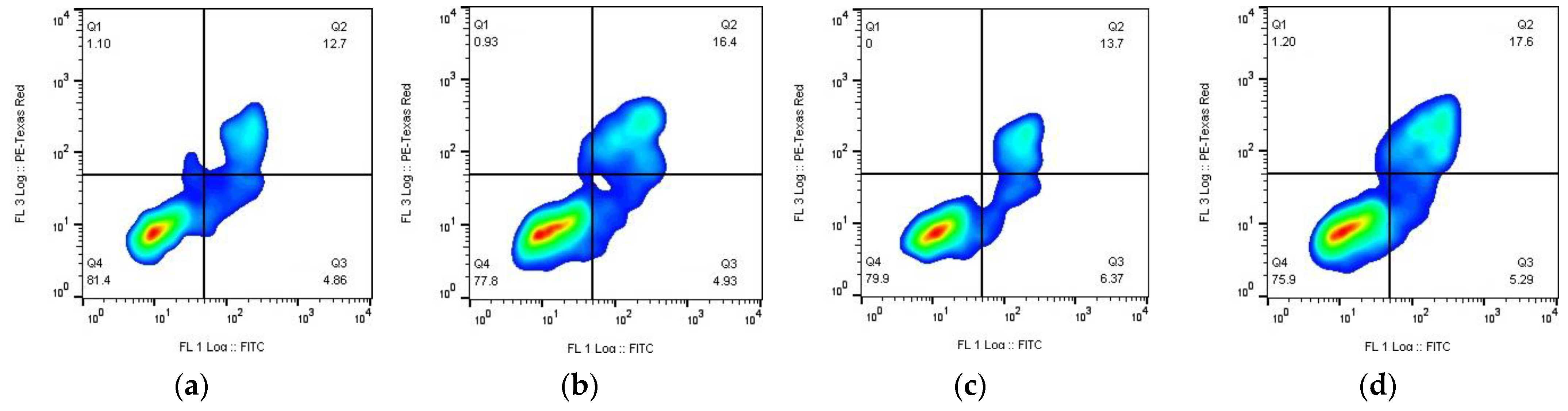
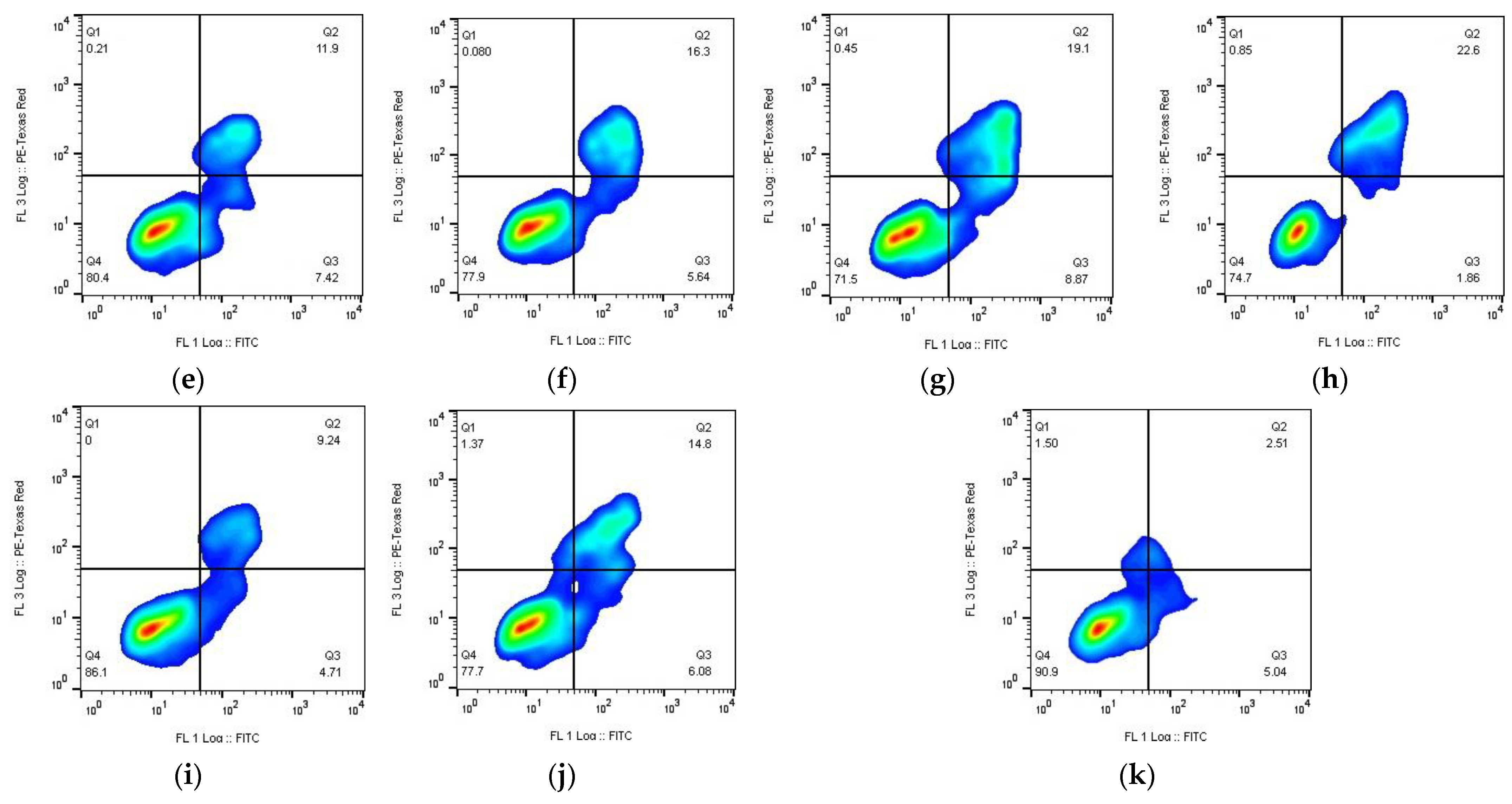
3.7. Apoptosis Assay
- Substances (early apoptosis (Q3))—Kpt3 (8.9%) > Kpt2 (7.4%) > Kpt1 (6.4%) > Kpt (4.9%) > Kpt4 (4.7%);
- NPs (early apoptosis (Q3))—Kpt4 (6.1%) > Kpt2 (5.6%) > Kpt1 (5.3%) > Kpt (4.9%) > Kpt3 (1.9%);
- Substances (late apoptosis/necrosis (Q2))—Kpt3 (19.1%) > Kpt1 (13.7%) > Kpt (12.7 %) > Kpt2 (11.9%) > Kpt4 (9.24%);
- NPs (late apoptosis/necrosis (Q2))—Kpt3 (22.6%) > Kpt1 (17.6%) > Kpt (16.4%) > Kpt2 (16.3%) > Kpt4 (14.8%).
