Advancements in Polymeric Nanocarriers to Mediate Targeted Therapy against Triple-Negative Breast Cancer
Abstract
1. Introduction
2. Stimuli-Responsive Polymeric Nanoparticles
2.1. pH-Responsive Polymeric Nanoparticles
2.2. Thermo/Temperature-Responsive Polymeric Nanoparticles
2.3. Redox-Responsive Polymeric Nanoparticles
2.4. Light-Responsive Polymeric Nanoparticles
3. Polymeric Nanoparticles for Combating Triple-Negative Breast Cancer
4. Polymeric Nanoparticles for Triple-Negative Breast Cancer Immunotherapy
5. Polymeric Nanoparticles Combating Cancer-Stem Cells in Triple-Negative Breast Cancer
6. Polymeric Nanoparticles for Triple-Negative Breast Cancer Metastasis
7. Conclusions, Current Challenges and Prospects
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhang, Z.; Zhou, L.; Xie, N.; Nice, E.C.; Zhang, T.; Cui, Y.; Huang, C. Overcoming cancer therapeutic bottleneck by drug repurposing. Signal Transduct. Target. Ther. 2020, 5, 113. [Google Scholar] [CrossRef]
- Rhee, S.Y.; Hong, S.M.; Chon, S.; Ahn, K.J.; Kim, S.H.; Baik, S.H.; Park, Y.S.; Nam, M.S.; Lee, K.W.; Woo, J.T.; et al. Hypoglycemia and Medical Expenses in Patients with Type 2 Diabetes Mellitus: An Analysis Based on the Korea National Diabetes Program Cohort. PLoS ONE 2016, 11, e0148630. [Google Scholar] [CrossRef]
- Gala, U.H.; Miller, D.A.; Williams, R.O. Harnessing the therapeutic potential of anticancer drugs through amorphous solid dispersions. Biochim. Biophys. Acta Rev. Cancer 2020, 1873, 188319. [Google Scholar] [CrossRef]
- Sheikh, A.; Md, S.; Kesharwani, P. Aptamer grafted nanoparticle as targeted therapeutic tool for the treatment of breast cancer. Biomed. Pharmacother. 2022, 146, 112530. [Google Scholar] [CrossRef]
- Madamsetty, V.S.; Tavakol, S.; Moghassemi, S.; Dadashzadeh, A.; Schneible, J.D.; Fatemi, I.; Shirvani, A.; Zarrabi, A.; Azedi, F.; Dehshahri, A.; et al. Chitosan: A versatile bio-platform for breast cancer theranostics. J. Control Release 2021, 341, 733–752. [Google Scholar] [CrossRef]
- Alharbi, M.; Alqefari, A.; Alhawday, Y.; Alghammas, A.; Hershan, A.; Abdulmonem, W.; Alsudais, M. Association of menstrual and reproductive factors with thyroid cancer in Saudi female patients. J. Umm Al-Qura Univ. Med. Sci. 2021, 7, 11–13. [Google Scholar] [CrossRef]
- Sporikova, Z.; Koudelakova, V.; Trojanec, R.; Hajduch, M. Genetic Markers in Triple-Negative Breast Cancer. Clin. Breast Cancer 2018, 18, e841–e850. [Google Scholar] [CrossRef]
- Singh, S.K.; Singh, S.; Wlillard, J.; Singh, R. Drug delivery approaches for breast cancer. Int. J. Nanomed. 2017, 12, 6205–6218. [Google Scholar] [CrossRef]
- Rocchi, P.; So, A.; Kojima, S.; Signaevsky, M.; Beraldi, E.; Fazli, L.; Hurtado-Coll, A.; Yamanaka, K.; Gleave, M. Heat Shock Protein 27 Increases after Androgen Ablation and Plays a Cytoprotective Role in Hormone-Refractory Prostate Cancer. Cancer Res. 2004, 64, 6595–6602. [Google Scholar] [CrossRef]
- Sheikh, A.; Kesharwani, P. An insight into aptamer engineered dendrimer for cancer therapy. Eur. Polym. J. 2021, 159, 110746. [Google Scholar] [CrossRef]
- Singh, V.; Kesharwani, P. Recent advances in microneedles-based drug delivery device in the diagnosis and treatment of cancer. J. Control Release 2021, 338, 394–409. [Google Scholar] [CrossRef]
- Ahmad, A.; Buzby, S.; Ni, C.; Shah, S.I. Effect of Nb and Sc Doping on the Phase Transformation of Sol–Gel Processed TiO2 Nanoparticles. J. Nanosci. Nanotechnol. 2008, 8, 2410–2418. [Google Scholar] [CrossRef]
- Fatima, M.; Sheikh, A.; Hasan, N.; Sahebkar, A.; Riadi, Y.; Kesharwani, P. Folic acid conjugated poly(amidoamine) dendrimer as a smart nanocarriers for tracing, imaging, and treating cancers over-expressing folate receptors. Eur. Polym. J. 2022, 170, 111156. [Google Scholar] [CrossRef]
- Ahmed, S.A.; Hasan, N.; Altass, H.M.; Bera, A.; Alsantali, R.I.; Pan, N.; Alzahrani, A.Y.A.; Bagchi, D.; Al-Fahemi, J.H.; Khder, A.S.; et al. Tetracycline Encapsulated in Au Nanoparticle-Decorated ZnO Nanohybrids for Enhanced Antibacterial Activity. ACS Appl. Nano Mater. 2022, 5, 4484–4492. [Google Scholar] [CrossRef]
- Singh, D.; Kesharwani, P.; Alhakamy, N.A.; Siddique, H.R. Accentuating CircRNA-miRNA-Transcription Factors Axis: A Conundrum in Cancer Research. Front. Pharmacol. 2022, 12, 3904. [Google Scholar] [CrossRef]
- Sheikh, A.; Alhakamy, N.A.; Md, S.; Kesharwani, P. Recent Progress of RGD Modified Liposomes as Multistage Rocket Against Cancer. Front. Pharmacol. 2022, 12, 4024. [Google Scholar] [CrossRef]
- Sheikh, A.; Md, S.; Alhakamy, N.A.; Kesharwani, P. Recent development of aptamer conjugated chitosan nanoparticles as cancer therapeutics. Int. J. Pharm. 2022, 620, 121751. [Google Scholar] [CrossRef]
- Peng, J.; Chen, J.; Xie, F.; Bao, W.; Xu, H.; Wang, H.; Xu, Y.; Du, Z. Herceptin-conjugated paclitaxel loaded PCL-PEG worm-like nanocrystal micelles for the combinatorial treatment of HER2-positive breast cancer. Biomaterials 2019, 222, 119420. [Google Scholar] [CrossRef]
- Kesharwani, P.; Tekade, R.K.; Gajbhiye, V.; Jain, K.; Jain, N.K. Cancer targeting potential of some ligand-anchored poly(propylene imine) dendrimers: A comparison. Nanomed. Nanotechnol. Biol. Med. 2011, 7, 295–304. [Google Scholar] [CrossRef]
- Fouad, O.A.; Khder, A.E.R.S.; Dai, Q.; El-Shall, M.S. Structural and catalytic properties of ZnO and Al2O3 nanostructures loaded with metal nanoparticles. J. Nanopart. Res. 2011, 13, 7075–7083. [Google Scholar] [CrossRef]
- Kesharwani, P.; Tekade, R.K.; Jain, N.K. Generation dependent cancer targeting potential of poly(propyleneimine) dendrimer. Biomaterials 2014, 35, 5539–5548. [Google Scholar] [CrossRef] [PubMed]
- Kesharwani, P.; Tekade, R.K.; Jain, N.K. Generation dependent safety and efficacy of folic acid conjugated dendrimer based anticancer drug formulations. Pharm. Res. 2015, 32, 1438–1450. [Google Scholar] [CrossRef] [PubMed]
- Soni, N.; Soni, N.; Pandey, H.; Maheshwari, R.; Kesharwani, P.; Tekade, R.K. Augmented delivery of gemcitabine in lung cancer cells exploring mannose anchored solid lipid nanoparticles. J. Colloid Interface Sci. 2016, 481, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Hassan, D.; Aldawsari, H.M.; Molugulu, N.; Shukla, R.; Kesharwani, P. Immune checkpoint inhibitors: A promising anticancer therapy. Drug Discov. Today 2020, 25, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Chadar, R.; Afsana; Kesharwani, P. Nanotechnology-based siRNA delivery strategies for treatment of triple negative breast cancer. Int. J. Pharm. 2021, 605, 120835. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.; Weiner, L.S.; Hartman, S.J.; Horvath, S.; Jeste, D.; Mischel, P.S.; Kado, D.M. Breast cancer treatment and its effects on aging. J. Geriatr. Oncol. 2019, 10, 346–355. [Google Scholar] [CrossRef]
- Singh, S.; Numan, A.; Maddiboyina, B.; Arora, S.; Riadi, Y.; Md, S.; Alhakamy, N.A.; Kesharwani, P. The emerging role of immune checkpoint inhibitors in the treatment of triple-negative breast cancer. Drug Discov. Today 2021, 26, 1721–1727. [Google Scholar] [CrossRef]
- Akram, D.; Ahmad, S.; Sharmin, E.; Ahmad, S. Silica Reinforced Organic–Inorganic Hybrid Polyurethane Nanocomposites From Sustainable Resource. Macromol. Chem. Phys. 2010, 211, 412–419. [Google Scholar] [CrossRef]
- Ashfaq, M.; Shah, S.; Rasul, A.; Hanif, M.; Khan, H.U.; Khames, A.; Abdelgawad, M.A.; Ghoneim, M.M.; Ali, M.Y.; Abourehab, M.A.S.; et al. Enhancement of the Solubility and Bioavailability of Pitavastatin through a Self-Nanoemulsifying Drug Delivery System (SNEDDS). Pharmaceutics 2022, 14, 482. [Google Scholar] [CrossRef]
- Abd El-Aziz, E.A.E.D.; Elgayar, S.F.; Mady, F.M.; Abourehab, M.A.S.; Hasan, O.A.; Reda, L.M.; Alaaeldin, E. The Potential of Optimized Liposomes in Enhancement of Cytotoxicity and Apoptosis of Encapsulated Egyptian Propolis on Hep-2 Cell Line. Pharmaceutics 2021, 13, 2184. [Google Scholar] [CrossRef]
- Sheikh, A.; Md, S.; Kesharwani, P. RGD engineered dendrimer nanotherapeutic as an emerging targeted approach in cancer therapy. J. Control Release 2021, 340, 221–242. [Google Scholar] [CrossRef]
- Bhagwat, G.S.; Athawale, R.B.; Gude, R.P.; Md, S.; Alhakamy, N.A.; Fahmy, U.A.; Kesharwani, P. Formulation and Development of Transferrin Targeted Solid Lipid Nanoparticles for Breast Cancer Therapy. Front. Pharmacol. 2020, 11, 614290. [Google Scholar] [CrossRef]
