Target Prediction of 5,10,15,20-Tetrakis(4′-Sulfonatophenyl)-Porphyrin Using Molecular Docking
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Molecular Target Selection and Similarity Virtual Screening
2.3. Pharmacodynamic, Pharmacogenomic, and Pharmacokinetic Predictions
2.4. Molecular Docking
| Target | Identification Code | GPS | Blind Molecular Docking | |
|---|---|---|---|---|
| GPD (x y z) | GPM (x y z) | |||
| BCL-2 | PDB 2XA0 [40] | 0.375 Å | 112; 106; 96 | 33.111, −12.383, −15.527 |
| BCL-B | PDB 4B4S [41] | 0.375 Å | 112; 126; 116 | −11.425, 20.511, 7.450 |
| BCL-xL | PDB 3WIZ [42] | 0.519 Å | 126; 100; 126 | 40.398, 2.687, −22.529 |
| MCL-1 | PDB 3WIX [42] | 0.375 Å | 108; 122; 98 | −9.969, 2.268, −48.521 |
| A1 | PDB 5UUL [43] | 0.375 Å | 116; 124; 102 | −9.348, 5.397, −5.604 |
| BCL-W | PDB 2Y6W [44] | 0.419 Å | 100; 124; 92 | −22.718, 9.756, −4.104 |
| β-catenin | PDB 1LUJ [45] | 0.853 Å | 78; 70; 126 | 23.902, 31.829, 33.523 |
| NFKB | PDB 1SVC [46] | 0.536 Å | 126; 126; 126 | 40.385, 8.741, 38.710 |