4. Conclusions
5. Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dilruba, S.; Kalayda, G.V. Platinum-Based Drugs: Past, Present and Future. Cancer Chemother. Pharmacol. 2016, 77, 1103–1124. [Google Scholar] [CrossRef] [PubMed]
- Carboplatin Monograph for Professionals. Available online: https://www.drugs.com/monograph/carboplatin.html (accessed on 23 October 2022).
- Oun, R.; Moussa, Y.E.; Wheate, N.J. The Side Effects of Platinum-Based Chemotherapy Drugs: A Review for Chemists. Dalton Trans. 2018, 47, 6645–6653. [Google Scholar] [CrossRef] [PubMed]
- Štarha, P.; Vančo, J.; Trávníček, Z. Platinum Iodido Complexes: A Comprehensive Overview of Anticancer Activity and Mechanisms of Action. Coord. Chem. Rev. 2019, 380, 103–135. [Google Scholar] [CrossRef]
- Lippert, B.; Sanz Miguel, P.J. More of a Misunderstanding than a Real Mismatch? Platinum and Its Affinity for Aqua, Hydroxido, and Oxido Ligands. Coord. Chem. Rev. 2016, 327–328, 333–348. [Google Scholar] [CrossRef]
- Rottenberg, S.; Disler, C.; Perego, P. The Rediscovery of Platinum-Based Cancer Therapy. Nat. Rev. Cancer 2021, 21, 37–50. [Google Scholar] [CrossRef]
- Wen, X.; Buckley, B.; McCandlish, E.; Goedken, M.J.; Syed, S.; Pelis, R.; Manautou, J.E.; Aleksunes, L.M. Transgenic Expression of the Human MRP2 Transporter Reduces Cisplatin Accumulation and Nephrotoxicity in Mrp2-Null Mice. Am. J. Pathol. 2014, 184, 1299–1308. [Google Scholar] [CrossRef]
- Manzanares, D.; Ceña, V. Endocytosis: The Nanoparticle and Submicron Nanocompounds Gateway into the Cell. Pharmaceutics 2020, 12, 371. [Google Scholar] [CrossRef]
- Sokol, M.; Yabbarov, N.; Mollaeva, M.; Chirkina, M.; Mollaev, M.; Zabolotsky, A.; Kuznetsov, S.; Nikolskaya, E. Alpha-Fetoprotein Mediated Targeting of Polymeric Nanoparticles to Treat Solid Tumors. Nanomedicine 2022. [Google Scholar] [CrossRef]
- Nikolskaya, E.; Krugliy, B.; Tereshchenko, O.G.; Popov, R.; Gruznova, O.; Yabbarov, N.; Shvets, V.I.; Séverin, E. The in Vivo Study of Antitumor Activity of the Actinomycin D Protein-Vector Drug Delivery. Allergol. Immunol. 2017, 18, 14–18. [Google Scholar]
- Wu, J. The Enhanced Permeability and Retention (EPR) Effect: The Significance of the Concept and Methods to Enhance Its Application. J. Pers. Med. 2021, 11, 771. [Google Scholar] [CrossRef]
- Elmowafy, E.M.; Tiboni, M.; Soliman, M.E. Biocompatibility, Biodegradation and Biomedical Applications of Poly(Lactic Acid)/Poly(Lactic-Co-Glycolic Acid) Micro and Nanoparticles. J. Pharm. Investig. 2019, 49, 347–380. [Google Scholar] [CrossRef]
- Nikolskaya, E.; Sokol, M.; Mollaeva, M.; Gruznova, O.; Mollaev, M.; Yabbarov, N.; Tereshchenko, O.; Popov, R.; Severin, E. The Comparative Study of Influence of Lactic and Glycolic Acids Copolymers Type on Properties of Daunorubicin Loaded Nanoparticles and Drug Release. Acta Bioeng. Biomech. 2018, 20, 65–77. [Google Scholar] [CrossRef]
- Wang, Y.; Qin, B.; Xia, G.; Choi, S.H. FDA’s Poly (Lactic-Co-Glycolic Acid) Research Program and Regulatory Outcomes. AAPS J. 2021, 23, 92. [Google Scholar] [CrossRef] [PubMed]
- Gavas, S.; Quazi, S.; Karpiński, T.M. Nanoparticles for Cancer Therapy: Current Progress and Challenges. Nanoscale Res. Lett. 2021, 16, 173. [Google Scholar] [CrossRef] [PubMed]
- Rezvantalab, S.; Drude, N.I.; Moraveji, M.K.; Güvener, N.; Koons, E.K.; Shi, Y.; Lammers, T.; Kiessling, F. PLGA-Based Nanoparticles in Cancer Treatment. Front. Pharmacol. 2018, 9, 1260. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Zhou, Y.; Liu, L.; Xu, Y.; Chen, Q.; Wang, Y.; Wu, S.; Deng, Y.; Zhang, J.; Shao, A. Nanoparticle-Based Drug Delivery in Cancer Therapy and Its Role in Overcoming Drug Resistance. Front. Mol. Biosci. 2020, 7, 193. [Google Scholar] [CrossRef]