- Devi, L.; Gupta, R.; Jain, S.K.; Singh, S.; Kesharwani, P. Synthesis, characterization and in vitro assessment of colloidal gold nanoparticles of Gemcitabine with natural polysaccharides for treatment of breast cancer. J. Drug Deliv. Sci. Technol. 2020, 56, 101565. [Google Scholar] [CrossRef]
- Jain, A.; Sharma, G.; Kushwah, V.; Garg, N.K.; Kesharwani, P.; Ghoshal, G.; Singh, B.; Shivhare, U.S.; Jain, S.; Katare, O.P. Methotrexate and beta-carotene loaded-lipid polymer hybrid nanoparticles: A preclinical study for breast cancer. Nanomedicine 2017, 12, 1851–1872. [Google Scholar] [CrossRef]
- Mady, F.M.; Ibrahim, S.R.; Abourehab, M.A.; Adv Biomed, J.; Sci, P. Development and evaluation of alginate-gum blend mucoadhesive microspheres for controlled release of Metformin Hydrochloride. J. Adv. Biomed. Pharm. Sci. 2021, 4, 111–118. [Google Scholar] [CrossRef]
- Abourehab, M.A.S.; Khames, A.; Genedy, S.; Mostafa, S.; Khaleel, M.A.; Omar, M.M.; El Sisi, A.M. Sesame Oil-Based Nanostructured Lipid Carriers of Nicergoline, Intranasal Delivery System for Brain Targeting of Synergistic Cerebrovascular Protection. Pharmaceutics 2021, 13, 581. [Google Scholar] [CrossRef]
- Yao, D.; Li, S.; Zhu, X.; Wu, J.; Tian, H. Tumor-cell targeting polydiacetylene micelles encapsulated with an antitumor drug for the treatment of ovarian cancer. Chem. Commun. 2017, 53, 1233–1236. [Google Scholar] [CrossRef]
- Croce, C.M. Oncogenes and cancer. N. Engl. J. Med. 2008, 358, 502–511. [Google Scholar] [CrossRef]
- Shim, G.; Kim, D.; Le, Q.-V.; Park, G.T.; Kwon, T.; Oh, Y.-K. Nonviral Delivery Systems For Cancer Gene Therapy: Strategies And Challenges. Curr. Gene Ther. 2018, 18, 3–20. [Google Scholar] [CrossRef]
- Ozpolat, B.; Sood, A.K.; Lopez-Berestein, G. Liposomal siRNA nanocarriers for cancer therapy. Adv. Drug Deliv. Rev. 2014, 66, 110–116. [Google Scholar] [CrossRef]
- Jain, A.; Jain, A.; Parajuli, P.; Mishra, V.; Ghoshal, G.; Singh, B.; Shivhare, U.S.; Katare, O.P.; Kesharwani, P. Recent advances in galactose-engineered nanocarriers for the site-specific delivery of siRNA and anticancer drugs. Drug Discov. Today 2017, 23, 960–973. [Google Scholar] [CrossRef] [PubMed]
- Mishra, V.; Kesharwani, P.; Jain, N.K. siRNA nanotherapeutics: A Trojan horse approach against HIV. Drug Discov. Today 2014, 19, 1913–1920. [Google Scholar] [CrossRef] [PubMed]
- Blanco, E.; Shen, H.; Ferrari, M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 2015, 33, 941–951. [Google Scholar] [CrossRef] [PubMed]
- Kesharwani, P.; Gothwal, A.; Iyer, A.K.; Jain, K.; Chourasia, M.K.; Gupta, U. Dendrimer nanohybrid carrier systems: An expanding horizon for targeted drug and gene delivery. Drug Discov. Today 2018, 23, 300–314. [Google Scholar] [CrossRef]
- Shi, J.; Kantoff, P.W.; Wooster, R.; Farokhzad, O.C. Cancer nanomedicine: Progress, challenges and opportunities. Nat. Rev. Cancer 2016, 17, 20–37. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Hirst, D.G.; O’Sullivan, J.M. Gold nanoparticles as novel agents for cancer therapy. BJR 2014, 85, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Tong, R.; Chiang, H.H.; Kohane, D.S. Photoswitchable nanoparticles for in vivo cancer chemotherapy. Proc. Natl. Acad. Sci. USA 2013, 110, 19048–19053. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Sun, W.; Zhong, J.; Yang, Q.; Zhu, X.; Zhou, Z.; Zhang, Z.; Huang, Y. Multistage Nanovehicle Delivery System Based on Stepwise Size Reduction and Charge Reversal for Programmed Nuclear Targeting of Systemically Administered Anticancer Drugs. Adv. Funct. Mater. 2015, 25, 4101–4113. [Google Scholar] [CrossRef]
- Wong, C.; Stylianopoulos, T.; Cui, J.; Martin, J.; Chauhan, V.P.; Jiang, W.; Popovíc, Z.; Jain, R.K.; Bawendi, M.G.; Fukumura, D. Multistage nanoparticle delivery system for deep penetration into tumor tissue. Proc. Natl. Acad. Sci. USA 2011, 108, 2426–2431. [Google Scholar] [CrossRef]
- Ruan, S.; He, Q.; Gao, H. Matrix metalloproteinase triggered size-shrinkable gelatin-gold fabricated nanoparticles for tumor microenvironment sensitive penetration and diagnosis of glioma. Nanoscale 2015, 7, 9487–9496. [Google Scholar] [CrossRef]
- Kaur, J.; Gulati, M.; Jha, N.K.; Disouza, J.; Patravale, V.; Dua, K.; Singh, S.K. Recent advances in developing polymeric micelles for treating cancer: Breakthroughs and bottlenecks in their clinical translation. Drug Discov. Today 2022, 27, 1495–1512. [Google Scholar] [CrossRef] [PubMed]
- Bashir, M.; Ahmad, J.; Asif, M.; Khan, S.U.D.; Irfan, M.; Ibrahim, A.Y.; Asghar, S.; Khan, I.U.; Iqbal, M.S.; Haseeb, A.; et al. Nanoemulgel, an Innovative Carrier for Diflunisal Topical Delivery with Profound Anti-Inflammatory Effect: In vitro and in vivo Evaluation. Int. J. Nanomed. 2021, 16, 1457–1472. [Google Scholar] [CrossRef] [PubMed]
- Islam, N.; Irfan, M.; Zahoor, A.F.; Iqbal, M.S.; Syed, H.K.; Khan, I.U.; Rasul, A.; Khan, S.U.D.; Alqahtani, A.M.; Ikram, M.; et al. Improved Bioavailability of Ebastine through Development of Transfersomal Oral Films. Pharmaceutics 2021, 13, 1315. [Google Scholar] [CrossRef] [PubMed]
- Gouda, A.A.; Amin, A.H.; Ali, I.S.; Al Malah, Z. Green Dispersive Micro Solid-Phase Extraction using Multiwalled Carbon Nanotubes for Preconcentration and Determination of Cadmium and Lead in Food, Water, and Tobacco Samples. Curr. Anal. Chem. 2018, 16, 381–392. [Google Scholar] [CrossRef]
- Begines, B.; Ortiz, T.; Pérez-Aranda, M.; Martínez, G.; Merinero, M.; Argüelles-Arias, F.; Alcudia, A. Polymeric Nanoparticles for Drug Delivery: Recent Developments and Future Prospects. Nanomaterials 2020, 10, 1403. [Google Scholar] [CrossRef]
- Singh, S.; Aldawsari, H.M.; Alam, A.; Alqarni, M.H.S.; Ranjan, S.; Kesharwani, P. Synthesis and antimicrobial activity of vancomycin–conjugated zinc coordination polymer nanoparticles against methicillin-resistant staphylococcus aureus. J. Drug Deliv. Sci. Technol. 2022, 70, 103255. [Google Scholar] [CrossRef]
- Farhoudi, L.; Kesharwani, P.; Majeed, M.; Johnston, T.P.; Sahebkar, A. Polymeric nanomicelles of curcumin: Potential applications in cancer. Int. J. Pharm. 2022, 617, 121622. [Google Scholar] [CrossRef]
- Singh, V.; Md, S.; Alhakamy, N.A.; Kesharwani, P. Taxanes loaded polymersomes as an emerging polymeric nanocarrier for cancer therapy. Eur. Polym. J. 2022, 162, 110883. [Google Scholar] [CrossRef]
- Ghosh, B.; Biswas, S. Polymeric micelles in cancer therapy: State of the art. J. Control Release 2021, 332, 127–147. [Google Scholar] [CrossRef]
- Singh, V.; Sahebkar, A.; Kesharwani, P. Poly (propylene imine) dendrimer as an emerging polymeric nanocarrier for anticancer drug and gene delivery. Eur. Polym. J. 2021, 158, 110683. [Google Scholar] [CrossRef]
- Wang, J.; Mao, W.; Lock, L.L.; Tang, J.; Sui, M.; Sun, W.; Cui, H.; Xu, D.; Shen, Y. The Role of Micelle Size in Tumor Accumulation, Penetration, and Treatment. ACS Nano 2015, 9, 7195–7206. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Alrobaian, M.M.; Molugulu, N.; Agrawal, N.; Numan, A.; Kesharwani, P. Pyramid-Shaped PEG-PCL-PEG Polymeric-Based Model Systems for Site-Specific Drug Delivery of Vancomycin with Enhance Antibacterial Efficacy. ACS Omega 2020, 5, 11935–11945. [Google Scholar] [CrossRef] [PubMed]
- Amjad, M.W.; Kesharwani, P.; Mohd Amin, M.C.I.; Iyer, A.K. Recent advances in the design, development, and targeting mechanisms of polymeric micelles for delivery of siRNA in cancer therapy. Prog. Polym. Sci. 2017, 64, 154–181. [Google Scholar] [CrossRef]
- Kaditi, E.; Mountrichas, G.; Pispas, S. Amphiphilic block copolymers by a combination of anionic polymerization and selective post-polymerization functionalization. Eur. Polym. J. 2011, 47, 415–434. [Google Scholar] [CrossRef]
- Shin, D.H.; Tam, Y.T.; Kwon, G.S. Polymeric micelle nanocarriers in cancer research. Front. Chem. Sci. Eng. 2016 103 2016, 10, 348–359. [Google Scholar] [CrossRef]
- Loureiro, J.A.; Gomes, B.; Coelho, M.A.N.; Do Carmo Pereira, M.; Rocha, S. Targeting nanoparticles across the blood–brain barrier with monoclonal antibodies. Nanomedicine 2014, 9, 709–722. [Google Scholar] [CrossRef] [PubMed]
- Jhaveri, A.M.; Torchilin, V.P. Multifunctional polymeric micelles for delivery of drugs and siRNA. Front. Pharmacol. 2014, 5, 77. [Google Scholar] [CrossRef]
- Ghezzi, M.; Pescina, S.; Padula, C.; Santi, P.; Del Favero, E.; Cantù, L.; Nicoli, S. Polymeric micelles in drug delivery: An insight of the techniques for their characterization and assessment in biorelevant conditions. J. Control Release 2021, 332, 312–336. [Google Scholar] [CrossRef]
