Itraconazole Amorphous Solid Dispersion Tablets: Formulation and Compaction Process Optimization Using Quality by Design Principles and Tools
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Hot-Melt Extrusion (HME)
2.2.2. ITZ-KOL Amorphous Solid Dispersions
2.3. Compaction of ITZ-KOL-ASDs
2.4. Quality by Design Principles and Tools
2.4.1. Quality Target Product Profile (QTPP)
2.4.2. Identification of CQAs, CMAs and CPPs of the Tableting Process
2.4.3. Risk Assessment
2.4.4. ITZ-KOL-ASD Tablet Formulation Development
2.4.5. Design of Experiments (DoEs)
2.5. Characterisation of Powders and Tablets
2.5.1. True Density
2.5.2. USP1062 Plots
2.5.3. Hardness TESTING
2.5.4. Disintegration
2.5.5. Friability
2.5.6. Dissolution
2.5.7. Scanning Electron Microscopy (SEM)
3. Results
3.1. Characterisation of ITZ-KOL-ASD and Excipients
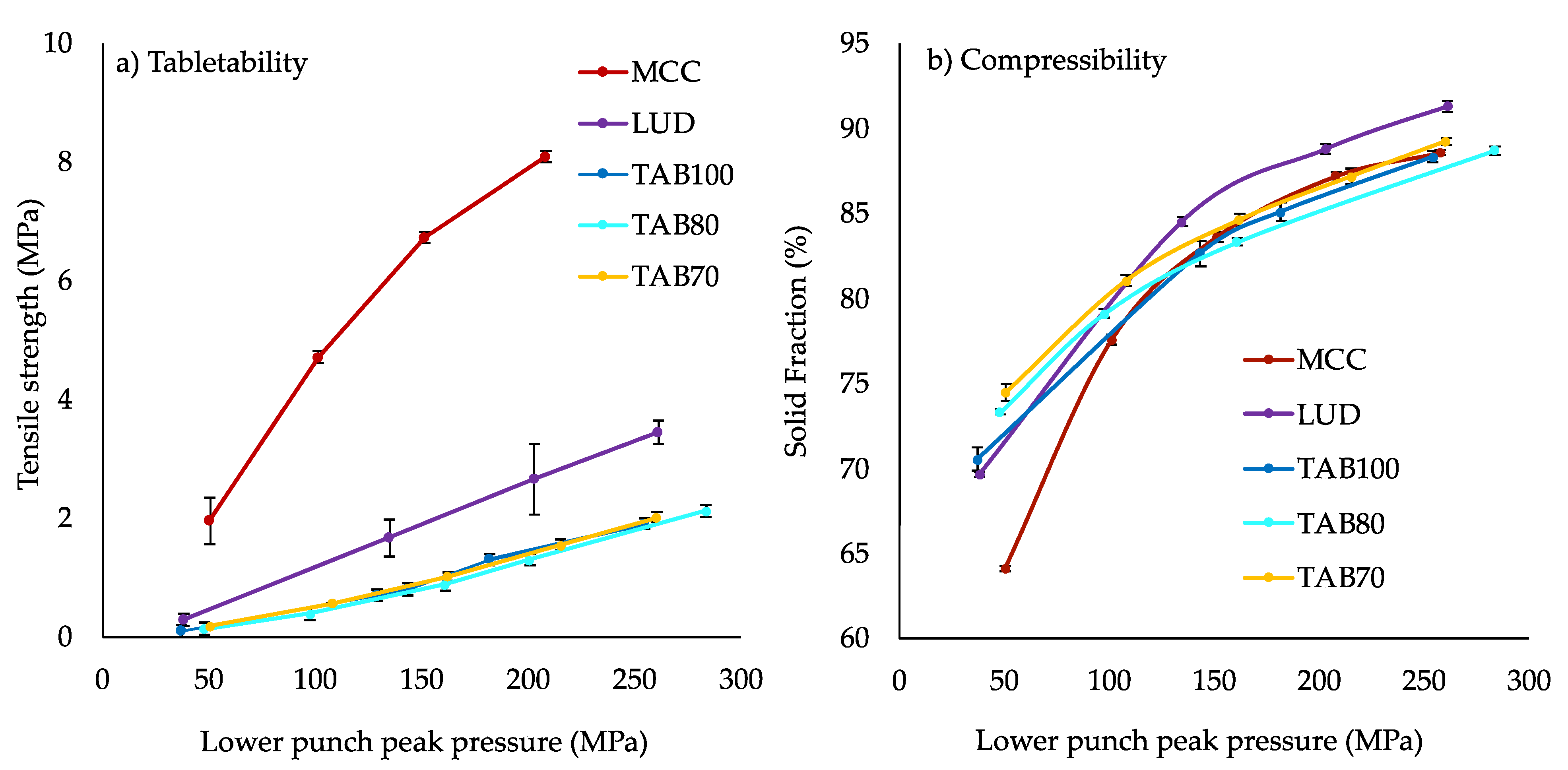
3.1.1. Compaction of ITZ-KOL-ASDs and Their Physical Mixture
3.1.2. Compaction of Pure Excipients
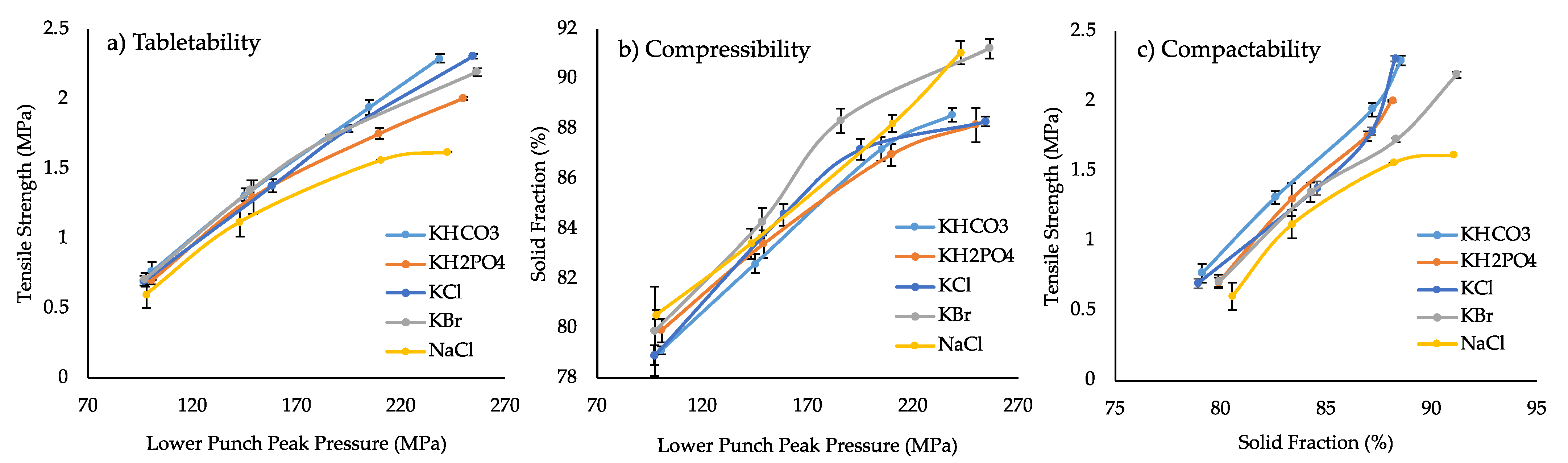
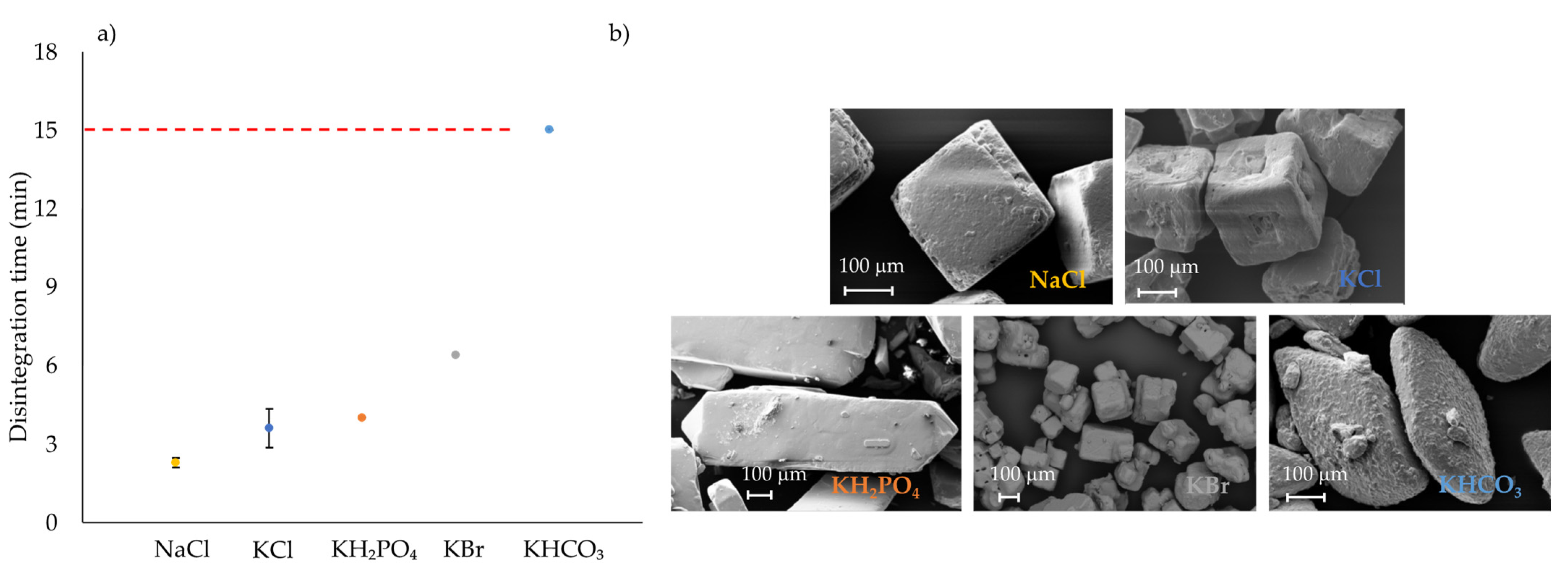
3.1.3. Compaction and Disintegration of Formulations Containing Inorganic Salts
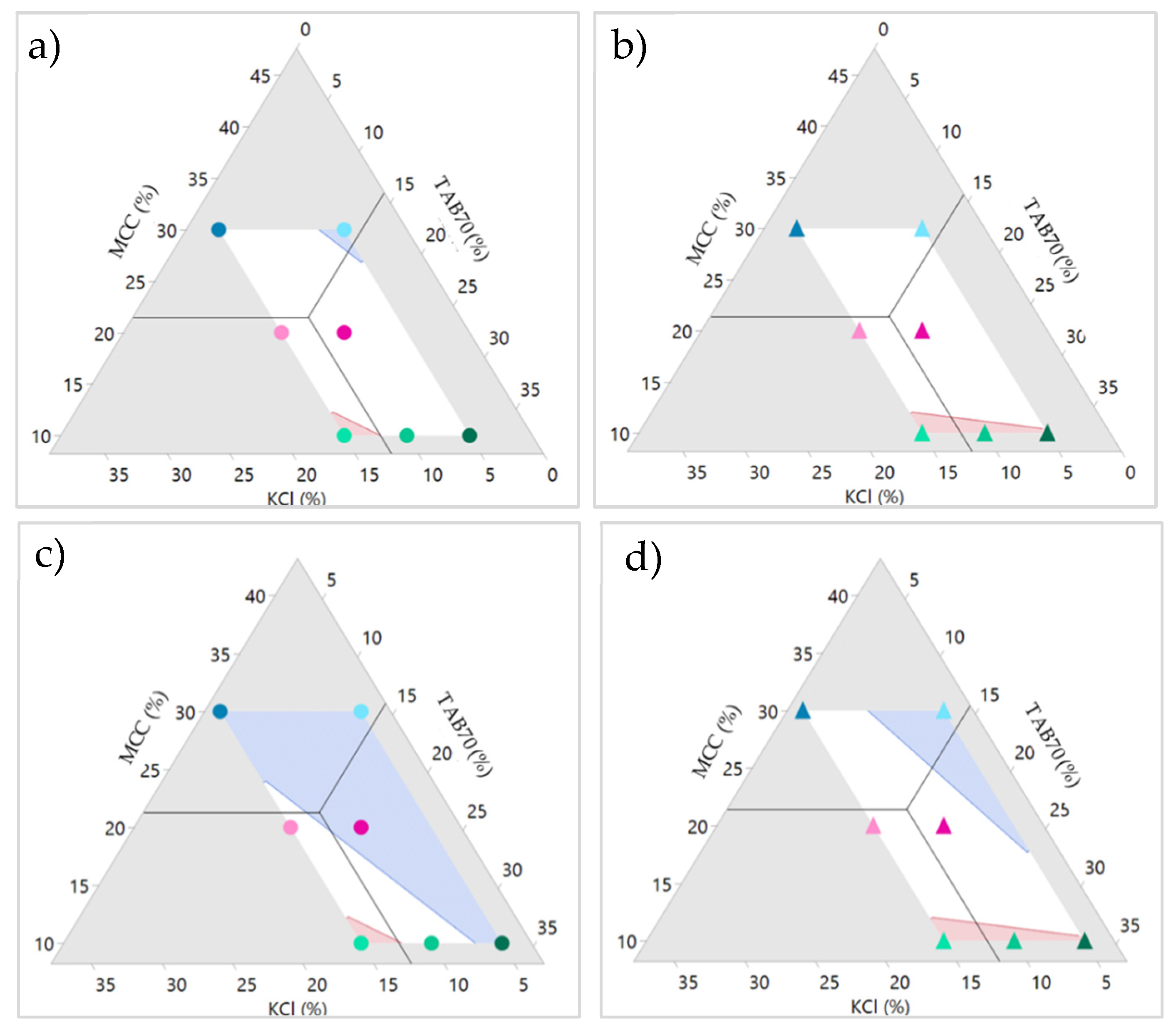
3.2. Formulation Mixture Design
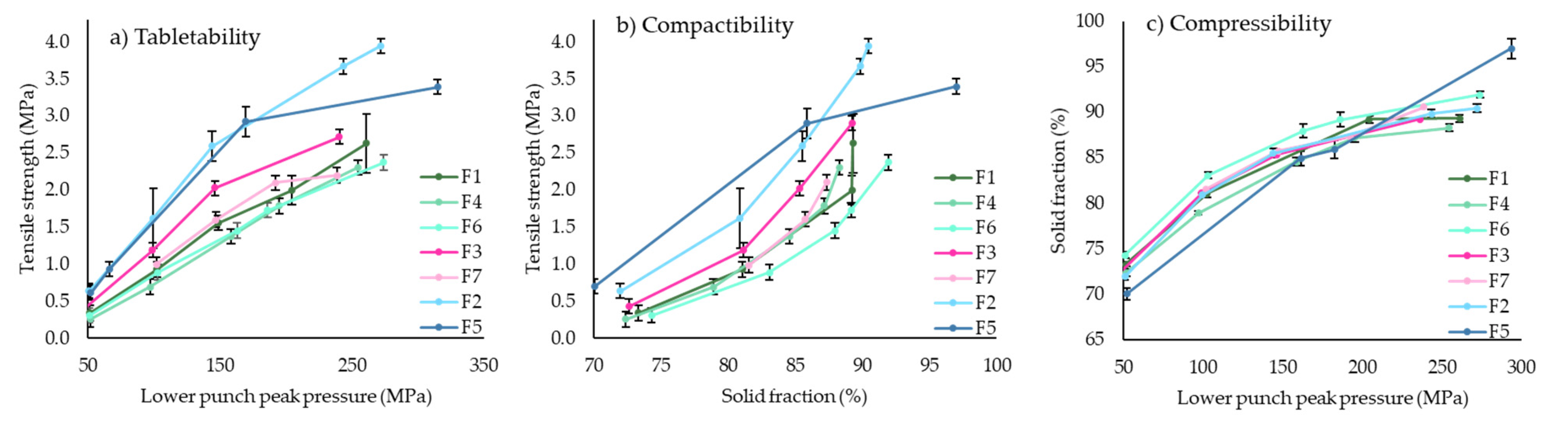
3.2.1. Compaction of Round Tablets
