Potent Virucidal Activity In Vitro of Photodynamic Therapy with Hypericum Extract as Photosensitizer and White Light against Human Coronavirus HCoV-229E
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Cultures
2.2. Viruses
2.3. Compounds
2.4. Cell Viability Assay
2.5. Treatments and Infections
2.6. Photosensitizers-Degradation
2.7. Flow Cytometry
2.8. Statistics
3. Results
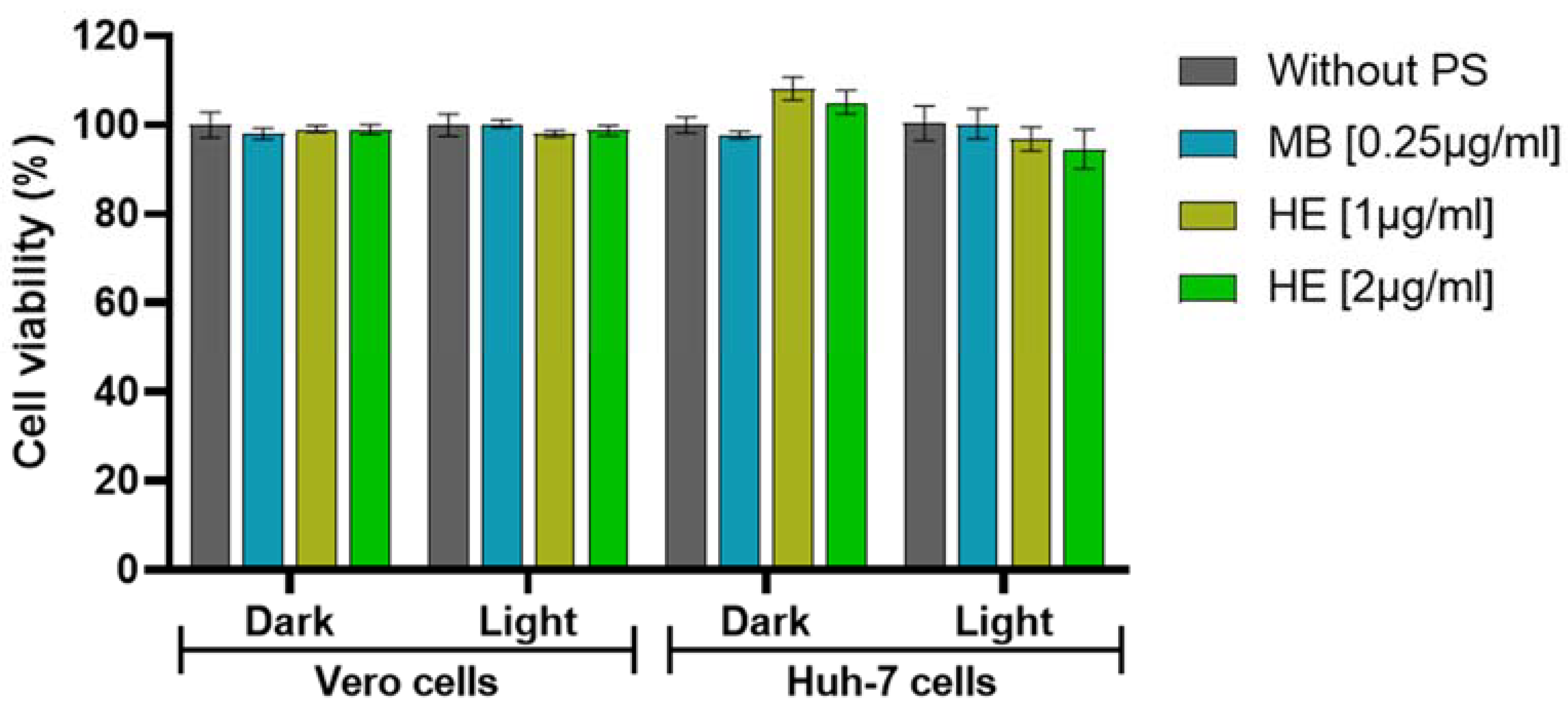
3.1. Effect of aPDT in Vero and Huh-7 Cells
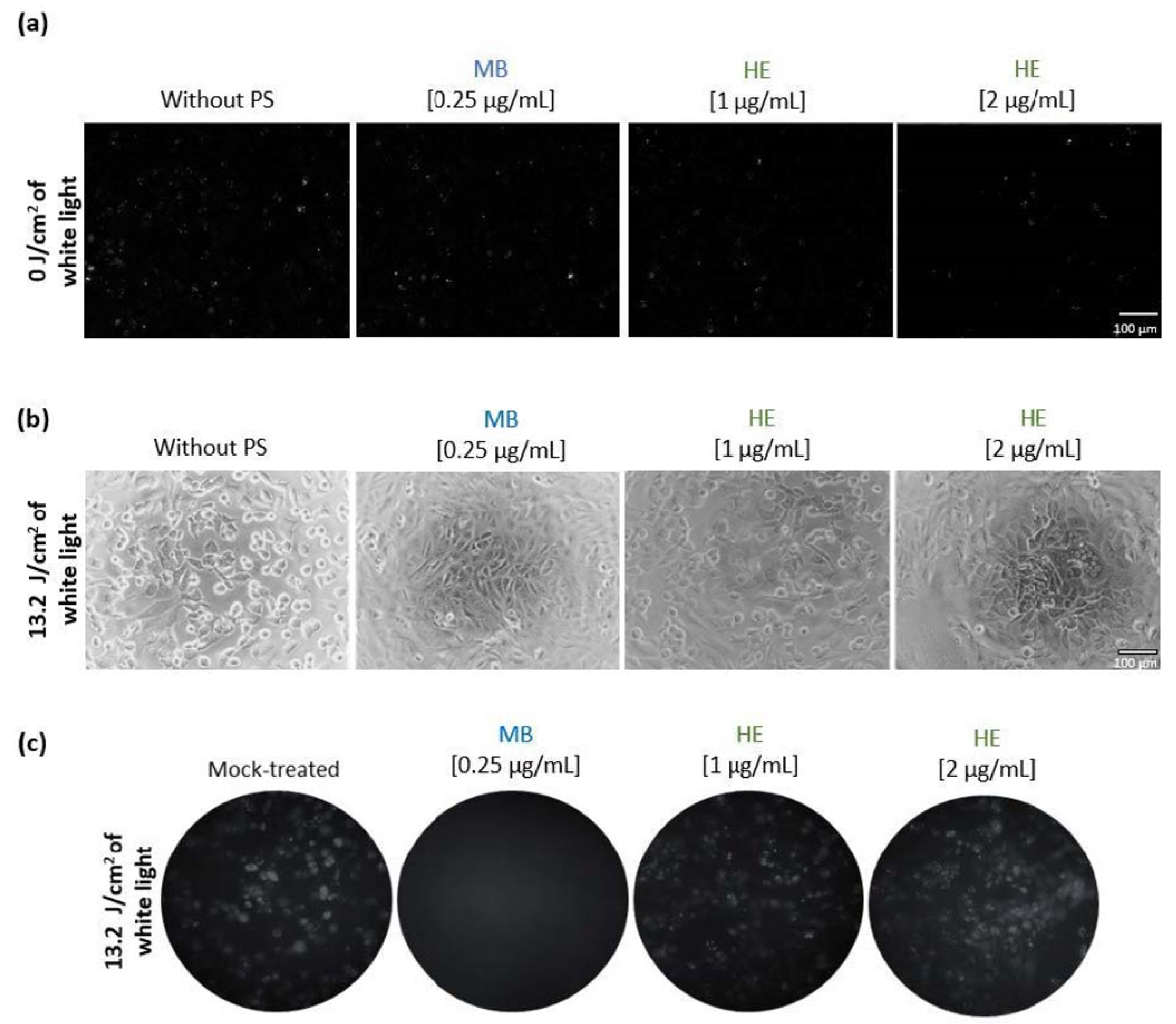
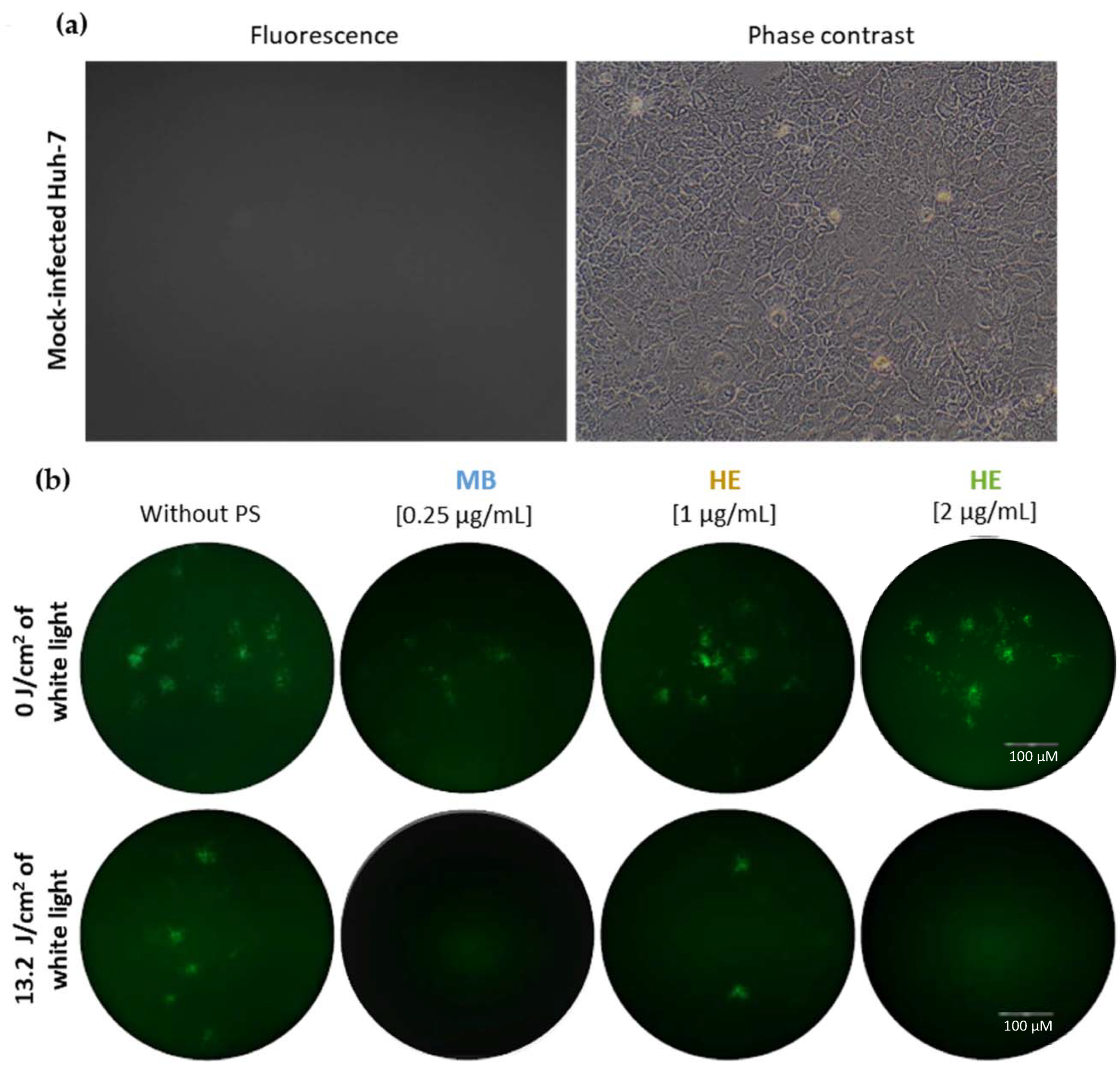
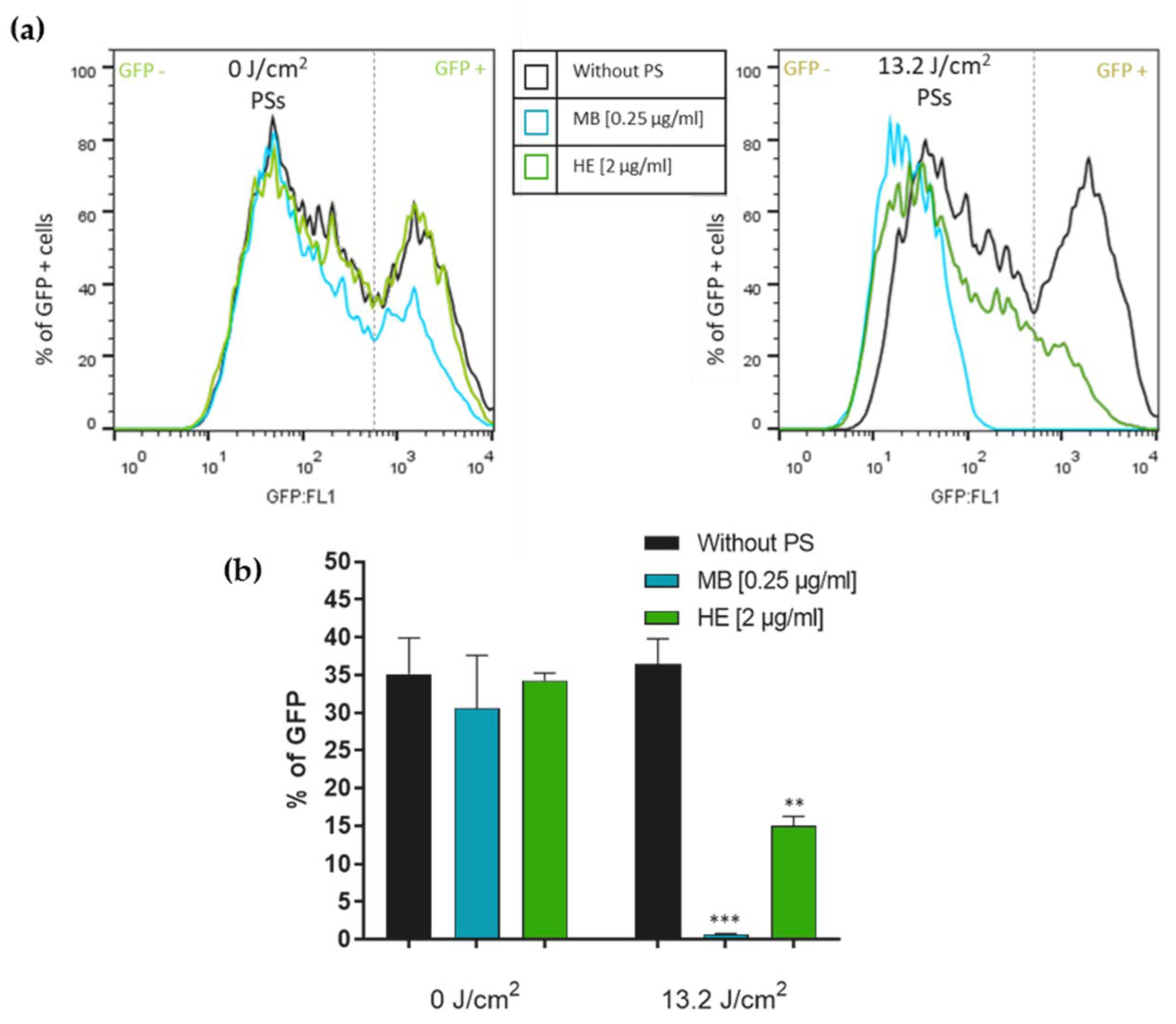
3.2. Effect of Irradiated and Non-Irradiated HE and MB against HSV-1 Infection
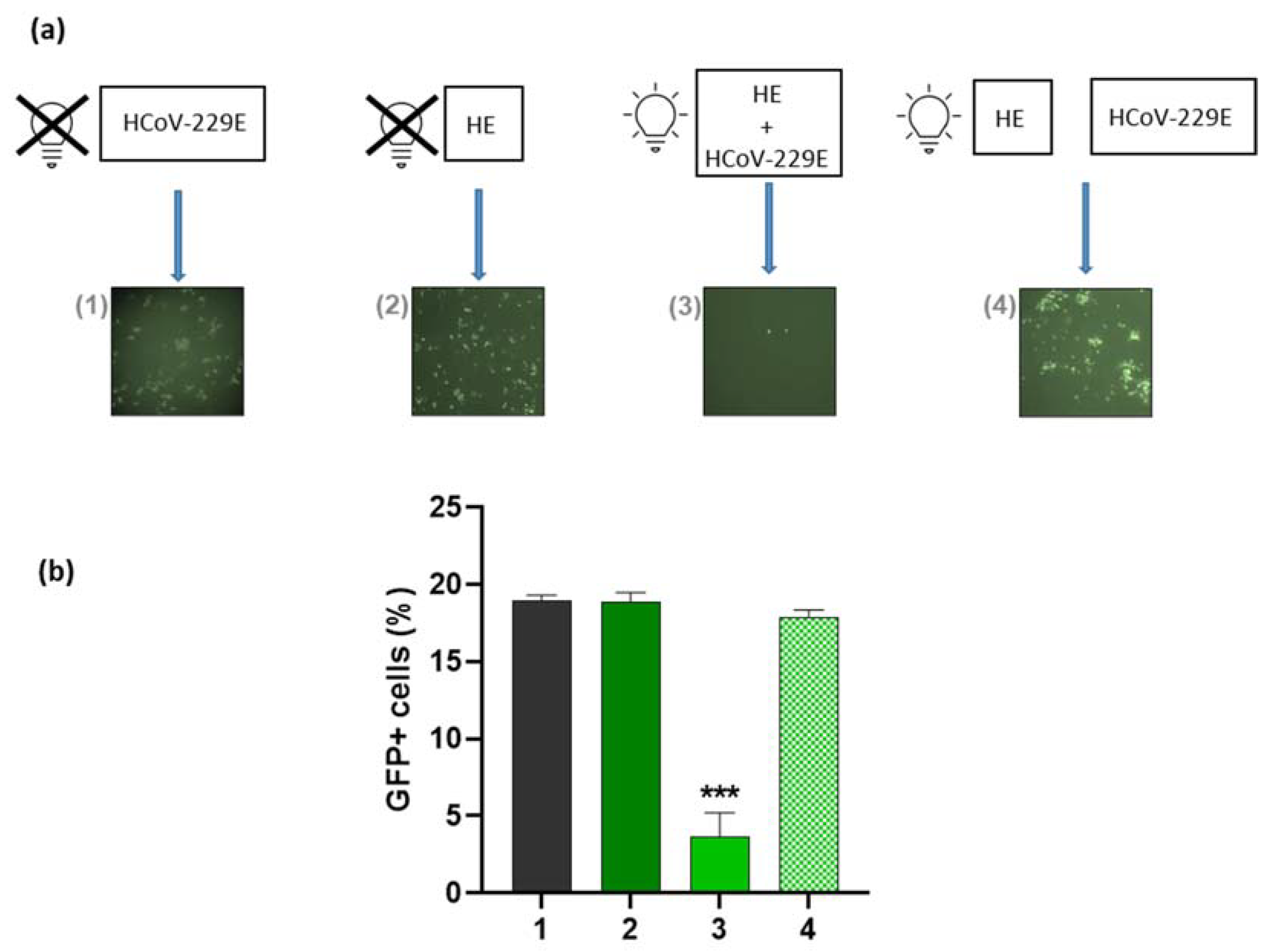
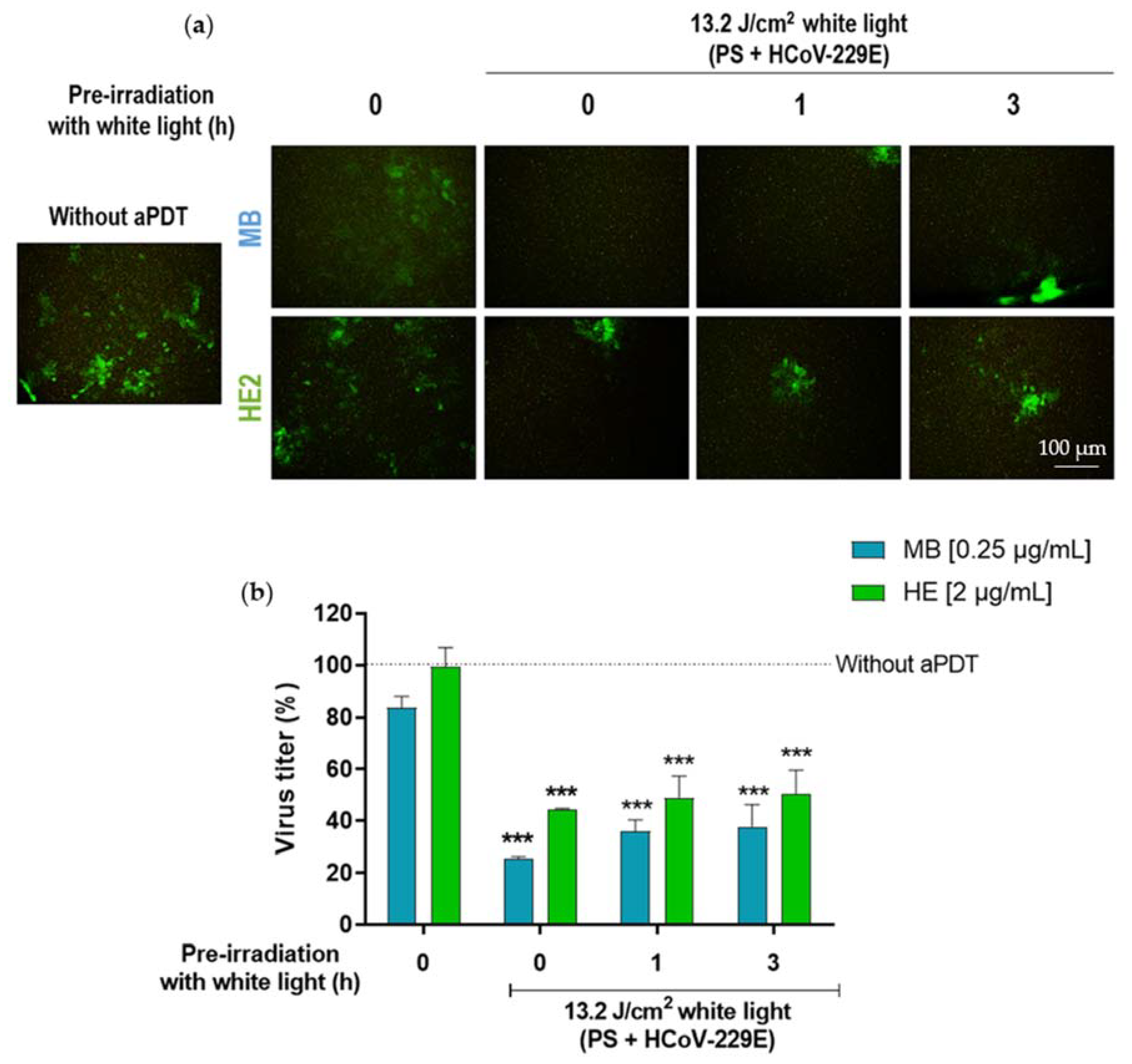
3.3. Virucidal Effect of HE against HCoV-229E
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
Abbreviations
References
- Hu, B.; Guo, H.; Zhou, P.; Shi, Z.L. Characteristics of SARS-CoV-2 and COVID-19. Nat. Rev. Microbiol. 2020, 19, 141–154. [Google Scholar] [CrossRef] [PubMed]
- Carrasco, L.; del Río, J.A. Virus Patógenos; Carrasco, L., Ed.; Editorial Hélice, Fundación BBVA: Madrid, Spain, 2006; ISBN 8493410608. [Google Scholar]
- Paules, C.I.; Marston, H.D.; Fauci, A.S. Coronavirus Infections-More Than Just the Common Cold. JAMA 2020, 323, 707–708. [Google Scholar] [CrossRef] [PubMed]
- Ahsan, W.; Javed, S.; Al Bratty, M.; Alhazmi, H.A.; Najmi, A. Treatment of SARS-CoV-2: How far have we reached? Drug Discov. Ther. 2020, 14, 67–72. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization WHO Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int/ (accessed on 15 February 2022).
- Centers for Disease Control and Prevention (CDC). COVID-19 Treatments and Medications. Available online: https://www.cdc.gov/coronavirus/2019-ncov/your-health/treatments-for-severe-illness.html (accessed on 7 November 2021).
- Asselah, T.; Durantel, D.; Pasmant, E.; Lau, G.; Schinazi, R.F. COVID-19: Discovery, diagnostics and drug development. J. Hepatol. 2021, 74, 168–184. [Google Scholar] [CrossRef]