- Meng, X.Y.; Li, J.J.; Ni, T.J.; Lu, X.; He, T.; Men, Z.N.; Liu, J.S.; Shen, T. Electro-responsive brain-targeting mixed micelles based on Pluronic F127 and d-α-tocopherol polyethylene glycol succinate–ferrocene. Colloids Surf. A Physicochem. Eng. Asp. 2020, 601, 124986. [Google Scholar] [CrossRef]
- Nair, H.A.; Rajawat, G.S.; Nagarsenker, M.S. Stimuli-responsive micelles: A nanoplatform for therapeutic and diagnostic applications. Drug Target. Stimuli Sensitive Drug Deliv. Syst. 2018, 2018, 303–342. [Google Scholar] [CrossRef]
- Kaur, J.; Mishra, V.; Singh, S.K.; Gulati, M.; Kapoor, B.; Chellappan, D.K.; Gupta, G.; Dureja, H.; Anand, K.; Dua, K.; et al. Harnessing amphiphilic polymeric micelles for diagnostic and therapeutic applications: Breakthroughs and bottlenecks. J. Control Release 2021, 334, 64–95. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Ma, B.; Hu, J.; Yu, T.; Zhuang, W.; Yang, L.; Li, G.; Wang, Y. Dual-Responsive Doxorubicin-Conjugated Polymeric Micelles with Aggregation-Induced Emission Active Bioimaging and Charge Conversion for Cancer Therapy. Bioconjug. Chem. 2018, 29, 4050–4061. [Google Scholar] [CrossRef] [PubMed]
- Lue, S.J.; Chen, C.-H.; Shih, C.-M. Tuning of Lower Critical Solution Temperature (LCST) of Poly(N-Isopropylacrylamide-co-Acrylic acid) Hydrogels. J. Macromol. Sci. 2011, 50, 563–579. [Google Scholar] [CrossRef]
- Bardajee, G.R.; Pourjavadi, A.; Ghavami, S.; Soleyman, R.; Jafarpour, F. UV-prepared salep-based nanoporous hydrogel for controlled release of tetracycline hydrochloride in colon. J. Photochem. Photobiol. B Biol. 2011, 102, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, R.V.; Boppana, R.; Krishna Mohan, G.; Mutalik, S.; Kalyane, N.V. pH-responsive interpenetrating network hydrogel beads of poly(acrylamide)-g-carrageenan and sodium alginate for intestinal targeted drug delivery: Synthesis, in vitro and in vivo evaluation. J. Colloid Interface Sci. 2012, 367, 509–517. [Google Scholar] [CrossRef]
- Li, G.; Song, S.; Zhang, T.; Qi, M.; Liu, J. pH-sensitive polyelectrolyte complex micelles assembled from CS-g-PNIPAM and ALG-g-P(NIPAM-co-NVP) for drug delivery. Int. J. Biol. Macromol. 2013, 62, 203–210. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhang, L.; Yang, T.; Wu, H. Stimuli-responsive polymeric micelles for drug delivery and cancer therapy. Int. J. Nanomed. 2018, 13, 2921–2942. [Google Scholar] [CrossRef]
- Huo, M.; Yuan, J.; Tao, L.; Wei, Y. Redox-responsive polymers for drug delivery: From molecular design to applications. Polym. Chem. 2014, 5, 1519–1528. [Google Scholar] [CrossRef]
- Bae, J.; Maurya, A.; Shariat-Madar, Z.; Murthy, S.N.; Jo, S. Novel Redox-Responsive Amphiphilic Copolymer Micelles for Drug Delivery: Synthesis and Characterization. AAPS J. 2015, 17, 1357–1368. [Google Scholar] [CrossRef]
- Wang, X.; Xuan, Z.; Zhu, X.; Sun, H.; Li, J.; Xie, Z. Near-infrared photoresponsive drug delivery nanosystems for cancer photo-chemotherapy. J. Nanobiotechnol. 2020, 18, 108. [Google Scholar] [CrossRef]
- Xiang, J.; Tong, X.; Shi, F.; Yan, Q.; Yu, B.; Zhao, Y. Near-infrared light-triggered drug release from UV-responsive diblock copolymer-coated upconversion nanoparticles with high monodispersity. J. Mater. Chem. B 2018, 6, 3531–3540. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Wang, G. NIR light-responsive nanocarriers for controlled release. J. Photochem. Photobiol. C Photochem. Rev. 2021, 47, 100420. [Google Scholar] [CrossRef]
- Yoo, J.; Park, C.; Yi, G.; Lee, D.; Koo, H. Active targeting strategies using biological ligands for nanoparticle drug delivery systems. Cancers 2019, 11, 640. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Lin, C.M.; Xu, Z.; Miao, L.; Wang, Y.; Huang, L. Co-delivery of cisplatin and rapamycin for enhanced anticancer therapy through synergistic effects and microenvironment modulation. ACS Nano 2014, 8, 4996–5009. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.Y.; Silvestre, O.F.; Huang, X.; Min, K.H.; Howard, G.P.; Hida, N.; Jin, A.J.; Carvajal, N.; Lee, S.W.; Hong, J.I.; et al. Versatile RNA interference nanoplatform for systemic delivery of RNAs. ACS Nano 2014, 8, 4559–4570. [Google Scholar] [CrossRef]
- Xu, X.; Xie, K.; Zhang, X.Q.; Pridgen, E.M.; Park, G.Y.; Cui, D.S.; Shi, J.; Wu, J.; Kantoff, P.W.; Lippard, S.J.; et al. Enhancing tumor cell response to chemotherapy through nanoparticle-mediated codelivery of siRNA and cisplatin prodrug. Proc. Natl. Acad. Sci. USA 2013, 110, 18638–18643. [Google Scholar] [CrossRef]
- Miao, L.; Guo, S.; Zhang, J.; Kim, W.Y.; Huang, L. Nanoparticles with Precise Ratiometric Co-Loading and Co-Delivery of Gemcitabine Monophosphate and Cisplatin for Treatment of Bladder Cancer. Adv. Funct. Mater. 2014, 24, 6601–6611. [Google Scholar] [CrossRef]
- Pacardo, D.B.; Ligler, F.S.; Gu, Z. Programmable nanomedicine: Synergistic and sequential drug delivery systems. Nanoscale 2015, 7, 3381–3391. [Google Scholar] [CrossRef]
- Li, Y.-T.; Qian, X.-J.; Yu, Y.; Li, Z.-H.; Wu, R.-Y.; Ji, J.; Jiao, L.; Li, X.; Kong, P.-F.; Chen, W.-D.; et al. EGFR tyrosine kinase inhibitors promote pro-caspase-8 dimerization that sensitizes cancer cells to DNA-damaging therapy. Oncotarget 2015, 6, 17491–17500. [Google Scholar] [CrossRef][Green Version]
- He, Y.; Su, Z.; Xue, L.; Xu, H.; Zhang, C. Co-delivery of erlotinib and doxorubicin by pH-sensitive charge conversion nanocarrier for synergistic therapy. J. Control Release 2016, 229, 80–92. [Google Scholar] [CrossRef]
- Ghildiyal, M.; Zamore, P.D. Small silencing RNAs: An expanding universe. Nat. Rev. Genet. 2009, 102, 94–108. [Google Scholar] [CrossRef] [PubMed]
- Stinson, S.; Lackner, M.R.; Adai, A.T.; Yu, N.; Kim, H.J.; O’Brien, C.; Spoerke, J.; Jhunjhunwala, S.; Boyd, Z.; Januario, T.; et al. TRPS1 targeting by miR-221/222 promotes the epithelial-to-mesenchymal transition in breast cancer. Sci. Signal. 2011, 4, ra41. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Liu, Z.; Zhao, Y.; Ding, Y.; Liu, H.; Xi, Y.; Xiong, W.; Li, G.; Lu, J.; Fodstad, O.; et al. MicroRNA-125b confers the resistance of breast cancer cells to paclitaxel through suppression of pro-apoptotic Bcl-2 antagonist killer 1 (Bak1) expression. J. Biol. Chem. 2010, 285, 21496–21507. [Google Scholar] [CrossRef]
- Galardi, S.; Mercatelli, N.; Giorda, E.; Massalini, S.; Frajese, G.V.; Ciafrè, S.A.; Farace, M.G. miR-221 and miR-222 expression affects the proliferation potential of human prostate carcinoma cell lines by targeting p27Kip1. J. Biol. Chem. 2007, 282, 23716–23724. [Google Scholar] [CrossRef]
- Shah, M.Y.; Calin, G.A. MicroRNAs miR-221 and miR-222: A new level of regulation in aggressive breast cancer. Genome Med. 2011, 3, 56. [Google Scholar] [CrossRef] [PubMed]
- Garofalo, M.; Di Leva, G.; Romano, G.; Nuovo, G.; Suh, S.S.; Ngankeu, A.; Taccioli, C.; Pichiorri, F.; Alder, H.; Secchiero, P.; et al. miR-221&222 regulate TRAIL resistance and enhance tumorigenicity through PTEN and TIMP3 downregulation. Cancer Cell 2009, 16, 498–509. [Google Scholar] [CrossRef] [PubMed]
- Le Sage, C.; Nagel, R.; Egan, D.A.; Schrier, M.; Mesman, E.; Mangiola, A.; Anile, C.; Maira, G.; Mercatelli, N.; Ciafrè, S.A.; et al. Regulation of the p27(Kip1) tumor suppressor by miR-221 and miR-222 promotes cancer cell proliferation. EMBO J. 2007, 26, 3699–3708. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, J.J.; Huang, L. Nanoparticles targeted with NGR motif deliver c-myc siRNA and doxorubicin for anticancer therapy. Mol. Ther. 2010, 18, 828–834. [Google Scholar] [CrossRef]
- Shi, S.; Han, L.; Deng, L.; Zhang, Y.; Shen, H.; Gong, T.; Zhang, Z.; Sun, X. Dual drugs (microRNA-34a and paclitaxel)-loaded functional solid lipid nanoparticles for synergistic cancer cell suppression. J. Control Release 2014, 194, 228–237. [Google Scholar] [CrossRef]
- Cheng, J.; Teply, B.A.; Sherifi, I.; Sung, J.; Luther, G.; Gu, F.X.; Levy-Nissenbaum, E.; Radovic-Moreno, A.F.; Langer, R.; Farokhzad, O.C. Formulation of functionalized PLGA-PEG nanoparticles for in vivo targeted drug delivery. Biomaterials 2007, 28, 869–876. [Google Scholar] [CrossRef]
- Pack, D.W.; Hoffman, A.S.; Pun, S.; Stayton, P.S. Design and development of polymers for gene delivery. Nat. Rev. Drug Discov. 2005, 4, 581–593. [Google Scholar] [CrossRef] [PubMed]
