The Ethanolic Extract of Gomphrena celosioides Mart. Does Not Alter Reproductive Performance or Embryo-Fetal Development, nor Does It Cause Chromosomal Damage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material of G. celosioides Extract
2.2. Animals
2.3. Experimental Design
2.4. Biological Tests
2.5. Biometric Parameters
2.6. Reproductive Performance and Embryo-Fetal Development
2.7. Micronucleus in Peripheral Blood
2.8. Splenic Phagocytosis
2.9. Statistical Analysis
3. Results
3.1. Biometric Parameters
3.2. Reproductive Performance
3.3. Embryofetal Development and Placental Efficiency
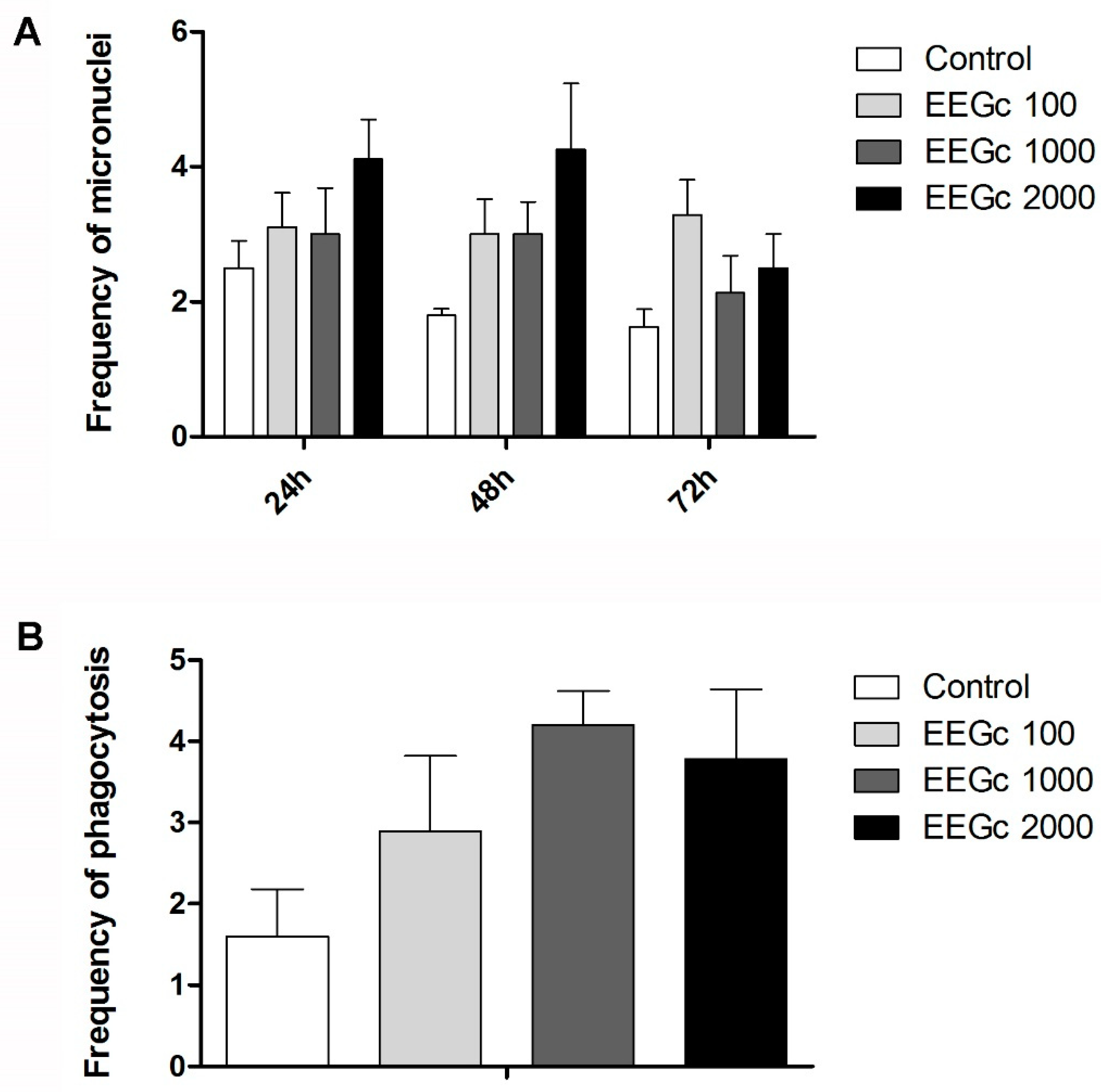
3.4. Toxicogenetics
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Myers, N.; Mittermeier, R.A.; Mittermeier, C.G.; da Fonseca, G.A.; Kent, J. Biodiversity hotspots for conservation priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef] [PubMed]
- de Paula Vasconcelos, P.C.; Tirloni, C.A.S.; Palozi, R.A.C.; Leitão, M.M.; Carneiro, M.T.S.; Schaedler, M.I.; Silva, A.O.; Souza, R.I.C.; Salvador, M.J.; Junior, A.G.; et al. Diuretic herb Gomphrena celosioides Mart. (Amaranthaceae) promotes sustained arterial pressure reduction and protection from cardiac remodeling on rats with renovascular hypertension. J. Ethnopharmacol. 2018, 224, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Jardim Botânico do Rio de Janeiro. Flora do Brasil; Amaranthaceae in Flora do Brasil 2020 em construção. 2019. Available online: http://floradobrasil.jbrj.gov.br/reflora/floradobrasil/FB15428 (accessed on 5 October 2019).