- Panda, M.K.; Panda, S.K.; Singh, Y.D.; Jit, B.P.; Behara, R.K.; Dhal, N.K. Role of Nanoparticles and Nanomaterials in Drug Delivery: An Overview. In Advances in Pharmaceutical Biotechnology: Recent Progress and Future Applications; Patra, J.K., Shukla, A.C., Das, G., Eds.; Springer: Singapore, 2020; pp. 247–265. ISBN 9789811521959. [Google Scholar]
- de Moraes Profirio, D.; Pessine, F.B.T. Formulation of Functionalized PLGA Nanoparticles with Folic Acid-Conjugated Chitosan for Carboplatin Encapsulation. Eur. Polym. J. 2018, 108, 311–321. [Google Scholar] [CrossRef]
- Arshad, A.; Yang, B.; Bienemann, A.S.; Barua, N.U.; Wyatt, M.J.; Woolley, M.; Johnson, D.E.; Edler, K.J.; Gill, S.S. Convection-Enhanced Delivery of Carboplatin PLGA Nanoparticles for the Treatment of Glioblastoma. PLoS ONE 2015, 10, e0132266. [Google Scholar] [CrossRef]
- Sadhukha, T.; Prabha, S. Encapsulation in Nanoparticles Improves Anti-Cancer Efficacy of Carboplatin. AAPS PharmSciTech 2014, 15, 1029–1038. [Google Scholar] [CrossRef]
- Simón-Vázquez, R.; Tsapis, N.; Lorscheider, M.; Rodríguez, A.; Calleja, P.; Mousnier, L.; de Miguel Villegas, E.; González-Fernández, Á.; Fattal, E. Improving Dexamethasone Drug Loading and Efficacy in Treating Arthritis through a Lipophilic Prodrug Entrapped into PLGA-PEG Nanoparticles. Drug Deliv. Transl. Res. 2022, 12, 1270–1284. [Google Scholar] [CrossRef]
- Johnstone, T.C.; Lippard, S.J. The Effect of Ligand Lipophilicity on the Nanoparticle Encapsulation of Pt(IV) Prodrugs. Inorg. Chem. 2013, 52, 9915–9920. [Google Scholar] [CrossRef] [PubMed]
- Novel Lipophilic Platinum(II) Compounds of Salicylate Derivatives. Available online: https://technology.matthey.com/journal (accessed on 30 September 2022).
- Megy, S.; Aguero, S.; Da Costa, D.; Lamrayah, M.; Berthet, M.; Primard, C.; Verrier, B.; Terreux, R. Molecular Dynamics Studies of Poly(Lactic Acid) Nanoparticles and Their Interactions with Vitamin E and TLR Agonists Pam1CSK4 and Pam3CSK4. Nanomaterials 2020, 10, 2209. [Google Scholar] [CrossRef]
- Abd-algaleel, S.A.; Abdel-Bar, H.M.; Metwally, A.A.; Hathout, R.M. Evolution of the Computational Pharmaceutics Approaches in the Modeling and Prediction of Drug Payload in Lipid and Polymeric Nanocarriers. Pharmaceuticals 2021, 14, 645. [Google Scholar] [CrossRef] [PubMed]
- Hathout, R.M.; Mahmoud, O.A.; Ali, D.S.; Mamdouh, M.; Metwally, A.A. Modeling Drugs-PLGA Nanoparticles Interactions Using Gaussian Processes: Pharmaceutics Informatics Approach. J. Clust. Sci. 2022, 33, 2031–2036. [Google Scholar] [CrossRef]
- Stipa, P.; Marano, S.; Galeazzi, R.; Minnelli, C.; Laudadio, E. Molecular Dynamics Simulations of Quinine Encapsulation into Biodegradable Nanoparticles: A Possible New Strategy against Sars-CoV-2. Eur. Polym. J. 2021, 158, 110685. [Google Scholar] [CrossRef]
- Dobhal, A.; Srivastav, A.; Dandekar, P.; Jain, R. Influence of Lactide vs Glycolide Composition of Poly (Lactic-Co-Glycolic Acid) Polymers on Encapsulation of Hydrophobic Molecules: Molecular Dynamics and Formulation Studies. J. Mater. Sci. Mater. Med. 2021, 32, 126. [Google Scholar] [CrossRef]
- Horishny, V.; Geronikaki, A.; Kartsev, V.; Matiychuk, V.; Petrou, A.; Pogodin, P.; Poroikov, V.; Papadopoulou, T.A.; Vizirianakis, I.S.; Kostic, M.; et al. Synthesis, Biological Evaluation and Molecular Docking Studies of 5-Indolylmethylen-4-Oxo-2-Thioxothiazolidine Derivatives. Molecules 2022, 27, 1068. [Google Scholar] [CrossRef]
- Main Info. Available online: http://way2drug.com/passonline/info.php (accessed on 30 September 2022).