- Kingston, R.E.; Chen, C.A.; Rose, J.K. Calcium Phosphate Transfection. Curr. Protoc. Mol. Biol. 2003, 63, 9.1.1–9.1.11. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, E.H.; Kunou, M.; Nagaoka, M.; Kundu, A.K.; Hoshiba, T.; Akaike, T. High-efficiency gene delivery for expression in mammalian cells by nanoprecipitates of Ca-Mg phosphate. Gene 2004, 341, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Giger, E.V.; Puigmartí-Luis, J.; Schlatter, R.; Castagner, B.; Dittrich, P.S.; Leroux, J.C. Gene delivery with bisphosphonate-stabilized calcium phosphate nanoparticles. J. Control Release 2011, 150, 87–93. [Google Scholar] [CrossRef]
- Roy, I.; Mitra, S.; Maitra, A.; Mozumdar, S. Calcium phosphate nanoparticles as novel non-viral vectors for targeted gene delivery. Int. J. Pharm. 2003, 250, 25–33. [Google Scholar] [CrossRef]
- Li, J.; Yang, Y.; Huang, L. Calcium phosphate nanoparticles with an asymmetric lipid bilayer coating for siRNA delivery to the tumor. J. Control Release 2012, 158, 108–114. [Google Scholar] [CrossRef]
- Zhou, Z.; Kennell, C.; Lee, J.Y.; Leung, Y.K.; Tarapore, P. Calcium phosphate-polymer hybrid nanoparticles for enhanced triple negative breast cancer treatment via co-delivery of paclitaxel and miR-221/222 inhibitors. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 403–410. [Google Scholar] [CrossRef]
- Morton, S.W.; Lee, M.J.; Deng, Z.J.; Dreaden, E.C.; Siouve, E.; Shopsowitz, K.E.; Shah, N.J.; Yaffe, M.B.; Hammond, P.T. A Nanoparticle-Based Combination Chemotherapy Delivery System for Enhanced Tumor Killing by Dynamic Rewiring of Signaling Pathways. Sci. Signal. 2014, 7, ra44. [Google Scholar] [CrossRef]
- Zhou, Z.; Kennell, C.; Jafari, M.; Lee, J.Y.; Ruiz-Torres, S.J.; Waltz, S.E.; Lee, J.H. Sequential delivery of erlotinib and doxorubicin for enhanced triple negative Breast cancer treatment using polymeric nanoparticle. Int. J. Pharm. 2017, 530, 300–307. [Google Scholar] [CrossRef]
- Ng, K.K.; Lovell, J.F.; Zheng, G. Lipoprotein-Inspired Nanoparticles for Cancer Theranostics. Acc. Chem. Res. 2011, 44, 1105. [Google Scholar] [CrossRef]
- Silverstein, S.C.; Steinman, R.M.; Cohn, Z.A. Endocytosis. Annu. Rev. Biochem. 1977, 46, 669–722. [Google Scholar] [CrossRef] [PubMed]
- Bobrin, V.A.; Lin, Y.; He, J.; Qi, Y.; Gu, W.; Monteiro, M.J. Therapeutic Delivery of Polymeric Tadpole Nanostructures with High Selectivity to Triple Negative Breast Cancer Cells. Biomacromolecules 2020, 21, 4457–4468. [Google Scholar] [CrossRef]
- Naoum, G.E.; Tawadros, F.; Farooqi, A.A.; Qureshi, M.Z.; Tabassum, S.; Buchsbaum, D.J.; Arafat, W. Role of nanotechnology and gene delivery systems in TRAIL-based therapies. Ecancermedicalscience 2016, 10, 660. [Google Scholar] [CrossRef] [PubMed]
- Soria, J.C.; Márk, Z.; Zatloukal, P.; Szima, B.; Albert, I.; Juhász, E.; Pujol, J.L.; Kozielski, J.; Baker, N.; Smethurst, D.; et al. Randomized phase II study of dulanermin in combination with paclitaxel, carboplatin, and bevacizumab in advanced non-small-cell lung cancer. J. Clin. Oncol. 2011, 29, 4442–4451. [Google Scholar] [CrossRef]
- Nigam, N.; Grover, A.; Goyal, S.; Katiyar, S.P.; Bhargava, P.; Wang, P.C.; Sundar, D.; Kaul, S.C.; Wadhwa, R. Targeting Mortalin by Embelin Causes Activation of Tumor Suppressor p53 and Deactivation of Metastatic Signaling in Human Breast Cancer Cells. PLoS ONE 2015, 10, e0138192. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.L.; Jiang, L.; Qu, F.; Wang, Z.Y.; Zhao, L.M. Inhibitory effect of Embelin on human acute T cell lymphoma Jurkat cells through activation of the apoptotic pathway. Oncol. Lett. 2015, 10, 921–926. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, D.; Hu, J.; Ding, P.; Chen, M. Hyaluronic acid-coated pH sensitive poly (β-amino ester) nanoparticles for co-delivery of embelin and TRAIL plasmid for triple negative breast cancer treatment. Int. J. Pharm. 2020, 573, 118637. [Google Scholar] [CrossRef]
- Tang, S.; Yin, Q.; Su, J.; Sun, H.; Meng, Q.; Chen, Y.; Chen, L.; Huang, Y.; Gu, W.; Xu, M.; et al. Inhibition of metastasis and growth of breast cancer by pH-sensitive poly (β-amino ester) nanoparticles co-delivering two siRNA and paclitaxel. Biomaterials 2015, 48, 1–15. [Google Scholar] [CrossRef]
- Tang, S.; Yin, Q.; Zhang, Z.; Gu, W.; Chen, L.; Yu, H.; Huang, Y.; Chen, X.; Xu, M.; Li, Y. Co-delivery of doxorubicin and RNA using pH-sensitive poly (β-amino ester) nanoparticles for reversal of multidrug resistance of breast cancer. Biomaterials 2014, 35, 6047–6059. [Google Scholar] [CrossRef]
- Badwaik, V.; Liu, L.; Gunasekera, D.; Kulkarni, A.; Thompson, D.H. Mechanistic Insight into Receptor-Mediated Delivery of Cationic-β-Cyclodextrin:Hyaluronic Acid-Adamantamethamidyl Host:Guest pDNA Nanoparticles to CD44(+) Cells. Mol. Pharm. 2016, 13, 1176–1184. [Google Scholar] [CrossRef]
- Lin, W.J.; Lee, W.C.; Shieh, M.J. Hyaluronic acid conjugated micelles possessing CD44 targeting potential for gene delivery. Carbohydr. Polym. 2017, 155, 101–108. [Google Scholar] [CrossRef]
- Ghosh, S.C.; Neslihan Alpay, S.; Klostergaard, J. CD44: A validated target for improved delivery of cancer therapeutics. Expert Opin. Ther. Targets 2012, 16, 635–650. [Google Scholar] [CrossRef] [PubMed]
- Rather, R.A.; Bhagat, M. Cancer Chemoprevention and Piperine: Molecular Mechanisms and Therapeutic Opportunities. Front. Cell Dev. Biol. 2018, 6, 10. [Google Scholar] [CrossRef] [PubMed]
- Lai, L.H.; Fu, Q.H.; Liu, Y.; Jiang, K.; Guo, Q.M.; Chen, Q.Y.; Yan, B.; Wang, Q.Q.; Shen, J.G. Piperine suppresses tumor growth and metastasis in vitro and in vivo in a 4T1 murine breast cancer model. Acta Pharmacol. Sin. 2012, 33, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Do, M.T.; Kim, H.G.; Choi, J.H.; Khanal, T.; Park, B.H.; Tran, T.P.; Jeong, T.C.; Jeong, H.G. Antitumor efficacy of piperine in the treatment of human HER2-overexpressing breast cancer cells. Food Chem. 2013, 141, 2591–2599. [Google Scholar] [CrossRef]
- Greenshields, A.L.; Doucette, C.D.; Sutton, K.M.; Madera, L.; Annan, H.; Yaffe, P.B.; Knickle, A.F.; Dong, Z.; Hoskin, D.W. Piperine inhibits the growth and motility of triple-negative breast cancer cells. Cancer Lett. 2015, 357, 129–140. [Google Scholar] [CrossRef]
- Khajuria, A.; Zutshi, U.; Bedi, K.L. Permeability characteristics of piperine on oral absorption—An active alkaloid from peppers and a bioavailability enhancer. Indian J. Exp. Biol. 1998, 36, 46–50. [Google Scholar]
- Jeong, K.; Kang, C.S.; Kim, Y.; Lee, Y.D.; Kwon, I.C.; Kim, S. Development of highly efficient nanocarrier-mediated delivery approaches for cancer therapy. Cancer Lett. 2016, 374, 31–43. [Google Scholar] [CrossRef]
- Rezvantalab, S.; Drude, N.I.; Moraveji, M.K.; Güvener, N.; Koons, E.K.; Shi, Y.; Lammers, T.; Kiessling, F. PLGA-based nanoparticles in cancer treatment. Front. Pharmacol. 2018, 9, 1260. [Google Scholar] [CrossRef]
- Rad, J.G.; Hoskin, D.W. Delivery of apoptosis-inducing piperine to triple-negative breast cancer cells via co-polymeric nanoparticles. Anticancer Res. 2020, 40, 689–694. [Google Scholar] [CrossRef]
- Cabral, H.; Matsumoto, Y.; Mizuno, K.; Chen, Q.; Murakami, M.; Kimura, M.; Terada, Y.; Kano, M.R.; Miyazono, K.; Uesaka, M.; et al. Accumulation of sub-100 nm polymeric micelles in poorly permeable tumours depends on size. Nat. Nanotechnol. 2011, 6, 815–823. [Google Scholar] [CrossRef] [PubMed]
- Ulbrich, K.; Šubr, V. Structural and chemical aspects of HPMA copolymers as drug carriers. Adv. Drug Deliv. Rev. 2010, 62, 150–166. [Google Scholar] [CrossRef] [PubMed]
- Kopeček, J.; Kopečková, P. HPMA copolymers: Origins, early developments, present, and future. Adv. Drug Deliv. Rev. 2010, 62, 122–149. [Google Scholar] [CrossRef] [PubMed]
- Hutanu, D.; Frishberg, M.D.; Guo, L.; Darie, C.C. Recent Applications of Polyethylene Glycols (PEGs) and PEG Derivatives. Mod. Chem. Appl. 2014, 2, 1000132. [Google Scholar] [CrossRef]
- Bobde, Y.; Patel, T.; Paul, M.; Biswas, S.; Ghosh, B. PEGylated N-(2 hydroxypropyl) methacrylamide polymeric micelles as nanocarriers for the delivery of doxorubicin in breast cancer. Colloids Surf. B Biointerfaces 2021, 204, 111833. [Google Scholar] [CrossRef]
- Choi, C.K.K.; Li, J.; Wei, K.; Xu, Y.J.; Ho, L.W.C.; Zhu, M.; To, K.K.W.; Choi, C.H.J.; Bian, L. A Gold@Polydopamine Core-Shell Nanoprobe for Long-Term Intracellular Detection of MicroRNAs in Differentiating Stem Cells. J. Am. Chem. Soc. 2015, 137, 7337–7346. [Google Scholar] [CrossRef]