- Senna, L.; Siqueira, J.C.; Marchioretto, M.S. Gomphrena in Lista de Espécies da Flora do Brasil. Jardim Botânico do Rio de Janeiro. 2015. Available online: http://floradobrasil.jbrj.gov.br/jabot/floradobrasil/FB15428 (accessed on 7 July 2019).
- Takim, F.O.; Olawoyin, O.K.; A Olanrewaju, W. Growth and Development of Gomphrena celosioides Mart under Screen House Conditions in Ilorin, Southern Guinea Savanna Zone of Nigeria. Agrosearch 2013, 13, 59. [Google Scholar] [CrossRef] [Green Version]
- Chassagne, F.; Deharo, E.; Punley, H.; Bourdy, G. Treatment and management of liver diseases by Khmer traditional healers practicing in Phnom Penh area, Cambodia. J. Ethnopharmacol. 2017, 202, 38–53. [Google Scholar] [CrossRef] [PubMed]
- Prachi, N.C.; Kumar, D.; Kansana, M.S. Medicinal plants of muzaffarnagar district used in treatment of urinary tract and kidney stone. Indian J. Tradit. Knowl. 2009, 8, 191–195. [Google Scholar]
- Sharma, N.; Vijayvergia, R. Study of primary metabolites and antimicrobial activity of Gomphrena celosioides mart. Int. J. Pharma. Bio. Sci. 2011, 2, 581–586. [Google Scholar]
- de Paula Vasconcelos, P.C.; Spessotto, D.R.; Marinho, J.V.; Salvador, M.J.; Junior, A.G.; Kassuya, C.A. Mechanisms underlying the diuretic effect of Gomphrena celosioides Mart. (Amaranthaceae). J. Ethnopharmacol. 2017, 202, 85–91. [Google Scholar] [CrossRef]
- Macorini, L.F.B.; Radai, J.A.S.; Maris, R.S.; Silva-Filho, S.E.; Leitao, M.M.; de Andrade, S.F.; Gelves, D.I.A.; Salvador, M.J.; Arena, A.C.; Kassuya, C.A.L. Antiarthritic and Antihyperalgesic Properties of Ethanolic Extract from. Evid. Based Complement. Alternat. Med. 2020, 2020, 4170589. [Google Scholar] [CrossRef]
- Olutola Dosumu, O.; Onocha, P.; Ekundayo, O.; Ali, M. Isolation of aurantiamides from Gomphrena celosioides C. Mart. Iran J. Pharm. Res. 2014, 13, 143–147. [Google Scholar]
- Burkill, H.M. The Useful Plants of West Tropical Africa, 2nd ed.; University Press of Virginia, Box 3608 University Station: Charlottesville, VA, USA, 1985; Volume 1, p. 22903. [Google Scholar]
- Kpodar, M.S.; Karou, S.D.; Katawa, G.; Anani, K.; Gbekley, H.E.; Adjrah, Y.; Tchacondo, T.; Batawila, K.; Simpore, J. An ethnobotanical study of plants used to treat liver diseases in the Maritime region of Togo. J. Ethnopharmacol. 2016, 181, 263–273. [Google Scholar] [CrossRef]
- Oluwabunmi, I.J.; Abiola, T. Gastroprotective effect of methanolic extract of Gomphrena celosioides on indomethacin induced gastric ulcer in Wistar albino rats. Int. J. Appl. Basic Med. Res. 2015, 5, 41–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Moura, R.M.; Pereira, P.S.; Januário, A.H.; França, S.e.C.; Dias, D.A. Antimicrobial screening and quantitative determination of benzoic acid derivative of Gomphrena celosioides by TLC-densitometry. Chem. Pharm. Bull. 2004, 52, 1342–1344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghonime, M.; Emara, M.; Shawky, R.; Soliman, H.; El-Domany, R.; Abdelaziz, A. Immunomodulation of RAW 264.7 murine macrophage functions and antioxidant activities of 11 plant extracts. Immunol. Investig. 2015, 44, 237–252. [Google Scholar] [CrossRef]
- Promraksa, B.; Phetcharaburanin, J.; Namwat, N.; Techasen, A.; Boonsiri, P.; Loilome, W. Evaluation of anticancer potential of Thai medicinal herb extracts against cholangiocarcinoma cell lines. PLoS ONE 2019, 14, e0216721. [Google Scholar] [CrossRef]
