Phytochemical Characterization and Biological Evaluation of Origanum vulgare L. Essential Oil Formulated as Polymeric Micelles Drug Delivery Systems
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Gas Chromatography-Mass Spectroscopy (GC-MS) Quantitative Analysis of the Volatile Compounds
2.3. Determination of the Antioxidant Activity
2.3.1. 2,2-Diphenyl-1-picrylhydrazyl (DPPH) Radical Scavenging Assay
2.3.2. 2,2-Azino-bis-3-ethylbenzothiazoline-6-sulfonic Acid (ABTS) Radical Scavenging Assay
2.3.3. Cupric Ion Reducing Antioxidant Capacity (CUPRAC) Assay
2.3.4. Oxygen Radical Absorption Capacity (ORAC) Assay
2.4. Preparation and Characterization of the Polymeric Micelles Drug Delivery System
2.4.1. Materials and Preparation of the Poloxamer-Based Binary Hydrogels
2.4.2. Physicochemical Characterization of OEO-PbH and B-PbH
2.5. Antimicrobial Activity Evaluation
2.5.1. Bacterial Strains
2.5.2. Determination of the Minimal Inhibitory Concentration (MIC), Minimal Bactericidal Concentration (MBC), and Minimal Fungal Concentration (MFC)
2.6. In Vitro Evaluation of OEO Formulated as Polymeric Micelles Drug Delivery Systems
2.6.1. Cell Culture
2.6.2. Cell Viability Assessment by MTT Assay
2.6.3. Cell Migration Assessment by Scratch Assay
2.6.4. Cell Cytotoxicity Assessment by LDH Assay
2.6.5. Hoechst Staining
2.7. Immunomodulatory Effects of OEO-PbH on Human DCs Culture
2.7.1. In Vitro Culture of Human DCs
2.7.2. Cell Viability and Apoptosis Assay
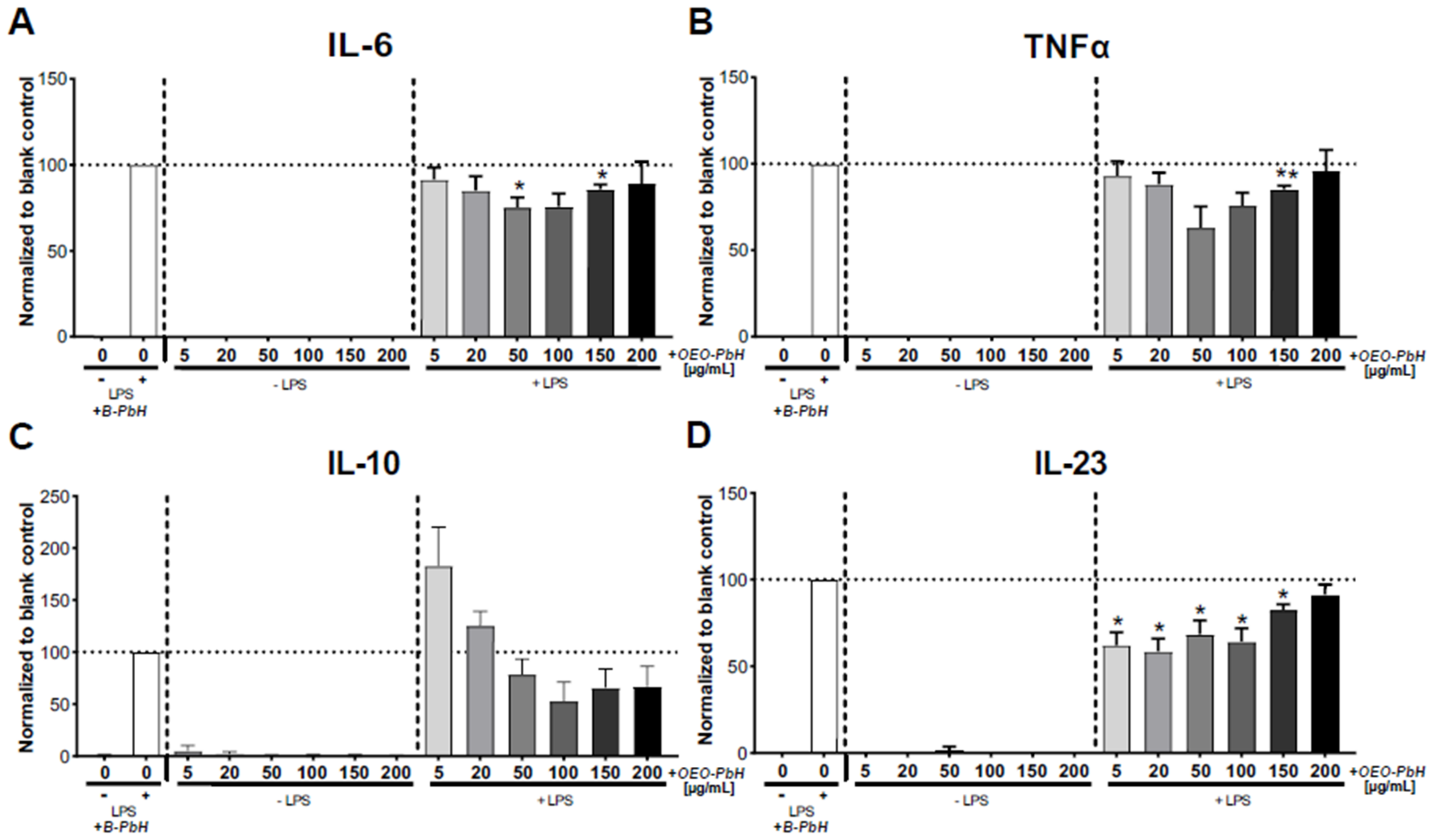
2.7.3. Cytokine Measurements
2.8. Statistical Analysis
3. Results
3.1. GC-MS Characterization of OEO
3.2. The Antioxidant Activity of OEO
3.3. Physicochemical Characterization of OEO-PbH and B-PbH
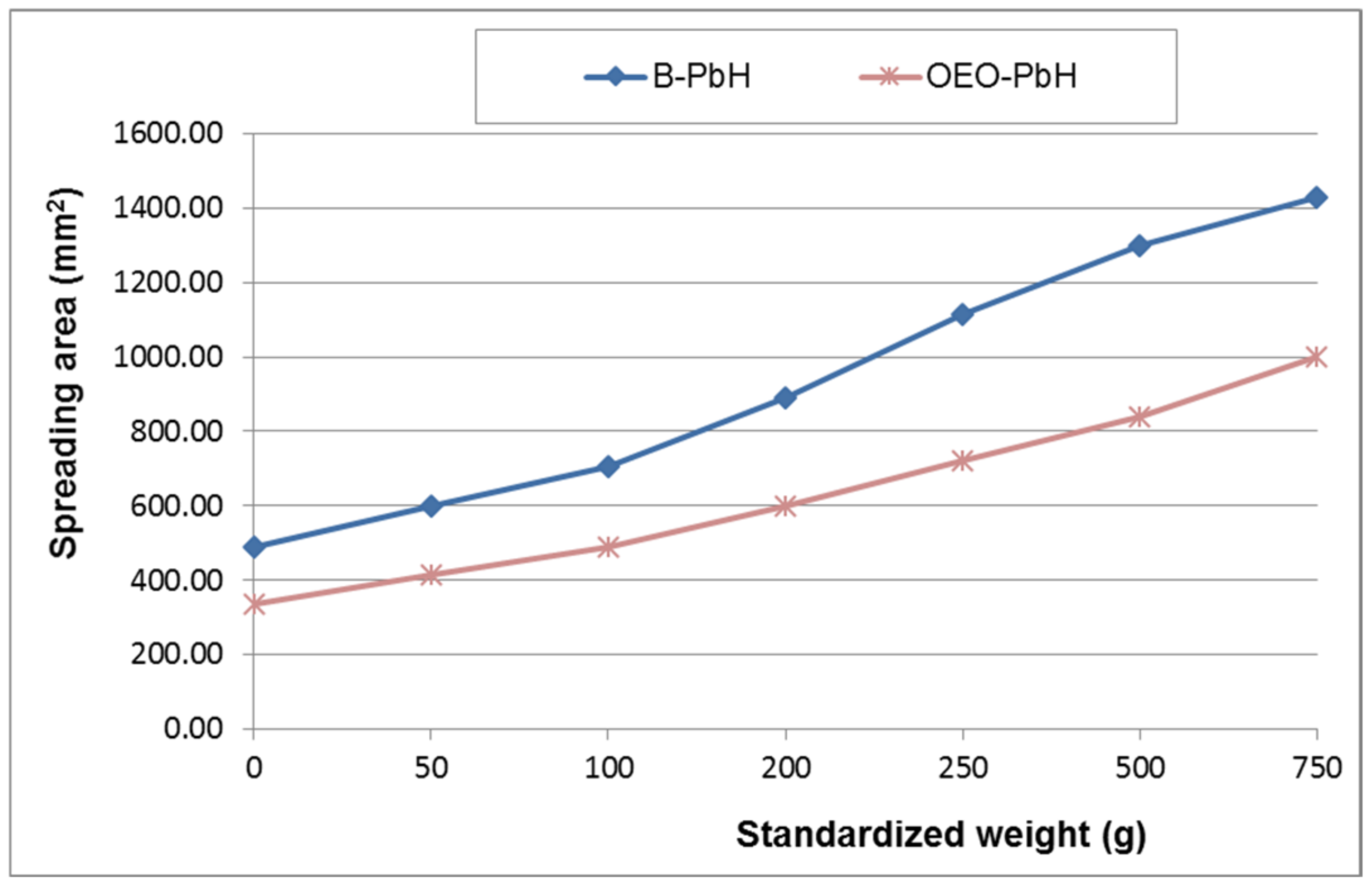
3.3.1. Macroscopic Examination
3.3.2. Determination of pH
3.3.3. Rheological Characterization
3.3.4. Particle Size and Zeta Potential Determination
3.4. The Antimicrobial Activity of OEO and OEO-PbH
3.5. The Antiproliferative/Cytotoxic Activity of OEO-PbH
3.5.1. MTT Assay
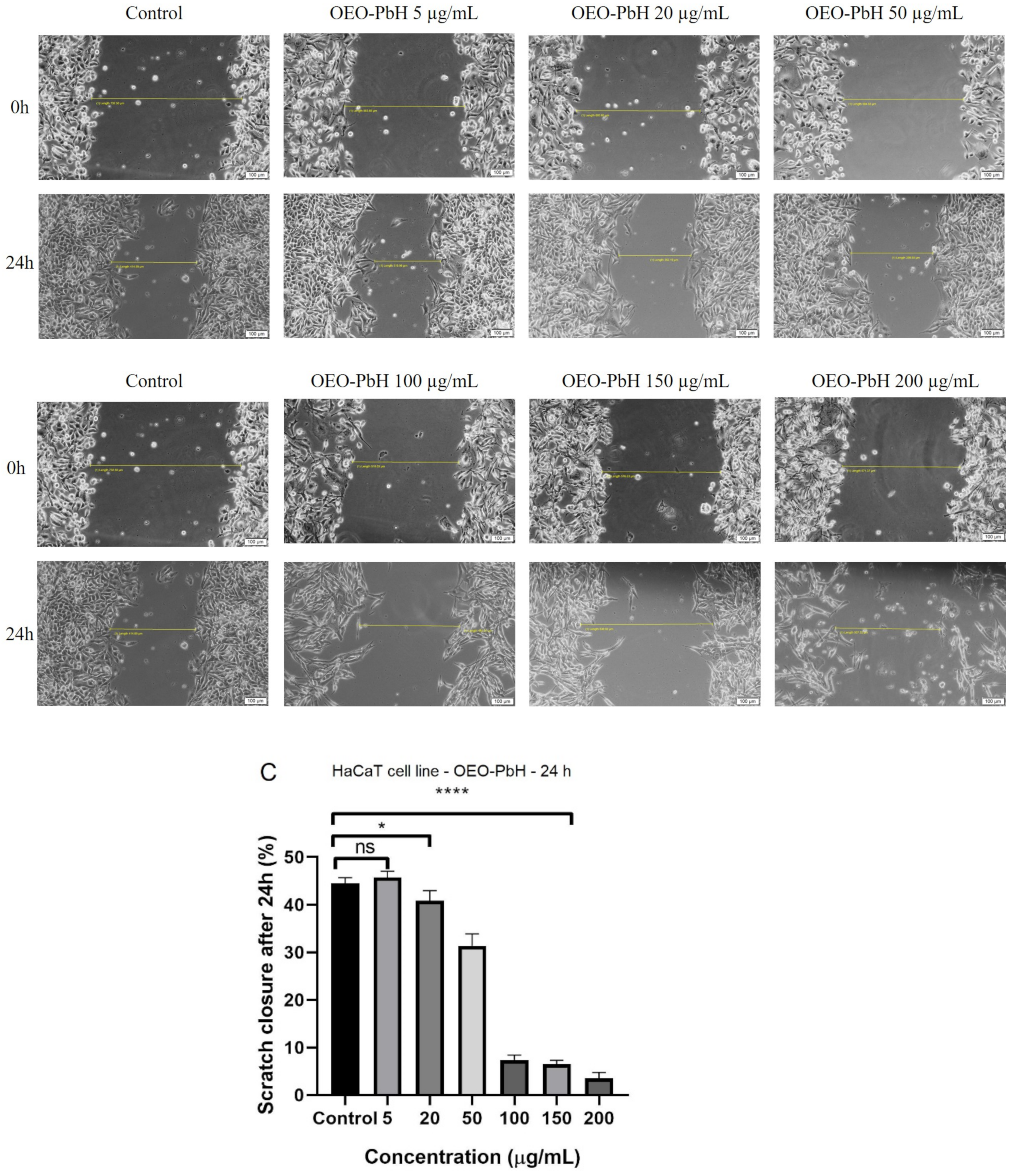
3.5.2. Scratch Assay
3.5.3. LDH Assay
3.5.4. Nuclear Staining
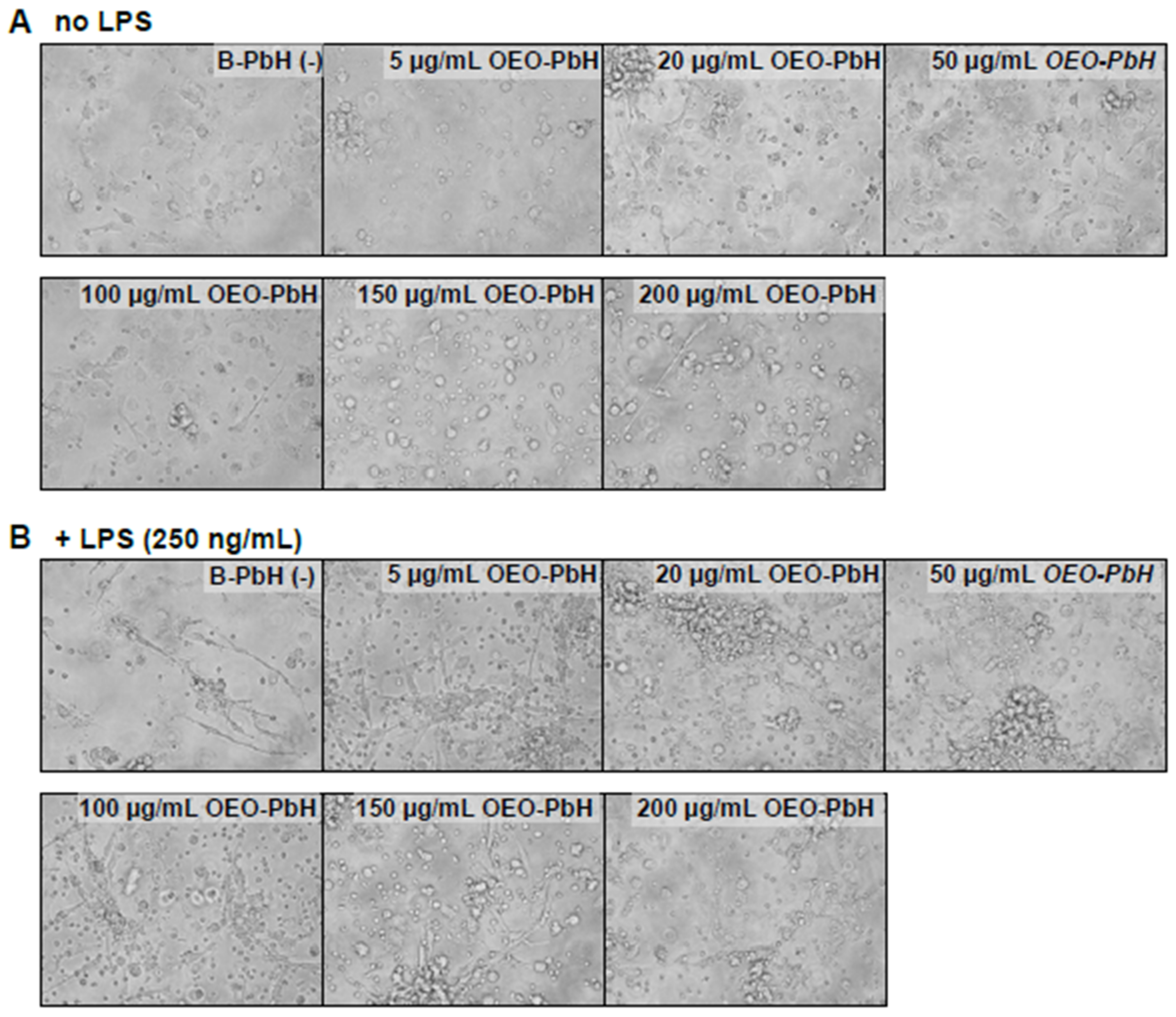
3.6. Immunomodulatory Effects on Human DCs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lopes, C.; Lazzarini, J.; Junior, J.; Baracat, E. Phytotherapy: Yesterday, Today, and Forever? Rev. Assoc. Med. Bras. 2018, 64, 765–768. [Google Scholar] [CrossRef]
- Colalto, C. What Phytotherapy Needs: Evidence-Based Guidelines for Better Clinical Practice. Phytother. Res. 2017, 32, 413–425. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, T.S.; Moreira, C.Z.; Cária, N.Z.; Victoriano, G.; Silva, W.F., Jr.; Magalhães, J.C. Phytotherapy: An Introduction to Its History, Use and Application. Rev. Bras. Plantas Med. 2014, 16, 290–298. [Google Scholar] [CrossRef] [Green Version]
- Raut, J.S.; Karuppayil, S.M. A Status Review on the Medicinal Properties of Essential Oils. Ind. Crops Prod. 2014, 62, 250–264. [Google Scholar] [CrossRef]
- Lombrea, A.; Antal, D.; Ardelean, F.; Avram, S.; Pavel, I.Z.; Vlaia, L.; Mut, A.; Diaconeasa, Z.; Dehelean, C.A.; Soica, C.; et al. A Recent Insight Regarding the Phytochemistry and Bioactivity of Origanum vulgare L. Essential Oil. Int. J. Mol. Sci. 2020, 21, 9653. [Google Scholar] [CrossRef]
- Yakup, M.S.; Berkay, Y.; Bahare, A.; Boyunegmez, T.; Chidambaram, T.; Venil, K.; Das, G.; Imran, M. Phytochemical Constituents, Biological Activities, and Health-Promoting Effects of the Genus Origanum. Phytother. Res. 2021, 35, 95–121. [Google Scholar] [CrossRef]
- Oniga, I.; Puscas, C.; Silaghi-Dumitrescu, R.; Olah, N.K.; Sevastre, B.; Marica, R.; Marcus, I.; Sevastre-Berghian, A.C.; Benedec, D.; Pop, C.E.; et al. Origanum vulgare ssp. Vulgare: Chemical Composition and Biological Studies. Molecules 2018, 23, 2077. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fikry, S.; Khalil, N.; Salama, O. Chemical Profiling, Biostatic and Biocidal Dynamics of Origanum vulgare L. Essential Oil. AMB Express 2019, 9, 41. [Google Scholar] [CrossRef]
- Skoufogianni, E.; Solomou, A.D.; Danalatos, N.G. Ecology, Cultivation and Utilization of the Aromatic Greek Oregano (Origanum vulgare L.): A Review. Not. Bot. Horti Agrobot. 2019, 47, 545–552. [Google Scholar] [CrossRef] [Green Version]
- Moghrovyan, A.; Sahakyan, N.; Babayan, A.; Chichoyan, N.; Petrosyan, M.; Trchounian, A. Essential Oil and Ethanol Extract of Oregano (Origanum vulgare L.) from Armenian Flora as a Natural Source of Terpenes, Flavonoids and Other Phytochemicals with Antiradical, Antioxidant, Metal Chelating, Tyrosinase Inhibitory and Antibacterial Activity. Curr. Pharm. Des. 2019, 25, 1809–1816. [Google Scholar] [CrossRef] [PubMed]