- Metwally, A.A.; Hathout, R.M. Computer-Assisted Drug Formulation Design: Novel Approach in Drug Delivery. Mol. Pharm. 2015, 12, 2800–2810. [Google Scholar] [CrossRef]
- Gad, H.A.; Hathout, R.M. Can the Docking Experiments Select the Optimum Natural Bio-Macromolecule for Doxorubicin Delivery? J. Clust. Sci. 2021, 32, 1747–1751. [Google Scholar] [CrossRef]
- Ossama, M.; Hathout, R.M.; Attia, D.A.; Mortada, N.D. Enhanced Allicin Cytotoxicity on HEPG-2 Cells Using Glycyrrhetinic Acid Surface-Decorated Gelatin Nanoparticles. ACS Omega 2019, 4, 11293–11300. [Google Scholar] [CrossRef]
- Hathout, R.M.; Abdelhamid, S.G.; Metwally, A.A. Chloroquine and Hydroxychloroquine for Combating COVID-19: Investigating Efficacy and Hypothesizing New Formulations Using Bio/Chemoinformatics Tools. Inform. Med. Unlocked 2020, 21, 100446. [Google Scholar] [CrossRef] [PubMed]
- Hathout, R.M.; Abdelhamid, S.G.; El-Housseiny, G.S.; Metwally, A.A. Comparing Cefotaxime and Ceftriaxone in Combating Meningitis through Nose-to-Brain Delivery Using Bio/Chemoinformatics Tools. Sci. Rep. 2020, 10, 21250. [Google Scholar] [CrossRef] [PubMed]
- Hill, M.; Cunningham, R.N.; Hathout, R.M.; Johnston, C.; Hardy, J.G.; Migaud, M.E. Formulation of Antimicrobial Tobramycin Loaded PLGA Nanoparticles via Complexation with AOT. J. Funct. Biomater. 2019, 10, 26. [Google Scholar] [CrossRef]
- Magaz, A.; Ashton, M.D.; Hathout, R.M.; Li, X.; Hardy, J.G.; Blaker, J.J. Electroresponsive Silk-Based Biohybrid Composites for Electrochemically Controlled Growth Factor Delivery. Pharmaceutics 2020, 12, 742. [Google Scholar] [CrossRef]
- Shah, S.A.A.; Firlak, M.; Berrow, S.R.; Halcovitch, N.R.; Baldock, S.J.; Yousafzai, B.M.; Hathout, R.M.; Hardy, J.G. Electrochemically Enhanced Drug Delivery Using Polypyrrole Films. Materials 2018, 11, 1123. [Google Scholar] [CrossRef]
- Bernhardt, G.; Brunner, H.; Gruber, N.; Lottner, C.; Pushpan, S.K.; Tsuno, T.; Zabel, M. Carboplatin Derivatives with Superior Antitumor Activity Compared to the Parent Compound. Inorg. Chim. Acta 2004, 357, 4452–4466. [Google Scholar] [CrossRef]
- Pasini, A. A New Synthetic Method for Diaminomalonatoplatinum Type Complexes and the Unexpected Behaviour of [PtCl2(Trans-Dach)]. Inorg. Chim. Acta 1988, 151, 19–20. [Google Scholar] [CrossRef]
- Tietze, L.F.; Eicher, T. Aufbau organischer Moleküle: Abschnitt 3.1.1–3.1.5. In Reaktionen und Synthesen im Organisch-Chemischen Praktikum und Forschungslaboratorium; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 1991; pp. 169–216. ISBN 978-3-527-60171-4. [Google Scholar]
- Mollaeva, M.; Yabbarov, N.; Sokol, M.; Chirkina, M.; Mollaev, M.; Zabolotskii, A.; Seregina, I.; Bolshov, M.; Kaplun, A.; Nikolskaya, E. Optimization, Characterization and Pharmacokinetic Study of Meso-Tetraphenylporphyrin Metal Complex-Loaded PLGA Nanoparticles. Int. J. Mol. Sci. 2021, 22, 12261. [Google Scholar] [CrossRef]
- Mollaeva, M.; Nikolskaya, E.; Mollaev, M.; Sokol, M.; Zabolotsky, A.; Gruznova, O.; Chirkina, M.; Schvets, V.; Lobanov, A.; Yabbarov, N. Polymer Particles Containing Fe-Based Metalloporphyrin as a Highly Efficient Stimulator of Reactive Oxygen Species Formation In Vitro and In Vivo. Russ. Chem. Bull. 2019, 68, 2216–2224. [Google Scholar] [CrossRef]