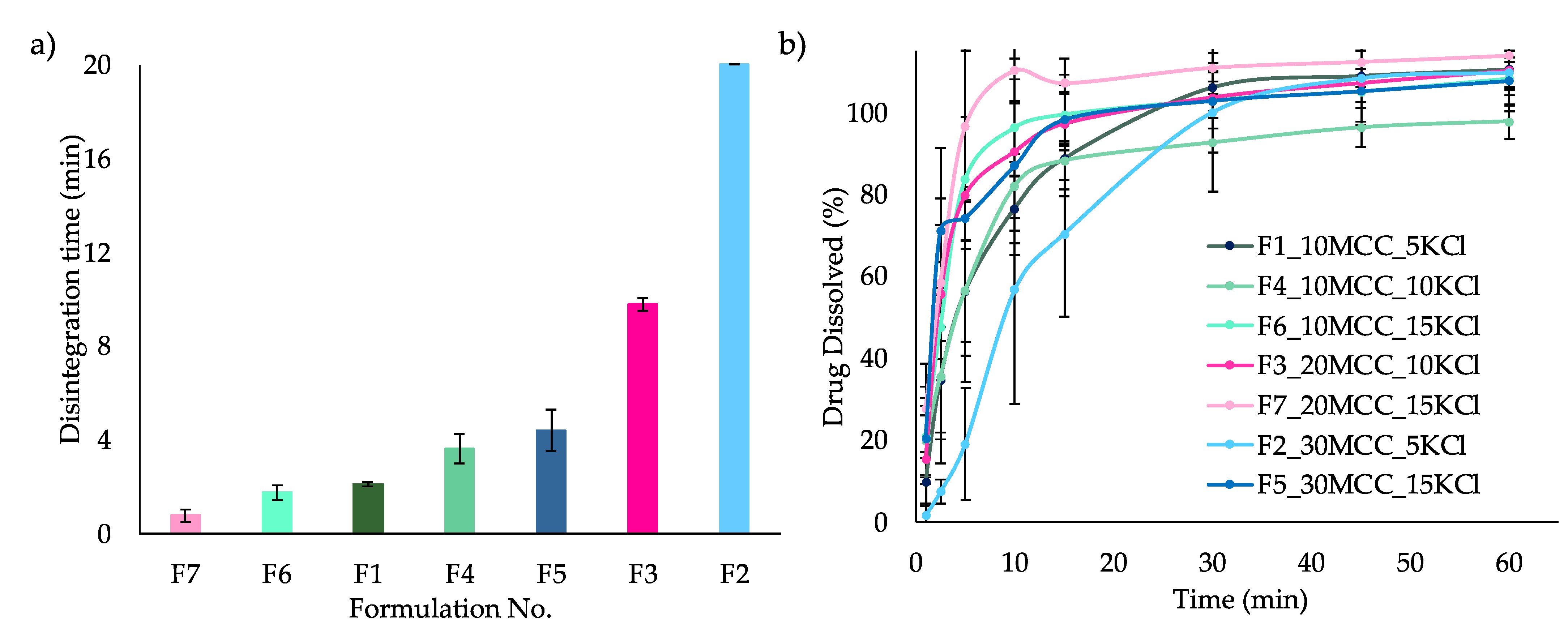
3.2.2. Disintegration and Dissolution of ITZ-KOL-ASD Round Tablets
3.3. Round Versus Oblong ITZASD Tablets
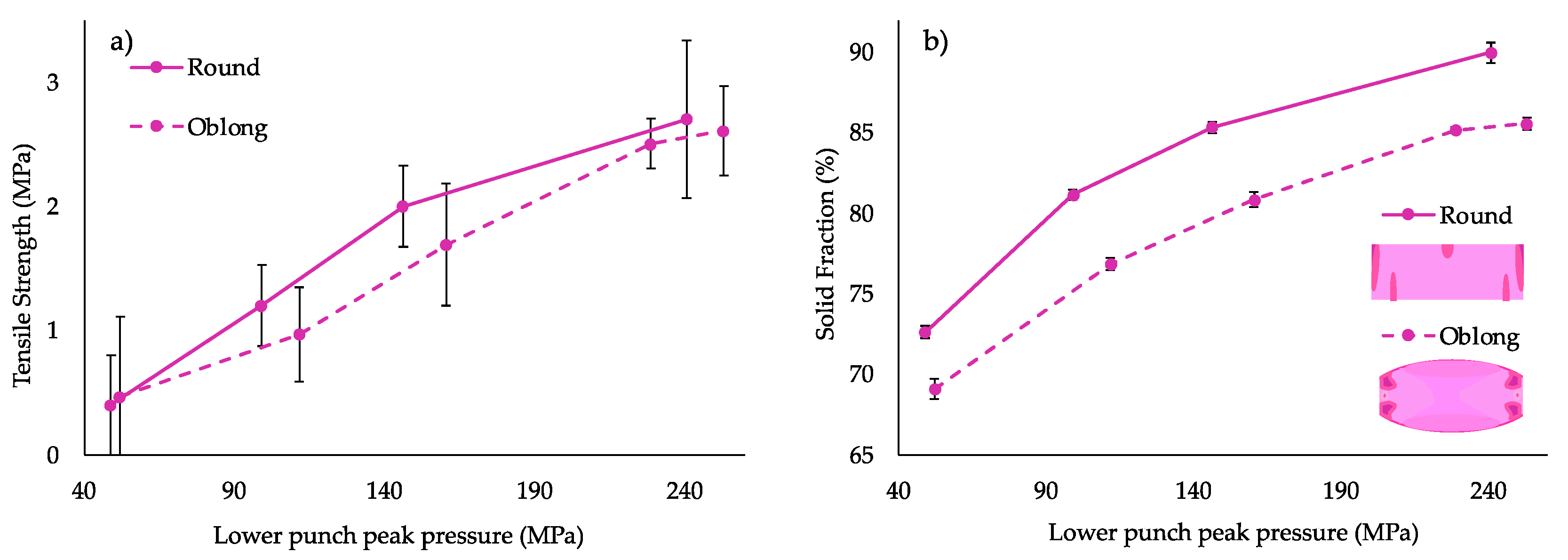
3.3.1. Compaction Study
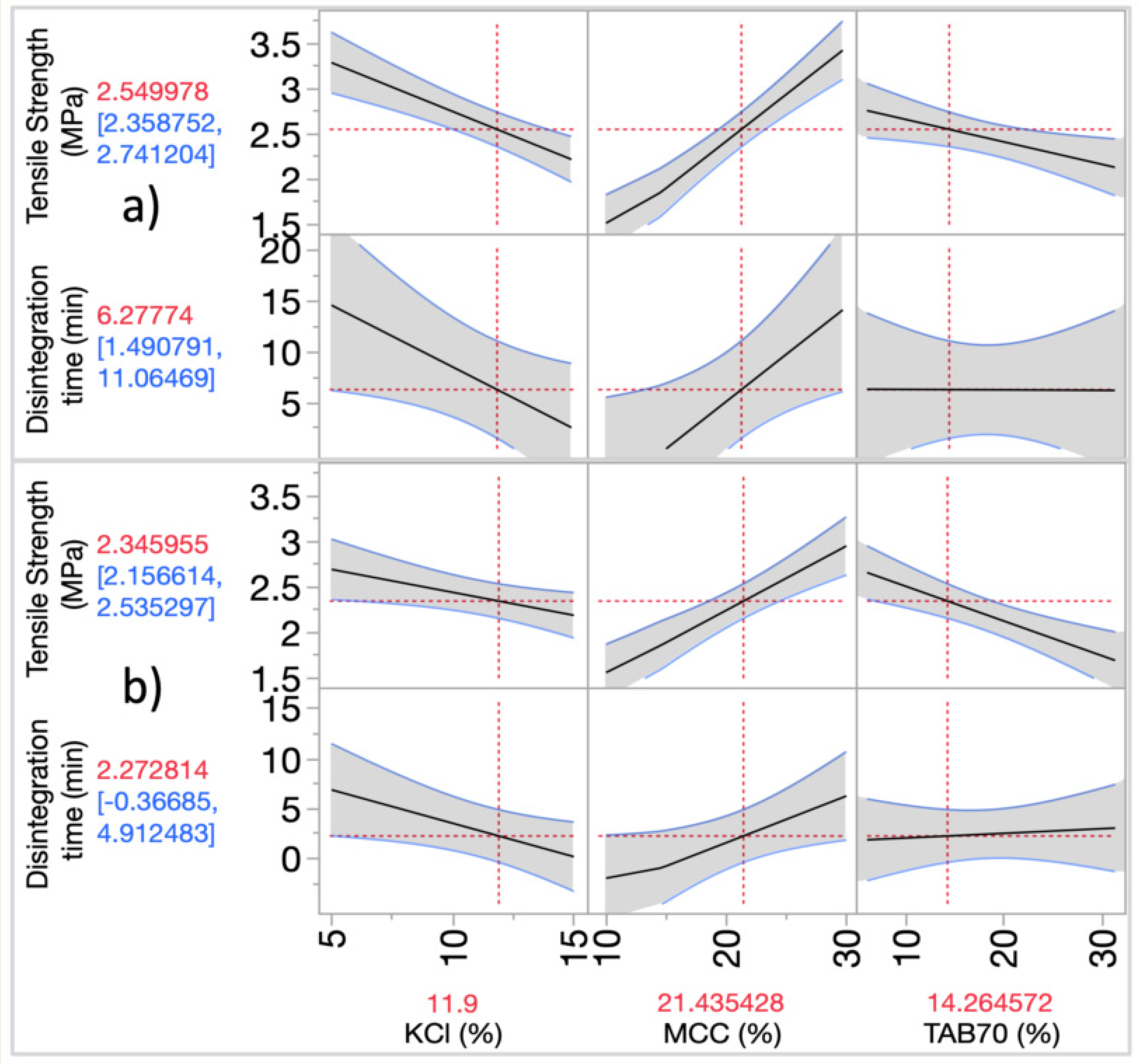
3.3.2. Modelling the Effects of MAs on CQAs
3.3.3. Design Space
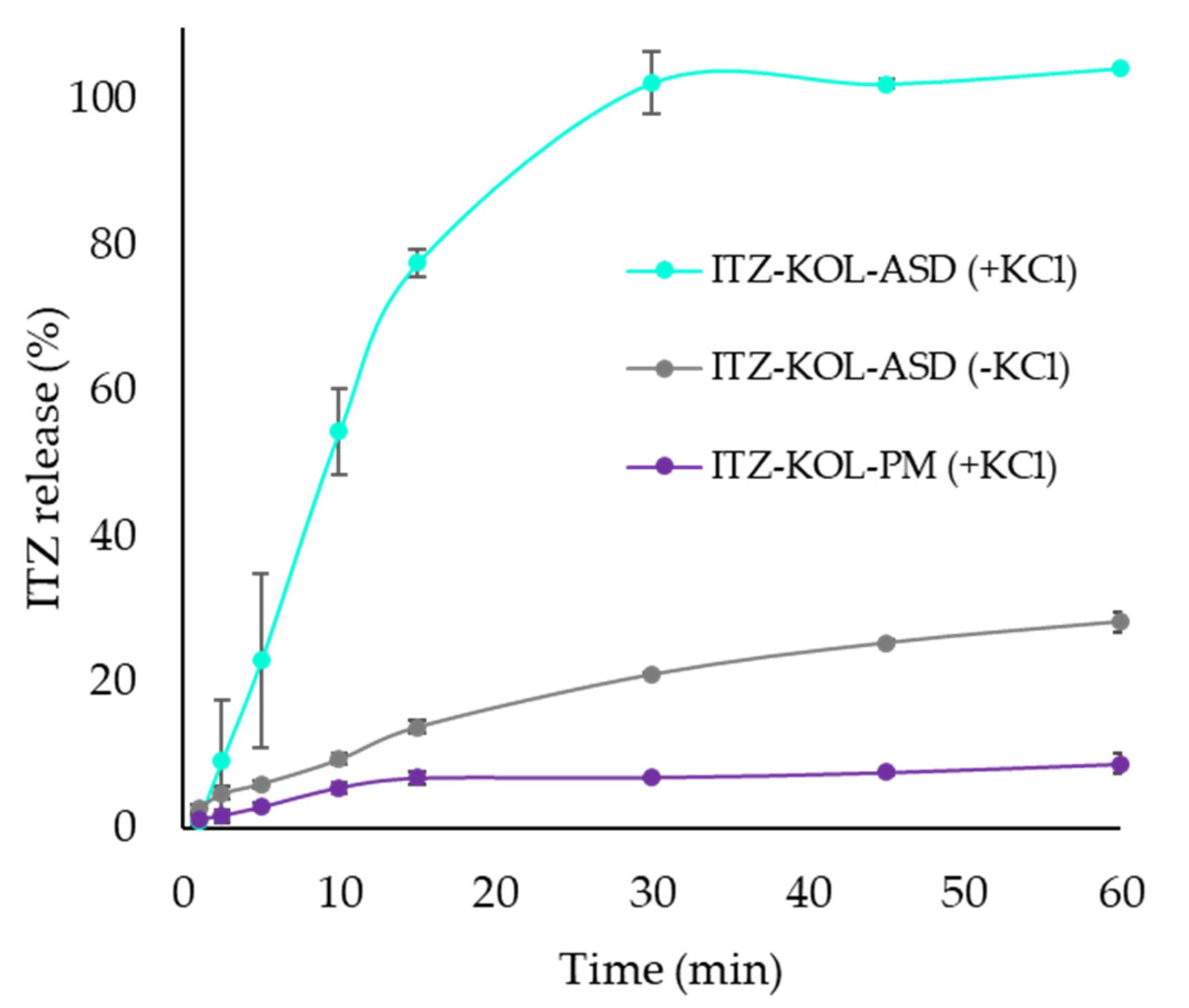
3.4. Dissolution Study of ITZ-KOL-ASD Round Tablets
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alzahrani, A.; Nyavanandi, D.; Mandati, P.; Youssef, A.; Narala, S.; Bandari, S.; Repka, M. A systematic and robust assessment of hot-melt extrusion-based amorphous solid dispersions: Theoretical prediction to practical implementation. Int. J. Pharm. 2022, 624, 121951. [Google Scholar] [CrossRef]
- Butreddy, A.; Bandari, S.; Repka, M. Quality-by-design in hot melt extrusion based amorphous solid dispersions: An industrial perspective on product development. Eur. J. Pharm. Sci. 2021, 158, 105655. [Google Scholar] [CrossRef]
- Verreck, G.; Six, K.; Van Den Mooter, G.; Baert, L.; Peeters, J.; Brewster, M. Characterization of solid dispersions of itraconazole and hydroxypropylmethylcellulose prepared by melt extrusion—Part I. Int. J. Pharm. 2003, 251, 165–174. [Google Scholar] [CrossRef]