- Ličina, B.Z.; Stefanović, O.D.; Vasić, S.M.; Radojević, I.D.; Dekić, M.S.; Čomić, L.R. Biological Activities of the Extracts from Wild Growing Origanum vulgare L. Food Control 2013, 33, 498–504. [Google Scholar] [CrossRef]
- Lukas, B.; Schmiderer, C.; Novak, J. Essential Oil Diversity of European Origanum vulgare L. (Lamiaceae). Phytochemistry 2015, 119, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Parker, T.L. Anti-Inflammatory, Tissue Remodeling, Immunomodulatory, and Anticancer Activities of Oregano (Origanum vulgare) Essential Oil in a Human Skin Disease Model. Biochim. Open 2017, 4, 73–77. [Google Scholar] [CrossRef]
- Fournomiti, M.; Kimbaris, A.; Mantzourani, I.; Plessas, S.; Theodoridou, I.; Papaemmanouil, V.; Kapsiotis, I.; Panopoulou, M.; Stavropoulou, E.; Bezirtzoglou, E.E.; et al. Antimicrobial Activity of Essential Oils of Cultivated Oregano (Origanum vulgare), Sage (Salvia officinalis), and Thyme (Thymus vulgaris) against Clinical Isolates of Escherichia coli, Klebsiella oxytoca, and Klebsiella pneumoniae. Microb. Ecol. Health Dis. 2015, 26, 23289. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, E.C.A.; Leuthier, L.L.; Almeida Junior, A.; Nunes, J.M.F.F.; Sampaio, F.C.; Farias, I.A.P. Physicochemical Characteristics and Antimicrobial Activity of Origanum vulgare L. Essential Oil and Carvacrol on Cariogenic Bacteria: An in Vitro and in Silico Study. Nat. Prod. Res. 2022. [Google Scholar] [CrossRef]
- Giordani, R.; Regli, P.; Kaloustian, J.; Mikaïl, C.; Abou, L.; Portugal, H. Antifungal Effect of Various Essential Oils against Candida albicans. Potentiation of Antifungal Action of Amphotericin B by Essential Oil from Thymus Vulgaris. Phytother. Res. 2004, 18, 990–995. [Google Scholar] [CrossRef]
- Santoro, G.F.; Cardoso, M.G.; Guimarães, L.G.L.; Salgado, A.P.S.P.; Menna-Barreto, R.F.S.; Soares, M.J. Effect of Oregano (Origanum vulgare L.) and Thyme (Thymus vulgaris L.) Essential Oils on Trypanosoma Cruzi (Protozoa: Kinetoplastida) Growth and Ultrastructure. Parasitol. Res. 2007, 100, 783–790. [Google Scholar] [CrossRef]
- Balusamy, S.R.; Perumalsamy, H.; Huq, M.A.; Balasubramanian, B. Anti-Proliferative Activity of Origanum vulgare Inhibited Lipogenesis and Induced Mitochondrial Mediated Apoptosis in Human Stomach Cancer Cell Lines. Biomed. Pharmacother. 2018, 108, 1835–1844. [Google Scholar] [CrossRef]
- Emire, Z.; Yabalak, E. Can Origanum Be a Hope for Cancer Treatment? A Review on the Potential of Origanum Species in Preventing and Treating Cancers. Int. J. Environ. Health Res. 2022. [Google Scholar] [CrossRef] [PubMed]
- Perumalsamy, H.; Shanmugam, R.; Kim, J.R.; Anandapadmanaban, G.; Huq, M.A.; Dua, K.; Chellappan, D.K.; Yoon, T.H.; Balusamy, S.R. Nanoemulsion and Encapsulation Strategy of Hydrophobic Oregano Essential Oil Increased Human Prostate Cancer Cell Death via Apoptosis by Attenuating Lipid Metabolism. Bioinorg. Chem. Appl. 2022, 2022, 9569226. [Google Scholar] [CrossRef]
- Kryeziu, T.L.; Haloci, E.; Loshaj-Shala, A.; Bagci, U.; Oral, A.; Stefkov, G.J.; Zimmer, A.; Basholli-Salihu, M. Nanoencapsulation of Origanum vulgare Essential Oil into Liposomes with Anticancer Potential. Pharmazie 2022, 77, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Silva, F.; Guimarães, G.; Silva, E.; Sousa-Neto, B.; Machado, F.; Quintans-Junior, L.; Arcanjo, D.; Oliveira, F.; Oliveira, R. Anti-Inflammatory and Anti-Ulcer Activities of Carvacrol, a Monoterpene Present in the Essential Oil of Oregano. J. Med. Food 2012, 15, 984–991. [Google Scholar] [CrossRef]
- Demirci, F.; Teralı, K.; Karadağ, A.E.; Biltekin, S.N.; Sakallı, E.A.; Demirci, B.; Koşar, M.; Başer, K.H.C. In Vitro and in Silico Evaluation of ACE2 and LOX Inhibitory Activity of Origanum Essential Oils and Carvacrol. Planta Med. 2022. [Google Scholar] [CrossRef]
- Orhan, I.; Mesaik, M.; Jabeen, A.; Kan, Y. Immunomodulatory Properties of Various Natural Compounds and Essential Oils through Modulation of Human Cellular Immune Response. Ind. Crops Prod. 2016, 81, 117–122. [Google Scholar] [CrossRef]
- Valdivieso-Ugarte, M.; Gomez-Llorente, C.; Plaza-Diaz, J.; Gil, A. Antimicrobial, Antioxidant, and Immunomodulatory Properties of Essential Oils: A Systematic Review. Nutrients 2019, 11, 2786. [Google Scholar] [CrossRef] [Green Version]
- Bodratti, A.M.; Alexandridis, P. Formulation of Poloxamers for Drug Delivery. J. Funct. Biomater. 2018, 9, 11. [Google Scholar] [CrossRef] [Green Version]