- Ramalho, M.J.; Loureiro, J.A.; Coelho, M.A.N.; Pereira, M.C. Factorial Design as a Tool for the Optimization of PLGA Nanoparticles for the Co-Delivery of Temozolomide and O6-Benzylguanine. Pharmaceutics 2019, 11, 401. [Google Scholar] [CrossRef]
- Abd El Hady, W.E.; Mohamed, E.A.; Soliman, O.A.E.-A.; El-Sabbagh, H.M. In Vitro-in Vivo Evaluation of Chitosan-PLGA Nanoparticles for Potentiated Gastric Retention and Anti-Ulcer Activity of Diosmin. Int. J. Nanomed. 2019, 14, 7191–7213. [Google Scholar] [CrossRef] [PubMed]
- Dobrovolskaia, M.A.; Clogston, J.D.; Neun, B.W.; Hall, J.B.; Patri, A.K.; McNeil, S.E. Method for Analysis of Nanoparticle Hemolytic Properties In Vitro. Nano Lett. 2008, 8, 2180–2187. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Huo, M.; Zhou, J.; Zou, A.; Li, W.; Yao, C.; Xie, S. DDSolver: An Add-In Program for Modeling and Comparison of Drug Dissolution Profiles. AAPS J. 2010, 12, 263–271. [Google Scholar] [CrossRef]
- Kumar, P.; Nagarajan, A.; Uchil, P.D. Analysis of Cell Viability by the MTT Assay. Cold Spring Harb. Protoc. 2018, 2018. [Google Scholar] [CrossRef] [PubMed]
- Alassadi, S.; Pisani, M.J.; Wheate, N.J. A Chemical Perspective on the Clinical Use of Platinum-Based Anticancer Drugs. Dalton Trans. 2022, 51, 10835–10846. [Google Scholar] [CrossRef] [PubMed]
- van der Vijgh, W.J.F. Clinical Pharmacokinetics of Carboplatin. Clin. Pharmacokinet. 1991, 21, 242–261. [Google Scholar] [CrossRef] [PubMed]
- Stewart, D.J. Mechanisms of Resistance to Cisplatin and Carboplatin. Crit. Rev. Oncol./Hematol. 2007, 63, 12–31. [Google Scholar] [CrossRef]
- Wagstaff, A.J.; Ward, A.; Benfield, P.; Heel, R.C. Carboplatin. Drugs 1989, 37, 162–190. [Google Scholar] [CrossRef]
- Ghosh, A.K.; Osswald, H.L.; Glauninger, K.; Agniswamy, J.; Wang, Y.-F.; Hayashi, H.; Aoki, M.; Weber, I.T.; Mitsuya, H. Probing Lipophilic Adamantyl Group as the P1-Ligand for HIV-1 Protease Inhibitors: Design, Synthesis, Protein X-ray Structural Studies, and Biological Evaluation. J. Med. Chem. 2016, 59, 6826–6837. [Google Scholar] [CrossRef]
- Hansen, M.; Jacobsen, S.E.; Plunkett, S.; Liebscher, G.E.; McCorvy, J.D.; Bräuner-Osborne, H.; Kristensen, J.L. Synthesis and Pharmacological Evaluation of N-Benzyl Substituted 4-Bromo-2,5-Dimethoxyphenethylamines as 5-HT2A/2C Partial Agonists. Bioorg. Med. Chem. 2015, 23, 3933–3937. [Google Scholar] [CrossRef]
- Tacke, M. Benzyl-Substituted Carbene–Metal Complexes: Potential for Novel Antibiotics and Anticancer Drugs? J. Organomet. Chem. 2015, 782, 17–21. [Google Scholar] [CrossRef]
- Blakemore, D.C.; Castro, L.; Churcher, I.; Rees, D.C.; Thomas, A.W.; Wilson, D.M.; Wood, A. Organic Synthesis Provides Opportunities to Transform Drug Discovery. Nat. Chem. 2018, 10, 383–394. [Google Scholar] [CrossRef] [PubMed]
- Ruirui, Z.; He, J.; Xu, X.; Li, S.; Peng, H.; Deng, Z.; Huang, Y. PLGA-Based Drug Delivery System for Combined Therapy of Cancer: Research Progress. Mater. Res. Express 2021, 8, 122002. [Google Scholar] [CrossRef]
- Bauer, C.A.; Schneider, G.; Göller, A.H. Machine Learning Models for Hydrogen Bond Donor and Acceptor Strengths Using Large and Diverse Training Data Generated by First-Principles Interaction Free Energies. J. Cheminform. 2019, 11, 59. [Google Scholar] [CrossRef]
- Du, X.; Li, Y.; Xia, Y.-L.; Ai, S.-M.; Liang, J.; Sang, P.; Ji, X.-L.; Liu, S.-Q. Insights into Protein–Ligand Interactions: Mechanisms, Models, and Methods. Int. J. Mol. Sci. 2016, 17, 144. [Google Scholar] [CrossRef]