- Démuth, B.; Nagy, Z.K.; Balogh, A.; Vigh, T.; Marosi, G.; Verreck, G.; Van Assche, I.; Brewster, M.E. Downstream processing of polymer-based amorphous solid dispersions to generate tablet formulations. Int. J. Pharm. 2015, 486, 268–286. [Google Scholar] [CrossRef]
- Crowley, M.M.; Zhang, F.; Repka, M.A.; Thumma, S.; Upadhye, S.B.; Kumar Battu, S.; Mcginity, J.W.; Martin, C. Pharmaceutical Applications of Hot-Melt Extrusion: Part I. Drug. Dev. Ind. Pharm 2007, 33, 909–926. [Google Scholar] [CrossRef]
- Maniruzzaman, M.; Boateng, J.; Snowden, M.; Douroumis, D. A Review of Hot-Melt Extrusion: Process Technology to Pharmaceutical Products. ISRN Pharm. 2012, 2012, 1–9. [Google Scholar] [CrossRef]
- Repka, M.; Bandari, S.; Kallakunta, V.; Vo, A.; Mcfall, H.; Pimparade, M.; Bhagurkar, A. Melt extrusion with poorly soluble drugs—An integrated review. Int. J. Pharm. 2018, 535, 68–85. [Google Scholar] [CrossRef]
- Censi, R.; Gigliobianco, M.; Casadidio, C.; Di Martino, P. Hot Melt Extrusion: Highlighting Physicochemical Factors to Be Investigated While Designing and Optimizing a Hot Melt Extrusion Process. Pharmaceutics 2008, 10, 89. [Google Scholar] [CrossRef]
- Ren, Y.; Mei, L.; Zhou, L.; Guo, G. Recent Perspectives in Hot Melt Extrusion-Based Polymeric Formulations for Drug Delivery: Applications and Innovations. AAPS Pharm. Sci. Tech. 2019, 20, 92. [Google Scholar] [CrossRef]