- Stasi, C.; Fallani, S.; Voller, F.; Silvestri, C. Treatment for COVID-19: An overview. Eur. J. Pharmacol. 2020, 889, 173644. [Google Scholar] [CrossRef]
- García-Lledó, A.; Gómez-Pavón, J.; Del Castillo, J.G.; Hernández-Sampelayo, T.; Martín-Delgado, M.C.; Martín Sánchez, F.J.; Martínez-Sellés, M.; García, J.M.M.; Guillén, S.M.; Rodríguez-Artalejo, F.; et al. Pharmacological treatment of COVID-19: An opinion paper. Rev. Española Quimioter. 2022, 35, 115. [Google Scholar] [CrossRef]
- Lebedeva, N.S.; Gubarev, Y.A.; Koifman, M.O.; Koifman, O.I. The application of porphyrins and their analogues for inactivation of viruses. Molecules 2020, 25, 4368. [Google Scholar] [CrossRef]
- Correia, J.H.; Rodrigues, J.A.; Pimenta, S.; Dong, T.; Yang, Z. Photodynamic therapy review: Principles, photosensitizers, applications, and future directions. Pharmaceutics 2021, 13, 1332. [Google Scholar] [CrossRef]
- Kwiatkowski, S.; Knap, B.; Przystupski, D.; Saczko, J.; Kędzierska, E.; Knap-Czop, K.; Kotlińska, J.; Michel, O.; Kotowski, K.; Kulbacka, J. Photodynamic therapy—Mechanisms, photosensitizers and combinations. Biomed. Pharmacother. 2018, 106, 1098–1107. [Google Scholar] [CrossRef]
- Agostinis, P.; Berg, K.; Cengel, K.A.; Foster, T.H.; Girotti, A.W.; Gollnick, S.O.; Hahn, S.M.; Hamblin, M.R.; Juzeniene, A.; Kessel, D.; et al. Photodynamic therapy of cancer: An update. CA. Cancer J. Clin. 2011, 61, 250–281. [Google Scholar] [CrossRef]
- Guan, M.; Chu, G.; Jin, J.; Liu, C.; Cheng, L.; Guo, Y.; Deng, Z.; Wang, Y. A combined cyanine/carbomer gel enhanced photodynamic antimicrobial activity and wound healing. Nanomaterials 2022, 12, 2173. [Google Scholar] [CrossRef]