- Almeida, M.; Magalhães, M.; Veiga, F.; Figueiras, A. Poloxamers, Poloxamines and Polymeric Micelles: Definition, Structure and Therapeutic Applications in Cancer. J. Polym. Res. 2018, 25, 31. [Google Scholar] [CrossRef]
- Pontes-Quero, G.M.; Esteban-Rubio, S.; Pérez Cano, J.; Aguilar, M.R.; Vázquez-Lasa, B. Oregano Essential Oil Micro- and Nanoencapsulation with Bioactive Properties for Biotechnological and Biomedical Applications. Front. Bioeng. Biotechnol. 2021, 9, 703684. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, A.; Cristiano, M.C.; Fresta, M.; Paolino, D. The Challenge of Nanovesicles for Selective Topical Delivery for Acne Treatment: Enhancing Absorption Whilst Avoiding Toxicity. Int. J. Nanomed. 2020, 15, 9197–9210. [Google Scholar] [CrossRef] [PubMed]
- Fathalla, Z.M.A.; Vangala, A.; Longman, M.; Khaled, K.A.; Hussein, A.K.; El-Garhy, O.H.; Alany, R.G. Poloxamer-Based Thermoresponsive Ketorolac Tromethamine in Situ Gel Preparations: Design, Characterisation, Toxicity and Transcorneal Permeation Studies. Eur. J. Pharm. Biopharm. 2017, 114, 119–134. [Google Scholar] [CrossRef] [PubMed]
- Russo, E.; Villa, C. Poloxamer Hydrogels for Biomedical Applications. Pharmaceutics 2019, 11, 671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Marian, E.; Vicas, L.G.; Jurca, T.; Muresan, M.; Pallag, A.; Stan, R.L.; Sevastre, B.; Diaconeasa, Z.; Ionescu, C.M.L.; Hangan, A.C. Salivia officinalis L. and Verbascum phlomoides L. Chemical, Antimicrobial, Antioxidant and Antitumor Investigations. Rev. Chim. 2018, 69, 365–370. [Google Scholar] [CrossRef]
- Acosta, M.; Arnao, M.B.; Cano, A. Estimation of Free Radical-Quenching Activity of Leaf Pigment Extracts. Phytochem. Anal. 2001, 12, 138–143. [Google Scholar] [CrossRef]
- Özyürek, M.; Bektaşoğlu, B.; Güçlü, K.; Güngör, N.; Apak, R. Simultaneous Total Antioxidant Capacity Assay of Lipophilic and Hydrophilic Antioxidants in the Same Acetone–Water Solution Containing 2% Methyl-β-Cyclodextrin Using the Cupric Reducing Antioxidant Capacity (CUPRAC) Method. Anal. Chim. Acta 2008, 630, 28–39. [Google Scholar] [CrossRef]
- Özyürek, M.; Güçlü, K.; Tütem, E.; Başkan, K.; Erçağ, E.; Çelik, S.; Baki, S.; Yıldız, L.; Karaman, S.; Apak, R. A Comprehensive Review of CUPRAC Methodology. Anal. Methods 2011, 3, 2439–2453. [Google Scholar] [CrossRef]
- Anastasiou, T.I.; Mandalakis, M.; Krigas, N.; Vézignol, T.; Lazari, D.; Katharios, P.; Dailianis, T.; Antonopoulou, E. Comparative Evaluation of Essential Oils from Medicinal-Aromatic Plants of Greece: Chemical Composition, Antioxidant Capacity and Antimicrobial Activity against Bacterial Fish Pathogens. Molecules 2020, 25, 148. [Google Scholar] [CrossRef] [Green Version]
- Schmolka, I.R. Artificial Skin I. Preparation and Properties of Pluronic F-127 Gels for Treatment of Burns. J. Biomed. Mater. Res. 1972, 6, 571–582. [Google Scholar] [CrossRef]
- Directorate for the Quality of Medicines & Healthcare of the Council of Europe. Potentiometric Determination of PH. In European Pharmacopeia; EDQM Council of Europe: Strasbourg, France, 2021. [Google Scholar]
- Directorate for the Quality of Medicines & Healthcare of the Council of Europe. Measurement of Consistency by Penetrometry. In European Pharmacopeia; EDQM Council of Europe: Strasbourg, France, 2020; p. 337. [Google Scholar]
- Popa, E.A.; Popovici, I.; Braha, S.L. Forme Farmaceutice Bioadezive. In Tehnologie Farmaceutică; Polirom: Iasi, Romania, 2017; Volume 2, pp. 780–782. [Google Scholar]
- Arendrup, M.C.; Cuenca-Estrella, M.; Lass-Flörl, C.; Hope, W.; EUCAST-AFST. EUCAST Technical Note on the EUCAST Definitive Document EDef 7.2: Method for the Determination of Broth Dilution Minimum Inhibitory Concentrations of Antifungal Agents for Yeasts EDef 7.2 (EUCAST-AFST). Clin. Microbiol. Infect. 2012, 18, 7–8. [Google Scholar] [CrossRef] [Green Version]
- Clinical and Laboratory Standards Institute. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2018. [Google Scholar]
- Muntean, D.; Licker, M.; Alexa, E.; Popescu, I.; Jianu, C.; Buda, V.; Dehelean, C.A.; Ghiulai, R.; Horhat, F.; Horhat, D.; et al. Evaluation of Essential Oil Obtained from Mentha×piperita L. against Multidrug-Resistant Strains. Infect. Drug Resist. 2019, 12, 2905–2914. [Google Scholar] [CrossRef]
- Jianu, C.; Stoin, D.; Cocan, I.; David, I.; Pop, G.; Lukinich-Gruia, A.T.; Mioc, M.; Mioc, A.; Soica, C.; Muntean, D.; et al. In Silico and In Vitro Evaluation of the Antimicrobial and Antioxidant Potential of Mentha × Smithiana R. GRAHAM Essential Oil from Western Romania. Foods 2021, 10, 815. [Google Scholar] [CrossRef]