- Simões, M.; Pinto, R.; Simões, S. Hot-melt extrusion in the pharmaceutical industry: Toward filing a new drug application. Drug. Disc. Today 2019, 24, 1749–1768. [Google Scholar] [CrossRef]
- Miller, D.; Dinunzio, J.; Yang, W.; Mcginity, J.; Williams, R. Enhanced In Vivo Absorption of Itraconazole via Stabilization of Supersaturation Following Acidic-to-Neutral pH Transition. Drug Dev. Ind. Pharm. 2008, 34, 890–902. [Google Scholar] [CrossRef] [PubMed]
- FDA. Available online: https://www.janssenlabels.com/package-insert/product-monograph/prescribing-information/SPORANOX-Capsules-pi.pdf (accessed on 22 September 2022).
- Schönfeld, B.; Westedt, U.; Wagner, K. Compression of amorphous solid dispersions prepared by hot-melt extrusion, spray drying and vacuum drum drying. Int. J. Pharm. 2021, 3, 100102. [Google Scholar] [CrossRef] [PubMed]
- ICH Q8. Available online: https://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Quality/Q8_R1/Step4/Q8_R2_Guideline.pdf (accessed on 22 September 2022).
- ICH Q9. Available online: https://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Quality/Q9/Step4/Q9_Guideline.pdf (accessed on 22 September 2022).