- Kim, B.J.; Park, T.; Moon, H.C.; Park, S.Y.; Hong, D.; Ko, E.H.; Kim, J.Y.; Hong, J.W.; Han, S.W.; Kim, Y.G.; et al. Cytoprotective Alginate/Polydopamine Core/Shell Microcapsules in Microbial Encapsulation. Angew. Chem. Int. Ed. 2014, 53, 14443–14446. [Google Scholar] [CrossRef]
- Park, J.; Brust, T.F.; Lee, H.J.; Lee, S.C.; Watts, V.J.; Yeo, Y. Polydopamine-based simple and versatile surface modification of polymeric nano drug carriers. ACS Nano 2014, 8, 3347–3356. [Google Scholar] [CrossRef]
- Chang, D.; Gao, Y.; Wang, L.; Liu, G.; Chen, Y.; Wang, T.; Tao, W.; Mei, L.; Huang, L.; Zeng, X. Polydopamine-based surface modification of mesoporous silica nanoparticles as pH-sensitive drug delivery vehicles for cancer therapy. J. Colloid Interface Sci. 2016, 463, 279–287. [Google Scholar] [CrossRef]
- Liu, Y.; Ai, K.; Liu, J.; Deng, M.; He, Y.; Lu, L. Dopamine-Melanin Colloidal Nanospheres: An Efficient Near-Infrared Photothermal Therapeutic Agent for In Vivo Cancer Therapy. Adv. Mater. 2013, 25, 1353–1359. [Google Scholar] [CrossRef]
- Black, K.C.L.; Yi, J.; Rivera, J.G.; Zelasko-Leon, D.C.; Messersmith, P.B. Polydopamine-enabled surface functionalization of gold nanorods for cancer cell-targeted imaging and photothermal therapy. Nanomedicine 2012, 8, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Sharker, S.M.; Kim, S.M.; Lee, J.E.; Choi, K.H.; Shin, G.; Lee, S.; Lee, K.D.; Jeong, J.H.; Lee, H.; Park, S.Y. Functionalized biocompatible WO3 nanoparticles for triggered and targeted in vitro and in vivo photothermal therapy. J. Control Release 2015, 217, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Su, S.; Zhang, R.; Shao, L.; Zhang, Y.; Wang, B.; Li, Y.; Chen, L.; Yu, Q.; Wu, Y.; et al. Precision combination therapy for triple negative breast cancer via biomimetic polydopamine polymer core-shell nanostructures. Biomaterials 2017, 113, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, P.F.; Gulfam, M.; Monteiro, C.J.; Travanut, A.; Abelha, T.F.; Pearce, A.K.; Jerôme, C.; Grabowska, A.M.; Clarke, P.A.; Collins, H.M.; et al. Synthesis of micellar-like terpolymer nanoparticles with reductively-cleavable cross-links and evaluation of efficacy in 2D and 3D models of triple negative breast cancer. J. Control Release 2020, 323, 549–564. [Google Scholar] [CrossRef] [PubMed]
- Teesalu, T.; Sugahara, K.N.; Ruoslahti, E. Tumor-penetrating peptides. Front. Oncol. 2013, 3, 216. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, P.; Haldar, M.K.; Karandish, F.; Confeld, M.; Hossain, R.; Borowicz, P.; Gange, K.; Xia, L.; Sarkar, K.; Mallik, S. Tissue-Penetrating, Hypoxia-Responsive Echogenic Polymersomes For Drug Delivery To Solid Tumors. Chem. A Eur. J. 2018, 24, 12490–12494. [Google Scholar] [CrossRef]
- Sugahara, K.N.; Teesalu, T.; Karmali, P.P.; Kotamraju, V.R.; Agemy, L.; Girard, O.M.; Hanahan, D.; Mattrey, R.F.; Ruoslahti, E. Tissue-penetrating delivery of compounds and nanoparticles into tumors. Cancer Cell 2009, 16, 510–520. [Google Scholar] [CrossRef]
- Mamnoon, B.; Loganathan, J.; Confeld, M.I.; De Fonseka, N.; Feng, L.; Froberg, J.; Choi, Y.; Tuvin, D.M.; Sathish, V.; Mallik, S. Targeted Polymeric Nanoparticles for Drug Delivery to Hypoxic, Triple-Negative Breast Tumors. ACS Appl. Biol. Mater. 2021, 4, 1450–1460. [Google Scholar] [CrossRef]
- Coyle, C.; Cafferty, F.H.; Vale, C.; Langley, R.E. Metformin as an adjuvant treatment for cancer: A systematic review and meta-analysis. Ann. Oncol. 2016, 27, 2184–2195. [Google Scholar] [CrossRef]
- Wu, D.; Hu, D.; Chen, H.; Shi, G.; Fetahu, I.S.; Wu, F.; Rabidou, K.; Fang, R.; Tan, L.; Xu, S.; et al. Glucose-regulated phosphorylation of TET2 by AMPK reveals a pathway linking diabetes to cancer. Nature 2018, 559, 637–641. [Google Scholar] [CrossRef]
- Markowicz-Piasecka, M.; Huttunen, J.; Sikora, J.; Huttunen, K.M. Sulfenamide derivatives can improve transporter-mediated cellular uptake of metformin and induce cytotoxicity in human breast adenocarcinoma cell lines. Bioorg. Chem. 2019, 87, 321–334. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Towsey, M.; Xie, J.; Zhang, J.; Roe, P. Using multi-label classification for acoustic pattern detection and assisting bird species surveys. Appl. Acoust. 2016, 110, 91–98. [Google Scholar] [CrossRef]
- Yu, J.; Zhang, J.; Xing, H.; Yang, Z.; Cai, C.; Zhang, C.; Zhao, X.; Wei, M.; Yang, L.; Ding, P. Guanidinylated bioresponsive poly(amido amine)s designed for intranuclear gene delivery. Int. J. Nanomed. 2016, 11, 4011. [Google Scholar] [CrossRef] [PubMed]
- Shi, K.; Zhao, Y.; Miao, L.; Satterlee, A.; Haynes, M.; Luo, C.; Musetti, S.; Huang, L. Dual Functional LipoMET Mediates Envelope-type Nanoparticles to Combinational Oncogene Silencing and Tumor Growth Inhibition. Mol. Ther. 2017, 25, 1567–1579. [Google Scholar] [CrossRef]
- Lin, Y.; Li, Y.; Zeng, Y.; Tian, B.; Qu, X.; Yuan, Q.; Song, Y. Pharmacology, Toxicity, Bioavailability, and Formulation of Magnolol: An Update. Front. Pharmacol. 2021, 12, 394. [Google Scholar] [CrossRef]
- Xia, T.; Zhang, J.; Han, L.; Jin, Z.; Wang, J.; Li, X.; Man, S.; Liu, C.; Gao, W. Protective effect of magnolol on oxaliplatin-induced intestinal injury in mice. Phyther. Res. 2019, 33, 1161–1172. [Google Scholar] [CrossRef]
- Liu, Y.; Tong, Y.; Yang, X.; Li, F.; Zheng, L.; Liu, W.; Wu, J.; Ou, R.; Zhang, G.; Hu, M.; et al. Novel histone deacetylase inhibitors derived from Magnolia officinalis significantly enhance TRAIL-induced apoptosis in non-small cell lung cancer. Pharmacol. Res. 2016, 111, 113–125. [Google Scholar] [CrossRef]
- Chei, S.; Oh, H.J.; Song, J.H.; Seo, Y.J.; Lee, K.; Lee, B.Y. Magnolol Suppresses TGF-β-Induced Epithelial-to-Mesenchymal Transition in Human Colorectal Cancer Cells. Front. Oncol. 2019, 9, 752. [Google Scholar] [CrossRef]
- Chen, M.C.; Lee, C.F.; Huang, W.H.; Chou, T.C. Magnolol suppresses hypoxia-induced angiogenesis via inhibition of HIF-1α/VEGF signaling pathway in human bladder cancer cells. Biochem. Pharmacol. 2013, 85, 1278–1287. [Google Scholar] [CrossRef]
- Li, M.; Zhang, F.; Wang, X.; Wu, X.; Zhang, B.; Zhang, N.; Wu, W.; Wang, Z.; Weng, H.; Liu, S.; et al. Magnolol inhibits growth of gallbladder cancer cells through the p53 pathway. Cancer Sci. 2015, 106, 1341–1350. [Google Scholar] [CrossRef]
- Nabekura, T.; Hiroi, T.; Kawasaki, T.; Uwai, Y. Effects of natural nuclear factor-kappa B inhibitors on anticancer drug efflux transporter human P-glycoprotein. Biomed. Pharmacother. 2015, 70, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Sun, C.; Huang, L.; Liu, M.; Li, L.; Wang, X.; Wang, L.; Sun, S.; Xu, H.; Ma, G.; et al. Magnolol-loaded cholesteryl biguanide conjugate hydrochloride nanoparticles for triple-negative breast cancer therapy. Int. J. Pharm. 2022, 615, 121509. [Google Scholar] [CrossRef] [PubMed]
- Miao, L.; Newby, J.M.; Lin, C.M.; Zhang, L.; Xu, F.; Kim, W.Y.; Forest, M.G.; Lai, S.K.; Milowsky, M.I.; Wobker, S.E.; et al. The Binding Site Barrier Elicited by Tumor-Associated Fibroblasts Interferes Disposition of Nanoparticles in Stroma-Vessel Type Tumors. ACS Nano 2016, 10, 9243–9258. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.; Upadhyay, P.; Ghosh, A.; Bose, A.; Gupta, P.; Chattopadhyay, S.; Chattopadhyay, D.; Adhikary, A. Hyaluronic acid engrafted metformin loaded graphene oxide nanoparticle as CD44 targeted anti-cancer therapy for triple negative breast cancer. Biochim. Biophys. Acta Gen. Subj. 2021, 1865, 129841. [Google Scholar] [CrossRef] [PubMed]
- Depan, D.; Shah, J.; Misra, R.D.K. Controlled release of drug from folate-decorated and graphene mediated drug delivery system: Synthesis, loading efficiency, and drug release response. Mater. Sci. Eng. C 2011, 31, 1305–1312. [Google Scholar] [CrossRef]
- Pan, Y.; Sahoo, N.G.; Li, L. The application of graphene oxide in drug delivery. Expert Opin. Drug Deliv. 2012, 9, 1365–1376. [Google Scholar] [CrossRef]
- Layek, R.K.; Nandi, A.K. A review on synthesis and properties of polymer functionalized graphene. Polymer 2013, 54, 5087–5103. [Google Scholar] [CrossRef]
- Basu, A.; Upadhyay, P.; Ghosh, A.; Chattopadhyay, D.; Adhikary, A. Folic-Acid-Adorned PEGylated Graphene Oxide Interferes with the Cell Migration of Triple Negative Breast Cancer Cell Line, MDAMB-231 by Targeting miR-21/PTEN Axis through NFκB. ACS Biomater. Sci. Eng. 2019, 5, 373–389. [Google Scholar] [CrossRef]