| Fas | PDB 1DDF [47] | 0.375 Å | 126; 94; 84 | 1.736, −1.315, 2.392 |
| EIF2AK1 | AF-Q9BQI3-F1 [31,32] | 0.525 Å | 126; 126; 126 | 11.643, −1.149, −3.074 |
| HSA (blind) | PDB 1N5U [48] | 0.658 Å | 126; 78; 126 | 24.950, 5.672, 19.674 |
2.5. UV–Vis Absorption Spectroscopy
3. Results
3.1. Small Molecules and Similarity Report
3.2. Pharmacodynamic, Pharmacogenomic, and Pharmacokinetic Predictions
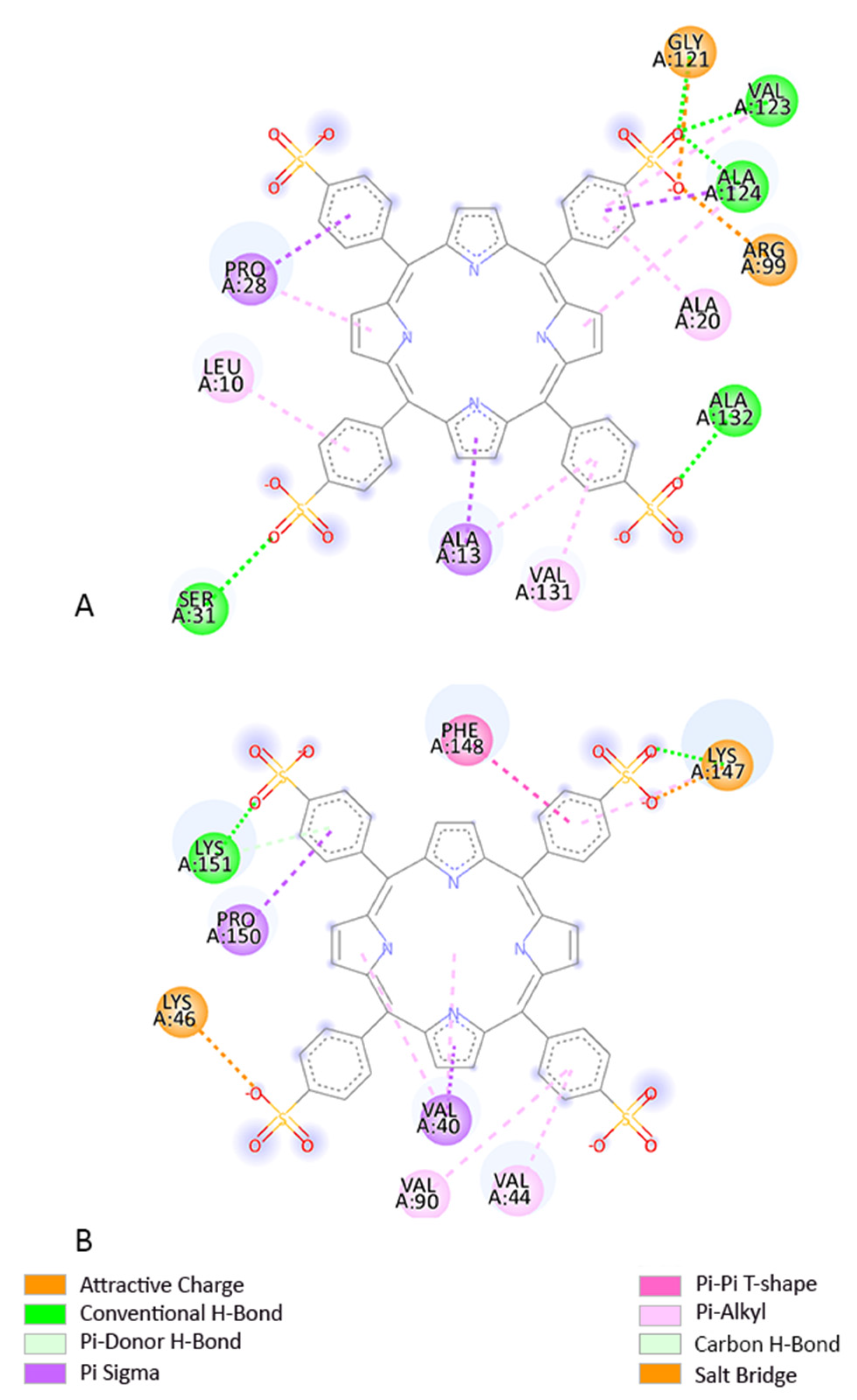
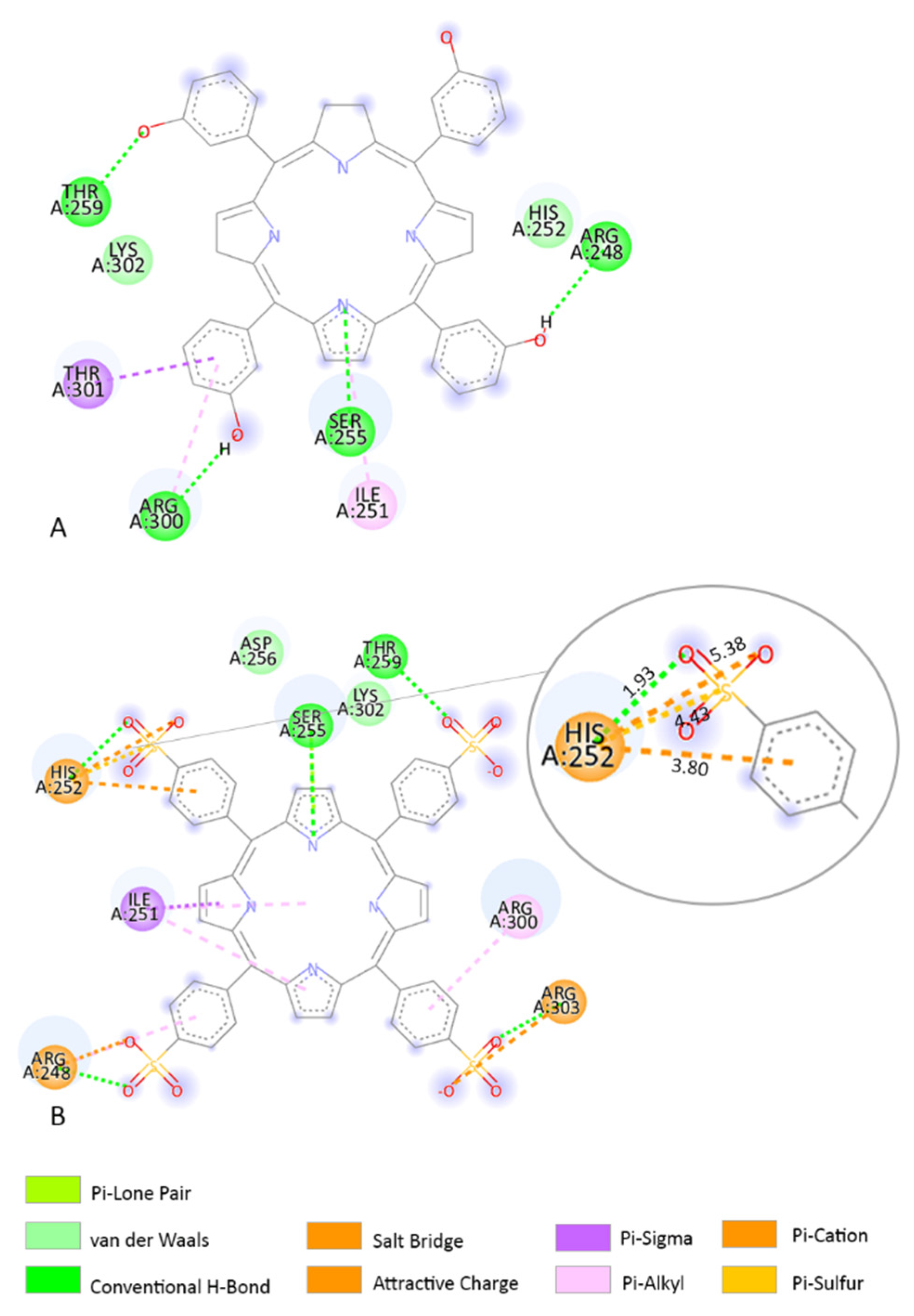