- Yu, L.; Amidon, G.; Khan, M.; Hoag, S.; Polli, J.; Raju, G.; Woodcock, J. Understanding Pharmaceutical Quality by Design. AAPS J. 2014, 16, 771–783. [Google Scholar] [CrossRef]
- DInunzio, J.; Miller, D.; Yang, W.; Mcginity, J.; Williams, R. Amorphous Compositions Using Concentration Enhancing Polymers for Improved Bioavailability of Itraconazole. Mol. Pharm. 2008, 5, 968–980. [Google Scholar] [CrossRef] [PubMed]
- Solanki, N.; Lam, K.; Tahsin, M.; Gumaste, S.; Shah, A.; Serajuddin, A. Effects of Surfactants on Itraconazole-HPMCAS Solid Dispersion Prepared by Hot-Melt Extrusion I: Miscibility and Drug Release. J. Pharm. Sci. 2019, 108, 1453–1465. [Google Scholar] [CrossRef]
- Mishra, S.; Richter, M.; Mejia, L.; Sauer, A. Downstream Processing of Itraconazole: HPMCAS Amorphous Solid Dispersion: From Hot-Melt Extrudate to Tablet Using a Quality by Design Approach. Pharmaceutics 2022, 14, 1429. [Google Scholar] [CrossRef]
- Zhong, Y.; Jing, G.; Tian, B.; Huang, H.; Zhang, Y.; Gou, J.; Tang, X.; He, H.; Wang, Y. Supersaturation induced by Itraconazole/Soluplus® micelles provided high GI absorption in vivo. Asian J. Pharm. Sci. 2016, 11, 255–264. [Google Scholar] [CrossRef][Green Version]
- Hughey, J.R.; Keen, J.M.; Miller, D.A.; Kolter, K.; Langley, N.; McGinity, J.W. The use of inorganic salts to improve the dissolution characteristics of tablets containing Soluplus-based solid dispersions. Eur. J. Pharm. Sci. 2013, 48, 758–766. [Google Scholar] [CrossRef]
- Mitchell, K.; Ford, J.L.; Armstrong, D.J.; Elliott, P.N.C.; Rostron, C.; Hogan, J.E. The influence of additives on the cloud point, disintegration and dissolution of hydroxypropylmethylcellulose gels and matrix tablets. Int. J. Pharm. 1990, 66, 233–242. [Google Scholar] [CrossRef]
- Kajiyama, A.; Takagi, H.; Moribe, K.; Yamamoto, K. Improvement of HPMC Tablet Disintegration by the Addition of Inorganic Salts. Chem. Pharm. Bull. 2008, 56, 598–601. [Google Scholar] [CrossRef][Green Version]
- Takano, R.; Maurer, R.; Jacob, L.; Stowasser, F.; Stillhart, C.; Page, S. Formulating Amorphous Solid Dispersions: Impact of Inorganic Salts on Drug Release from Tablets Containing Itraconazole-HPMC Extrudate. Mol. Pharm. 2019, 17, 2768–2778. [Google Scholar] [CrossRef] [PubMed]
- Cao, Q.R.; Choi, Y.W.; Cui, J.H.; Lee, B.J. Formulation, release characteristics and bioavailability of novel monolithic hydroxypropylmethylcellulose matrix tablets containing acetaminophen. Control. Release 2005, 108, 351–361. [Google Scholar] [CrossRef] [PubMed]
- FDA. 1062 Tablet Compression Characterisation—Second Supplement to USP 40–NF 35 General Chapters: The United States Pharmacopoeial Convention; FAD: Silver Spring, MA, USA, 2020. [Google Scholar]
- Van Buskirk, G.; Asotra, S.; Balducci, C.; Basu, P.; Didonato, G.; Dorantes, A.; Eickhoff, W.; Ghosh, T.; González, M.; Henry, T.; et al. Best Practices for the Development, Scale-up, and Post-approval Change Control of IR and MR Dosage Forms in the Current Quality-by-Design Paradigm. AAPS Pharm. Sci. Tech. 2014, 15, 665–693. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, I.; Pinto, C.; Moreira, C.; Saviano, A.; Lourenço, F. Design of Experiments (DoE) applied to Pharmaceutical and Analytical Quality by Design (QbD). Braz. J. Pharm. Sci. 2018, 54. [Google Scholar] [CrossRef]
- ZoomlabTM. Available online: https://myapps.basf.com/zoomlab (accessed on 23 September 2022).
- Leane, M.; Pitt, K.; Reynolds, G. A proposal for a drug product Manufacturing Classification System (MCS) for oral solid dosage forms. Pharm. Dev. Technol. 2015, 20, 12–21. [Google Scholar] [CrossRef]
- Berton, P.; Kelley, S.; Bridges, N.; Klingshirn, M.; Huddleston, J.; Willauer, H.; Baldwin, J.; Moody, M.; Rogers, R. Water in Solutions of Chaotropic and Kosmotropic Salts: A Differential Scanning Calorimetry Investigation. J. Chem. Eng. 2019, 64, 4781–4792. [Google Scholar] [CrossRef]
- Rantanen, J.; Khinast, J. The Future of Pharmaceutical Manufacturing Sciences. J. Pharm. Sci. 2015, 104, 3612–3638. [Google Scholar] [CrossRef]
- Pharmaexcipients. Available online: https://www.pharmaexcipients.com/wp-content/uploads/2020/03/MEGGLE_Tablettose_brochure.pdf (accessed on 23 September 2022).
- Pitt, K.; Heasley, M. Determination of the tensile strength of elongated tablets. Powder Technol. 2013, 238, 169–175. [Google Scholar] [CrossRef]
- Eiliazadeh, B.; Briscoe, B.; Sheng, Y.; Pitt, K. Investigating Density Distributions for Tablets of Different Geometry During the Compaction of Pharmaceuticals. Part. Sci. Technol. 2003, 21, 303–316. [Google Scholar] [CrossRef]
- Snee, R.D.; Hoerl, W.R. Strategies for Formulation Development A step by Step Guide Using JMP®; SAS Institute Inc.: Cary, CA, USA, 2016; pp. 66–67. [Google Scholar]
- Dinunzio, J.C.; Schilling, S.U.; Coney, A.W.; Hughey, J.R.; Kaneko, N.; Mcginity, J.W. Use of highly compressible Ceolus™ microcrystalline cellulose for improved dosage form properties containing a hydrophilic solid dispersion. Drug Dev. Ind. Pharm. 2012, 38, 180–189. [Google Scholar] [CrossRef]
- Nguyen, H.V.; Park, C.; Oh, E.; Lee, B. Improving the dissolution rate of a poorly water-soluble drug via adsorption onto pharmaceutical diluents. J. Drug Del. Sci. Technol. 2016, 35, 146–154. [Google Scholar] [CrossRef]
- Carlin, B.A.C. Direct Compression and the Role of Fillers-Binders; Augsburger, L.L., Hoag, S.W., Eds.; Taylor & Francis Group: Baton Rouge, LA, USA, 2008; pp. 173–216. [Google Scholar]
- Vromans, H.; De Boer, A.; Bolhuis, G.; Lerk, C.; Kussendrager, K.; Bosch, H. Studies on tableting properties of lactose. Pharm. Weekbl. 1985, 7, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Pharma. BASF. Available online: https://pharma.basf.com/products/kollidon-30-1f (accessed on 23 September 2022).