- Veena, P.; Perivela, L.; Raghavan, S.S. Furosemide in postpartum management of severe preeclampsia: A randomized controlled trial. Hypertens Pregnancy 2017, 36, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, M.; Morita, T.; Kodama, Y.; Sofuni, T.; Ishidate, M., Jr. The micronucleus assay with mouse peripheral blood reticulocytes using acridine orange-coated slides. Mutat. Res. Lett. 1990, 245, 245–249. [Google Scholar] [CrossRef]
- Oliveira, R.J.; Salles, M.J.; da Silva, A.F.; Kanno, T.Y.; Lourenço, A.C.; Freiria, G.A.; Matiazi, H.J.; Ribeiro, L.R.; Mantovani, M.S. Effects of the polysaccharide beta-glucan on clastogenicity and teratogenicity caused by acute exposure to cyclophosphamide in mice. Regul Toxicol. Pharmacol. 2009, 53, 164–173. [Google Scholar] [CrossRef]
- Gonçalves, C.A.; Siqueira, J.M.; Carollo, C.A.; Mauro, M.e.O.; de Davi, N.; Cunha-Laura, A.L.; Monreal, A.C.; Castro, A.H.; Fernandes, L.; Chagas, R.R.; et al. Gestational exposure to Byrsonima verbascifolia: Teratogenicity, mutagenicity and immunomodulation evaluation in female Swiss mice. J. Ethnopharmacol. 2013, 150, 843–850. [Google Scholar] [CrossRef]
- David, N.; Mauro, M.e.O.; Gonçalves, C.A.; Pesarini, J.R.; Strapasson, R.L.; Kassuya, C.A.; Stefanello, M.; Cunha-Laura, A.L.; Monreal, A.C.; Oliveira, R.J. Gochnatia polymorpha ssp. floccosa: Bioprospecting of an anti-inflammatory phytotherapy for use during pregnancy. J. Ethnopharmacol. 2014, 154, 370–379. [Google Scholar] [CrossRef]
- Oliveira, R.J.; Mantovani, M.S.; Pesarini, J.R.; Mauro, M.O.; da Silva, A.F.; Souza, T.R.; Ribeiro, L.R. 6-Dimethylaminopurine and cyclohexamide are mutagenic and alter reproductive performance and intrauterine development in vivo. Genet Mol. Res. 2015, 14, 834–849. [Google Scholar] [CrossRef]
- Ishikawa, R.B.; Vani, J.M.; das Neves, S.C.; Rabacow, A.P.M.; Kassuya, C.A.L.; Croda, J.; Cardoso, C.A.L.; Monreal, A.C.D.F.; Antoniolli, A.C.M.B.; Cunha-Laura, A.L.; et al. The safe use of Doliocarpus dentatus in the gestational period: Absence of changes in maternal reproductive performance, embryo-fetal development and DNA integrity. J. Ethnopharmacol. 2018, 217, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Vani, J.M.; de Carvalho Schweich, L.; de Oliveira, K.R.W.; Auharek, S.A.; Cunha-Laura, A.L.; Antoniolli-Silva, A.C.M.B.; Nazario, C.E.D.; Oliveira, R.J. Evaluation of the effects of the larvicides temephos on reproductive performance, embryofetal development and DNA integrity of Swiss mice. Pestic Biochem. Physiol. 2018, 148, 22–27. [Google Scholar] [CrossRef] [PubMed]
- OECD. OECD Test No. 421: Reproduction/Developmental Toxicity Screening Test. Reprod./Dev. Toxic. Screen. Test 2015, 421, 14. [Google Scholar] [CrossRef]
- ANVISA—Agência Nacional de Vigilância Sanitária. Guia Para a Condução de Estudos não Clínicos de Segurança Necessários ao Desenvolvimento de Medicamentos. Brasília; 2010. Available online: https://www.gov.br/anvisa/pt-br/centraisdeconteudo/publicacoes/medicamentos/pesquisa-clinica/manuais-e-guias/guia-para-a-conducao-de-estudos-nao-clinicos-de-toxicologia-e-seguranca-farmacologica-necessarios-ao-desenvolvimento-de-medicamentos-versao-2.pdf/view (accessed on 7 July 2019).