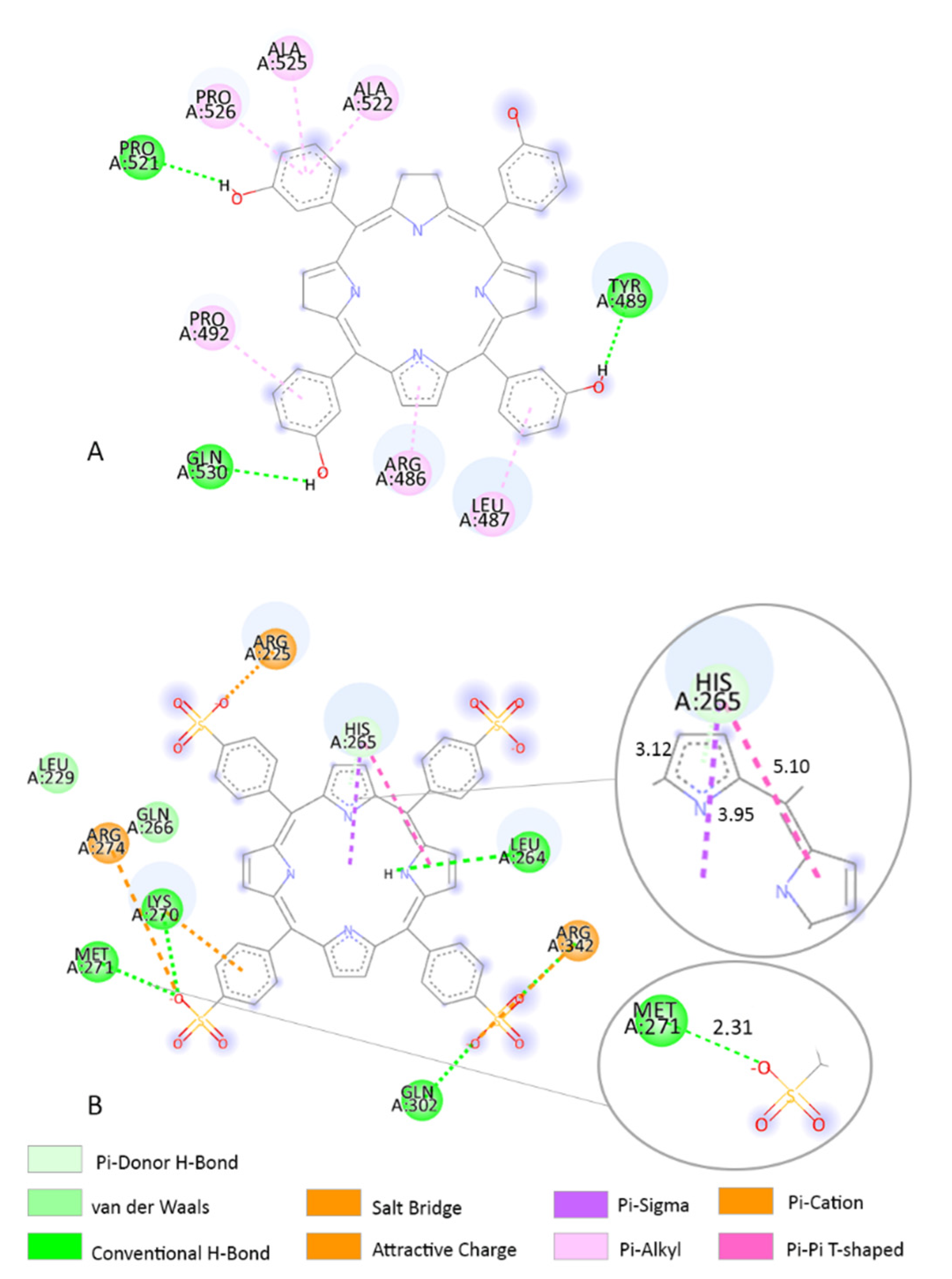
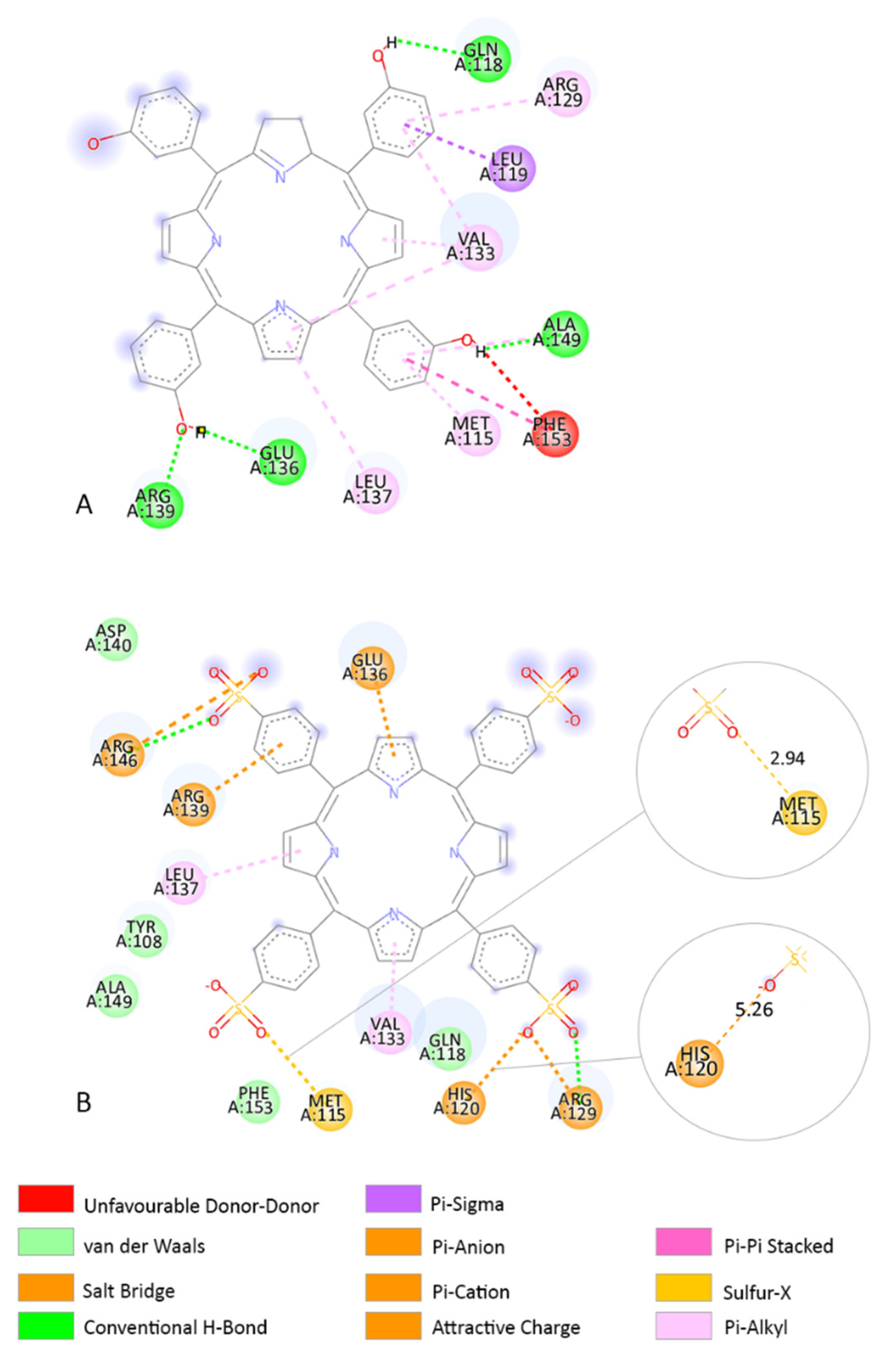
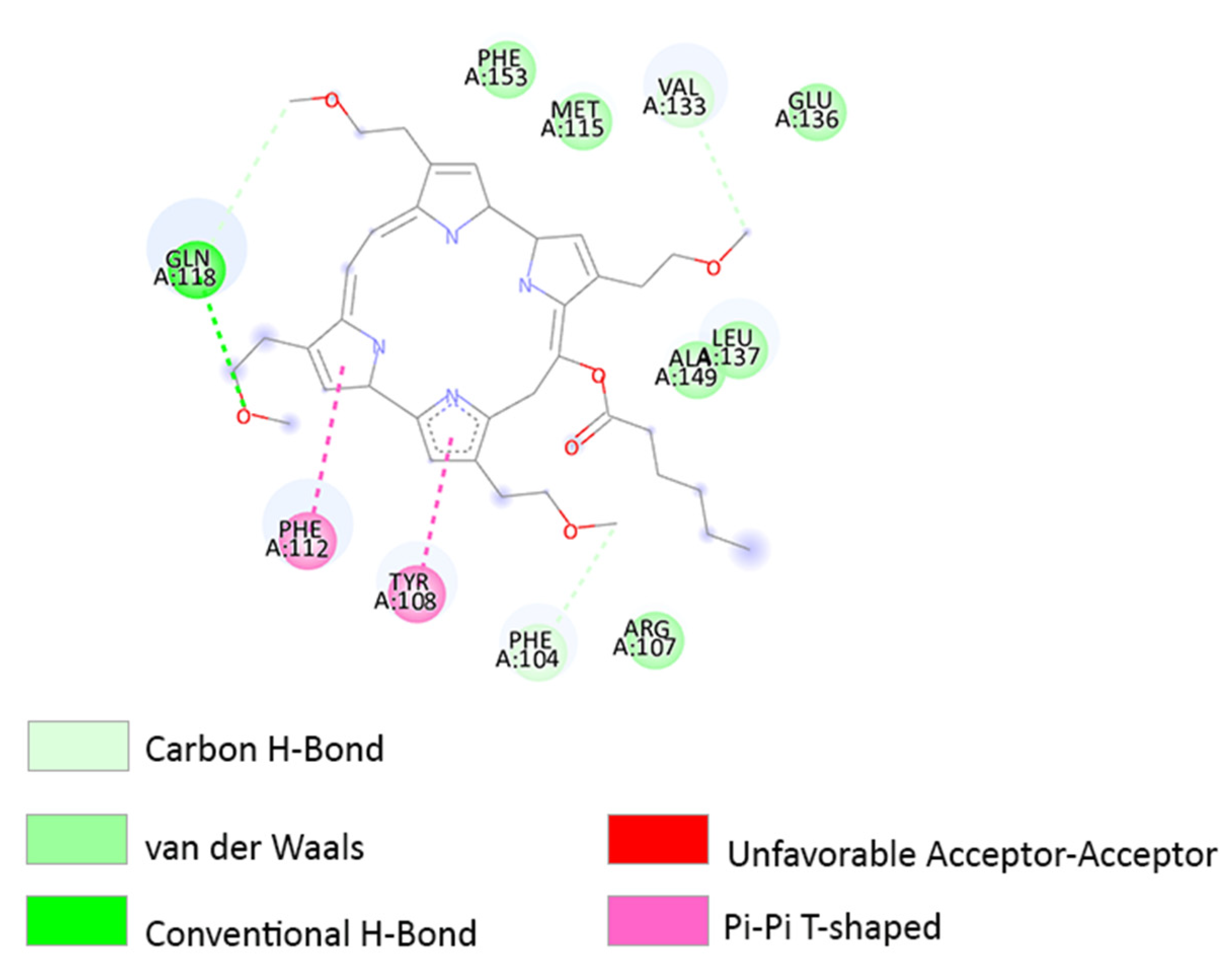
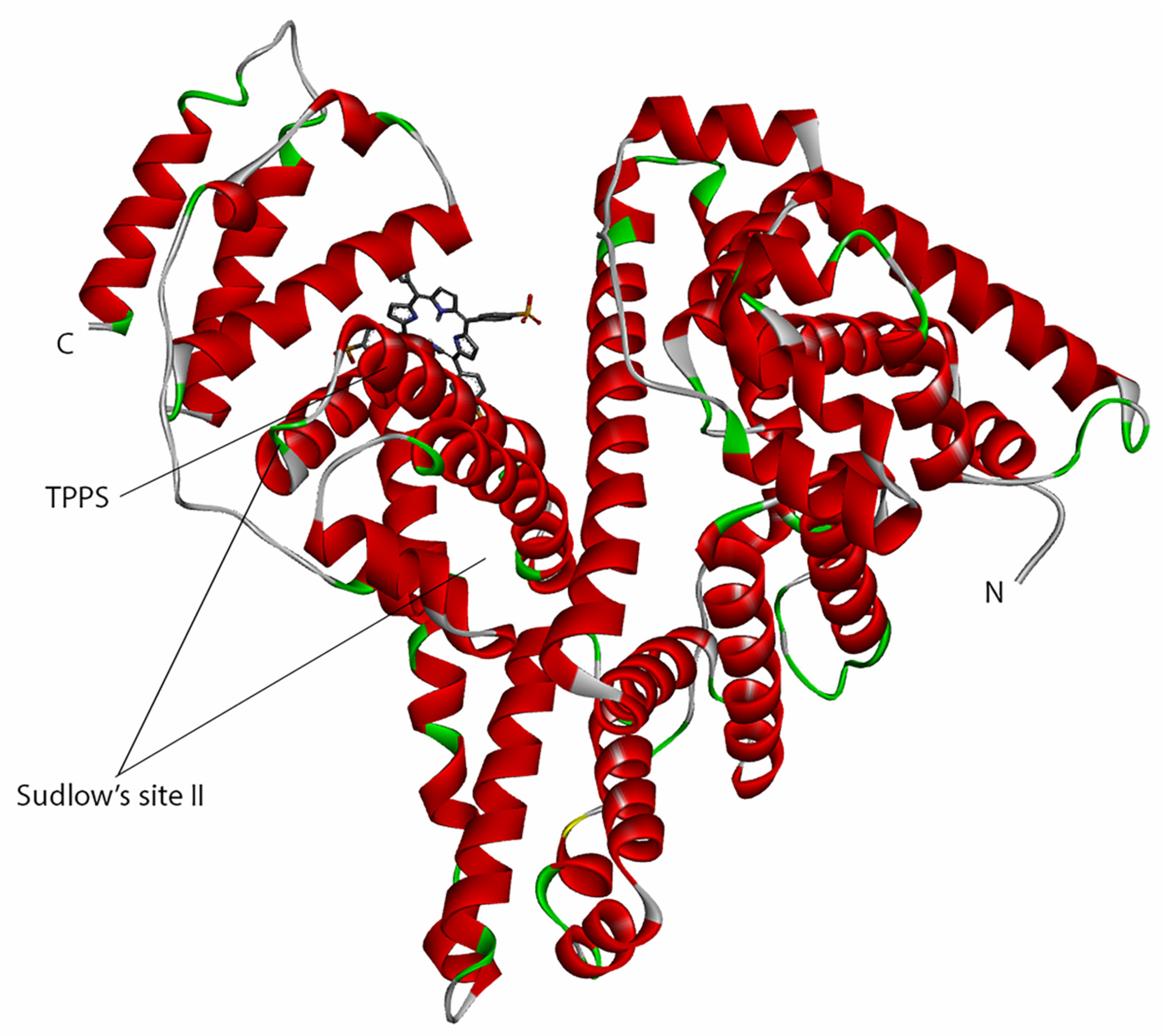
3.3. Molecular Docking
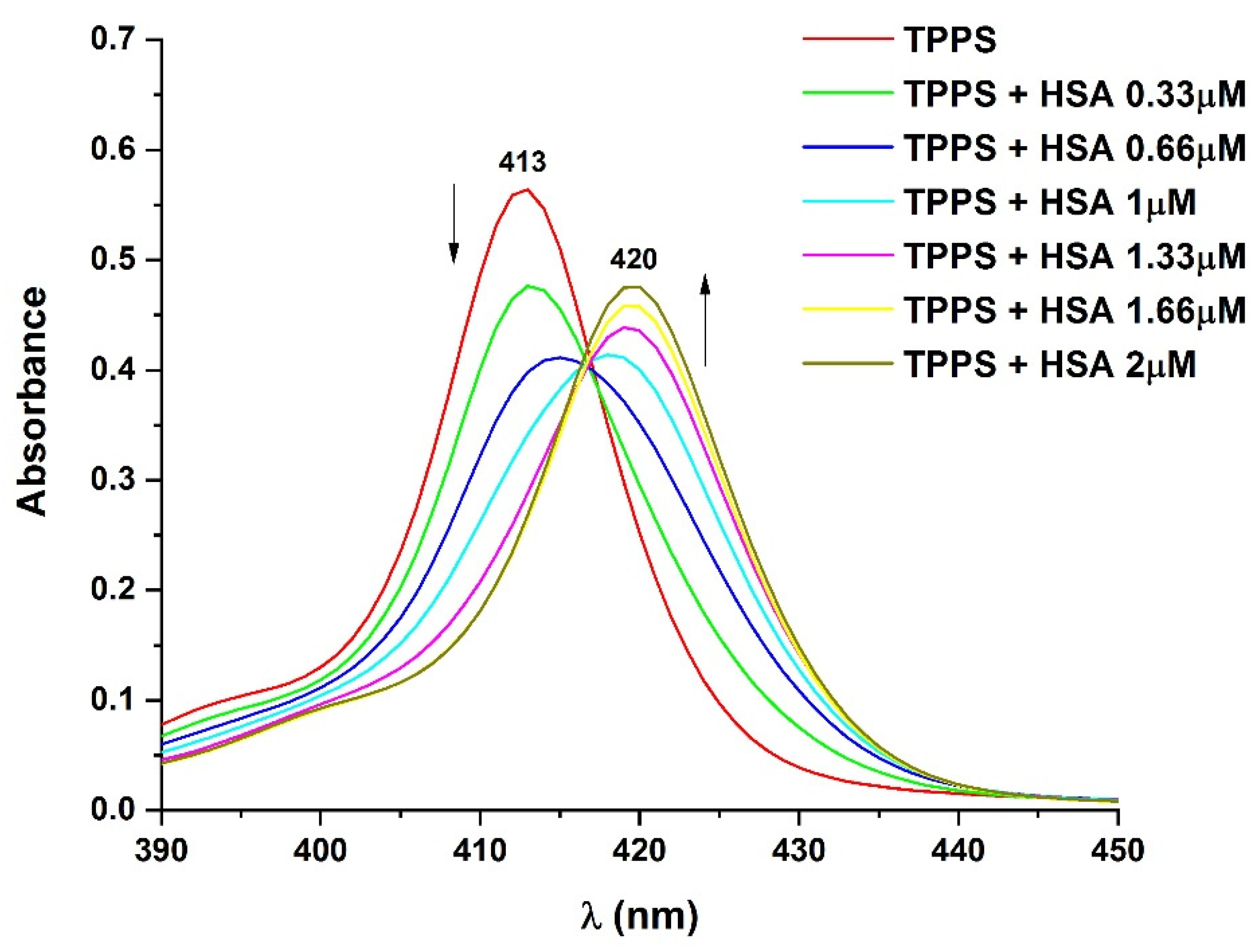
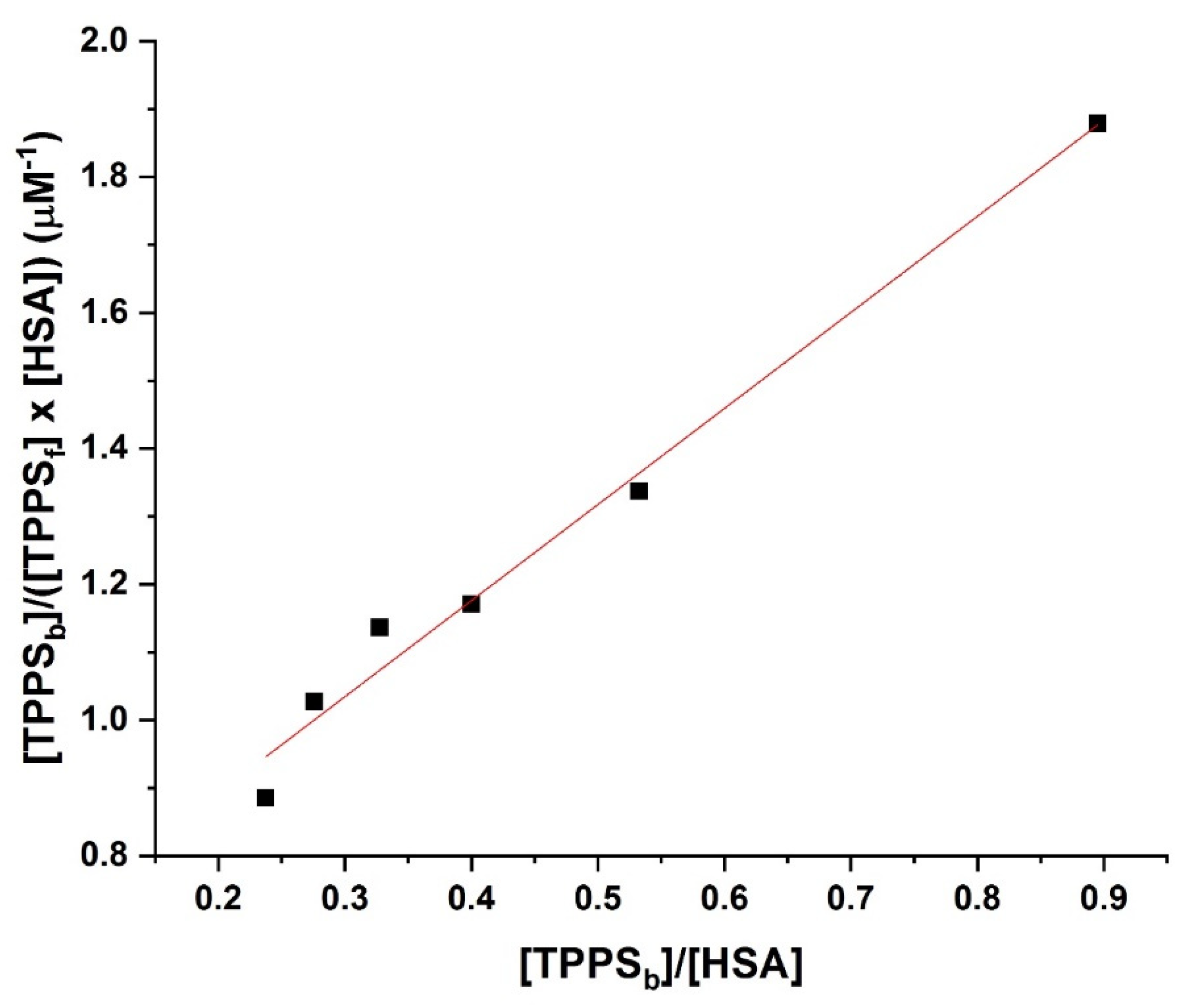
3.4. Determination of the Binding Affinity through UV–Vis Absorption Spectroscopy
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cancer Today. Available online: http://gco.iarc.fr/today/home (accessed on 25 January 2022).