- Bridges, N.; Gutowski, K.; Rogers, R. Investigation of aqueous biphasic systems formed from solutions of chaotropic salts with kosmotropic salts (salt–salt ABS). Green Chem. 2007, 9, 177–183. [Google Scholar] [CrossRef]
- Xi, H.; Ren, J.; Novak, J.; Kemp, E.; Johnson, G.; Klinzing, G.; Johnson, M.; Xu, W. The Effect of Inorganic Salt on Disintegration of Tablets with High Loading of Amorphous Solid Dispersion Containing Copovidone. Pharm. Res. 2020, 37, 70. [Google Scholar] [CrossRef] [PubMed]
- Amidon, G.E.; Secreast, P.J.; Mudie, D. Particle, Powder, and Compact Characterization. Elsevier Sci. Technol. 2008, 163–186. [Google Scholar] [CrossRef]
- Sun, C. Mechanism of moisture induced variations in true density and compaction properties of microcrystalline cellulose. Int. J. Pharm. 2008, 346, 93–101. [Google Scholar] [CrossRef]

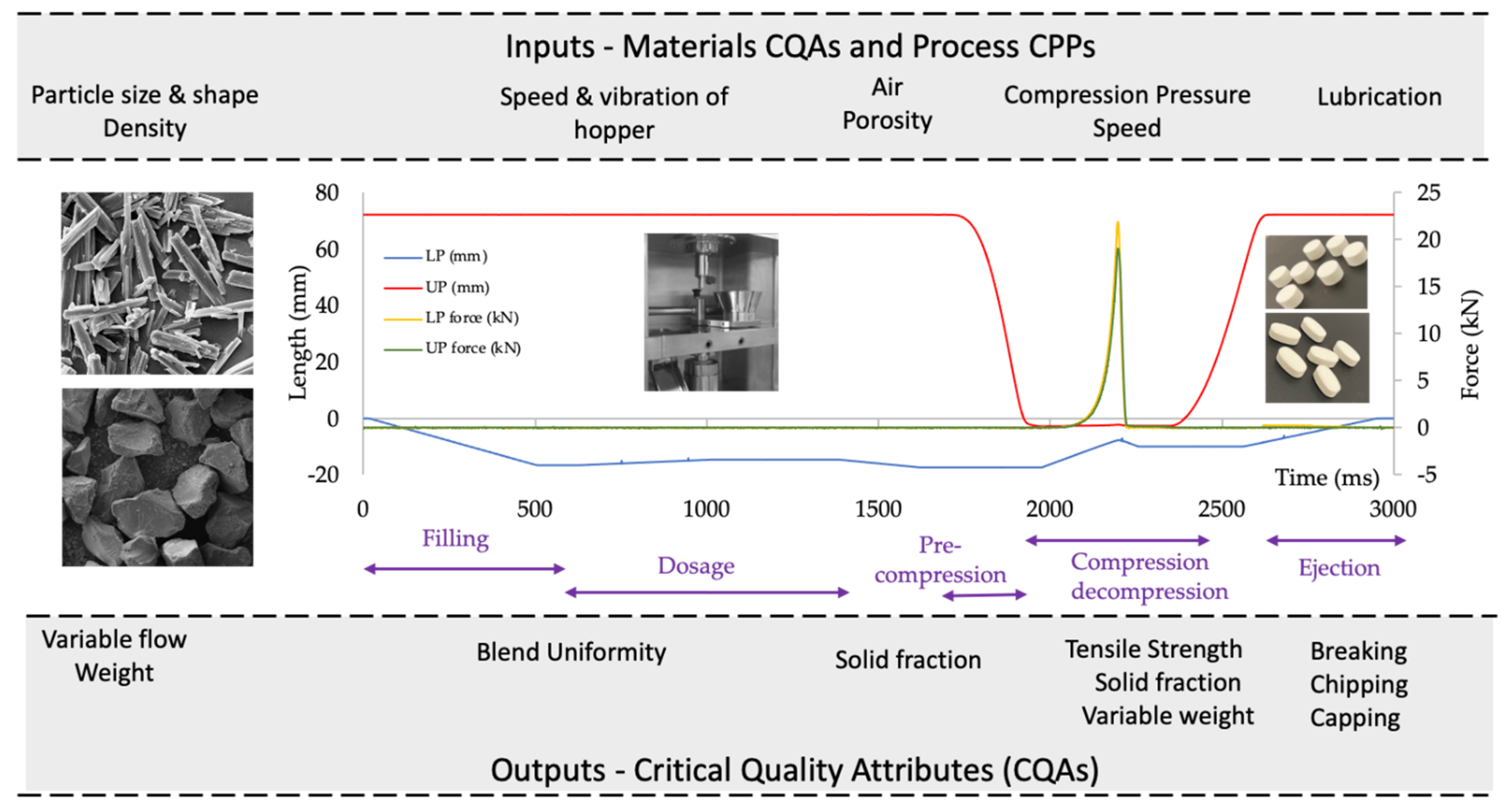
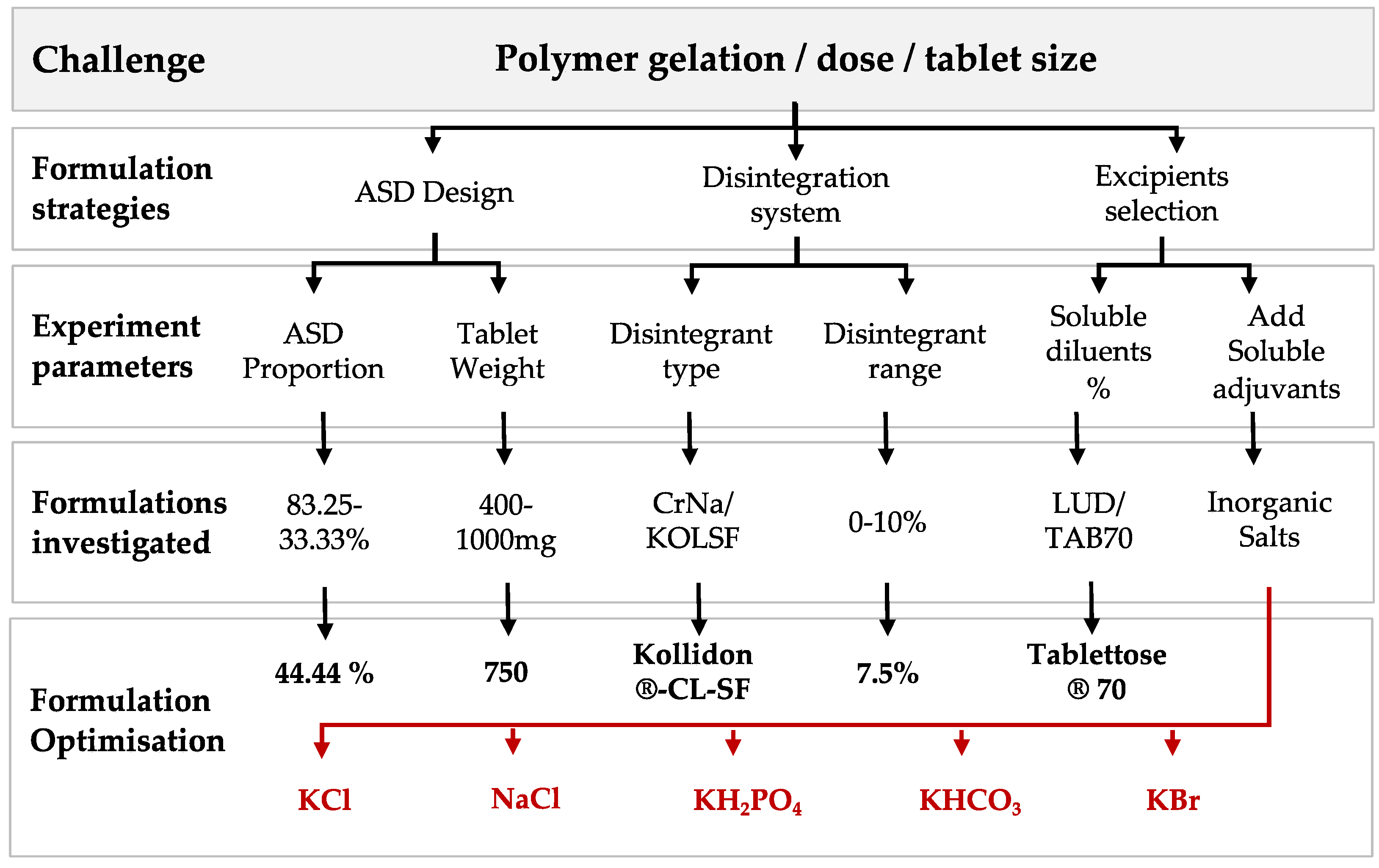
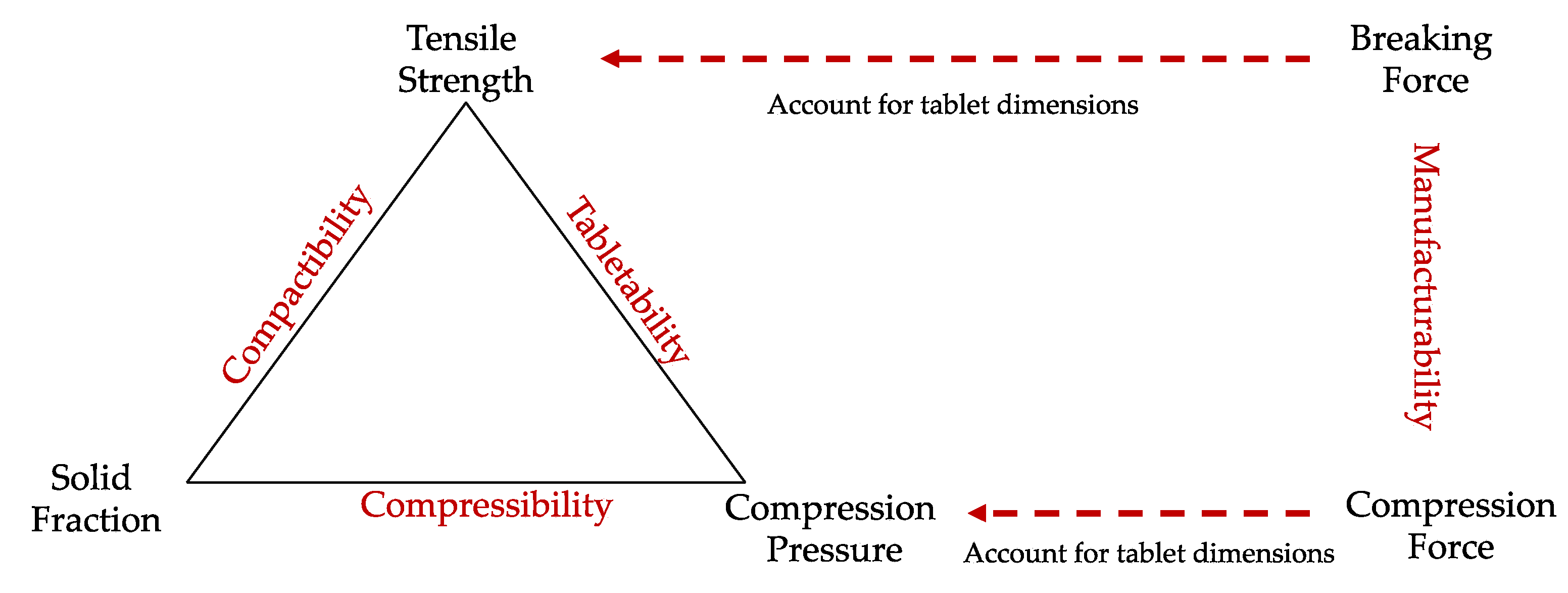
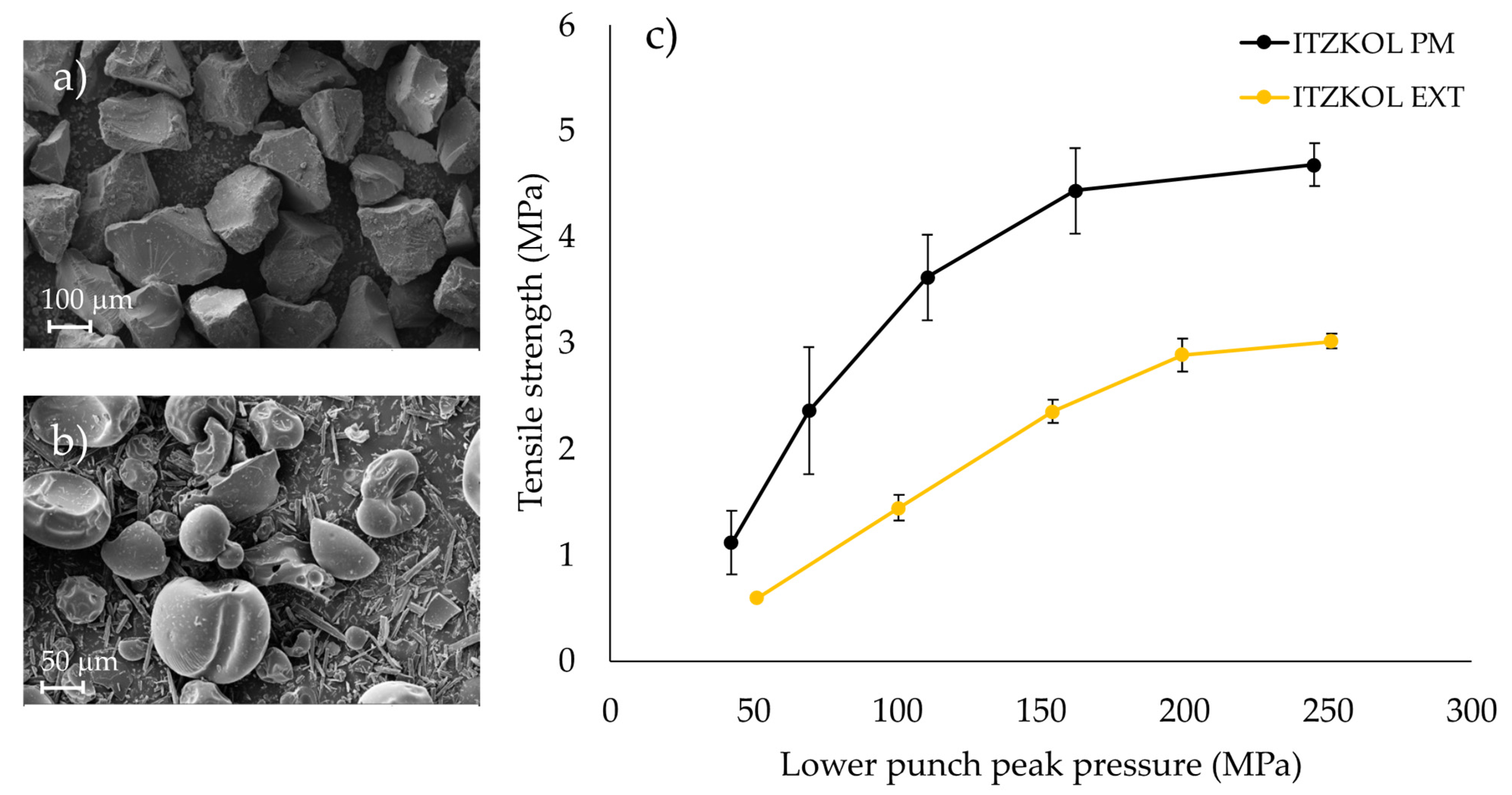
| Quality Attributes | Target |
|---|---|
| Intended use | Antifungal medication |
| ITZ-KOL-ASD 1 | Itraconazole amorphous solid dispersion with 150 < PSD < 180 μm |
| Dosage strength | 100 mg |
| Dosage form | Uncoated tablets |
| Route | Oral administration |
| Appearance | Flat faced round or convex oblong tablets containing ITZ-KOL-ASD |
| Dosage weight | 750 mg ± 5% |
| Assay | 90–110% |
| Solubility | >80% in 15 min |
| Disintegration time | <15 min |
| Friability | <1% |
| Tensile strength | >1.7 MPa (target 2.0 MPa) |
| Solid Fraction | 85 ± 0.05% |
| Index Number | Risk Area | Potential Failure Mode | Potential Failure Effects | S | O | D | RPN * | Mitigations | Revised Ranking | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S | O | D | RPN | |||||||||
| 1 | API | Concentration, PSD, morphology, impurity | Inaccurate dose | 7 | 4 | 2 | 56 | Standard operation procedure for dispensing API accurately. Calibration of balance. Check certificate of analysis. Verification chemical identity and physical chemical properties | 7 | 4 | 1 | 28 |
| 2 | Polymer matrix | Residual water | API crystallisation | 7 | 4 | 2 | 56 | Perform thermogravimetric analysis to determine water content. Control storage conditions (temperature and humidity) | 7 | 1 | 1 | 7 |