- OECD. Test No. 425: Acute Oral Toxicity: Up-and-down Procedure; OECD Publishing: Paris, France, 2008. [Google Scholar] [CrossRef] [Green Version]
- Russell, W.M.S.; Burch, R.L. The Principles of Humane Experimental Technique; Methuen & Co.: London, UK, 1959. [Google Scholar]
- Vani, J.M.; Monreal, M.T.F.D.; Auharek, S.A.; Cunha-Laura, A.L.; de Arruda, E.J.; Lima, A.R.; da Silva, C.M.; Antoniolli-Silva, A.C.M.B.; de Lima, D.P.; Beatriz, A.; et al. The mixture of cashew nut shell liquid and castor oil results in an efficient larvicide against Aedes aegypti that does not alter embryo-fetal development, reproductive performance or DNA integrity. PLoS ONE 2018, 13, e0193509. [Google Scholar] [CrossRef] [PubMed]
- Barrow, M.V.; Taylor, W.J. A rapid method for detecting malformations in rat fetuses. J. Morphol. 1969, 127, 291–305. [Google Scholar] [CrossRef]
- Oliveira, R.J.; Oliva, S.U.; Daroz, G.A.; Rubio, E.M. Fertility assessment and possible external structural defects on progeny from male rats chronically exposed to arsenic. Rev. Bras. Toxicol. 2005, 18, 57. [Google Scholar]
- Damasceno, D.C. Anomalias Congênitas: Estudos Experimentais. CoopMed 2008. [Google Scholar]
- Taylor, P. Practical Teratology; Academic Press: London, UK, 1986. [Google Scholar]
- Manson, J.C.; Clarke, A.R.; Hooper, M.L.; Aitchison, L.; McConnell, I.; Hope, J. 129/Ola mice carrying a null mutation in PrP that abolishes mRNA production are developmentally normal. Mol. Neurobiol. 1994, 8, 121–127. [Google Scholar] [CrossRef]
- Wise, L.D.; Beck, S.L.; Beltrame, D.; Beyer, B.K.; Chahoud, I.; Clark, R.L.; Clark, R.; Druga, A.M.; Feuston, M.H.; Guittin, P.; et al. Terminology of developmental abnormalities in common laboratory mammals (version 1). Teratology 1997, 55, 249–292. [Google Scholar] [CrossRef]
- Staples, R.E.; Schnell, V.L. Refinements in Rapid Clearing Technic in the Koh-Alizarin Red S Method for fetal bone. Stain. Technol. 1964, 39, 61–63. [Google Scholar] [PubMed]
- Soulimane-Mokhtari, N.A.; Guermouche, B.; Yessoufou, A.; Saker, M.; Moutairou, K.; Hichami, A.; Merzouk, H.; Khan, N.A. Modulation of lipid metabolism by n-3 polyunsaturated fatty acids in gestational diabetic rats and their macrosomic offspring. Clin. Sci. 2005, 109, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, P.C.; Santos, E.A.; Schneider, B.U.; Matuo, R.; Pesarini, J.R.; Cunha-Laura, A.L.; Monreal, A.C.; Lima, D.P.; Antoniolli, A.C.; Oliveira, R.J. Diaryl sulfide analogs of combretastatin A-4: Toxicogenetic, immunomodulatory and apoptotic evaluations and prospects for use as a new chemotherapeutic drug. Environ. Toxicol. Pharmacol. 2015, 40, 715–721. [Google Scholar] [CrossRef]
- Schneider, B.U.; Meza, A.; Beatriz, A.; Pesarini, J.R.; Carvalho, P.C.; Mauro, M.e.O.; Karaziack, C.B.; Cunha-Laura, A.L.; Monreal, A.C.; Matuo, R.; et al. Cardanol: Toxicogenetic assessment and its effects when combined with cyclophosphamide. Genet. Mol. Biol. 2016, 39, 279–289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haseman, J.K.; Hogan, M.D. Selection of the experimental unit in teratology studies. Teratology 1975, 12, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Tuha, A.; Gurbie, Y.; Hailu, H.G. Evaluation of Knowledge and Practice of Pharmacy Professionals regarding the Risk of Medication Use during Pregnancy in Dessie Town, Northeast Ethiopia: A Cross-Sectional Study. J. Pregnancy 2019, 2019, 2186841. [Google Scholar] [CrossRef] [Green Version]