- Fiorillo, M.; Verre, A.F.; Iliut, M.; Peiris-Pagés, M.; Ozsvari, B.; Gandara, R.; Cappello, A.R.; Sotgia, F.; Vijayaraghavan, A.; Lisanti, M.P. Graphene oxide selectively targets cancer stem cells, across multiple tumor types: Implications for non-toxic cancer treatment, via “differentiation-based nano-therapy”. Oncotarget 2015, 6, 3553. [Google Scholar] [CrossRef]
- Fang, L.; Zhao, Z.; Wang, J.; Xiao, P.; Sun, X.; Ding, Y.; Zhang, P.; Wang, D.; Li, Y. Light-controllable charge-reversal nanoparticles with polyinosinic-polycytidylic acid for enhancing immunotherapy of triple negative breast cancer. Acta Pharm. Sin. B 2022, 12, 353–363. [Google Scholar] [CrossRef]
- Chen, C.; Guo, Q.; Fu, H.; Yu, J.; Wang, L.; Sun, Y.; Zhang, J.; Duan, Y. Asynchronous blockade of PD-L1 and CD155 by polymeric nanoparticles inhibits triple-negative breast cancer progression and metastasis. Biomaterials 2021, 275, 120988. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Alakhova, D.Y.; Zhao, X.; Band, V.; Batrakova, E.V.; Kabanov, A.V. Eradication of cancer stem cells in triple negative breast cancer using doxorubicin/pluronic polymeric micelles. Nanomed. Nanotechnol. Biol. Med. 2020, 24, 102124. [Google Scholar] [CrossRef] [PubMed]
- Gener, P.; Montero, S.; Xandri-Monje, H.; Díaz-Riascos, Z.V.; Rafael, D.; Andrade, F.; Martínez-Trucharte, F.; González, P.; Seras-Franzoso, J.; Manzano, A.; et al. ZileutonTM loaded in polymer micelles effectively reduce breast cancer circulating tumor cells and intratumoral cancer stem cells. Nanomed. Nanotechnol. Biol. Med. 2020, 24, 102106. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Zhang, J.; Xu, R.; Li, Q.; Shen, Q.; Zhu, G. Nanoparticles based on polymers modified with pH-sensitive molecular switch and low molecular weight heparin carrying Celastrol and ferrocene for breast cancer treatment. Int. J. Biol. Macromol. 2021, 183, 2215–2226. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S.; Ghosh, A.; Maiti, S.; Ahir, M.; Debnath, G.H.; Gupta, P.; Bhattacharjee, M.; Ghosh, S.; Chattopadhyay, S.; Mukherjee, P.; et al. Delivery of thymoquinone through hyaluronic acid-decorated mixed Pluronic® nanoparticles to attenuate angiogenesis and metastasis of triple-negative breast cancer. J. Control. Release 2020, 322, 357–374. [Google Scholar] [CrossRef]
- Cé, R.; Couto, G.K.; Pacheco, B.Z.; Dallemole, D.R.; Paschoal, J.D.; Pacheco, B.S.; Guterres, S.S.; Seixas, F.; Collares, T.; Pohlmann, A.R. Folic acid-doxorubicin polymeric nanocapsules: A promising formulation for the treatment of triple-negative breast cancer. Eur. J. Pharm. Sci. 2021, 165, 105943. [Google Scholar] [CrossRef]
- Jin, F.; Qi, J.; Liu, D.; You, Y.; Shu, G.; Du, Y.; Wang, J.; Xu, X.; Ying, X.; Ji, J.; et al. Cancer-cell-biomimetic Upconversion nanoparticles combining chemo-photodynamic therapy and CD73 blockade for metastatic triple-negative breast cancer. J. Control. Release 2021, 337, 90–104. [Google Scholar] [CrossRef]
- Zhou, P.; Qin, J.; Zhou, C.; Wan, G.; Liu, Y.; Zhang, M.; Yang, X.; Zhang, N.; Wang, Y. Multifunctional nanoparticles based on a polymeric copper chelator for combination treatment of metastatic breast cancer. Biomaterials 2019, 195, 86–99. [Google Scholar] [CrossRef]
- Barroso-Sousa, R.; Keenan, T.E.; Pernas, S.; Exman, P.; Jain, E.; Garrido-Castro, A.C.; Hughes, M.; Bychkovsky, B.; Umeton, R.; Files, J.L.; et al. Tumor Mutational Burden and PTEN Alterations as Molecular Correlates of Response to PD-1/L1 Blockade in Metastatic Triple-Negative Breast Cancer. Clin. Cancer Res. 2020, 26, 2565–2572. [Google Scholar] [CrossRef]
- Savas, P.; Loi, S. Expanding the Role for Immunotherapy in Triple-Negative Breast Cancer. Cancer Cell 2020, 37, 623–624. [Google Scholar] [CrossRef]
- Tan, T.; Wang, Y.; Wang, J.; Wang, Z.; Wang, H.; Cao, H.; Li, J.; Li, Y.; Zhang, Z.; Wang, S. Targeting peptide-decorated biomimetic lipoproteins improve deep penetration and cancer cells accessibility in solid tumor. Acta Pharm. Sin. B 2020, 10, 529–545. [Google Scholar] [CrossRef] [PubMed]
- Kwapisz, D. Pembrolizumab and atezolizumab in triple-negative breast cancer. Cancer Immunol. Immunother. 2021, 70, 607–617. [Google Scholar] [CrossRef] [PubMed]
- Schmid, P.; Cortes, J.; Pusztai, L.; McArthur, H.; Kümmel, S.; Bergh, J.; Denkert, C.; Park, Y.H.; Hui, R.; Harbeck, N.; et al. Pembrolizumab for Early Triple-Negative Breast Cancer. N. Engl. J. Med. 2020, 382, 810–821. [Google Scholar] [CrossRef]
- Liu, J.; Iqbal, S.; Du, X.J.; Yuan, Y.; Yang, X.; Li, H.J.; Wang, J. Ultrafast charge-conversional nanocarrier for tumor-acidity-activated targeted drug elivery. Biomater. Sci. 2018, 6, 350–355. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Du, Y.; Liu, J.; Sun, H.; Wang, J.; Li, R.; Kim, D.; Hyeon, T.; Ling, D. Responsive Assembly of Upconversion Nanoparticles for pH-Activated and Near-Infrared-Triggered Photodynamic Therapy of Deep Tumors. Adv. Mater. 2018, 30, 1802808. [Google Scholar] [CrossRef]
- Dai, L.; Li, X.; Duan, X.; Li, M.; Niu, P.; Xu, H.; Cai, K.; Yang, H. A pH/ROS Cascade-Responsive Charge-Reversal Nanosystem with Self-Amplified Drug Release for Synergistic Oxidation-Chemotherapy. Adv. Sci. 2019, 6, 1801807. [Google Scholar] [CrossRef]
- Wang, C.; Sun, W.; Wright, G.; Wang, A.Z.; Gu, Z. Inflammation-Triggered Cancer Immunotherapy by Programmed Delivery of CpG and Anti-PD1 Antibody. Adv. Mater. 2016, 28, 8912–8920. [Google Scholar] [CrossRef]
- Phuengkham, H.; Song, C.; Um, S.H.; Lim, Y.T. Implantable Synthetic Immune Niche for Spatiotemporal Modulation of Tumor-Derived Immunosuppression and Systemic Antitumor Immunity: Postoperative Immunotherapy. Adv. Mater. 2018, 30, 1706719. [Google Scholar] [CrossRef]
- Deng, H.; Tan, S.; Gao, X.; Zou, C.; Xu, C.; Tu, K.; Song, Q.; Fan, F.; Huang, W.; Zhang, Z. Cdk5 knocking out mediated by CRISPR-Cas9 genome editing for PD-L1 attenuation and enhanced antitumor immunity. Acta Pharm. Sin. B 2020, 10, 358–373. [Google Scholar] [CrossRef]
- Wang, D.; Wang, T.; Liu, J.; Yu, H.; Jiao, S.; Feng, B.; Zhou, F.; Fu, Y.; Yin, Q.; Zhang, P.; et al. Acid-Activatable Versatile Micelleplexes for PD-L1 Blockade-Enhanced Cancer Photodynamic Immunotherapy. Nano Lett. 2016, 16, 5503–5513. [Google Scholar] [CrossRef]
- Li, Z.; Ho, W.; Bai, X.; Li, F.; Chen, Y.J.; Zhang, X.Q.; Xu, X. Nanoparticle depots for controlled and sustained gene delivery. J. Control. Release 2020, 322, 622–631. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Li, D.; Cao, Z.; Xiong, M.; Yang, X.; Wang, J. Facile Hydrophobization of siRNA with Anticancer Drug for Non-Cationic Nanocarrier-Mediated Systemic Delivery. Nano Lett. 2019, 19, 2688–2693. [Google Scholar] [CrossRef] [PubMed]
- Stubelius, A.; Lee, S.; Almutairi, A. The Chemistry of Boronic Acids in Nanomaterials for Drug Delivery. Acc. Chem. Res. 2019, 52, 3108–3119. [Google Scholar] [CrossRef] [PubMed]
- Meng, Z.; Zhou, X.; Xu, J.; Han, X.; Dong, Z.; Wang, H.; Zhang, Y.; She, J.; Xu, L.; Wang, C.; et al. Light-Triggered In Situ Gelation to Enable Robust Photodynamic-Immunotherapy by Repeated Stimulations. Adv. Mater. 2019, 31, 1900927. [Google Scholar] [CrossRef]
- Zhang, P.; Zhai, Y.; Cai, Y.; Zhao, Y.; Li, Y. Nanomedicine-Based Immunotherapy for the Treatment of Cancer Metastasis. Adv. Mater. 2019, 31, 1904156. [Google Scholar] [CrossRef]
- Hwang, S.Y.; Park, S.; Kwon, Y. Recent therapeutic trends and promising targets in triple negative breast cancer. Pharmacol. Ther. 2019, 199, 30–57. [Google Scholar] [CrossRef]
- Emens, L.A. Breast cancer immunotherapy: Facts and hopes. Clin. Cancer Res. 2018, 24, 511–520. [Google Scholar] [CrossRef]
- Voorwerk, L.; Slagter, M.; Horlings, H.M.; Sikorska, K.; van de Vijver, K.K.; de Maaker, M.; Nederlof, I.; Kluin, R.J.C.; Warren, S.; Ong, S.F.; et al. Immune induction strategies in metastatic triple-negative breast cancer to enhance the sensitivity to PD-1 blockade: The TONIC trial. Nat. Med. 2019 256 2019, 25, 920–928. [Google Scholar] [CrossRef]
- Adams, S.; Loi, S.; Toppmeyer, D.; Cescon, D.W.; De Laurentiis, M.; Nanda, R.; Winer, E.P.; Mukai, H.; Tamura, K.; Armstrong, A.; et al. Pembrolizumab monotherapy for previously untreated, PD-L1-positive, metastatic triple-negative breast cancer: Cohort B of the phase II KEYNOTE-086 study. Ann. Oncol. 2019, 30, 405–411. [Google Scholar] [CrossRef]
- Gao, J.; Zheng, Q.; Xin, N.; Wang, W.; Zhao, C. CD155, an onco-immunologic molecule in human tumors. Cancer Sci. 2017, 108, 1934–1938. [Google Scholar] [CrossRef]