| 3 | Excipients | Out of specifications | Impact on critical quality attributes of product | 7 | 2 | 2 | 28 | Check supplier’s certificate of analysis. If necessary, perform analytical tests. | 7 | 1 | 1 | 7 |
| 8 | Mill | Particle size distribution (PSD) | Impact on dissolution profile | 10 | 7 | 4 | 280 | Determine the particle size distribution | 10 | 4 | 1 | 40 |
| 4 | Blender | Time, speed, type | Content uniformity | 10 | 4 | 4 | 160 | Perform content uniformity validation to identify optimum time and speed of mixing. Selected blender type, Turbula | 10 | 4 | 1 | 40 |
| 5 | STYL’One Nano Hopper | Powder flow, calculated die fill level not achieved | Content uniformity, weight variation | 7 | 5 | 4 | 140 | Angle of repose to determine flow properties or increase hopper vibrations | 7 | 4 | 1 | 28 |
| 9 | Compaction | Powder flow, PSD, formulation excipients, machine calibration, profile type, speed, compression pressure, tablet size and weight | Tensile Strength, solid fraction, Disintegration and Dissolution | 10 | 7 | 4 | 280 | Measure manufacturability profile. Perform DoE to optimise formulation and compaction conditions | 10 | 4 | 2 | 80 |
| 10 | Personnel | Sample labelling, data collection, lab skills and competence | Product quality | 7 | 4 | 1 | 28 | Training programme aided by the use of SOPs | 7 | 1 | 1 | 7 |
| 11 | Storage | Sample change with time | Variability on product quality attributes | 7 | 4 | 1 | 28 | Keep samples in controlled storage conditions for temperature and humidity | 7 | 1 | 1 | 7 |
| Component | Functionality | % |
|---|---|---|
| ITZ ASD | Active | 44.4 |
| Avicel® pH102 | Diluent | 10 |
| Tablettose® 70 | Diluent | 27.6 |
| Kollidon®-CL-SF | Disintegrant | 7.5 |
| Inorganic Salt | Soluble Adjuvant | 10 |
| Magnesium Stearate | Lubricant | 0.5 |
| Component (%) | Formulation No. | Ternary Diagram | ||||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | ||
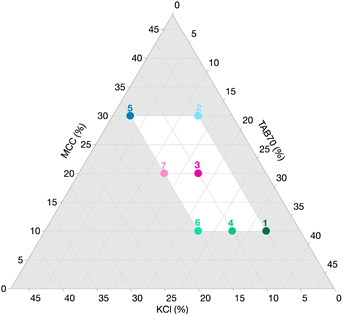 | ||||||||
| ITZ ASD | 44.4 | |||||||
| KCl | 5 | 5 | 10 | 10 | 15 | 15 | 15 | |
| MCC | 10 | 30 | 20 | 10 | 30 | 10 | 20 | |
| TAB70 | 32.6 | 12.6 | 17.6 | 27.6 | 2.6 | 22.6 | 12.6 | |
| KOL-CL-SF | 7.5 | |||||||
| MgSt | 0.5 | |||||||
| Round | Oblong | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Formulation No. | KCl (%) | MCC (%) | TAB70 (%) | Tensile Strength (MPa) | Solid Fraction (%) | Disintegration Time (min) | Tensile Strength (MPa) | Solid Fraction (%) | Disintegration Time (min) |