- Krieg, S.A.; Shahine, L.K.; Lathi, R.B. Environmental exposure to endocrine-disrupting chemicals and miscarriage. Fertil Steril 2016, 106, 941–947. [Google Scholar] [CrossRef] [Green Version]
- Gug, C.; Rațiu, A.; Navolan, D.; Drăgan, I.; Groza, I.M.; Păpurică, M.; Vaida, M.A.; Mozoș, I.; Jurcă, M.C. Incidence and Spectrum of Chromosome Abnormalities in Miscarriage Samples: A Retrospective Study of 330 Cases. Cytogenet. Genome Res. 2019, 158, 171–183. [Google Scholar] [CrossRef]
- Sabolović Rudman, S.; Djaković, I.; Gall, V.; Djaković, Ž.; Košec, V. Pregnancy outcome in gestational diabetes compared to body mass index. Acta Clin. Croat. 2019, 58, 37–41. [Google Scholar] [CrossRef] [Green Version]
- Filali Khattabi, Z.; Biolcati, M.; Fois, A.; Chatrenet, A.; Laroche, D.; Attini, R.; Cheve, M.T.; Piccoli, G.B. Chronic kidney disease in preeclamptic patients: Not found unless searched for-Is a nephrology evaluation useful after an episode of preeclampsia? J. Nephrol. 2019, 32, 977–987. [Google Scholar] [CrossRef]
- Salama, M.; Rezk, M.; Gaber, W.; Hamza, H.; Marawan, H.; Gamal, A.; Abdallah, S. Methyldopa versus nifedipine or no medication for treatment of chronic hypertension during pregnancy: A multicenter randomized clinical trial. Pregnancy Hypertens. 2019, 17, 54–58. [Google Scholar] [CrossRef]
- Duzenli, M.A.; Ozdemir, K.; Aygul, N.; Soylu, A.; Tokac, M. Comparison of increased aspirin dose versus combined aspirin plus clopidogrel therapy in patients with diabetes mellitus and coronary heart disease and impaired antiplatelet response to low-dose aspirin. Am. J. Cardiol. 2008, 102, 396–400. [Google Scholar] [CrossRef] [PubMed]
- Yokoi, R.; Hayashi, M.; Tamura, T.; Kobayashi, K.; Kuroda, J.; Kusama, H.; Kagami, H.; Ono, T. Embryonic mortality and intrauterine growth retardation (IUGR) associated with placental alterations in pregnant rats treated with methyl methanesulfonate (MMS) at the peri-implantation stage. J. Toxicol. Sci. 2008, 33, 585–598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teng, C.; Jia, J.; Wang, Z.; Sharma, V.K.; Yan, B. Size-dependent maternal-fetal transfer and fetal developmental toxicity of ZnO nanoparticles after oral exposures in pregnant mice. Ecotoxicol. Environ. Saf. 2019, 182, 109439. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, R.J.; da Cruz Leite Santos, N.; Pesarini, J.R.; de Oliveira, B.C.; Berno, C.R.; de Araújo, F.H.S.; da Silveira, I.O.M.F.; Nascimento, R.O.; Brochado Antoniolli-Silva, A.C.M.; Duenhas Monreal, A.C.; et al. Assessment of genetic integrity, splenic phagocytosis and cell death potential of (Z)-4-((1,5-dimethyl-3-oxo-2-phenyl-2,3dihydro-1H-pyrazol-4-yl) amino)-4-oxobut-2-enoic acid and its effect when combined with commercial chemotherapeutics. Genet. Mol. Biol. 2018, 41, 154–166. [Google Scholar] [CrossRef]
- Wilson, M.E.; Ford, S.P. Comparative aspects of placental efficiency. Reprod. Suppl. 2001, 58, 223–232. [Google Scholar] [CrossRef]
- Fowden, A.L.; Sferruzzi-Perri, A.N.; Coan, P.M.; Constancia, M.; Burton, G.J. Placental efficiency and adaptation: Endocrine regulation. J. Physiol. 2009, 587, 3459–3472. [Google Scholar] [CrossRef]
- Guimarães, C.F.; Meirelles, M.G.; Fernandes, C.B. Placental efficiency in the equine species: Which factors may be related? Braz. J. Vet. Res. Anim. Sci. 2015, 52, 98–105. [Google Scholar] [CrossRef] [Green Version]
- Moraes-Souza, R.Q.; Soares, T.S.; Carmo, N.O.; Damasceno, D.C.; Campos, K.E.; Volpato, G.T. Adverse effects of Croton urucurana B. exposure during rat pregnancy. J. Ethnopharmacol. 2017, 199, 328–333. [Google Scholar] [CrossRef] [Green Version]