- Hall, R.; Dixon, T.; Dickson, A. On Calculating Free Energy Differences Using Ensembles of Transition Paths. Front. Mol. Biosci. 2020, 7, 106. [Google Scholar] [CrossRef]
- Forrey, C.; Douglas, J.F.; Gilson, M.K. The Fundamental Role of Flexibility on the Strength of Molecular Binding. Soft Matter 2012, 8, 6385–6392. [Google Scholar] [CrossRef]
- Poroikov, V.V.; Filimonov, D.A.; Gloriozova, T.A.; Lagunin, A.A.; Druzhilovskiy, D.S.; Rudik, A.V.; Stolbov, L.A.; Dmitriev, A.V.; Tarasova, O.A.; Ivanov, S.M.; et al. Computer-Aided Prediction of Biological Activity Spectra for Organic Compounds: The Possibilities and Limitations. Russ. Chem. Bull. 2019, 68, 2143–2154. [Google Scholar] [CrossRef]
- Filimonov, D.; Poroikov, V. Probabilistic Approaches in Activity Prediction. In Chemoinformatics Approaches to Virtual Screening; Royal Society of Chemistry: London, UK, 2008; pp. 182–216. ISBN 978-0-85404-144-2. [Google Scholar]
- Calvert, H. The Clinical Development of Carboplatin—A Personal Perspective. Inorg. Chim. Acta 2019, 498, 118987. [Google Scholar] [CrossRef]
- Samimi, S.; Maghsoudnia, N.; Eftekhari, R.B.; Dorkoosh, F. Chapter 3—Lipid-Based Nanoparticles for Drug Delivery Systems. In Characterization and Biology of Nanomaterials for Drug Delivery; Mohapatra, S.S., Ranjan, S., Dasgupta, N., Mishra, R.K., Thomas, S., Eds.; Micro and Nano Technologies; Elsevier: Amsterdam, The Netherlands, 2019; pp. 47–76. ISBN 978-0-12-814031-4. [Google Scholar]
- Gaio, E.; Guerrini, A.; Ballestri, M.; Varchi, G.; Ferroni, C.; Martella, E.; Columbaro, M.; Moret, F.; Reddi, E. Keratin Nanoparticles Co-Delivering Docetaxel and Chlorin E6 Promote Synergic Interaction between Chemo- and Photo-Dynamic Therapies. J. Photochem. Photobiol. B 2019, 199, 111598. [Google Scholar] [CrossRef]
- Park, K.E.; Noh, Y.-W.; Kim, A.; Lim, Y.T. Hyaluronic Acid-Coated Nanoparticles for Targeted Photodynamic Therapy of Cancer Guided by near-Infrared and MR Imaging. Carbohydr. Polym. 2017, 157, 476–483. [Google Scholar] [CrossRef] [PubMed]
- Lucero-Acuña, A.; Gutiérrez-Valenzuela, C.A.; Esquivel, R.; Guzmán-Zamudio, R. Mathematical Modeling and Parametrical Analysis of the Temperature Dependency of Control Drug Release from Biodegradable Nanoparticles. RSC Adv. 2019, 9, 8728–8739. [Google Scholar] [CrossRef] [PubMed]
- Mollaeva, M.; Nikolskaya, E.; Beganovskaya, V.; Sokol, M.; Chirkina, M.; Obydennyi, S.; Belykh, D.; Startseva, O.; Mollaev, M.; Yabbarov, N. Oxidative Damage Induced by Phototoxic Pheophorbide a 17-Diethylene Glycol Ester Encapsulated in PLGA Nanoparticles. Antioxidants 2021, 10, 1985. [Google Scholar] [CrossRef] [PubMed]
- Dash, S.; Murthy, P.N.; Nath, L.; Chowdhury, P. Kinetic Modeling on Drug Release from Controlled Drug Delivery Systems. Acta Pol. Pharm. 2010, 67, 217–223. [Google Scholar]
- Yoo, J.; Won, Y.-Y. Phenomenology of the Initial Burst Release of Drugs from PLGA Microparticles. ACS Biomater. Sci. Eng. 2020, 6, 6053–6062. [Google Scholar] [CrossRef]
- Costa, P.; Sousa Lobo, J.M. Modeling and Comparison of Dissolution Profiles. Eur. J. Pharm. Sci. 2001, 13, 123–133. [Google Scholar] [CrossRef]
- Siepmann, J.; Peppas, N.A. Higuchi Equation: Derivation, Applications, Use and Misuse. Int. J. Pharm. 2011, 418, 6–12. [Google Scholar] [CrossRef]