- Fecker, R.; Buda, V.; Alexa, E.; Avram, S.; Pavel, I.Z.; Muntean, D.; Cocan, I.; Watz, C.; Minda, D.; Dehelean, C.A.; et al. Phytochemical and Biological Screening of Oenothera biennis L. Hydroalcoholic Extract. Biomolecules 2020, 10, 818. [Google Scholar] [CrossRef] [PubMed]
- Kis, B.; Pavel, I.Z.; Avram, S.; Moaca, E.A.; Herrero San Juan, M.; Schwiebs, A.; Radeke, H.H.; Muntean, D.; Diaconeasa, Z.; Minda, D.; et al. Antimicrobial Activity, in Vitro Anticancer Effect (MCF-7 Breast Cancer Cell Line), Antiangiogenic and Immunomodulatory Potentials of Populus nigra L. Buds Extract. BMC Complement. Med. Ther. 2022, 22, 74. [Google Scholar] [CrossRef]
- Minda, D.; Ghiulai, R.; Banciu, C.D.; Pavel, I.Z.; Danciu, C.; Racoviceanu, R.; Soica, C.; Budu, O.D.; Muntean, D.; Diaconeasa, Z.; et al. Phytochemical Profile, Antioxidant and Wound Healing Potential of Three Artemisia Species: In Vitro and In Ovo Evaluation. Appl. Sci. 2022, 12, 1359. [Google Scholar] [CrossRef]
- Hut, E.F.; Radulescu, M.; Pilut, N.; Macasoi, I.; Berceanu, D.; Coricovac, D.; Pinzaru, I.; Cretu, O.; Dehelean, C. Two Antibiotics, Ampicillin and Tetracycline, Exert Different Effects in Ht-29 Colorectal Adenocarcinoma Cells in Terms of Cell Viability and Migration Capacity. Curr. Oncol. 2021, 28, 2466–2480. [Google Scholar] [CrossRef] [PubMed]
- Tomanić, D.; Božin, B.; Kladar, N.; Stanojević, J.; Čabarkapa, I.; Stilinović, N.; Apić, J.; Božić, D.D.; Kovačević, Z. Environmental Bovine Mastitis Pathogens: Prevalence, Antimicrobial Susceptibility, and Sensitivity to Thymus vulgaris L., Thymus serpyllum L., and Origanum vulgare L. Essential Oils. Antibiotics 2022, 11, 1077. [Google Scholar] [CrossRef]
- Campos, E.V.R.; Proença, P.L.F.; Oliveira, J.L.; Pereira, A.E.S.; De Morais Ribeiro, L.N.; Fernandes, F.O.; Gonçalves, K.C.; Polanczyk, R.A.; Pasquoto-Stigliani, T.; Lima, R.; et al. Carvacrol and Linalool Co-Loaded in β-Cyclodextrin-Grafted Chitosan Nanoparticles as Sustainable Biopesticide Aiming Pest Control. Sci. Rep. 2018, 8, 7623. [Google Scholar] [CrossRef] [Green Version]
- Sousa, L.G.V.; Castro, J.; Cavaleiro, C.; Salgueiro, L.; Tomás, M.; Palmeira-Oliveira, R.; Martinez-Oliveira, J.; Cerca, N. Synergistic Effects of Carvacrol, α-Terpinene, γ-Terpinene, ρ-Cymene and Linalool against Gardnerella Species. Sci. Rep. 2022, 12, 4417. [Google Scholar] [CrossRef]
- Becer, E.; Altundag, E.M.; Başer, K.H.C.; Vatansever, H.S. Cytotoxic Activity and Antioxidant Effects of Origanum Onites Essential Oil and Its Two Major Contents, Carvacrol and p-Cymene on Human Colorectal (HCT116) and Hepatocelluler Carcinoma (HepG2) Cell Lines. J. Essent. Oil Res. 2022, 1–10. [Google Scholar] [CrossRef]
- Moghrovyan, A.; Parseghyan, L.; Sevoyan, G.; Darbinyan, A.; Sahakyan, N.; Gaboyan, M.; Karabekian, Z.; Voskanyan, A. Antinociceptive, Anti-Inflammatory, and Cytotoxic Properties of Origanum vulgare Essential Oil, Rich with β-Caryophyllene and β-Caryophyllene Oxide. Korean J. Pain 2022, 35, 140–151. [Google Scholar] [CrossRef]
- Rodrigues, M.R.A.; Krause, L.C.; Caramão, E.B.; Dos Santos, J.G.; Dariva, C.; De Oliveira, J.V. Chemical Composition and Extraction Yield of the Extract of Origanum vulgare Obtained from Sub- and Supercritical CO2. J. Agric. Food Chem. 2004, 52, 3042–3047. [Google Scholar] [CrossRef]
- Figiel, A.; Szumny, A.; Gutiérrez-Ortíz, A.; Carbonell-Barrachina, Á.A. Composition of Oregano Essential Oil (Origanum vulgare) as Affected by Drying Method. J. Food Eng. 2010, 98, 240–247. [Google Scholar] [CrossRef]
- Ruben, O.; Valeria, N.; Ruben, G.N. Antioxidant Activity of Fractions from Oregano Essential Oils Obtained by Molecular Distillation. Food Chem. 2014, 156, 212–219. [Google Scholar] [CrossRef]
- Khan, M.; Khan, S.T.; Khan, N.A.; Mahmood, A. The Composition of the Essential Oil and Aqueous Distillate of Origanum vulgare L. Growing in Saudi Arabia and Evaluation of Their Antibacterial Activity. Arab. J. Chem. 2018, 11, 1189–1200. [Google Scholar] [CrossRef]
- De Martino, L.; De Feo, V.; Formisano, C.; Mignola, E.; Senatore, F. Chemical Composition and Antimicrobial Activity of the Essential Oils from Three Chemotypes of Origanum vulgare L. ssp. Hirtum (Link) Ietswaart Growing Wild in Campania (Southern Italy). Molecules 2009, 8, 2735–2746. [Google Scholar] [CrossRef] [Green Version]
- Karaman, M.; Bogavac, M.; Radovanović, B.; Sudji, J.; Tešanović, K.; Janjušević, L. Origanum vulgare Essential Oil Affects Pathogens Causing Vaginal Infections. J. Appl. Microbiol. 2017, 122, 1177–1185. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, B.; Marques, A.; Ramos, C.; Serrano, C.; Matos, O.; Neng, N.; Nogueira, J.; Saraiva, J.; Nunes, M. Chemical Composition and Bioactivity of Different Oregano (Origanum vulgare) Extracts and Essential Oil. J. Sci. Food Agric. 2013, 93, 2707–2714. [Google Scholar] [CrossRef]