- Sibley, C.P.; Turner, M.A.; Cetin, I.; Ayuk, P.; Boyd, C.A.; D’Souza, S.W.; Glazier, J.D.; Greenwood, S.L.; Jansson, T.; Powell, T. Placental phenotypes of intrauterine growth. Pediatr. Res. 2005, 58, 827–832. [Google Scholar] [CrossRef]
- Brett, K.E.; Ferraro, Z.M.; Yockell-Lelievre, J.; Gruslin, A.; Adamo, K.B. Maternal-fetal nutrient transport in pregnancy pathologies: The role of the placenta. Int. J. Mol. Sci. 2014, 15, 16153–16185. [Google Scholar] [CrossRef] [Green Version]
- Fowden, A.L.; Forhead, A.J. Endocrine mechanisms of intrauterine programming. Reproduction 2004, 127, 515–526. [Google Scholar] [CrossRef] [PubMed]
- Pessatto, L.R.; Auharek, S.A.; Gonçalves, C.A.; de David, N.; Monreal, A.C.; Kassuya, C.A.; Antoniolli-Silva, A.C.; Stefanello, M.; Oliveira, R.J. Effects of dichloromethane and butanol fractions of Gochnatia polymorpha subsp. floccosa in maternal reproductive outcome, embryo-fetal development and DNA integrity in mice. J. Ethnopharmacol. 2017, 200, 205–208. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, R.J.; Pesarini, J.R.; Sparça Salles, M.J.; Nakamura Kanno, T.Y.; Dos Santos Lourenço, A.C.; da Silva Leite, V.; da Silva, A.F.; Matiazi, H.J.; Ribeiro, L.R.; Mantovani, M.S. Effects of β-glucan polysaccharide revealed by the dominant lethal assay and micronucleus assays, and reproductive performance of male mice exposed to cyclophosphamide. Genet. Mol. Biol. 2014, 37, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Vani, J.M.; de Carvalho Schweich-Adami, L.; Auharek, S.A.; Antoniolli-Silva, A.C.M.B.; Oliveira, R.J. Pyriproxyfen does not cause microcephaly or malformations in a preclinical mammalian model. Environ. Sci. Pollut. Res. Int. 2021, 28, 4585–4593. [Google Scholar] [CrossRef] [PubMed]
| Parameters | Experimental Groups | |||
|---|---|---|---|---|
| Control | EEGc 100 | EEGc 1000 | EEGc 2000 | |
| Initial weight (g) | 34.05 ± 1.95 a | 31.89 ± 0.98 a | 33.31 ± 1.17 a | 32.66 ± 0.90 a |
| Final weight (g) | 52.19 ± 1.71 a | 49.41 ± 2.49 a | 54.02 ± 2.06 a | 51.33 ± 1.45 a |
| Weight gain (g) | 18.14 ± 1.69 a | 17.52 ± 2.83 a | 20.71 ± 1.67 a | 18.67 ± 1.24 a |
| Uterus weight (g) | 18.19 ± 0.67 a | 16.27 ± 1.65 a | 18.23 ± 1.35 a | 17.59 ± 0.98 a |
| Net weight (g) | −0.052 ± 1.47 a | 1.25 ± 1.35 a | 2.47 ± 0.64 a | 1.07 ± 0.69 a |
| Absolute Weight (g) | ||||
| Heart | 0.18 ± 0.0099 a | 0.19 ± 0.0083 a | 0.20 ± 0.0133 a | 0.17 ± 0.0047 a |
| Lung | 0.25 ± 0.0119 a | 0.24 ± 0.0123 a | 0.26 ± 0.0104 a | 0.25 ± 0.0087 a |
| Spleen | 0.15 ± 0.0129 a | 0.14 ± 0.0102 a | 0.15 ± 0.0085 a | 0.14 ± 0.1077 a |
| Liver | 2.14 ± 0.0801 a | 2.25 ± 0.0938 a | 2.29 ± 0.0843 a | 2.14 ± 0.0739 a |
| Kidneys | 0.45 ± 0.0293 a | 0.38 ± 0.0169 a | 0.40 ± 0.0094 a | 0.40 ± 0.0151 a |
| Relative Weight | ||||
| Heart | 0.0034 ± 0.0002 a | 0.0039 ± 0.0002 a | 0.0036 ± 0.0002 a | 0.0034 ± 0.0000 a |
| Lung | 0.0049 ± 0.0002 a | 0.0049 ± 0.0003 a | 0.0049 ± 0.0003 a | 0.0049 ± 0.0002 a |
| Spleen | 0.0029 ± 0.0002 a | 0.0028 ± 0.0001 a | 0.0028 ± 0.0002 a | 0.0027 ± 0.0002 a |
| Liver | 0.041 ± 0.0023 a | 0.046 ± 0.0018 a | 0.043 ± 0.0017 a | 0.042 ± 0.0015 a |
| Kidneys | 0.0087 ± 0.0004 a | 0.0078 ± 0.0004 a | 0.0074 ± 0.0003 a | 0.0079 ± 0.0003 a |
| Parameters | Experimental Groups (mg/kg) | |||
|---|---|---|---|---|
| Control | EEGc 100 | EEGc 1000 | EEGc 2000 | |