- Luo, X.; Zhang, B.; Zhang, Y.; Meng, Z.; Li, P.; Jiang, X.; Xiao, J.; Lin, C.; Su, W. Rose bengal-modified gold nanorods for PTT/PDT antibacterial synergistic therapy. Photodiagnosis Photodyn. Ther. 2022, 39, 102988. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Bielsa, A.; Gracia-Cazaña, T.; Robres, P.; Lopez, C.; Calvo-Priego, M.D.; Aspiroz, C.; Gilaberte, Y. Combination of photodynamic therapy and oral antifungals for the treatment of onychomycosis. Pharmaceuticals 2022, 15, 722. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Xiao, Z.; Feng, Y.; Wang, Q.; Teng, M. Mechanism of a new photosensitizer (TBZPy) in the treatment of high-risk human papillomavirus-related diseases. Photodiagnosis Photodyn. Ther. 2022, 37, 102591. [Google Scholar] [CrossRef]
- Pérez-Laguna, V.; Gilaberte, Y.; Millán-Lou, M.I.; Agut, M.; Nonell, S.; Rezusta, A.; Hamblin, M.R. A combination of photodynamic therapy and antimicrobial compounds to treat skin and mucosal infections: A systematic review. Photochem. Photobiol. Sci. 2019, 18, 1020–1029. [Google Scholar] [CrossRef] [PubMed]
- Mariewskaya, K.A.; Tyurin, A.P.; Chistov, A.A.; Korshun, V.A.; Alferova, V.A.; Ustinov, A.V. Photosensitizing antivirals. Molecules 2021, 26, 3971. [Google Scholar] [CrossRef] [PubMed]
- Barroso, R.A.; Navarro, R.; Tim, C.R.; de Paula Ramos, L.; de Oliveira, L.D.; Araki, Â.T.; Fernandes, K.G.C.; Macedo, D.; Assis, L. Antimicrobial photodynamic therapy against Propionibacterium acnes biofilms using hypericin (Hypericum perforatum) photosensitizer: In vitro study. Lasers Med. Sci. 2021, 36, 1235–1240. [Google Scholar] [CrossRef] [PubMed]
- Almeida, A.; Faustino, M.A.F.; Neves, M.G.P.M.S. Antimicrobial photodynamic therapy in the control of COVID-19. Antibiotics 2020, 9, 320. [Google Scholar] [CrossRef]
- Sabino, C.P.; Ball, A.R.; Baptista, M.S.; Dai, T.; Hamblin, M.R.; Ribeiro, M.S.; Santos, A.L.; Sellera, F.P.; Tegos, G.P.; Wainwright, M. Light-based technologies for management of COVID-19 pandemic crisis. J. Photochem. Photobiol. B Biol. 2020, 212, 111999. [Google Scholar] [CrossRef]