- Naidoo, C.; Kruger, C.A.; Abrahamse, H. Photodynamic Therapy for Metastatic Melanoma Treatment: A Review. Technol. Cancer Res. Treat 2018, 17, 1533033818791795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baldea, I.; Filip, A.G. Photodynamic Therapy in Melanoma—An Update. J. Physiol. Pharmacol. 2012, 63, 109–118. [Google Scholar] [PubMed]
- Baldea, I.; Giurgiu, L.; Teacoe, I.D.; Olteanu, D.E.; Olteanu, F.C.; Clichici, S.; Filip, G.A. Photodynamic Therapy in Melanoma—Where Do We Stand? Curr. Med. Chem. 2018, 25, 5540–5563. [Google Scholar] [CrossRef] [PubMed]
- Agostinis, P.; Berg, K.; Cengel, K.A.; Foster, T.H.; Girotti, A.W.; Gollnick, S.O.; Hahn, S.M.; Hamblin, M.R.; Juzeniene, A.; Kessel, D.; et al. Photodynamic Therapy of Cancer: An Update. CA Cancer J. Clin. 2011, 61, 250–281. [Google Scholar] [CrossRef]
- Vo-Dinh, T. Biomedical Photonics Handbook; Oak Ridge National Laboratory: Oak Ridge, TN, USA, 2003; p. 984.
- Bellnier, D.A.; Greco, W.R.; Loewen, G.M.; Nava, H.; Oseroff, A.R.; Dougherty, T.J. Clinical Pharmacokinetics of the PDT Photosensitizers Porfimer Sodium (Photofrin), 2-[1-Hexyloxyethyl]-2-Devinyl Pyropheophorbide-a (Photochlor) and 5-ALA-Induced Protoporphyrin IX. Lasers Surg. Med. 2006, 38, 439–444. [Google Scholar] [CrossRef]
- Ethirajan, M.; Chen, Y.; Joshi, P.; Pandey, R.K. The Role of Porphyrin Chemistry in Tumor Imaging and Photodynamic Therapy. Chem. Soc. Rev. 2010, 40, 340–362. [Google Scholar] [CrossRef]
- Nistorescu, S.; Udrea, A.-M.; Badea, M.A.; Lungu, I.; Boni, M.; Tozar, T.; Dumitrache, F.; Maraloiu, V.-A.; Popescu, R.G.; Fleaca, C.; et al. Low Blue Dose Photodynamic Therapy with Porphyrin-Iron Oxide Nanoparticles Complexes: In Vitro Study on Human Melanoma Cells. Pharmaceutics 2021, 13, 2130. [Google Scholar] [CrossRef]
- Kwiatkowski, S.; Knap, B.; Przystupski, D.; Saczko, J.; Kędzierska, E.; Knap-Czop, K.; Kotlińska, J.; Michel, O.; Kotowski, K.; Kulbacka, J. Photodynamic Therapy-Mechanisms, Photosensitizers and Combinations. Biomed. Pharmacother. 2018, 106, 1098–1107. [Google Scholar] [CrossRef]
- Castano, A.P.; Demidova, T.N.; Hamblin, M.R. Mechanisms in Photodynamic Therapy: Part One-Photosensitizers, Photochemistry and Cellular Localization. Photodiagnosis Photodyn. Ther. 2004, 1, 279–293. [Google Scholar] [CrossRef] [Green Version]
- Aniogo, E.C.; George, B.P.A.; Abrahamse, H. Role of Bcl-2 Family Proteins in Photodynamic Therapy Mediated Cell Survival and Regulation. Molecules 2020, 25, 5308. [Google Scholar] [CrossRef]
- Broekgaarden, M.; Weijer, R.; van Gulik, T.M.; Hamblin, M.R.; Heger, M. Tumor Cell Survival Pathways Activated by Photodynamic Therapy: A Molecular Basis for Pharmacological Inhibition Strategies. Cancer Metastasis Rev. 2015, 34, 643–690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dougherty, T.J.; Gomer, C.J.; Henderson, B.W.; Jori, G.; Kessel, D.; Korbelik, M.; Moan, J.; Peng, Q. Photodynamic Therapy. JNCI J. Natl. Cancer Inst. 1998, 90, 889–905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sai, D.L.; Lee, J.; Nguyen, D.L.; Kim, Y.-P. Tailoring Photosensitive ROS for Advanced Photodynamic Therapy. Exp. Mol. Med. 2021, 53, 495–504. [Google Scholar] [CrossRef] [PubMed]
- Youle, R.J.; Strasser, A. The BCL-2 Protein Family: Opposing Activities That Mediate Cell Death. Nat. Rev. Mol. Cell Biol. 2008, 9, 47–59. [Google Scholar] [CrossRef]
- Kessel, D.; Castelli, M. Evidence That Bcl-2 Is the Target of Three Photosensitizers That Induce a Rapid Apoptotic Response¶. Photochem. Photobiol. 2001, 74, 318–322. [Google Scholar] [CrossRef]
- Mroz, P.; Yaroslavsky, A.; Kharkwal, G.B.; Hamblin, M.R. Cell Death Pathways in Photodynamic Therapy of Cancer. Cancers 2011, 3, 2516–2539. [Google Scholar] [CrossRef] [Green Version]
- Photodynamic Therapy with Redaporfin Targets the Endoplasmic Reticulum and Golgi Apparatus. EMBO J. 2018, 37, e98354. [CrossRef]
- Doo, D.W.; Meza-Perez, S.; Londoño, A.I.; Goldsberry, W.N.; Katre, A.A.; Boone, J.D.; Moore, D.J.; Hudson, C.T.; Betella, I.; McCaw, T.R.; et al. Inhibition of the Wnt/β-Catenin Pathway Enhances Antitumor Immunity in Ovarian Cancer. Ther. Adv. Med. Oncol. 2020, 12, 1758835920913798. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, N.; Gupta, S.; Feyes, D.K.; Mukhtar, H. Involvement of Fas (APO-1/CD-95) during Photodynamic-Therapy-Mediated Apoptosis in Human Epidermoid Carcinoma A431 Cells. J. Investig. Dermatol. 2000, 115, 1041–1046. [Google Scholar] [CrossRef] [Green Version]
- Avram, S.; Bologa, C.; Flonta, M.-L. Quantitative Structure-Activity Relationship by CoMFA for Cyclic Urea and Nonpeptide-Cyclic Cyanoguanidine Derivatives on Wild Type and Mutant HIV-1 Protease. J. Mol. Model. 2005, 11, 105–115. [Google Scholar] [CrossRef]
- Avram, S.; Buiu, C.; Duda-Seiman, D.; Duda-Seiman, C.; Borcan, F.; Mihailescu, D. Evaluation of the Pharmacological Descriptors Related to the Induction of Antidepressant Activity and Its Prediction by QSAR/QRAR Methods. Mini Rev. Med. Chem 2012, 12, 467–476. [Google Scholar] [CrossRef] [PubMed]
- Avram, S.; Svab, I.; Bologa, C.; Flonta, M.-L. Correlation between the Predicted and the Observed Biological Activity of the Symmetric and Nonsymmetric Cyclic Urea Derivatives Used as HIV-1 Protease Inhibitors. A 3D-QSAR-CoMFA Method for New Antiviral Drug Design. J. Cell. Mol. Med. 2003, 7, 287–296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bragina, M.E.; Daina, A.; Perez, M.A.S.; Michielin, O.; Zoete, V. The SwissSimilarity 2021 Web Tool: Novel Chemical Libraries and Additional Methods for an Enhanced Ligand-Based Virtual Screening Experience. Int. J. Mol. Sci. 2022, 23, 811. [Google Scholar] [CrossRef] [PubMed]