- Alway, B.; Sangchantra, R.; Stewart, P.J. Modelling the Dissolution of Diazepam in Lactose Interactive Mixtures. Int. J. Pharm. 1996, 130, 213–224. [Google Scholar] [CrossRef]
- Vasconcelos, A.; Vega, E.; Pérez, Y.; Gómara, M.J.; García, M.L.; Haro, I. Conjugation of Cell-Penetrating Peptides with Poly(Lactic-Co-Glycolic Acid)-Polyethylene Glycol Nanoparticles Improves Ocular Drug Delivery. Int. J. Nanomed. 2015, 10, 609–631. [Google Scholar] [CrossRef]
- Trongchuen, K.; Ounkaew, A.; Kasemsiri, P.; Hiziroglu, S.; Mongkolthanaruk, W.; Wannasutta, R.; Pongsa, U.; Chindaprasirt, P. Bioactive Starch Foam Composite Enriched with Natural Antioxidants from Spent Coffee Ground and Essential Oil. Starch 2018, 70, 1700238. [Google Scholar] [CrossRef]
- Heredia, N.S.; Vizuete, K.; Flores-Calero, M.J.; V., K.P.; Pilaquinga, F.; Kumar, B.; Debut, A. Comparative Statistical Analysis of the Release Kinetics Models for Nanoprecipitated Drug Delivery Systems Based on Poly(Lactic-Co-Glycolic Acid). PLoS ONE 2022, 17, e0264825. [Google Scholar] [CrossRef] [PubMed]
- Libi, S.; Calenic, B.; Astete, C.E.; Kumar, C.; Sabliov, C.M. Investigation on Hemolytic Effect of Poly(Lactic Co-Glycolic) Acid Nanoparticles Synthesized Using Continuous Flow and Batch Processes. Nanotechnol. Rev. 2017, 6, 209–220. [Google Scholar] [CrossRef]
- F04 Committee. Practice for Assessment of Hemolytic Properties of Materials; ASTM International: West Conshohocken, PA, USA, 2013. [Google Scholar]
- Karanam, V.; Marslin, G.; Krishnamoorthy, B.; Chellan, V.; Siram, K.; Natarajan, T.; Bhaskar, B.; Franklin, G. Poly (ε-Caprolactone) Nanoparticles of Carboplatin: Preparation, Characterization and In Vitro Cytotoxicity Evaluation in U-87 MG Cell Lines. Colloids Surf. B Biointerfaces 2015, 130, 48–52. [Google Scholar] [CrossRef] [PubMed]
- Akgül, S.; Chan, B.A.; Manders, P.M. Carboplatin Dose Calculations for Patients with Lung Cancer: Significant Dose Differences Found Depending on Dosing Equation Choice. BMC Cancer 2022, 22, 829. [Google Scholar] [CrossRef] [PubMed]
- Novohradsky, V.; Zanellato, I.; Marzano, C.; Pracharova, J.; Kasparkova, J.; Gibson, D.; Gandin, V.; Osella, D.; Brabec, V. Epigenetic and Antitumor Effects of Platinum(IV)-Octanoato Conjugates. Sci. Rep. 2017, 7, 3751. [Google Scholar] [CrossRef]
- Sun, Y.; Cao, Z.; Gou, S.; Hu, T. Novel Oxaliplatin Derivatives with 1-(Substituted Benzyl)Azetidine-3,3-Dicarboxylate Anions. Synthesis, Cytotoxicity, and Interaction with DNA. Chem. Biodivers. 2014, 11, 115–125. [Google Scholar] [CrossRef]
- Liu, F.; Gou, S.; Chen, F.; Fang, L.; Zhao, J. Study on Antitumor Platinum(II) Complexes of Chiral Diamines with Dicyclic Species as Steric Hindrance. J. Med. Chem. 2015, 58, 6368–6377. [Google Scholar] [CrossRef]
- Malik, M.A.; Lone, S.A.; Wani, M.Y.; Talukdar, M.I.A.; Dar, O.A.; Ahmad, A.; Hashmi, A.A. S-Benzyldithiocarbazate Imine Coordinated Metal Complexes Kill Candida Albicans by Causing Cellular Apoptosis and Necrosis. Bioorg. Chem. 2020, 98, 103771. [Google Scholar] [CrossRef]
- Chang, C.; Zhang, L.; Miao, Y.; Fang, B.; Yang, Z. Anticancer and Apoptotic-Inducing Effects of Rutin-Chitosan Nanoconjugates in Triple Negative Breast Cancer Cells. J. Clust. Sci. 2021, 32, 331–340. [Google Scholar] [CrossRef]
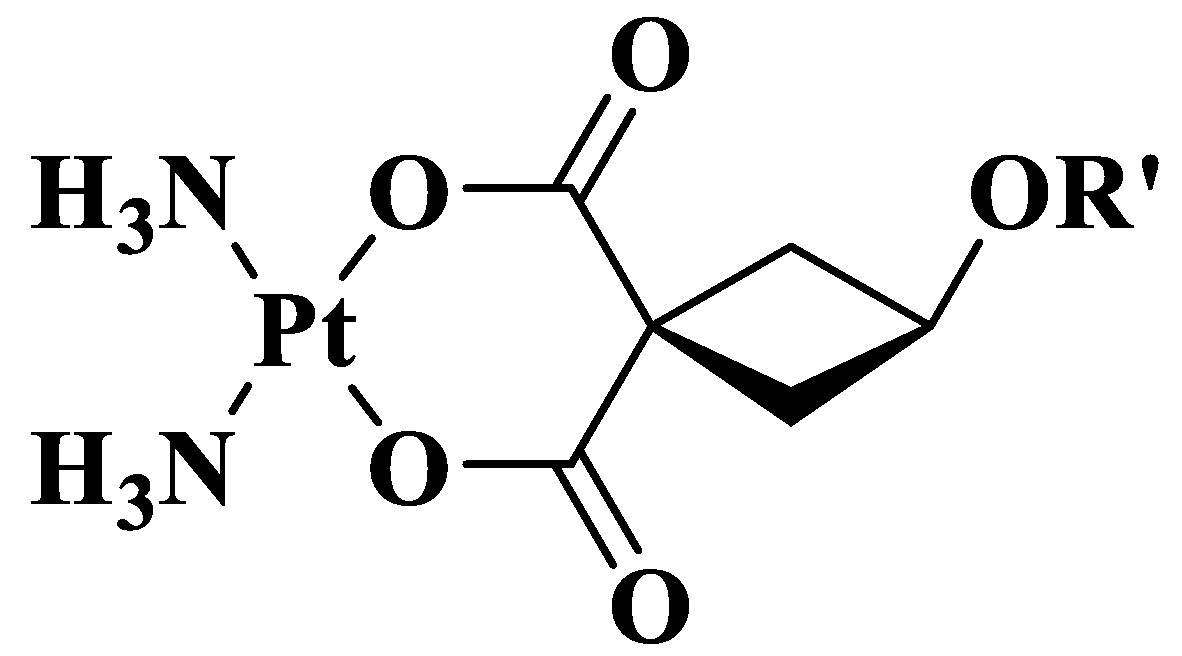
| Compound | Ligand | MW of Ligand | logP of Ligand * |
|---|---|---|---|