- García-Pérez, J.S.; Cuéllar-Bermúdez, S.P.; De la Cruz-Quiroz, R.; Arévalo-Gallegos, A.; Esquivel-Hernandez, D.A.; Rodríguez-Rodríguez, J.; García-García, R.; Iqbal, H.M.N.; Parra-Saldivar, R. Supercritical CO2-Based Tailor Made Valorization of Origanum vulgare L. Extracts: A Green Approach to Extract High-Value Compounds with Applied Perspectives. J. Environ. Manag. 2019, 232, 796–802. [Google Scholar] [CrossRef]
- Tapiero, J.; Salamanca, G.; Marín, C. Analysis of Volatile Compounds and Antioxidant Activity of the Essential Oil of Oregano (Origanum vulgare L.). Adv. Med. Plant Res. 2019, 7, 54–60. [Google Scholar] [CrossRef]
- Dutra, T.; Castro, J.; Menezes, J.; Ramos, T.; Nunes do Prado, I.; Junior, M.; Mikcha, J.; Filho, B. Bioactivity of Oregano (Origanum vulgare) Essential Oil against Alicyclobacillus Spp. Ind. Crops Prod. 2019, 129, 345–349. [Google Scholar] [CrossRef]
- Hashemi, S.; Khaneghah, A. Characterization of Novel Basil-Seed Gum Active Edible Films and Coatings Containing Oregano Essential Oil. Prog. Org. Coat. 2017, 110, 35–41. [Google Scholar] [CrossRef]
- Mechergui, K.; Jaouadi, W.; Coelho, J.P.; Khouja, M. Effect of Harvest Year on Production, Chemical Composition and Antioxidant Activities of Essential Oil of Oregano (Origanum vulgare Subsp Glandulosum (Desf.) Ietswaart) Growing in North Africa. Ind. Crops Prod. 2016, 90, 32–37. [Google Scholar] [CrossRef]
- Alinkina, E.S.; Misharina, T.A.; Fatkullina, L.D. Antiradical Properties of Oregano, Thyme, and Savory Essential Oils. Appl. Biochem. Microbiol. 2013, 49, 73–78. [Google Scholar] [CrossRef]
- Diniz do Nascimento, L.; Moraes, A.; Costa, K.; Galucio, J.; Taube, P.; Costa, C.; Cruz, J.; Andrade, E.; Faria, L. Bioactive Natural Compounds and Antioxidant Activity of Essential Oils from Spice Plants: New Findings and Potential Applications. Biomolecules 2020, 10, 988. [Google Scholar] [CrossRef]
- Oliveira, A.; Pinheiro, L.; Pereira, C.; Matias, W.; Gomes, R.; Chaves, O.; Souza, M.; Almeida, R.; Assis, T. Total Phenolic Content and Antioxidant Activity of Some Malvaceae Family Species. Antioxidants 2012, 1, 33–43. [Google Scholar] [CrossRef]
- Kulisic, T.; Radonic, A.; Katalinic, V.; Milos, M. Use of Different Methods for Testing Antioxidative Activity of Oregano Essential Oil. Food Chem. 2004, 85, 633–640. [Google Scholar] [CrossRef]
- Rostro-Alanis, M.J.; Báez-González, J.; Torres-Alvarez, C.; Parra-Saldívar, R.; Rodriguez-Rodriguez, J.; Castillo, S. Chemical Composition and Biological Activities of Oregano Essential Oil and Its Fractions Obtained by Vacuum Distillation. Molecules 2019, 24, 1904. [Google Scholar] [CrossRef] [Green Version]
- Ghadermazi, R.; Keramat, J.; Goli, S. Antioxidant Activity of Clove (Eugenia caryophyllata Thunb), Oregano (Oringanum vulgare L.) and Sage (Salvia officinalis L.) Essential Oils in Various Model Systems. Int. Food Res. J. 2017, 24, 1628–1635. [Google Scholar]
- Viuda-Martos, M.; Navajas, Y.R.; Zapata, E.S.; Fernández-lópez, J.; Pérez-Alvarez, J.A. Antioxidant Activity of Essential Oils of Five Spice Plants Widely Used in a Mediterranean Diet. Flavour Fragr. J. 2009, 25, 13–19. [Google Scholar] [CrossRef]
- Gavaric, N.; Mozina, S.S.; Kladar, N.; Bozin, B. Chemical Profile, Antioxidant and Antibacterial Activity of Thyme and Oregano Essential Oils, Thymol and Carvacrol and Their Possible Synergism. J. Essent. Oil Bear. Plants 2015, 18, 1013–1021. [Google Scholar] [CrossRef]
- Beirão, S.; Duarte, C.; Bourbon, A.I.; Pinheiro, A.C.; Teresa, A.; Moldão, M.; Isabel, M.; Januário, N.; Vicente, A.A.; Delgadillo, I.; et al. Effect of the Matrix System in the Delivery and in Vitro Bioactivity of Microencapsulated Oregano Essential Oil. J. Food Eng. 2012, 110, 190–199. [Google Scholar] [CrossRef]
- Asensio, C.M.; Grosso, N.R.; Juliani, H.R. Quality Characters, Chemical Composition and Biological Activities of Oregano (Origanum spp.) Essential Oils from Central and Southern Argentina. Ind. Crops Prod. 2015, 63, 203–213. [Google Scholar] [CrossRef]
- Bentayeb, K.; Vera, P.; Rubio, C.; Nerin, C. Adaptation of the ORAC Assay to the Common Laboratory Equipment and Subsequent Application to Antioxidant Plastic Films. Anal. Bioanal. Chem. 2009, 394, 903–910. [Google Scholar] [CrossRef]