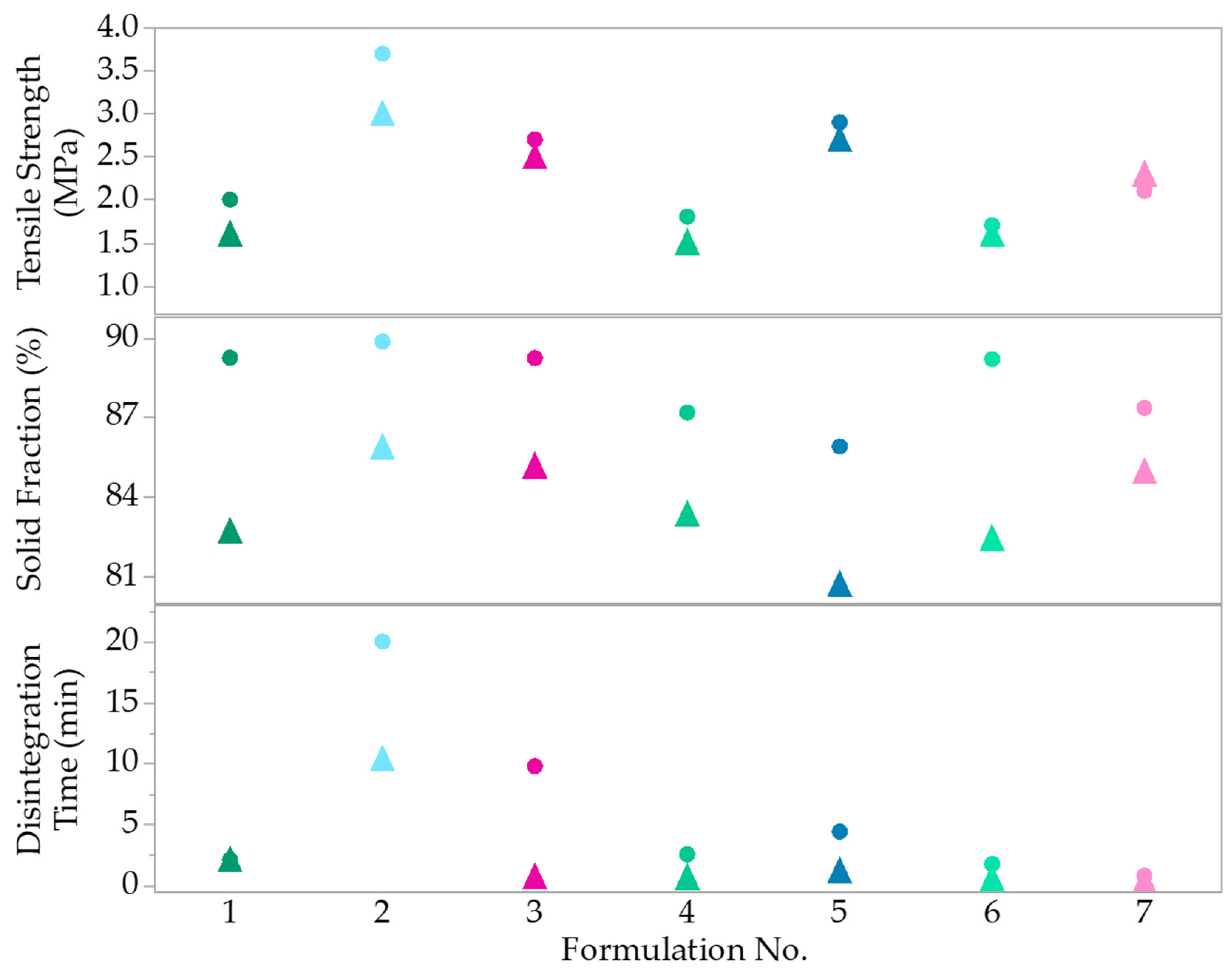
| 1 | 5 | 10 | 32.6 | 2.0 | 89.31 | 2.09 | 1.6 | 82.71 | 2.08 |
| 2 | 5 | 30 | 12.6 | 3.7 | 89.84 | 20 | 3 | 85.87 | 10.37 |
| 3 | 10 | 20 | 17.6 | 2.7 | 89.22 | 9.76 | 2.5 | 85.16 | 0.69 |
| 4 | 10 | 10 | 27.6 | 1.8 | 87.17 | 2.53 | 1.5 | 83.35 | 0.66 |
| 5 | 15 | 30 | 2.6 | 2.9 | 85.89 | 4.39 | 2.7 | 80.70 | 1.16 |
| 6 | 15 | 10 | 22.6 | 1.7 | 89.18 | 1.73 | 1.6 | 82.44 | 0.54 |
| 7 | 15 | 20 | 12.6 | 2.1 | 81.66 | 0.76 | 2.3 | 84.95 | 0.35 |
| Tensile Strength (MPa) | Disintegration Time (min) | |||
|---|---|---|---|---|
| Round | Oblong | Round | Oblong | |
| R2 Adj | 0.95 | 0.93 | 0.63 | 0.60 |
| F Ratio | 54.23 | 38.23 | 6.19 | 5.42 |
| Prob > F | 0.0013 * | 0.0025 * | 0.0596 | 0.0727 |
| CQAs | ITZ-KOL-ASD (+KCl) (%) | ITZ-KOL-ASD (-KCl) (%) | ITZ-KOL-PM (+KCl) (%) |
|---|---|---|---|
| Tensile Strength (MPa) | 2 | 3.2 | 4.4 |
| Solid Fraction (%) | 91 | 89 | 90 |
| Disintegration time (s) | 4 | >60 | 8 |
| Friability (%) | 0.23 | 0.26 | 0.25 |
| Dissolution @30 min | 100.0 | 21.0 | 7.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Triboandas, H.; Pitt, K.; Bezerra, M.; Ach-Hubert, D.; Schlindwein, W. Itraconazole Amorphous Solid Dispersion Tablets: Formulation and Compaction Process Optimization Using Quality by Design Principles and Tools. Pharmaceutics 2022, 14, 2398. https://doi.org/10.3390/pharmaceutics14112398
Triboandas H, Pitt K, Bezerra M, Ach-Hubert D, Schlindwein W. Itraconazole Amorphous Solid Dispersion Tablets: Formulation and Compaction Process Optimization Using Quality by Design Principles and Tools. Pharmaceutics. 2022; 14(11):2398. https://doi.org/10.3390/pharmaceutics14112398
Chicago/Turabian StyleTriboandas, Hetvi, Kendal Pitt, Mariana Bezerra, Delphine Ach-Hubert, and Walkiria Schlindwein. 2022. "Itraconazole Amorphous Solid Dispersion Tablets: Formulation and Compaction Process Optimization Using Quality by Design Principles and Tools" Pharmaceutics 14, no. 11: 2398. https://doi.org/10.3390/pharmaceutics14112398
APA StyleTriboandas, H., Pitt, K., Bezerra, M., Ach-Hubert, D., & Schlindwein, W. (2022). Itraconazole Amorphous Solid Dispersion Tablets: Formulation and Compaction Process Optimization Using Quality by Design Principles and Tools. Pharmaceutics, 14(11), 2398. https://doi.org/10.3390/pharmaceutics14112398