- Valerón Bergh, V.J.; Hjorth Tønnesen, H. Interaction between the Photosensitizer Lumichrome and Human Serum Albumin: Effect of Excipients. Pharm. Dev. Technol. 2017, 22, 992–1000. [Google Scholar] [CrossRef] [PubMed]
- Xiong, G.; Wu, Z.; Yi, J.; Fu, L.; Yang, Z.; Hsieh, C.; Yin, M.; Zeng, X.; Wu, C.; Lu, A.; et al. ADMETlab 2.0: An Integrated Online Platform for Accurate and Comprehensive Predictions of ADMET Properties. Nucleic Acids Res. 2021, 49, W5–W14. [Google Scholar] [CrossRef]
- Banerjee, P.; Dunkel, M.; Kemmler, E.; Preissner, R. SuperCYPsPred-a Web Server for the Prediction of Cytochrome Activity. Nucleic Acids Res. 2020, 48, W580–W585. [Google Scholar] [CrossRef]
- Nickel, J.; Gohlke, B.-O.; Erehman, J.; Banerjee, P.; Rong, W.W.; Goede, A.; Dunkel, M.; Preissner, R. SuperPred: Update on Drug Classification and Target Prediction. Nucleic Acids Res. 2014, 42, W26–W31. [Google Scholar] [CrossRef]
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. The Protein Data Bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef] [Green Version]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly Accurate Protein Structure Prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef]
- Varadi, M.; Anyango, S.; Deshpande, M.; Nair, S.; Natassia, C.; Yordanova, G.; Yuan, D.; Stroe, O.; Wood, G.; Laydon, A.; et al. AlphaFold Protein Structure Database: Massively Expanding the Structural Coverage of Protein-Sequence Space with High-Accuracy Models. Nucleic Acids Res. 2022, 50, D439–D444. [Google Scholar] [CrossRef]
- Abu-Dief, A.M.; Alotaibi, N.H.; S.Al-Farraj, E.; Qasem, H.A.; Alzahrani, S.; Mahfouz, M.K.; Abdou, A. Fabrication, Structural Elucidation, Theoretical, TD-DFT, Vibrational Calculation and Molecular Docking Studies of Some Novel Adenine Imine Chelates for Biomedical Applications. J. Mol. Liq. 2022, 365, 119961. [Google Scholar] [CrossRef]
- Alatawi, N.M.; Alsharief, H.H.; Alharbi, A.; Alhasani, M.; Attar, R.M.S.; Khalifa, M.E.; Abu-Dief, A.M.; El-Metwaly, N.M. Simulation for the Behavior of New Fe(III) and Cr(III)-Thiophenyl Complexes towards DNA Polymerase: Synthesis, Characterization, Eukaryotic DNA and Hartree–Fock Computation. Chem. Pap. 2022, 76, 3919–3935. [Google Scholar] [CrossRef]
- Kirthan, B.R.; Prabhakara, M.C.; Bhojya naik, H.S.; Viswanath, R.; Amith Nayak, P.H. Optoelectronic, Photocatalytic and Biological Studies of Mixed Ligand Cd(II) Complex and Its Fabricated CdO Nanoparticles. J. Mol. Struct. 2021, 1244, 130917. [Google Scholar] [CrossRef]
- Abu-Dief, A.M.; El-Metwaly, N.M.; Alzahrani, S.O.; Bawazeer, A.M.; Shaaban, S.; Adam, M.S.S. Targeting CtDNA Binding and Elaborated In-Vitro Assessments Concerning Novel Schiff Base Complexes: Synthesis, Characterization, DFT and Detailed in-Silico Confirmation. J. Mol. Liq. 2021, 322, 114977. [Google Scholar] [CrossRef]
- Open Babel: An Open Chemical Toolbox | Journal of Cheminformatics | Full Text. Available online: https://jcheminf.biomedcentral.com/articles/10.1186/1758-2946-3-33 (accessed on 16 March 2022).
- Morris, G.M.; Huey, R.; Olson, A.J. Using AutoDock for Ligand-Receptor Docking. In Current Protocols in Bioinformatics; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2008; Chapter 8, Unit 8.14. [Google Scholar] [CrossRef]
- Udrea, A.-M.; Dinache, A.; Pagès, J.-M.; Pirvulescu, R.A. Quinazoline Derivatives Designed as Efflux Pump Inhibitors: Molecular Modeling and Spectroscopic Studies. Molecules 2021, 26, 2374. [Google Scholar] [CrossRef] [PubMed]
- Ku, B.; Liang, C.; Jung, J.U.; Oh, B.-H. Evidence That Inhibition of BAX Activation by BCL-2 Involves Its Tight and Preferential Interaction with the BH3 Domain of BAX. Cell Res. 2011, 21, 627–641. [Google Scholar] [CrossRef]
- Rautureau, G.J.P.; Yabal, M.; Yang, H.; Huang, D.C.S.; Kvansakul, M.; Hinds, M.G. The Restricted Binding Repertoire of Bcl-B Leaves Bim as the Universal BH3-Only Prosurvival Bcl-2 Protein Antagonist. Cell Death Dis. 2012, 3, e443. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, Y.; Aikawa, K.; Nishida, G.; Homma, M.; Sogabe, S.; Igaki, S.; Hayano, Y.; Sameshima, T.; Miyahisa, I.; Kawamoto, T.; et al. Discovery of Potent Mcl-1/Bcl-XL Dual Inhibitors by Using a Hybridization Strategy Based on Structural Analysis of Target Proteins. J. Med. Chem. 2013, 56, 9635–9645. [Google Scholar] [CrossRef]
- Jenson, J.M.; Ryan, J.A.; Grant, R.A.; Letai, A.; Keating, A.E. Epistatic Mutations in PUMA BH3 Drive an Alternate Binding Mode to Potently and Selectively Inhibit Anti-Apoptotic Bfl-1. eLife 2017, 6, e25541. [Google Scholar] [CrossRef]
- Lee, E.F.; Dewson, G.; Smith, B.J.; Evangelista, M.; Pettikiriarachchi, A.; Dogovski, C.; Perugini, M.A.; Colman, P.M.; Fairlie, W.D. Crystal Structure of a BCL-W Domain-Swapped Dimer: Implications for the Function of BCL-2 Family Proteins. Structure 2011, 19, 1467–1476. [Google Scholar] [CrossRef] [Green Version]
- Graham, T.A.; Clements, W.K.; Kimelman, D.; Xu, W. The Crystal Structure of the β-Catenin/ICAT Complex Reveals the Inhibitory Mechanism of ICAT. Mol. Cell 2002, 10, 563–571. [Google Scholar] [CrossRef]
- Müller, C.W.; Rey, F.A.; Sodeoka, M.; Verdine, G.L.; Harrison, S.C. Structure of the NF-ΚB P50 Homodimer Bound to DNA. Nature 1995, 373, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Eberstadt, M.; Olejniczak, E.T.; Meadows, R.P.; Fesik, S.W. NMR Structure and Mutagenesis of the Fas (APO-1/CD95) Death Domain. Nature 1996, 384, 638–641. [Google Scholar] [CrossRef] [PubMed]