- Zhang, B.; Zheng, L.; Huang, Y.; Mo, Q.; Wang, X.; Qian, K. Detection of nucleic acid lesions during photochemical inactivation of RNA viruses by treatment with methylene blue and light using real-time PCR. Photochem. Photobiol. 2011, 87, 365–369. [Google Scholar] [CrossRef]
- Schikora, D.; Hepburn, J.; Plavin, S.R. Reduction of the viral load by non-invasive photodynamic therapy in early stages of COVID-19 infection. Am. J. Virol. Dis. 2020, 2, 1–5. [Google Scholar]
- Svyatchenko, V.A.; Nikonov, S.D.; Mayorov, A.P.; Gelfond, M.L.; Loktev, V.B. Antiviral photodynamic therapy: Inactivation and inhibition of SARS-CoV-2 in vitro using methylene blue and Radachlorin. Photodiagnosis Photodyn. Ther. 2021, 33, 102112. [Google Scholar] [CrossRef] [PubMed]
- Maury, W.; Price, J.P.; Brindley, M.A.; Oh, C.; Neighbors, J.D.; Wiemer, D.F.; Wills, N.; Carpenter, S.; Hauck, C.; Murphy, P.; et al. Identification of light-independent inhibition of human immunodeficiency virus-1 infection through bioguided fractionation of Hypericum perforatum. Virol. J. 2009, 6, 101. [Google Scholar] [CrossRef]
- Von Eggelkraut-Gottanka, S.G.; Abu Abed, S.; Müller, W.; Schmidt, P.C. Quantitative analysis of the active components and the by-products of eight dry extracts of Hypericum perforatum L. (St John’s wort). Phytochem. Anal. 2002, 13, 170–176. [Google Scholar] [CrossRef]
- Schmitt, L.A.; Liu, Y.; Murphy, P.A.; Birt, D.F. Evaluation of the light-sensitive cytotoxicity of Hypericum perforatum extracts, fractions, and pure compounds. J. Agric. Food Chem. 2006, 54, 2881–2890. [Google Scholar] [CrossRef] [PubMed]
- Wölfle, U.; Seelinger, G.; Schempp, C.M. Topical Application of St. John’s Wort (Hypericum perforatum). Planta Med. 2014, 80, 109–120. [Google Scholar] [CrossRef] [PubMed]
- de Andrade, G.P.; de Souza, T.F.M.; Cerchiaro, G.; da Silva Pinhal, M.A.; Ribeiro, A.O.; Girão, M.J.B.C. Hypericin in photobiological assays: An overview. Photodiagnosis Photodyn. Ther. 2021, 35, 102343. [Google Scholar] [CrossRef]