- Rowe, R.C.; Sheskey, P.J.; Qwen, S.C. Handbook of Pharmaceutical Excipients; Pharmaceutical Press: London, UK; American Pharmacists Association: Washington, DC, USA, 2006. [Google Scholar]
- Karavana, S.Y.; Rençbe, S.; Şenyiğit, Z.A.; Baloğlu, E. A New In-Situ Gel Formulation of Itraconazole for Vaginal Administration. Pharmacol. Pharm. 2012, 3, 417–426. [Google Scholar] [CrossRef] [Green Version]
- Lambers, H.; Piessens, S.; Bloem, A.; Pronk, H.; Finkel, P. Natural Skin Surface PH Is on Average below 5, Which Is Beneficial for Its Resident Flora. Int. J. Cosmet. Sci. 2006, 28, 359–370. [Google Scholar] [CrossRef]
- Laothaweerungsawat, N.; Neimkhum, W.; Anuchapreeda, S.; Sirithunyalug, J.; Chaiyana, W. Transdermal Delivery Enhancement of Carvacrol from Origanum vulgare L. Essential Oil by Microemulsion. Int. J. Pharm. 2020, 579, 119052. [Google Scholar] [CrossRef]
- Citu, I.M.; Borcan, F.; Zambori, C.; Tita, B.; Paunescu, V.; Ardelean, S. Influence of Crosslinking Agent—Chain Extender Ratio on the Properties of Hyperbranched Polyurethane Structures Used as Dendritic Drug Carrier. Rev. Chim. 2015, 66, 119–123. [Google Scholar]
- Salopek, B.; Krasic, D.; Filipovic, S. Measurement and Application of Zeta-Potential. Rud. Geolosko Naft. Zb. 1992, 4, 147–151. [Google Scholar]
- Silhavy, T.J.; Kahne, D.; Walker, S. The Bacterial Cell Envelope. Cold Spring Harb. Perspect. Biol. 2010, 2, a000414. [Google Scholar] [CrossRef] [PubMed]
- Özkalp, B.; Sevgi, F.; Özcan, M.; Özcan, M.M. The Antibacterial Activity of Essential Oil of Oregano (Origanum vulgare L.). J. Food Agric. Environ. 2010, 8, 272–274. [Google Scholar]
- Şahin, F.; Güllüce, M.; Daferera, D.; Sökmen, A.; Sökmen, M.; Polissiou, M.; Agar, G.; Özer, H. Biological Activities of the Essential Oils and Methanol Extract of Origanum vulgare ssp. Vulgare in the Eastern Anatolia Region of Turkey. Food Control 2004, 15, 549–557. [Google Scholar] [CrossRef]
- Peñalver, P.; Huerta, B.; Borge, C.; Astorga, R.; Romero, R.; Perea, A. Antimicrobial Activity of Five Essential Oils against Origin Strains of the Enterobacteriaceae Family. Apmis 2005, 113, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Rosato, A.; Sblano, S.; Salvagno, L.; Carocci, A.; Clodoveo, M.L.; Corbo, F.; Fracchiolla, G. Anti-Biofilm Inhibitory Synergistic Effects of Combinations of Essential Oils and Antibiotics. Antibiotics 2020, 9, 637. [Google Scholar] [CrossRef]
- D’Aquila, P.; Paparazzo, E.; Crudo, M.; Bonacci, S.; Procopio, A.; Passarino, G.; Bellizzi, D. Antibacterial Activity and Epigenetic Remodeling of Essential Oils from Calabrian Aromatic Plants. Nutrients 2022, 14, 391. [Google Scholar] [CrossRef]
- Ebani, V.V.; Nardoni, S.; Bertelloni, F.; Pistelli, L.; Mancianti, F. Antimicrobial Activity of Five Essential Oils against Bacteria and Fungi Responsible for Urinary Tract Infections. Molecules 2018, 23, 1668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wijesundara, N.M.; Rupasinghe, H.P.V. Essential Oils from Origanum vulgare and Salvia Officinalis Exhibit Antibacterial and Anti-Biofilm Activities against Streptococcus Pyogenes. Microb. Pathog. 2018, 117, 118–127. [Google Scholar] [CrossRef] [PubMed]
- La Pergola, A.; Restuccia, C.; Napoli, E.; Bella, S.; Brighina, S.; Russo, A.; Suma, P. Commercial and Wild Sicilian Origanum vulgare Essential Oils: Chemical Composition, Antimicrobial Activity and Repellent Effects. J. Essent. Oil Res. 2017, 29, 451–460. [Google Scholar] [CrossRef]
- Bendahou, M.; Muselli, A.; Grignon-Dubois, M.; Benyoucef, M.; Desjobert, J.M.; Bernardini, A.F.; Costa, J. Antimicrobial Activity and Chemical Composition of Origanum Glandulosum Desf. Essential Oil and Extract Obtained by Microwave Extraction: Comparison with Hydrodistillation. Food Chem. 2008, 106, 132–139. [Google Scholar] [CrossRef]
- Yoncheva, K.; Benbassat, N.; Zaharieva, M.M.; Dimitrova, L.; Kroumov, A.; Spassova, I.; Kovacheva, D.; Najdenski, H.M. Improvement of the Antimicrobial Activity of Oregano Oil by Encapsulation in Chitosan—Alginate Nanoparticles. Molecules 2021, 26, 7017. [Google Scholar] [CrossRef]
- Kozics, K.; Bucková, M.; Puškárová, A.; Kalászová, V.; Cabicarová, T.; Pangallo, D. The Effect of Ten Essential Oils on Several Cutaneous Drug-Resistant Microorganisms and Their Cyto/Genotoxic and Antioxidant Properties. Molecules 2019, 24, 4570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Man, A.; Santacroce, L.; Jacob, R.; Mare, A.; Man, L. Antimicrobial Activity of Six Essential Oils against a Group of Human Pathogens: A Comparative Study. Pathogens 2019, 8, 15. [Google Scholar] [CrossRef] [Green Version]