- Wardell, M.; Wang, Z.; Ho, J.X.; Robert, J.; Ruker, F.; Ruble, J.; Carter, D.C. The Atomic Structure of Human Methemalbumin at 1.9 Å. Biochem. Biophys. Res. Commun. 2002, 291, 813–819. [Google Scholar] [CrossRef]
- Senge, M.O.; Brandt, J.C. Temoporfin (Foscan®, 5,10,15,20-Tetra(m-Hydroxyphenyl)Chlorin)—A Second-Generation Photosensitizer†,‡. Photochem. Photobiol. 2011, 87, 1240–1296. [Google Scholar] [CrossRef]
- Nistorescu, S.; Gradisteanu Pircalabioru, G.; Udrea, A.-M.; Simon, A.; Pascu, M.L.; Chifiriuc, M.-C. Laser-Irradiated Chlorpromazine as a Potent Anti-Biofilm Agent for Coating of Biomedical Devices. Coatings 2020, 10, 1230. [Google Scholar] [CrossRef]
- Kastritis, P.L.; Bonvin, A.M.J.J. On the Binding Affinity of Macromolecular Interactions: Daring to Ask Why Proteins Interact. J. R. Soc. Interface 2013, 10, 20120835. [Google Scholar] [CrossRef]
- Hirose, K. A Practical Guide for the Determination of Binding Constants. J. Incl. Phenom. Macrocycl. Chem. 2001, 39, 193–209. [Google Scholar] [CrossRef]
- Zhang, Y.; Görner, H. Photoprocesses of Xanthene Dyes Bound to Lysozyme or Serum Albumin. Photochem. Photobiol. 2009, 85, 677–685. [Google Scholar] [CrossRef]
- Rachakonda, S.; Kong, H.; Srinivas, N.; Garcia-Casado, Z.; Requena, C.; Fallah, M.; Heidenreich, B.; Planelles, D.; Traves, V.; Schadendorf, D.; et al. Telomere Length, Telomerase Reverse Transcriptase Promoter Mutations, and Melanoma Risk. Genes Chromosom. Cancer 2018, 57, 564–572. [Google Scholar] [CrossRef]
- Heidenreich, B.; Nagore, E.; Rachakonda, P.S.; Garcia-Casado, Z.; Requena, C.; Traves, V.; Becker, J.; Soufir, N.; Hemminki, K.; Kumar, R. Telomerase Reverse Transcriptase Promoter Mutations in Primary Cutaneous Melanoma. Nat. Commun. 2014, 5, 3401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benov, L. Photodynamic Therapy: Current Status and Future Directions. MPP 2015, 24, 14–28. [Google Scholar] [CrossRef] [PubMed]
- Vicente-Escobar, J.O.; García-Sánchez, M.Á.; Serratos, I.N.; Millán-Pacheco, C.; Tello-Solís, S.R. Binding of Two Tetrasulfophthalocyanines (Fe(III) and Metal-Free) to Lysozyme: Fluorescence Spectroscopic and Computational Approach. J. Fluoresc. 2021, 31, 787–796. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.F.; Harris, T.J.; Tran, S.; Evangelista, M.; Arulananda, S.; John, T.; Ramnac, C.; Hobbs, C.; Zhu, H.; Gunasingh, G.; et al. BCL-XL and MCL-1 Are the Key BCL-2 Family Proteins in Melanoma Cell Survival. Cell Death Dis. 2019, 10, 342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xue, L.; Chiu, S.; Oleinick, N.L. Differential Responses of Mcl-1 in Photosensitized Epithelial vs Lymphoid-Derived Human Cancer Cells. Oncogene 2005, 24, 6987–6992. [Google Scholar] [CrossRef] [Green Version]
- Song, J.; Chen, Q.; Xing, D. Enhanced Apoptotic Effects by Downregulating Mcl-1: Evidence for the Improvement of Photodynamic Therapy with Celecoxib. Exp. Cell Res. 2013, 319, 1491–1504. [Google Scholar] [CrossRef]
- Song, J.; Li, Y.; Wei, Y. Effect of the Prosurvival Protein MCL-1 on Photodynamic Therapy Induced Apoptosis. In Proceedings of the Seventh International Conference on Photonics and Imaging in Biology and Medicine, Wuhan, China, 11 December 2008; p. 72801T. [Google Scholar]
- Aledo, J.C.; Cantón, F.R.; Veredas, F.J. Sulphur Atoms from Methionines Interacting with Aromatic Residues Are Less Prone to Oxidation. Sci. Rep. 2015, 5, 16955. [Google Scholar] [CrossRef] [Green Version]
- Xue, L.; Chiu, S.; Oleinick, N.L. Photochemical Destruction of the Bcl-2 Oncoprotein during Photodynamic Therapy with the Phthalocyanine Photosensitizer Pc 4. Oncogene 2001, 20, 3420–3427. [Google Scholar] [CrossRef] [Green Version]
- Srivastava, M.; Ahmad, N.; Gupta, S.; Mukhtar, H. Involvement of Bcl-2 and Bax in Photodynamic Therapy-Mediated Apoptosis: Antisense Bcl-2 Oligonucleotide Sensitizes Rif 1 Cells to Photodynamic Therapy Apoptosis*. J. Biol. Chem. 2001, 276, 15481–15488. [Google Scholar] [CrossRef]
- Choi Kim, H.-R.; Luo, Y.; Li, G.; Kessel, D. Enhanced Apoptotic Response to Photodynamic Therapy after Bcl-2 Transfection. Cancer Res. 1999, 59, 3429–3432. [Google Scholar]
- Donohoe, C.; Senge, M.O.; Arnaut, L.G.; Gomes-da-Silva, L.C. Cell Death in Photodynamic Therapy: From Oxidative Stress to Anti-Tumor Immunity. Biochim. Et Biophys. Acta (BBA)—Rev. Cancer 2019, 1872, 188308. [Google Scholar] [CrossRef] [PubMed]
- Guevara, J.; Zahran, M.; Samaroo, D. Computational Studies of Glycosylated Photosensitizers with Plasma Proteins. FASEB J. 2020, 34, 1. [Google Scholar] [CrossRef]
- Zheng, L.; He, Y.; Lin, P.; Liu, L.; Yang, H.; Peng, Y.; Xie, S. Spectroscopic Analysis of the Interaction between Tetra-( p -Sulfoazophenyl-4-Aminosulfonyl)-Substituted Aluminum (III) Phthalocyanines and Serum Albumins. J. Innov. Opt. Health Sci. 2017, 10, 1650043. [Google Scholar] [CrossRef] [Green Version]
- Szafraniec, M.J. Interactions of Chlorophyll-Derived Photosensitizers with Human Serum Albumin Are Determined by the Central Metal Ion. J. Biomol. Struct. Dyn. 2021, 1–14. [Google Scholar] [CrossRef]
- Vicente-Escobar, J.O.; García-Sánchez, M.A.; González, F.; Cipagauta-Díaz, S.; Estrella González, A. A Spectroscopic and Molecular Docking Study of Interactions of Tetracarboxyphenyl Porphyrin and Chlorin E6 with Bovine Serum Albumin. Chem. Pap. 2021, 75, 4501–4515. [Google Scholar] [CrossRef]
| Compound Name and SMILES Code | 2D Structure |
|---|---|
| TPPS: O=S(=O)([O-])c9ccc(c7c1ccc(n1)c(c2ccc(S(=O)(=O)[O-])cc2)c3ccc([nH]3)c(c4ccc(S(=O)(=O)[O-])cc4)c5ccc(n5)c(c6ccc(S(=O)(=O)[O-])cc6)c8ccc7[nH]8)cc9 | 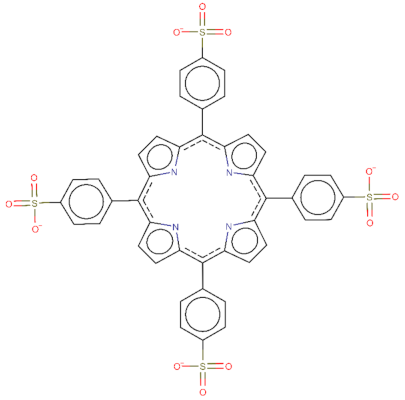 |