- Dong, X.; Zeng, Y.; Zhang, Z.; Fu, J.; You, L.; He, Y.; Hao, Y.; Gu, Z.; Yu, Z.; Qu, C.; et al. Hypericin-mediated photodynamic therapy for the treatment of cancer: A review. J. Pharm. Pharmacol. 2021, 73, 425–436. [Google Scholar] [CrossRef] [PubMed]
- Rezusta, A.; Lõpez-Chicõn, P.; Paz-Cristobal, M.P.; Alemany-Ribes, M.; Royo-Díez, D.; Agut, M.; Semino, C.; Nonell, S.; Revillo, M.J.; Aspiroz, C.; et al. In vitro fungicidal photodynamic effect of hypericin on Candida species. Photochem. Photobiol. 2012, 88, 613–619. [Google Scholar] [CrossRef]
- Nakabayashi, H.; Miyano, K.; Sato, J.; Yamane, T.; Taketa, K. Growth of human hepatoma cells lines with differentiated functions in chemically defined medium. Cancer Res. 1982, 42, 3858–3863. [Google Scholar]
- Reed, L.J.; Muench, H. A simple method of estimating fifty per cent endpoints. Am. J. Epidemiol. 1938, 27, 493–497. [Google Scholar] [CrossRef]
- Andreu, S.; Ripa, I.; Bello-Morales, R.; López-Guerrero, J.A. Nebulized CLODOS technology shows clear virucidal properties against the human coronavirus HCoV-229E at Non-Cytotoxic Doses. Viruses 2021, 13, 531. [Google Scholar] [CrossRef]
- Wagner, S.J.; Skripchenko, A.; Robinette, D.; Foley, J.W.; Cincotta, L. Factors affecting virus photoinactivation by a series of phenothiazine dyes. Photochem. Photobiol. 1998, 67, 343–349. [Google Scholar] [CrossRef]
- Ramalho, K.M.; Cunha, S.R.; Gonçalves, F.; Escudeiro, G.S.; Steiner-Oliveira, C.; Horliana, A.C.R.T.; de Eduardo, C.P. Photodynamic therapy and Acyclovir in the treatment of recurrent herpes labialis: A controlled randomized clinical trial. Photodiagnosis Photodyn. Ther. 2021, 33, 102093. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Laguna, V.; García-Malinis, A.J.; Aspiroz, C.; Rezustata, A.; Gilaberte, Y. Antimicrobial effects of photodynamic therapy. G. Ital. Dermatol. Venereol. 2018, 153, 833–846. [Google Scholar] [CrossRef] [PubMed]
- Jones, N.R.; Qureshi, Z.U.; Temple, R.J.; Larwood, J.P.J.; Greenhalgh, T.; Bourouiba, L. Two metres or one: What is the evidence for physical distancing in covid-19? BMJ 2020, 370, m3223. [Google Scholar] [CrossRef] [PubMed]