- O’Donnell, J.S.; Madore, J.; Li, X.Y.; Smyth, M.J. Tumor intrinsic and extrinsic immune functions of CD155. Semin. Cancer Biol. 2020, 65, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Tahara-Hanaoka, S.; Shibuya, K.; Kai, H.; Miyamoto, A.; Morikawa, Y.; Ohkochi, N.; Honda, S.I.; Shibuya, A. Tumor rejection by the poliovirus receptor family ligands of the DNAM-1 (CD226) receptor. Blood 2006, 107, 1491–1496. [Google Scholar] [CrossRef]
- Gilfillan, S.; Chan, C.J.; Cella, M.; Haynes, N.M.; Rapaport, A.S.; Boles, K.S.; Andrews, D.M.; Smyth, M.J.; Colonna, M. DNAM-1 promotes activation of cytotoxic lymphocytes by nonprofessional antigen-presenting cells and tumors. J. Exp. Med. 2008, 205, 2965–2973. [Google Scholar] [CrossRef]
- Li, X.Y.; Das, I.; Lepletier, A.; Addala, V.; Bald, T.; Stannard, K.; Barkauskas, D.; Liu, J.; Aguilera, A.R.; Takeda, K.; et al. CD155 loss enhances tumor suppression via combined host and tumor-intrinsic mechanisms. J. Clin. Investig. 2018, 128, 2613–2625. [Google Scholar] [CrossRef]
- Dougall, W.C.; Kurtulus, S.; Smyth, M.J.; Anderson, A.C. TIGIT and CD96: New checkpoint receptor targets for cancer immunotherapy. Immunol. Rev. 2017, 276, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Manieri, N.A.; Chiang, E.Y.; Grogan, J.L. TIGIT: A Key Inhibitor of the Cancer Immunity Cycle. Trends Immunol. 2017, 38, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Sun, Y.; Shen, M.; Song, K.; Yin, X.; Di, W.; Duan, Y. Enhanced Chemotherapeutic Efficacy of Paclitaxel Nanoparticles Co-delivered with MicroRNA-7 by Inhibiting Paclitaxel-Induced EGFR/ERK pathway Activation for Ovarian Cancer Therapy. ACS Appl. Mater. Interfaces 2018, 10, 7821–7831. [Google Scholar] [CrossRef]
- Tang, D.G. Understanding cancer stem cell heterogeneity and plasticity. Cell Res. 2012, 22, 457–472. [Google Scholar] [CrossRef]
- Singh, A.; Settleman, J. EMT, cancer stem cells and drug resistance: An emerging axis of evil in the war on cancer. Oncogene 2010, 29, 4741–4751. [Google Scholar] [CrossRef]
- DeVita, V.T.; Rosenberg, S.A. Two Hundred Years of Cancer Research. N. Engl. J. Med. 2012, 366, 2207–2214. [Google Scholar] [CrossRef]
- Valle, J.W.; Armstrong, A.; Newman, C.; Alakhov, V.; Pietrzynski, G.; Brewer, J.; Campbell, S.; Corrie, P.; Rowinsky, E.K.; Ranson, M. A phase 2 study of SP1049C, doxorubicin in P-glycoprotein-targeting pluronics, in patients with advanced adenocarcinoma of the esophagus and gastroesophageal junction. Investig. New Drugs 2011, 29, 1029–1037. [Google Scholar] [CrossRef] [PubMed]
- Alakhova, D.Y.; Zhao, Y.; Li, S.; Kabanov, A.V. Effect of Doxorubicin/Pluronic SP1049C on Tumorigenicity, Aggressiveness, DNA Methylation and Stem Cell Markers in Murine Leukemia. PLoS ONE 2013, 8, e72238. [Google Scholar] [CrossRef] [PubMed]
- Hussain, Z.; Thu, H.E.; Elsayed, I.; Abourehab, M.A.S.; Khan, S.; Sohail, M.; Sarfraz, R.M.; Farooq, M.A. Nano-scaled materials may induce severe neurotoxicity upon chronic exposure to brain tissues: A critical appraisal and recent updates on predisposing factors, underlying mechanism, and future prospects. J. Control. Release 2020, 328, 873–894. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.; Hall, R.R., III; Ahmed, A.U. Cancer Stem Cells: Cellular Plasticity, Niche, and its Clinical Relevance. J. Stem Cell Res. Ther. 2016, 6, 363. [Google Scholar] [CrossRef]
- Imran, A.; Butt, M.S.; Xiao, H.; Imran, M.; Rauf, A.; Mubarak, M.S.; Ramadan, M.F. Inhibitory effect of black tea (Camellia sinensis) theaflavins and thearubigins against HCT 116 colon cancer cells and HT 460 lung cancer cells. J. Food Biochem. 2019, 43, e12822. [Google Scholar] [CrossRef] [PubMed]
- Gener, P.; Gouveia, L.P.; Sabat, G.R.; de Sousa Rafael, D.F.; Fort, N.B.; Arranja, A.; Fernández, Y.; Prieto, R.M.; Ortega, J.S.; Arango, D.; et al. Fluorescent CSC models evidence that targeted nanomedicines improve treatment sensitivity of breast and colon cancer stem cells. Nanomedicine 2015, 11, 1883–1892. [Google Scholar] [CrossRef]
- Rafael, D.; Andrade, F.; Montero, S.; Gener, P.; Seras-Franzoso, J.; Martínez, F.; González, P.; Florindo, H.; Arango, D.; Sayós, J.; et al. Rational Design of a siRNA Delivery System: ALOX5 and Cancer Stem Cells as Therapeutic Targets. Precis. Nanomed. 2018, 1, 86–105. [Google Scholar] [CrossRef]
- Carey, L.; Winer, E.; Viale, G.; Cameron, D.; Gianni, L. Triple-negative breast cancer: Disease entity or title of convenience? Nat. Rev. Clin. Oncol. 2010, 712, 683–692. [Google Scholar] [CrossRef]
- Cordani, M.; Butera, G.; Pacchiana, R.; Masetto, F.; Mullappilly, N.; Riganti, C.; Donadelli, M. Mutant p53-Associated Molecular Mechanisms of ROS Regulation in Cancer Cells. Biomolecules 2020, 10, 361. [Google Scholar] [CrossRef]
- Prasad, S.; Gupta, S.C.; Tyagi, A.K. Reactive oxygen species (ROS) and cancer: Role of antioxidative nutraceuticals. Cancer Lett. 2017, 387, 95–105. [Google Scholar] [CrossRef]
- Fan, H.J.; Huang, S.T.; Chung, W.H.; Jan, J.L.; Lin, W.Y.; Chen, C.C. Degradation pathways of crystal violet by Fenton and Fenton-like systems: Condition optimization and intermediate separation and identification. J. Hazard. Mater. 2009, 171, 1032–1044. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Wang, Y.; Wu, R.; Xu, C.; Nie, J.J.; Zhao, N.; Yu, B.; Liu, Z.; Xu, F.J. Oxidation-Responsive Nanoassemblies for Light-Enhanced Gene Therapy. Small 2019, 15, 1904017. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.; Wu, Z.; Wang, L.; Kanai, Y.; He, X. CYP450s-Activity Relations of Celastrol to Interact with Triptolide Reveal the Reasons of Hepatotoxicity of Tripterygium wilfordii. Molecules 2019, 24, 2162. [Google Scholar] [CrossRef] [PubMed]
- Gannimani, R.; Walvekar, P.; Naidu, V.R.; Aminabhavi, T.M.; Govender, T. Acetal containing polymers as pH-responsive nano-drug delivery systems. J. Control. Release 2020, 328, 736–761. [Google Scholar] [CrossRef] [PubMed]
- Du, S.; Yu, Y.; Xu, C.; Xiong, H.; Yang, S.; Yao, J. LMWH and its derivatives represent new rational for cancer therapy: Construction strategies and combination therapy. Drug Discov. Today 2019, 24, 2096–2104. [Google Scholar] [CrossRef]
- Sharma, A.; Tandon, A.; Tovey, J.C.K.; Gupta, R.; Robertson, J.D.; Fortune, J.A.; Klibanov, A.M.; Cowden, J.W.; Rieger, F.G.; Mohan, R.R. Polyethylenimine-conjugated gold nanoparticles: Gene transfer potential and low toxicity in the cornea. Nanomed. Nanotechnol. Biol. Med. 2011, 7, 505–513. [Google Scholar] [CrossRef]
- Claesson-Welsh, L.; Welsh, M. VEGFA and tumour angiogenesis. J. Intern. Med. 2013, 273, 114–127. [Google Scholar] [CrossRef]
- Paul, C.D.; Mistriotis, P.; Konstantopoulos, K. Cancer cell motility: Lessons from migration in confined spaces. Nat. Rev. Cancer 2016 172 2016, 17, 131–140. [Google Scholar] [CrossRef]
- Burridge, K.; Wennerberg, K. Rho and Rac Take Center Stage. Cell 2004, 116, 167–179. [Google Scholar] [CrossRef]
- Kovac, B.; Teo, J.L.; Mäkelä, T.P.; Vallenius, T. Assembly of non-contractile dorsal stress fibers requires α-actinin-1 and Rac1 in migrating and spreading cells. J. Cell Sci. 2013, 126, 263–273. [Google Scholar] [CrossRef]
- Salmani, J.M.M.; Asghar, S.; Lv, H.; Zhou, J. Aqueous Solubility and Degradation Kinetics of the Phytochemical Anticancer Thymoquinone; Probing the Effects of Solvents, pH and Light. Molecules 2014, 19, 5925–5939. [Google Scholar] [CrossRef] [PubMed]
- Hijaz, M.; Das, S.; Mert, I.; Gupta, A.; Al-Wahab, Z.; Tebbe, C.; Dar, S.; Chhina, J.; Giri, S.; Munkarah, A.; et al. Folic acid tagged nanoceria as a novel therapeutic agent in ovarian cancer. BMC Cancer 2016, 16, 220. [Google Scholar] [CrossRef]
- Bazak, R.; Houri, M.; El Achy, S.; Kamel, S.; Refaat, T. Cancer active targeting by nanoparticles: A comprehensive review of literature. J. Cancer Res. Clin. Oncol. 2014, 141, 769–784. [Google Scholar] [CrossRef] [PubMed]
- Cé, R.; Lavayen, V.; Couto, G.K.; De Marchi, J.G.B.; Pacheco, B.Z.; Natividade, L.A.; Fracari, T.O.; Ciocheta, T.M.; de Cristo Soares Alves, A.; Jornada, D.S.; et al. Folic Acid-Doxorubicin-Double-Functionalized-Lipid-Core Nanocapsules: Synthesis, Chemical Structure Elucidation, and Cytotoxicity Evaluation on Ovarian (OVCAR-3) and Bladder (T24) Cancer Cell Lines. Pharm. Res. 2021, 38, 301–317. [Google Scholar] [CrossRef] [PubMed]
- Than, N.G.; Romero, R.; Tarca, A.L.; Draghici, S.; Erez, O.; Chaiworapongsa, T.; Mee Kim, Y.; Kim, S.K.; Vaisbuch, E.; Tromp, G. Mitochondrial manganese superoxide dismutase mRNA expression in human chorioamniotic membranes and its association with labor, inflammation, and infection. J. Matern.-Fet. Neonatal Med. 2010, 22, 1000–1013. [Google Scholar] [CrossRef] [PubMed]