| Temoporfin: C1CC2=NC1=C(C3=CC=C(N3)C(=C4C=CC(=N4)C(=C5C=CC(=C2C6=CC(=CC=C6)O)N5)C7=CC(=CC=C7)O)C8=CC(=CC=C8)O)C9=CC(=CC=C9)O | 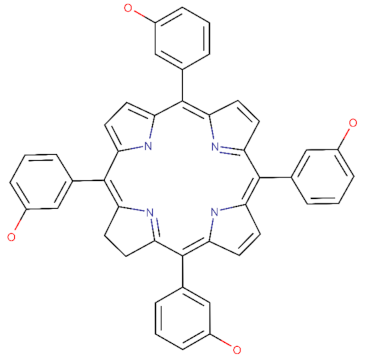 |
| CPO: O(C(=O)CCCCC)\C\1=C/2\NC(C=C\2CCOC)C2N=C(\C=C/c3[nH]c(cc3CCOC)C3=N\C(=C/1)\C(=C3)CCOC)C(=C2)CCOC | 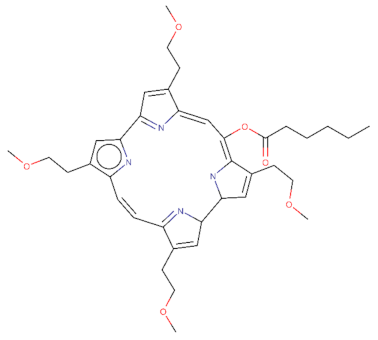 |
| Target | Fingerprint | TPPS | Temoporfin | CPO | |||
|---|---|---|---|---|---|---|---|
| Pred | Prob | Pred | Prob | Pred | Prob | ||
| CYP1A2 | MACCS | Inactive | 0.799 | inactive | 0.599 | Inactive | 0.771 |
| CYP1A2 | Morgan | Inactive | 0.563 | inactive | 0.531 | Inactive | 0.768 |
| CYP2C19 | MACCS | Inactive | 0.698 | inactive | 0.547 | Inactive | 0.779 |
| CYP2C19 | Morgan | Inactive | 0.829 | inactive | 0.847 | Inactive | 0.729 |
| CYP2C9 | MACCS | Active | 0.628 | active | 0.571 | Inactive | 0.607 |
| CYP2C9 | Morgan | Inactive | 0.6 | inactive | 0.673 | Inactive | 0.782 |
| CYP2D6 | MACCS | Inactive | 0.762 | inactive | 0.612 | Inactive | 0.59 |
| CYP2D6 | Morgan | Inactive | 0.802 | inactive | 0.509 | Inactive | 0.548 |
| CYP3A4 | MACCS | Inactive | 0.737 | inactive | 0.598 | Inactive | 0.698 |
| CYP3A4 | Morgan | Inactive | 0.711 | inactive | 0.623 | Inactive | 0.504 |
| Temoporfin | |||
|---|---|---|---|
| Target Name | Therapeutic Indication | Prob | Accuracy |
| DNA (apurinic or apyrimidinic site) lyase | Glioma, melanoma, ocular cancer, solid tumour/cancer | 96.37% | 91.11% |
| Beta-1 adrenergic receptor | Melanoma | 83.6% | 95.56% |
| C-X-C chemokine receptor type 4 | Acute lymphoblastic leukaemia, acute myeloid leukaemia, B-cell chronic lymphocytic leukaemia, breast cancer, haematological malignancy, melanoma, Merkel cell carcinoma, multiple myeloma, myelodysplastic syndrome, non-Hodgkin’s lymphoma pancreatic cancer, renal cell carcinoma, sarcoma solid tumour/cancer | 76.64% | 93.1% |
| Galectin-3 | Melanoma | 73.88% | 96.9% |
| Toll-like receptor 8 | Melanoma, solid tumour/cancer | 55.13% | 96.25% |
| TPPS | |||
| Target Name | Therapeutic indication | Prob | Accuracy |
| Telomerase reverse transcriptase | Acute myeloid leukaemia, brain cancer, breast cancer, head and neck cancer, liver cancer, melanoma, multiple myeloma, non-small-cell lung cancer, ovarian cancer, pancreatic cancer, prostate cancer, solid tumour/cancer | 79% | 90% |
| C-X-C chemokine receptor type 4 | Acute lymphoblastic leukaemia, acute myeloid leukaemia, B-cell chronic lymphocytic leukaemia, breast cancer, haematological malignancy, melanoma, Merkel cell carcinoma, multiple myeloma, myelodysplastic syndrome, non-Hodgkin’s lymphoma pancreatic cancer, renal cell carcinoma, sarcoma solid tumour/cancer | 74% | 93.1% |
| Histone deacetylase 1 | Acute myeloid leukaemia, breast cancer, colorectal cancer, cutaneous T-cell lymphoma, diffuse large B-cell lymphoma, hepatocellular carcinoma, leukaemia, melanoma, Merkel cell carcinoma, multiple myeloma, non-small-cell lung cancer, ovarian cancer, peripheral T-cell lymphoma, renal cell carcinoma, solid tumour/cancer | 60% | 96% |
| Galectin-3 | Melanoma | 55% | 96.9% |
| Prediction Probability | TPPS | Temoporfin | CPO |
|---|---|---|---|
| hERG blockers | 0.3–0.5 (−) | 0.3–0.5 (−) | 0.1–0.3 (−−) |
| Human hepatotoxicity | 0.9–1.0 (+++) | 0.1–0.3 (−−) | 0.3–0.5 (−) |
| Drug-induced liver injury | 0.1–0.3 (−−) | 0.9–1.0 (+++) | 0.9–1.0 (+++) |
| Carcinogenicity | 0.3–0.5 (−) | 0–0.1 (−−−) | 0.9–1.0 (+++) |
| Respiratory toxicity | 0.9–1.0 (+++) | 0.9–1.0 (+++) | 0.9–1.0 (+++) |
| Androgen receptor ligand-binding domain | 0–0.1 (−−−) | 0.1–0.3 (−−) | 0–0.1 (−−−) |
| Aryl hydrocarbon receptor | 0.3–0.5 (−) | 0.7–0.9 (++) | 0.9–1.0 (+++) |
| Oestrogen receptor ligand-binding domain | 0.7–0.9 (++) | 0.9–1.0 (+++) | 0.7–0.9 (++) |
| Peroxisome proliferator-activated receptor gamma | 0–0.1 (−−−) | 0.3–0.5 (−) | 0–0.1 (−−−) |
| Heat-shock factor response element | 0–0.1 (−−−) | 0.7–0.9 (++) | 0–0.1 (−−−) |
| Mitochondrial membrane potential | 0.7–0.9 (++) | 0.9–1.0 (+++) | 0.5–0.7 (+) |
| P53 | 0.5–0.7 (+) | 0.9–1.0 (+++) | 0.7–0.9 (+++) |
| Acute-toxicity rule | 0 alert | 0 alert | 0 alert |
| Target | TPPS EFEB (kcal/mol) | TPPS KI (nM) | Temoporfin EFEB (kcal/mol) | Temoporfin KI (nM) |
|---|---|---|---|---|
| BCL-2 | −7.90 | 1610 | −10.26 | 30.08 |
| BCL-B | −11.49 | 3.76 | −10.83 | 11.55 |
| BCL-xL | −8.81 | 349.53 | −9.19 | 184.88 |
| MCl-1 | −9.95 | 51.27 | −9.23 | 171.65 |
| A1 | −14.99 | 0.01023 | −10.63 | 16.05 |
| BCL-W | −9.05 | 233.04 | −9.92 | 53.25 |
| β-catenin | −9.81 | 64.39 | −7.35 | 4070 |
| NFKB | −10.92 | 9.78 | −9.84 | 61.66 |
| Fas | −7.69 | 2290 | −10.20 | 33.26 |
| EIF2AK1 | −10.99 | 8.77 | −11.44 | 4.13 |
| HSA | −8.35 | 761.92 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Udrea, A.-M.; Dinache, A.; Staicu, A.; Avram, S. Target Prediction of 5,10,15,20-Tetrakis(4′-Sulfonatophenyl)-Porphyrin Using Molecular Docking. Pharmaceutics 2022, 14, 2390. https://doi.org/10.3390/pharmaceutics14112390
Udrea A-M, Dinache A, Staicu A, Avram S. Target Prediction of 5,10,15,20-Tetrakis(4′-Sulfonatophenyl)-Porphyrin Using Molecular Docking. Pharmaceutics. 2022; 14(11):2390. https://doi.org/10.3390/pharmaceutics14112390
Chicago/Turabian StyleUdrea, Ana-Maria, Andra Dinache, Angela Staicu, and Speranta Avram. 2022. "Target Prediction of 5,10,15,20-Tetrakis(4′-Sulfonatophenyl)-Porphyrin Using Molecular Docking" Pharmaceutics 14, no. 11: 2390. https://doi.org/10.3390/pharmaceutics14112390
APA StyleUdrea, A.-M., Dinache, A., Staicu, A., & Avram, S. (2022). Target Prediction of 5,10,15,20-Tetrakis(4′-Sulfonatophenyl)-Porphyrin Using Molecular Docking. Pharmaceutics, 14(11), 2390. https://doi.org/10.3390/pharmaceutics14112390