- Yalçin, S.; Akan, H.; Çakilcioglu, U. Suruç İlçesindeki (Şanlıurfa-Türkiye) Aktarlarda Satılan Şifalı Bitkiler. Int. J. Nat. Life Sci. 2021, 5, 40–51. [Google Scholar] [CrossRef]
- Tang, J.; Colacino, J.M.; Larsen, S.H.; Spitzer, W. Virucidal activity of hypericin against enveloped and non-enveloped DNA and RNA viruses. Antiviral Res. 1990, 13, 313–325. [Google Scholar] [CrossRef]
- Hudson, J.B.; Delaey, E.; De Witte, P.A. Bromohypericins are potent photoactive antiviral agents. Photochem. Photobiol. 1999, 70, 820–822. [Google Scholar] [CrossRef]
- Andersen, D.O.; Weber, N.D.; Wood, S.G.; Hughes, B.G.; Murray, B.K.; North, J.A. In vitro virucidal activity of selected anthraquinones and anthraquinone derivatives. Antivir. Res. 1991, 16, 185–196. [Google Scholar] [CrossRef]
- Cagno, V.; Medaglia, C.; Cerny, A.; Cerny, T.; Cerny, E. Methylene Blue has a potent antiviral activity against SARS-CoV-2 in the absence of UV-activation in vitro. Sci. Rep. 2021, 11, 14295. [Google Scholar] [CrossRef] [PubMed]
- Bojadzic, D.; Alcazar, O.; Buchwald, P. Methylene blue inhibits the SARS-CoV-2 Spike–ACE2 protein-protein interaction–A mechanism that can contribute to its antiviral activity against COVID-19. Front. Pharmacol. 2021, 11, 2255. [Google Scholar] [CrossRef]
- Michútová, M.; Mrázová, V.; Kúdelová, M.; Smolinská, M.; Šupoliková, M.; Vrbová, M.; Golais, F. Herpes simplex viruses type 1 and 2 photoinactivated in the presence of methylene blue transform human and mouse cells in vitro. Acta Virol. 2017, 61, 308–315. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Delcanale, P.; Uriati, E.; Mariangeli, M.; Mussini, A.; Moreno, A.; Lelli, D.; Cavanna, L.; Bianchini, P.; Diaspro, A.; Abbruzzetti, S.; et al. The interaction of hypericin with SARS-CoV-2 reveals a multimodal antiviral activity. ACS Appl. Mater. Interfaces 2022, 14, 14025–14032. [Google Scholar] [CrossRef]