- Pandey, A.; Sonthalia, S. Skin Tags; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Akpinar, F.; Dervis, E. Association between Acrochordons and the Components of Metabolic Syndrome. Eur. J. Dermatol. 2012, 22, 106–110. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.E.; Osmun, W.E. Just a Pinch: Technique for Skin Tag Removal in Sensitive Areas. Can. Fam. Physician 2016, 62, 998–999. [Google Scholar] [PubMed]
- Antunes, A.; Rossel, B.; Adriaens, E. Efficacy Evaluation of the Pixie® Skin Tag Cryogenic Device on Skin Tags in a Prospective, Single-Blinded, Randomized, Comparative Clinical Trial. Dermatol. Ther. 2021, 11, 995–1007. [Google Scholar] [CrossRef] [PubMed]
- Avola, R.; Granata, G.; Geraci, C.; Napoli, E.; Graziano, A.C.E.; Cardile, V. Oregano (Origanum vulgare L.) Essential Oil Provides Anti-Inflammatory Activity and Facilitates Wound Healing in a Human Keratinocytes Cell Model. Food Chem. Toxicol. 2020, 144, 111586. [Google Scholar] [CrossRef]
- Kapustová, M.; Puškárová, A.; Bučková, M.; Granata, G.; Napoli, E.; Annušová, A.; Mesárošová, M.; Kozics, K.; Pangallo, D.; Geraci, C. Biofilm Inhibition by Biocompatible Poly(ε-Caprolactone) Nanocapsules Loaded with Essential Oils and Their Cyto/Genotoxicity to Human Keratinocyte Cell Line. Int. J. Pharm. 2021, 606, 120846. [Google Scholar] [CrossRef]
- Rojo-Ruvalcaba, B.E.; García-Cobián, T.A.; Pascoe-González, S.; Campos-Bayardo, T.I.; Guzmán-García, L.M.; Gil-Gálvez, M.C.; Escobar-Millan, Z.; Huerta-García, E.; García-Iglesias, T. Dose-Dependent Cytotoxicity of the Origanum vulgare and Carvacrol on Triple Negative Breast Cancer Cell Line. Proceedings 2020, 61, 6. [Google Scholar] [CrossRef]
- García-Salinas, S.; Elizondo-Castillo, H.; Arruebo, M.; Mendoza, G.; Irusta, S. Evaluation of the Antimicrobial Activity and Cytotoxicity of Different Components of Natural Origin Present in Essential Oils. Molecules 2018, 23, 1399. [Google Scholar] [CrossRef] [Green Version]
- Yao, N.; Xu, Q.; He, J.K.; Pan, M.; Hou, Z.F.; Liu, D.D.; Tao, J.P.; Huang, S.Y. Evaluation of Origanum vulgare Essential Oil and Its Active Ingredients as Potential Drugs for the Treatment of Toxoplasmosis. Front. Cell. Infect. Microbiol. 2021, 11, 793089. [Google Scholar] [CrossRef]
- Günes-Bayir, A.; Kocyigit, A.; Güler, E.M.; Bilgin, M.G.; Ergün, İ.S.; Dadak, A. Effects of Carvacrol on Human Fibroblast (WS-1) and Gastric Adenocarcinoma (AGS) Cells in Vitro and on Wistar Rats in Vivo. Mol. Cell. Biochem. 2018, 448, 237–249. [Google Scholar] [CrossRef]
- Günes-Bayir, A.; Kocyigit, A.; Guler, E.M.; Dadak, A. In Vitro Hormetic Effect Investigation of Thymol on Human Fibroblast and Gastric Adenocarcinoma Cells. Molecules 2020, 25, 3270. [Google Scholar] [CrossRef]
- Janani, K.; Teja, K.; Ajitha, P. Cytotoxicity of Oregano Essential Oil and Calcium Hydroxide on L929 Fibroblast Cell: A Molecular Level Study. J. Conserv. Dent. 2021, 24, 457. [Google Scholar] [CrossRef]
- Ranjitkar, S.; Zhang, D.; Sun, F.; Salman, S.; He, W.; Venkitanarayanan, K.; Tulman, E.R.; Tian, X. Cytotoxic Effects on Cancerous and Non-Cancerous Cells of Trans-Cinnamaldehyde, Carvacrol, and Eugenol. Sci. Rep. 2021, 11, 16281. [Google Scholar] [CrossRef]
- Su, B.; Wang, J.; Wang, X.; Jin, H.; Zhao, G.; Ding, Z.; Kang, Y.; Wang, B. The Effects of IL-6 and TNF-α as Molecular Adjuvants on Immune Responses to FMDV and Maturation of Dendritic Cells by DNA Vaccination. Vaccine 2008, 26, 5111–5122. [Google Scholar] [CrossRef]
- Di Cesare, A.; Di Meglio, P.; Nestle, F.O. The IL-23/Th17 Axis in the Immunopathogenesis of Psoriasis. J. Investig. Dermatol. 2009, 129, 1339–1350. [Google Scholar] [CrossRef] [Green Version]
- Tang, C.; Chen, S.; Qian, H.; Huang, W. Interleukin-23: As a Drug Target for Autoimmune Inflammatory Diseases. Immunology 2012, 135, 112–124. [Google Scholar] [CrossRef] [Green Version]
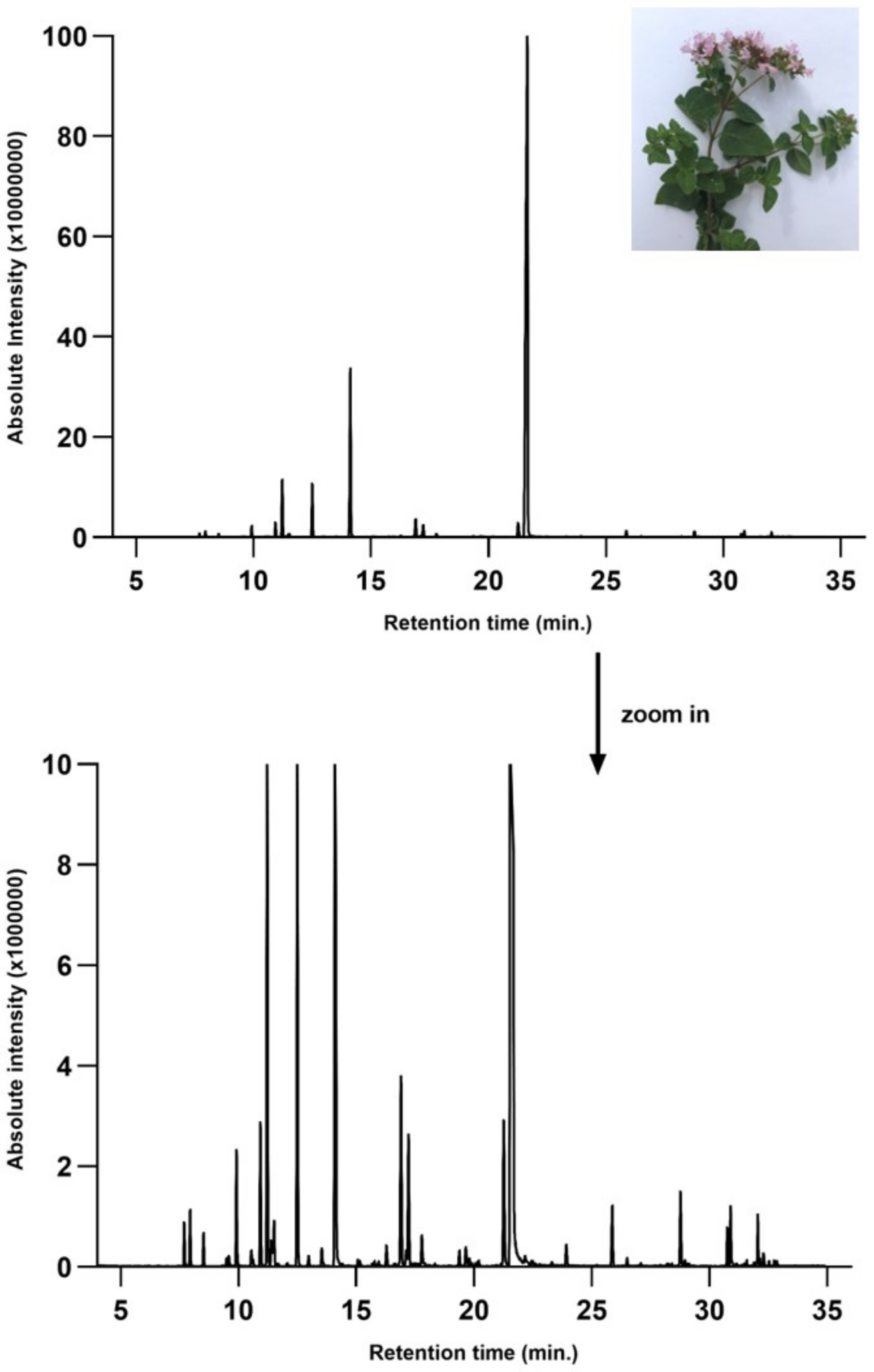






| Compound | Rt (min) | Concentration (% of Total Peak Areas) | Molecular Weight (g/mol) | Molecular Structure |
|---|---|---|---|---|
| α-thujene | 7.697 | 0.11 | 136 |  |
| α-pinene | 7.946 | 0.49 | 136 |  |
| Camphene | 8.518 | 0.15 | 136 | 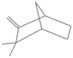 |
| β-pinene | 9.498 | 0.06 | 136 | 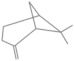 |
| 1-octen-3-ol | 9.578 | 0.11 | 128 | 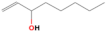 |
| β-myrcene | 9.911 | 1.06 | 136 |  |
| α-phellandrene | 10.54 | 0.19 | 136 | 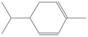 |
| n.i. | 10.616 | 0.06 | ||
| α-terpinene | 10.927 | 1.12 | 136 |  |
| p-cymene | 11.221 | 4.13 | 134 | 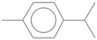 |