| Mating | 10 | 10 | 10 | 10 |
| Fertility rate (%) | 100 | 100 | 100 | 100 |
| Implants | 13.10 ± 0.78 a | 12.30 ± 1.22 a | 14.60 ± 0.98 a | 13.20 ± 0.87 a |
| Live fetuses | 12.50 ± 0.69 a | 11.50 ± 1.25 a | 12.70 ± 1.06 a | 12.30 ± 0.73 a |
| Dead fetuses | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.10 ± 0.10 a | 0.00 ± 0.00 a |
| Resorption | 0.60 ± 0.27 a | 0.90 ± 0.38 a | 1.80 ± 0.76 a | 0.90 ± 0.50 a |
| PILR (%) | 4.14 ± 1.94 a | 7.45 ± 2.87 a | 12.65 ± 5.43 a | 6.24 ± 2.93 a |
| RR (%) | 4.14 ± 1.94 a | 8.11 ± 3.09 a | 12.09 ± 5.35 a | 6.24 ± 2.93 a |
| Fetal viability (%) | 95.85 ± 1.94 a | 92.55 ± 2.87 a | 87.35 ± 5.43 a | 93.76 ± 2.93 a |
| Sexual reason | 2.36 ± 1.00 a | 1.68 ± 0.46 a | 1.41 ± 0.31 a | 1.36 ± 0.27 a |
| Parameters | Experimental Groups (mg/kg) | |||
|---|---|---|---|---|
| Control | EEGc 100 | EEGc 1000 | EEGc 2000 | |
| Fetal weight 1,* | 1.17 ± 0.13 a | 1.18 ± 0.16 a | 1.17 ± 0.12 a | 1.20 ± 0.009 a |
| Placenta weight 1,# | 0.07 ± 0.006 a | 0.07 ± 0.002 a | 0.07 ± 0.002 a | 0.08 ± 0.007 a |
| Placental index 1,# | 0.06 ± 0.005 a | 0.06 ± 0.001 a | 0.06 ± 0.001 a | 0.07 ± 0.005 a |
| Placental efficiency 1,# | 19.6 ± 0.59 c | 17.23 ± 0.43 a,b | 18.51 ± 0.44 b,c | 15.99 ± 0.35 a |
| %SGA 2 | 4.80 | 6.14 | 2.40 | 1.63 |
| %SUGA 2 | 92.80 | 86.84 | 95.20 | 98.37 |
| %LGA 2 | 2.40 | 7.02 | 2.40 | 0.00 |
| AJWPA—Oliveira | AWPA | AWPA | AWPA | |
| Parameters | Experimental Groups (mg/kg) | |||
|---|---|---|---|---|
| Control | EEGc 100 | EEGc 1000 | EEGc 2000 | |
| External Analysis | ||||
| Analyzed fetuses | 125 | 113 | 126 | 106 |
| Normal fetuses | 121 | 94 | 114 | 98 |
| % | 96.80 | 83.19 | 90.48 | 92.45 |
| Bilateral limb hyperflexion | 2 | 0 | 0 | 1 |
| % | 0.80 | 0.00 | 0.00 | 0.94 |
| Unilateral limb hyperflexion | 1 | 7 * | 10 * | 3 |
| % | 0.80 | 6.09 | 7.87 | 2.83 |
| Hyperkyphosis | 0 | 3 | 0 | 0 |
| % | 0.00 | 2.61 | 0.00 | 0.00 |
| Micromelia | 0 | 3 | 1 | 1 |
| % | 0.00 | 2.61 | 0.79 | 0.94 |
| Poorly rotating paw | 1 | 6 | 0 | 0 |
| % | 0.80 | 5.22 | 0.00 | 0.00 |
| Hind limb hyperextension | 0 | 4 | 2 | 4 |
| % | 0.00 | 3.48 | 1.57 | 3.77 |
| Anterior limb hyperextension | 0 | 0 | 0 | 1 |
| % | 0.00 | 0.00 | 0.00 | 0.94 |
| Limb hyperextension | 0 | 4 | 2 | 5 * |
| % | 0.00 | 3.48 | 1.57 | 4.72 |
| Folded tail | 0 | 1 | 0 | 0 |
| % | 0.00 | 0.87 | 0.00 | 0.00 |
| Paw hyperflexion | 0 | 1 | 0 | 0 |
| % | 0.00 | 0.87 | 0.00 | 0.00 |
| Frequency of external malformation | 4 | 19 | 12 | 8 |
| % external malformation | 3.20 | 16.81 | 9.52 | 7.55 |
| Parameters | Experimental Groups (mg/kg) | |||
|---|---|---|---|---|
| Control | EEGc 100 | EEGc 1000 | EEGc 2000 | |
| Visceral Analysis | ||||
| Analyzed fetuses | 62 | 54 | 61 | 59 |
| Normal fetuses | 22 | 26 | 19 | 19 |
| % | 35.48 | 48.15 | 31.15 | 32.2 |
| Mild hydrocephalus | 36 | 19 | 36 | 38 |
| % | 58.06 | 35.19 | 60 | 64.41 |
| Moderate hydrocephalus | 0 | 0 | 1 | 0 |
| % | 0 | 0 | 1.67 | 0 |
| Hidronefrose | 11 | 13 | 16 | 5 |
| % | 17.74 | 24.07 | 26.67 | 8.47 |
| Obstructed choana | 1 | 0 | 0 | 0 |
| % | 1.61 | 0 | 0 | 0 |
| Frequency of visceral malformation | 40 | 28 | 42 | 40 |
| % visceral malformation | 64.52 | 51.85 | 70.00 | 67.8 |
| Parameters | Experimental Groups (mg/kg) | |||
|---|---|---|---|---|
| Control | EEGc 100 | EEGc 1000 | EEGc 2000 | |
| Skeletal Analysis | ||||
| Analyzed fetuses | 63 | 59 | 65 | 47 |
| Normal fetuses | 7 | 3 | 2 | 8 |
| % | 11.11 | 5.18 | 3.08 | 17.02 |
| Skull | ||||
| Front OR | 4 | 7 | 8 | 2 |