- da Rocha Matos, A.; Caetano, B.C.; de Almeida Filho, J.L.; de Carvalho Martins, J.S.C.; de Oliveira, M.G.P.; das Chagas Sousa, T.; Horta, M.A.P.; Siqueira, M.M.; Fernandez, J.H. Identification of hypericin as a candidate repurposed therapeutic agent for COVID-19 and its potential Anti-SARS-CoV-2 activity. Front. Microbiol. 2022, 13, 828984. [Google Scholar] [CrossRef]
- Yalçın, S.; Yalçınkaya, S.; Ercan, F. Determination of Potential Drug Candidate Molecules of the Hypericum perforatum for COVID-19 Treatment. Curr. Pharmacol. Rep. 2021, 7, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Hilgenfeld, R. From SARS to MERS: Crystallographic studies on coronaviral proteases enable antiviral drug design. FEBS J. 2014, 281, 4085–4096. [Google Scholar] [CrossRef]
- Shivanika, C.; Deepak Kumar, S.; Ragunathan, V.; Tiwari, P.; Sumitha, A.; Brindha Devi, P. Molecular docking, validation, dynamics simulations, and pharmacokineticprediction of natural compounds against the SARS-CoV-2 main-protease. J. Biomol. Struct. Dyn. 2020, 40, 585–611. [Google Scholar] [CrossRef]
- Pitsillou, E.; Liang, J.; Karagiannis, C.; Ververis, K.; Darmawan, K.K.; Ng, K.; Hung, A.; Karagiannis, T.C. Interaction of small molecules with the SARS-CoV-2 main protease in silico and in vitro validation of potential lead compounds using an enzyme-linked immunosorbent assay. Comput. Biol. Chem. 2020, 89, 107408. [Google Scholar] [CrossRef]
- Agostinis, P.; Vantieghem, A.; Merlevede, W.; De Witte, P.A.M. Hypericin in cancer treatment: More light on the way. Int. J. Biochem. Cell Biol. 2002, 34, 221–241. [Google Scholar] [CrossRef]
- Laurent, D.; Baumann, F.; Benoit, A.G.; Mortelecqe, A.; Nitatpattana, N.; Desvignes, I.; Debitus, C.; Laille, M.; Gonzalez, J.P.; Chungue, E. Structure-activity relationships of dengue antiviral polycyclic quinones. Southeast Asian J. Trop. Med. Public Health 2005, 36, 901–905. [Google Scholar] [PubMed]