| Kpt1 | 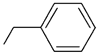 R’ = PhCH2 | 121.16 | 1.89 |
| Kpt2 | 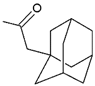 R’ = AdCH2C(O) | 209.27 | 3.06 |
| Kpt3 | 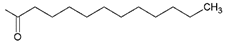 R’ = n-C11H23C(O) | 213.34 | 5.35 |
| Kpt4 | 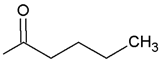 R’ = n-C4H9C(O) | 101.13 | 1.50 |
| Compound | Total Polar Surface Area | Number of H-Bond Acceptors | Number of H-Bond Donors | Molecular Globularity | Molecular Flexibility | logP (o/w) | Molecular Weight |
|---|---|---|---|---|---|---|---|
| Kpt | 80.18 | 2 | 0 | 0.17 | 2.72 | −1.80 | 370.25 |
| Kpt1 | 32.03 | 3 | 0 | 0.07 | 4.23 | 0.74 | 479.38 |
| Kpt2 | 48.53 | 3 | 0 | 0.10 | 4.72 | 1.52 | 565.51 |
| Kpt3 | 65.63 | 3 | 0 | 0.02 | 9.89 | 3.38 | 571.56 |
| Kpt4 | 63.05 | 3 | 0 | 0.07 | 5.43 | 0.29 | 473.37 |
| NPs | Size, nm | PDI | ζ-Potential, mV | DL, % | EE, % |
|---|---|---|---|---|---|
| Kpt-NP | 215 ± 38 | 0.243 ± 14 | −28.0 ± 3.2 | 0.16 ± 0.04 | 54 ± 3 |
| Kpt1-NP | 303 ± 26 | 0.204 ± 13 | −17.0 ± 2.1 | 0.21 ± 0.03 | 60 ± 2 |
| Kpt2-NP | 247 ± 45 | 0.277 ± 11 | −12.8 ± 1.7 | 0.39 ± 0.03 | 62 ± 3 |
| Kpt3-NP | 180 ± 37 | 0.127 ± 8 | −22.0 ± 2.3 | 1.07 ± 0.02 * | 92 ± 2 * |
| Kpt4-NP | 245 ± 51 | 0.245 ± 9 | −13.7 ± 1.9 | 0.30 ± 0.03 | 60 ± 1 |
| NPs | Zero Order | First Order | Higuchi | Hixson–Crowell | Korsmeyer–Peppas |
|---|---|---|---|---|---|
| R2 | R2 | R2 | R2 | R2 | |
| Kpt-NP | 0.2652 | 0.5951 | 0.6997 | 0.5271 | 0.9623 |
| Kpt1-NP | −0.0857 | 0.4432 | 0.6534 | 0.3173 | 0.9873 |
| Kpt2-NP | 0.4219 | 0.9042 | 0.8859 | 0.8291 | 0.9711 |
| Kpt3-NP | −0.1324 | 0.6951 | 0.6267 | 0.4219 | 0.9710 |
| Kpt4-NP | 0.0052 | 0.8239 | 0.6050 | 0.5170 | 0.9293 |
| Parameters | Kpt-NP | Kpt1-NP | Kpt2-NP | Kpt3-NP | Kpt4-NP |
| n | 0.35 | 0.21 | 0.32 | 0.21 | 0.24 |
| k | 19.7 | 24.0 | 33.7 | 35.3 | 32.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sokol, M.B.; Chirkina, M.V.; Yabbarov, N.G.; Mollaeva, M.R.; Podrugina, T.A.; Pavlova, A.S.; Temnov, V.V.; Hathout, R.M.; Metwally, A.A.; Nikolskaya, E.D. Structural Optimization of Platinum Drugs to Improve the Drug-Loading and Antitumor Efficacy of PLGA Nanoparticles. Pharmaceutics 2022, 14, 2333. https://doi.org/10.3390/pharmaceutics14112333
Sokol MB, Chirkina MV, Yabbarov NG, Mollaeva MR, Podrugina TA, Pavlova AS, Temnov VV, Hathout RM, Metwally AA, Nikolskaya ED. Structural Optimization of Platinum Drugs to Improve the Drug-Loading and Antitumor Efficacy of PLGA Nanoparticles. Pharmaceutics. 2022; 14(11):2333. https://doi.org/10.3390/pharmaceutics14112333
Chicago/Turabian StyleSokol, Maria B., Margarita V. Chirkina, Nikita G. Yabbarov, Mariia R. Mollaeva, Tatyana A. Podrugina, Anna S. Pavlova, Viktor V. Temnov, Rania M. Hathout, Abdelkader A. Metwally, and Elena D. Nikolskaya. 2022. "Structural Optimization of Platinum Drugs to Improve the Drug-Loading and Antitumor Efficacy of PLGA Nanoparticles" Pharmaceutics 14, no. 11: 2333. https://doi.org/10.3390/pharmaceutics14112333
APA StyleSokol, M. B., Chirkina, M. V., Yabbarov, N. G., Mollaeva, M. R., Podrugina, T. A., Pavlova, A. S., Temnov, V. V., Hathout, R. M., Metwally, A. A., & Nikolskaya, E. D. (2022). Structural Optimization of Platinum Drugs to Improve the Drug-Loading and Antitumor Efficacy of PLGA Nanoparticles. Pharmaceutics, 14(11), 2333. https://doi.org/10.3390/pharmaceutics14112333