| % | 6.35 | 11.86 | 12.50 | 4.26 |
| OR Interparietal | 0 | 1 | 0 | 1 |
| % | 0.00 | 1.69 | 0.00 | 2.13 |
| OR Palate | 6 | 5 | 7 | 3 |
| % | 9.52 | 8.47 | 10.94 | 6.38 |
| OR Presfenoid | 7 | 15 | 9 | 5 |
| % | 11.11 | 25.42 | 14.06 | 10.64 |
| OR Supra occipital | 0 | 1 | 0 | 0 |
| % | 0.00 | 1.69 | 0.00 | 0.00 |
| Body | ||||
| OR External center | 43 | 45 | 54 | 27 |
| % | 68.25 | 76.27 | 84.38 | 57.45 |
| Rib agenesis | 0 | 1 | 0 | 0 |
| % | 0.00 | 1.69 | 0.00 | 0.00 |
| Bifurcated rib | 0 | 1 | 0 | 0 |
| % | 0.00 | 1.69 | 0.00 | 0.00 |
| OR ribs | 0 | 0 | 0 | 1 |
| % | 0.00 | 0.00 | 0.00 | 2.13 |
| Abnormal sternebrio | 1 | 8 * | 3 | 1 |
| % | 1.59 | 13.56 | 9.69 | 2.13 |
| OR Manubrium | 0 | 0 | 1 | 0 |
| % | 0.00 | 0.00 | 1.56 | 0.00 |
| OR Xiphoid process | 7 | 15 | 2 | 0 |
| % | 11.11 | 25.42 | 3.13 | 0.00 |
| Limb | ||||
| Poorly positioned fibula | 0 | 2 | 1 | 3 |
| % | 0.00 | 3.39 | 1.56 | 6.38 |
| Phalange agnesia | 13 | 8 | 12 | 3 |
| % | 20.63 | 13.56 | 18.75 | 6.38 |
| OR Phalanx | 28 | 19 | 19 | 23 |
| % | 44.44 | 32.20 | 29.69 | 48.94 |
| Metacarpal agenesis | 0 | 1 | 0 | 0 |
| % | 0.00 | 1.69 | 0.00 | 0.00 |
| OR Metacarpal | 2 | 0 | 1 | 0 |
| % | 3.17 | 0.00 | 1.56 | 0.00 |
| OR Metatarsus | 1 | 0 | 0 | 0 |
| % | 1.59 | 0.00 | 0.00 | 0.00 |
| Rotation of the fibula over the tibia | 2 | 11 * | 5 | 6 |
| % | 3.17 | 18.64 | 7.81 | 12.77 |
| Frequency of visceral malformation | 56 | 56 | 63 | 39 |
| % visceral malformation | 88.89 | 94.91 | 96.92 | 82.98 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salustriano, F.R.; Monreal, A.C.D.; das Neves, S.C.; de Oliveira, G.M.; de Oliveira, D.D.M.; Vilela, M.L.B.; do Nascimento, V.A.; Martins, A.C.F.; Saroja, B.; Karuppusamy, A.; et al. The Ethanolic Extract of Gomphrena celosioides Mart. Does Not Alter Reproductive Performance or Embryo-Fetal Development, nor Does It Cause Chromosomal Damage. Pharmaceutics 2022, 14, 2369. https://doi.org/10.3390/pharmaceutics14112369
Salustriano FR, Monreal ACD, das Neves SC, de Oliveira GM, de Oliveira DDM, Vilela MLB, do Nascimento VA, Martins ACF, Saroja B, Karuppusamy A, et al. The Ethanolic Extract of Gomphrena celosioides Mart. Does Not Alter Reproductive Performance or Embryo-Fetal Development, nor Does It Cause Chromosomal Damage. Pharmaceutics. 2022; 14(11):2369. https://doi.org/10.3390/pharmaceutics14112369
Chicago/Turabian StyleSalustriano, Fabricia Rodrigues, Antonio Carlos Duenhas Monreal, Silvia Cordeiro das Neves, Giovana Martins de Oliveira, Diego Duarte Marques de Oliveira, Marcelo Luiz Brandão Vilela, Valter Aragão do Nascimento, Allana Cristina Faustino Martins, Baby Saroja, Arunachalam Karuppusamy, and et al. 2022. "The Ethanolic Extract of Gomphrena celosioides Mart. Does Not Alter Reproductive Performance or Embryo-Fetal Development, nor Does It Cause Chromosomal Damage" Pharmaceutics 14, no. 11: 2369. https://doi.org/10.3390/pharmaceutics14112369
APA StyleSalustriano, F. R., Monreal, A. C. D., das Neves, S. C., de Oliveira, G. M., de Oliveira, D. D. M., Vilela, M. L. B., do Nascimento, V. A., Martins, A. C. F., Saroja, B., Karuppusamy, A., Coelho, H. R. S., Kassuya, C. A. L., Gelves, D. I. A., Salvador, M. J., Oliveira, R. J., & Gomes, R. d. S. (2022). The Ethanolic Extract of Gomphrena celosioides Mart. Does Not Alter Reproductive Performance or Embryo-Fetal Development, nor Does It Cause Chromosomal Damage. Pharmaceutics, 14(11), 2369. https://doi.org/10.3390/pharmaceutics14112369