- Xu, Y.; Lu, C. Raman spectroscopic study on structure of human immunodeficiency virus (HIV) and hypericin-induced photosensitive damage of HIV. Sci. China Ser. C Life Sci. 2005, 48, 117–132. [Google Scholar] [CrossRef] [PubMed]
- Bonnett, R. Chemical Aspects of Photodynamic Therapy; CRC Press: London, UK, 2000; ISBN 9780429182013. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Praena, B.; Mascaraque, M.; Andreu, S.; Bello-Morales, R.; Abarca-Lachen, E.; Rapozzi, V.; Gilaberte, Y.; González, S.; López-Guerrero, J.A.; Juarranz, Á. Potent Virucidal Activity In Vitro of Photodynamic Therapy with Hypericum Extract as Photosensitizer and White Light against Human Coronavirus HCoV-229E. Pharmaceutics 2022, 14, 2364. https://doi.org/10.3390/pharmaceutics14112364
Praena B, Mascaraque M, Andreu S, Bello-Morales R, Abarca-Lachen E, Rapozzi V, Gilaberte Y, González S, López-Guerrero JA, Juarranz Á. Potent Virucidal Activity In Vitro of Photodynamic Therapy with Hypericum Extract as Photosensitizer and White Light against Human Coronavirus HCoV-229E. Pharmaceutics. 2022; 14(11):2364. https://doi.org/10.3390/pharmaceutics14112364
Chicago/Turabian StylePraena, Beatriz, Marta Mascaraque, Sabina Andreu, Raquel Bello-Morales, Edgar Abarca-Lachen, Valentina Rapozzi, Yolanda Gilaberte, Salvador González, José Antonio López-Guerrero, and Ángeles Juarranz. 2022. "Potent Virucidal Activity In Vitro of Photodynamic Therapy with Hypericum Extract as Photosensitizer and White Light against Human Coronavirus HCoV-229E" Pharmaceutics 14, no. 11: 2364. https://doi.org/10.3390/pharmaceutics14112364
APA StylePraena, B., Mascaraque, M., Andreu, S., Bello-Morales, R., Abarca-Lachen, E., Rapozzi, V., Gilaberte, Y., González, S., López-Guerrero, J. A., & Juarranz, Á. (2022). Potent Virucidal Activity In Vitro of Photodynamic Therapy with Hypericum Extract as Photosensitizer and White Light against Human Coronavirus HCoV-229E. Pharmaceutics, 14(11), 2364. https://doi.org/10.3390/pharmaceutics14112364