| D-limonene | 11.388 | 0.16 | 136 | 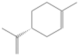 |
| β-phellandrene | 11.454 | 0.19 | 136 |  |
| Eucalyptol | 11.515 | 0.19 | 154 |  |
| Trans-β-ocimene * | 11.653 | 0.02 | 136 |  |
| Cis-β-ocimene | 12.055 | 0.05 | 136 |  |
| γ-terpinene | 12.498 | 4.08 | 136 | 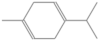 |
| n.i. | 12.967 | 0.21 | ||
| Terpinolene | 13.536 | 0.16 | 136 | 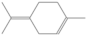 |
| Benzene, (2-methyl-1-propenyl)- | 13.737 | 0.05 | 132 |  |
| β-linalool | 14.113 | 6.46 | 154 |  |
| n.i. | 15.075 | 0.02 | ||
| α-campholenal * | 15.151 | 0.02 | 152 |  |
| n.i. | 15.687 | 0.01 | ||
| n.i. | 15.763 | 0.01 | ||
| Camphor | 15.951 | 0.03 | 152 | 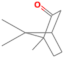 |
| Borneol | 16.897 | 0.99 | 154 | 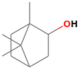 |
| Terpinen-4-ol | 17.219 | 0.97 | 154 |  |
| n.i. | 17.473 | 0.03 | ||
| α-terpineol | 17.8 | 0.27 | 154 |  |
| Thymol methyl eter | 19.373 | 0.2 | 164 |  |
| Carvone | 19.679 | 0.13 | 150 |  |
| Linalool acetate | 19.818 | 0.04 | 196 | 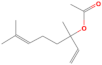 |
| Carvenone | 20.261 | 0.02 | 152 |  |
| Thymol | 21.264 | 1.7 | 150 |  |
| Carvacrol | 21.691 | 71.21 | 150 |  |
| n.i. | 22.171 | 0.14 | ||
| Thymol acetate | 23.915 | 0.15 | 192 | 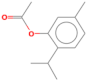 |
| Methyleugenol | 25.151 | 0.02 | 178 |  |
| Caryophyllene | 25.867 | 1.36 | 204 |  |
| α-bergamotene * | 26.286 | 0.03 | 204 | 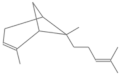 |
| Aromadendrene * | 26.491 | 0.17 | 204 |  |
| α-caryophyllene | 27.079 | 0.06 | 204 | 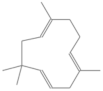 |
| n.i. | 28.239 | 0.08 | ||
| β-bisabolene * | 28.763 | 2.32 | 204 |  |
| n.i. | 28.954 | 0.06 | ||
| δ-cadinene | 29.107 | 0.07 | 204 |  |
| Spathulenol * | 30.748 | 0.15 | 220 |  |
| Caryophyllene oxide | 30.89 | 0.24 | 220 |  |
| α-cubebene * | 31.568 | 0.02 | 204 | 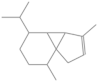 |
| γ-muurolene * | 32.043 | 0.2 | 204 |  |
| n.i. | 32.288 | 0.05 |
| ABTS TE μmol/mL | CUPRAC TE μmol/mL | ORAC TE μmol/mL | DPPH Scavenging Activity (%) | |
|---|---|---|---|---|
| OEO | 1304 ± 20 | 5320.1 ± 32 | 3752.7 ± 28 | 86 ± 28 |
| Sample | Particles Size (nm) | PDI | Zeta Potential (mV) |
|---|---|---|---|
| B-PbH | 28.4 ± 2.1 (19%) 285.3 ± 11.7 (75%) | 1.1059 | 21.45 ± 0.00 |
| OEO-PbH | 20.3 ± 1.9 (64%) 490.2 ± 14.4 (32%) | 0.858 | −2.52 ± 0.84 |
| OEO | 635.1 ± 12.3 (82%) | 0.697 | 0.00 ± 0.00 |
| Bacterial Strains | MIC MBC/MFC (μg/mL) | |
|---|---|---|
| OEO | OEO-PbH | |
| S. pyogenes ATCC 19615 | 5 5 | 5 5 |
| S. aureus ATCC 25923 | 5 5 | 10 10 |
| E. faecalis ATCC 51299 | 5 5 | 10 10 |
| E. coli ATCC 25922 | 40 40 | 80 80 |
| P. aeruginosa ATCC 27853 | 40 40 | 80 80 |
| C. albicans ATCC 10231 | 2.5 2.5 | 2.5 2.5 |
| C. parapsilosis ATCC 22019 | 2.5 2.5 | 2.5 2.5 |
| IC50 (μg/mL)—24 h | IC50 (μg/mL)—48 h | IC50 (μg/mL)—72 h |
|---|---|---|
| 93.26 | 82.65 | 29.69 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bora, L.; Burkard, T.; Juan, M.H.S.; Radeke, H.H.; Muț, A.M.; Vlaia, L.L.; Magyari-Pavel, I.Z.; Diaconeasa, Z.; Socaci, S.; Borcan, F.; et al. Phytochemical Characterization and Biological Evaluation of Origanum vulgare L. Essential Oil Formulated as Polymeric Micelles Drug Delivery Systems. Pharmaceutics 2022, 14, 2413. https://doi.org/10.3390/pharmaceutics14112413
Bora L, Burkard T, Juan MHS, Radeke HH, Muț AM, Vlaia LL, Magyari-Pavel IZ, Diaconeasa Z, Socaci S, Borcan F, et al. Phytochemical Characterization and Biological Evaluation of Origanum vulgare L. Essential Oil Formulated as Polymeric Micelles Drug Delivery Systems. Pharmaceutics. 2022; 14(11):2413. https://doi.org/10.3390/pharmaceutics14112413
Chicago/Turabian StyleBora, Larisa, Tobias Burkard, Martina Herrero San Juan, Heinfried H. Radeke, Ana Maria Muț, Lavinia Lia Vlaia, Ioana Zinuca Magyari-Pavel, Zorița Diaconeasa, Sonia Socaci, Florin Borcan, and et al. 2022. "Phytochemical Characterization and Biological Evaluation of Origanum vulgare L. Essential Oil Formulated as Polymeric Micelles Drug Delivery Systems" Pharmaceutics 14, no. 11: 2413. https://doi.org/10.3390/pharmaceutics14112413
APA StyleBora, L., Burkard, T., Juan, M. H. S., Radeke, H. H., Muț, A. M., Vlaia, L. L., Magyari-Pavel, I. Z., Diaconeasa, Z., Socaci, S., Borcan, F., Kis, B., Muntean, D., Dehelean, C. A., & Danciu, C. (2022). Phytochemical Characterization and Biological Evaluation of Origanum vulgare L. Essential Oil Formulated as Polymeric Micelles Drug Delivery Systems. Pharmaceutics, 14(11), 2413. https://doi.org/10.3390/pharmaceutics14112413