- Castano, A.P.; Demidova, T.N.; Hamblin, M.R. Mechanisms in photodynamic therapy: Part one—-photosensitizers, photochemistry and cellular localization. Photodiagnosis Photodyn. Ther. 2004, 1, 279–293. [Google Scholar] [CrossRef]
- Khaldoyanidi, S.K.; Glinsky, V.V.; Sikora, L.; Glinskii, A.B.; Mossine, V.V.; Quinn, T.P.; Glinsky, G.V.; Sriramarao, P. MDA-MB-435 Human Breast Carcinoma Cell Homo- and Heterotypic Adhesion under Flow Conditions Is Mediated in Part by Thomsen-Friedenreich Antigen-Galectin-3 Interactions. J. Biol. Chem. 2003, 278, 4127–4134. [Google Scholar] [CrossRef]
- Stagg, J.; Divisekera, U.; McLaughlin, N.; Sharkey, J.; Pommey, S.; Denoyer, D.; Dwyer, K.M.; Smyth, M.J. Anti-CD73 antibody therapy inhibits breast tumor growth and metastasis. Proc. Natl. Acad. Sci. USA 2010, 107, 1547–1552. [Google Scholar] [CrossRef]
- Boal, A.K.; Rosenzweig, A.C. Structural biology of copper trafficking. Chem. Rev. 2009, 109, 4760–4779. [Google Scholar] [CrossRef]
- Cho, H.Y.; Blum, R.A.; Sunderland, T.; Cooper, G.J.S.; Jusko, W.J. Pharmacokinetic and pharmacodynamic modeling of a copper-selective chelator (TETA) in healthy adults. J. Clin. Pharmacol. 2009, 49, 916–928. [Google Scholar] [CrossRef]





| Therapeutic Moiety | Polymer Used | Additional Chemical Moiety | Final Preparation | Type of Study | Cell Line Used | Animals Used | Outcomes Obtained | Ref |
|---|---|---|---|---|---|---|---|---|
| POLYMERIC NANOPARTICLES FOR THE TREATMENT OF TRIPLE-NEGATIVE BREAST CANCER | ||||||||
| Paclitaxel (PTX) and miRNAi-221/222 | PEG-PLGA | Calcium phosphate | miRNAi-221/222 encapsulated in calcium phosphate, PTX encapsulated in DOPA, both further encapsulated in PEG-PLGA nanoparticles | In vitro | MDA-MB-231 cell line | - | Synergistic action of combining a conventional chemotherapeutic drug (PTX) with RNA interference (miRNAi-221/222) was achieved. This combination helped in reducing the dose of potent cytotoxic drugs without compromising its cytotoxicity. | [107] |
| Doxorubicin (DXN), Erlotinib (ETB) | PLA-b-PEG | PLA-b-PEG nanoparticle | In vitro and in vivo | MDA-MB-231 cell line | R7 cell line containing FBV female mice | Formulated polymeric nanoparticles co-localized inside the tumor, showed improved therapeutic efficacy and minimum systemic toxicity by differentiating tumor tissues from the healthy ones | [109] | |
| Doxorubicin (DXN), | poly(N-isopropylacrylamide) | - | poly(N-isopropylacrylamide)-coated DXN-loaded tadpole-shaped nanostructure | In vitro | MDA-MB-231 cell line | - | Tadpole shape exhibited about 15 times increase in NPs cellular uptake in comparison to spherical-shaped NPs composed of same polymer. | [112]. |
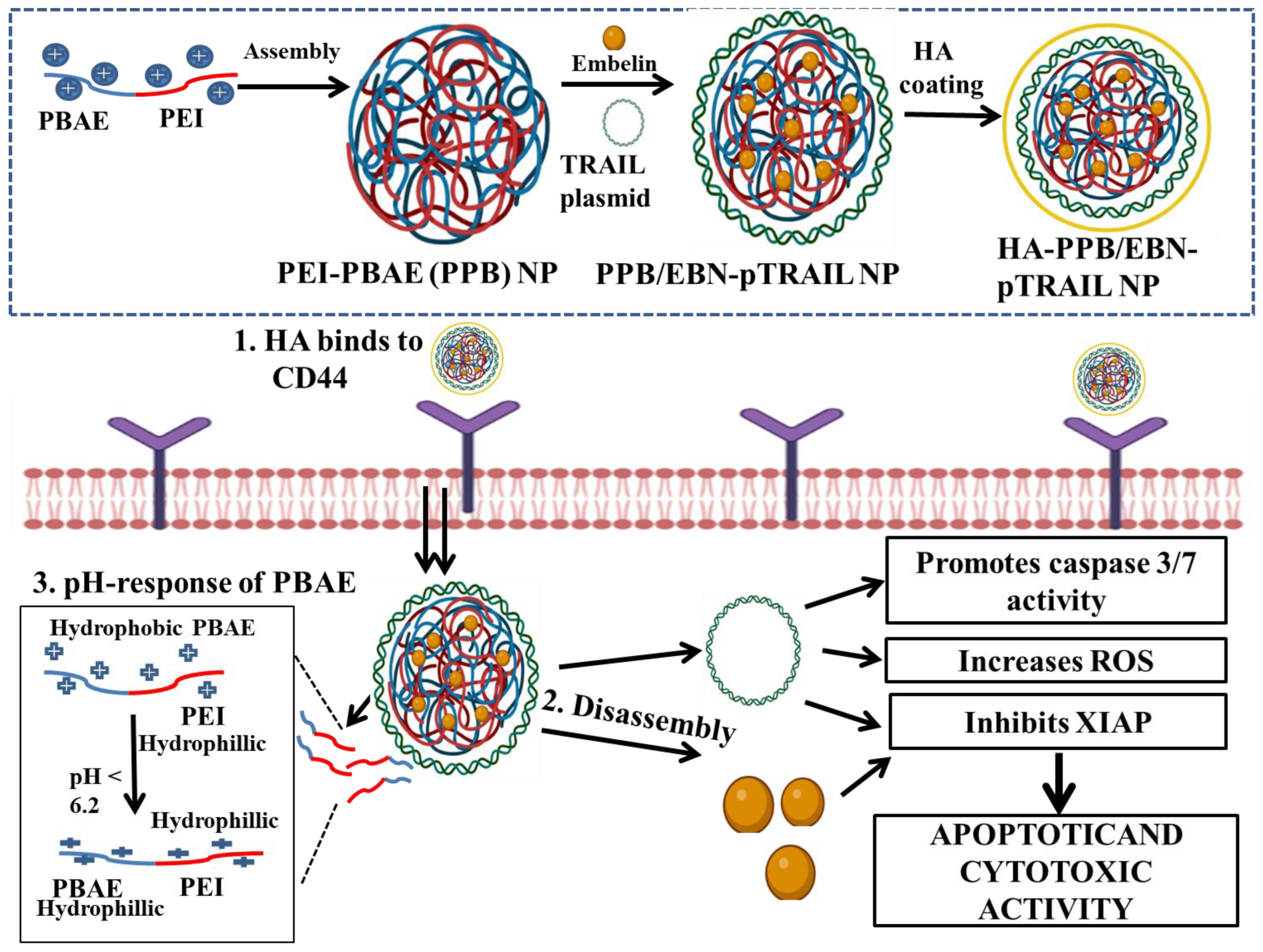
| Embelin (EBN) and TRAIL plasmid | PEI and PBAE | Hyaluronic acid (HA) | HA-coated PEI and PBAE nanoparticles (HA-PPB NPs) | In vitro | CD44 over-expressing MDA-MB-231 | - | HA-PPB NPs acted as a potent carrier for co-transporting therapeutic genes along with anti-cancer drugs. Combination of pTRAIL and EBN can be used as a potent synergistic therapy for TNBC treatment. | [117] |
| Piperine (PPN) | PEI–PLGA | PPN-loaded PLGA–mPEG co-polymers nanoparticle (PPP-NPs) | In vitro | BT-549 and MDA-MB-468 cell line | - | Polymeric nanoparticles helped in delivering hydrophobic PPN without comprising with its cytotoxicity | [130] | |
| Doxorubicin (DXN) | HPMA and mPEG | - | DXN -encapsulating HPMA-b-methoxy PEG co-block polymeric micelles | In vitro and in vivo | 4T1 murine and MDA-MB 231 and MCF-7 cell line | Female Wistar rats | Micelles formed using polymer with HPMA:mPEG and drug:polymer ratio of 175:1 and 1:10, respectively resulted in nanoparticles with particle size distribution of narrow range and highest drug loading, compared to other synthesized ranges of polymer. | [135] |
| Doxorubicin (DXN) and Paclitaxel (PTX) | (P MEO2MA-co-OEGMA-co-DMAEMA-b-PLGA) | Poly-dopamine (PDA) | DXN and PTX encapsulated encapsulating (P MEO2MA-co-OEGMA-co-DMAEMA-b-PLGA) co-polymer nanoparticles, surface modified with PDA | In vitro and in vivo | MDA-MB-231 tumor cell line | Female Balb/C mice | Surface-modified PDA prevented burst release of drug from the nanocomposite. Nanoparticles produced sufficient heat required for photothermal therapy, under localized NIR irradiation and thus precise thermo-responsive drug release was achieved. | [143] |
| Docetaxel (DCL) | Poly(ethyleneglycol)-b-poly (lactide)-co-poly(N3-alpha-ε-caprolactone) | Docetaxel-loaded micellar-like nanoparticles (MR-NPs) | In vitro | TNBC cell line (MDA-MB-231), breast cancer cell line (MCF7) and normal breast cell line (MCF10A) | - | Docetaxel-loaded MR-NPs with reducible cross-links exhibited greater efficacy in 2D, 3D in vitro TNBC models by acting against the abnormal cell biology of TNBC. | [144] | |
| Doxorubicin (DXN) | PLA-diazobenzene-PEG di-block co-polymer | iRGD peptide | DXN encapsulating self-assembled hypoxia-sensitive polymersomes (PMs), surface-conjugated with iRGD | In vitro and in vivo | MDA-MB-231 cell line | Female nude mice | Significant enhancement in drug release from the polymersomes in the hypoxic environment was observed | [148] |
| Cholesteryl biguanide-conjugated hydrochloride (CBCH) and Magnolol (MGL) | mPEG-PLGA | Aminoethyl anisamide ligand | AEAD-PEG-PLGA conjugated mPEG–PLGA-coated CBCH and MGL nanomicelles | In vitro and in vivo | Murine 4T1 TNBC cell line | Female Balb/c mouse model | An effective NP system for TNBC treatment was formulated | [162] |
| Metformin | PEG–PLGA | Hyaluronic acid (HA) nanoparticles | Metformin-encapsulated graphene oxide NPs | In vitro and in vivo | Murine 4T1 TNBC cell line | Female Balb/c mouse model | Novel graphene oxide nanoparticles successfully encapsulated metformin and exerted anti-cancer activity against TNBC cell line | [164] |
| POLYMERIC NANOPARTICLES FOR TRIPLE-NEGATIVE BREAST CANCER IMMUNOTHERAPY | ||||||||
| Polyinosinic–polycytidylic acid 17[Poly (I–C)] | PVA and PEI chlorin e6 (PEI-C-e6) | NIR light-regulated charge-reversal [Poly(I–C)] nanoparticles (NCRNPs-[Poly(I–C)]) | In vitro and in vivo | Murine 4T1 TNBC cell line | Female Balb/c mouse model | The NCRNPs-[Poly(I–C)] provide a promising strategy for the controlled release of nucleic acid-based immunomodulators that may improve the photodynamic cancer immunotherapy of TNBC | [170] | |
| CD155 siRNA and PD-L1 antibodies | mPEG-PLL-PLGA (PPGPL) | PPGPL-CD155si/P nanoparticles | In vitro and in vivo | Murine 4T1 cell line | 4T1 tumor bearing Female Balb/c mice | A potent combination approach for immunotherapy treating PD-L1/CD155+ TNBC. This formulation can be widely applied for treating CD155 and PD-L1 co-expressing cancers. | [171] | |
| POLYMERIC NANOPARTICLES FOR THE TREATMENT OF CANCER STEM-CELLS IN TRIPLE-NEGATIVE BREAST CANCER | ||||||||
| Doxorubicin (DXN). | Pluronic F127 and L61, | Doxorubicin-loaded Pluronic F127, L61 polymeric micelles | Basal MDA-MB-468 and claudin-low MDA-MB-231 TNBC cell lines | Female athymic mice | These discoveries encourage the involvement of Pluronic co-polymers in preventing the occurrence of drug-resistance. | [172] | ||
| Zileuton | Pluronic® F127 | - | Zileuton–Pluronic® F127 polymeric micelles | In vitro and in vivo | MDA-MB-231 tumor cell line | MDA-MB-231 tumor cell-bearing female athymic mice | Remarkable intra-tumoral reduction in CSC, reduction in CTCs and CSCs in the blood stream of tumor-bearing animal models | [173] |
| POLYMERIC NANOPARTICLES FOR THE TREATMENT OF TRIPLE-NEGATIVE BREAST CANCER METASTASIS | ||||||||
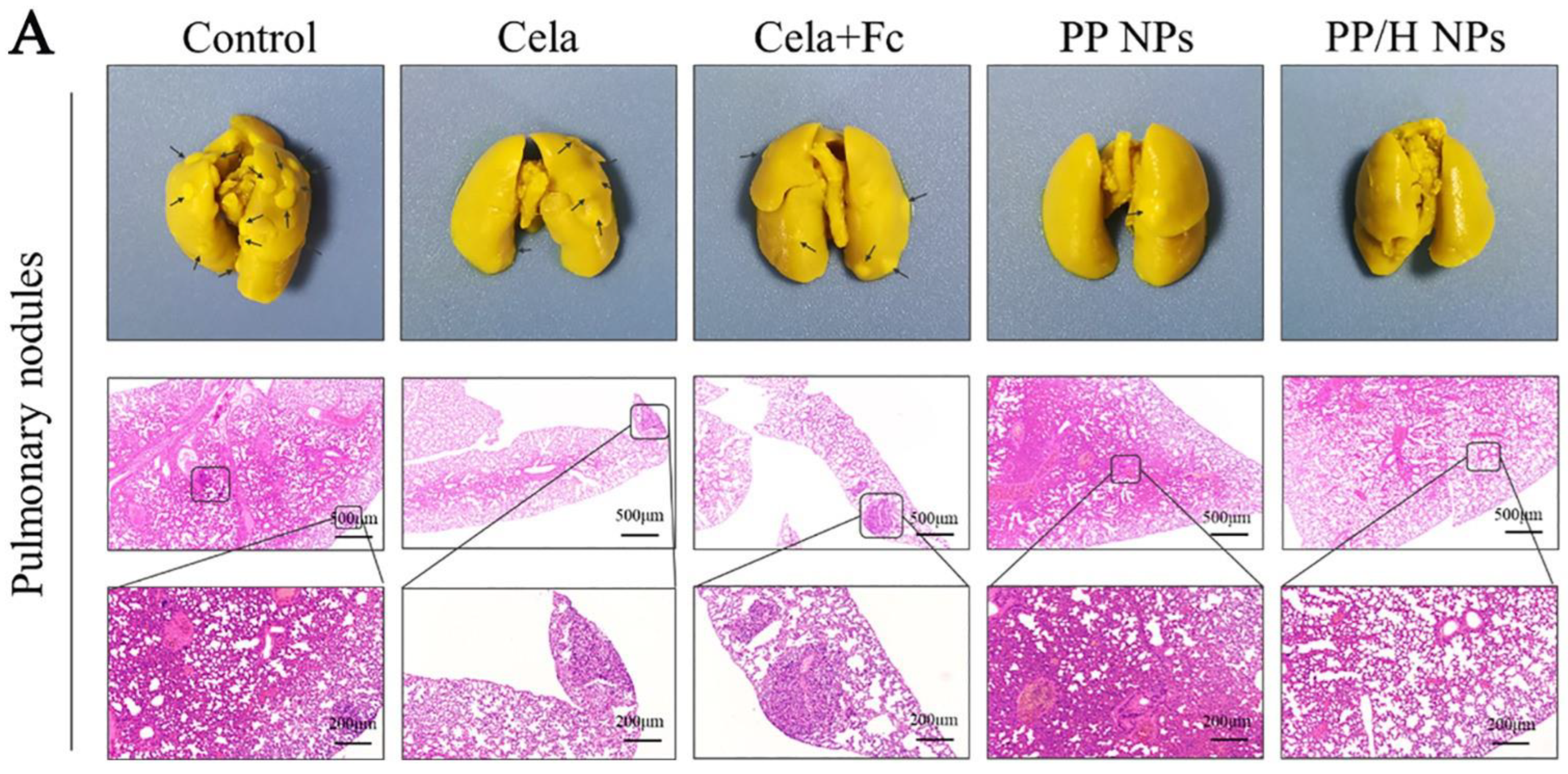
| Ferrocene (Frc) and Celastrol (Clt), | PEI-PLGA | Low molecular weight heparin (LMW-HR) for anti-metastatic activity | Amphiphilic, pH-sensitive, LMW-HR-coated nanoparticle | In vitro and in vivo | Murine 4T1 cell line | 3T3/4T1 tumor-bearing Female Balb/c mice | The cytotoxic effect of polymeric nanoparticles and anti-metastatic activity of LMW-HR enhanced the overall anti-tumor action of the developed nanoparticles. | [174] |
| Thymoquinone (TQN) | Pluronic block co-polymer | Hyaluronic acid (HA) | HA-coated TQN loaded, Pluronic nanoparticles (TQN-P-HA NPs) | In vitro and in vivo | 4T1 and MDA-MB-231 | 4T1-mammary tumor mice model and MDA-MB-231 chick embryos xenograft model | In TQN-P-HA NP-treated group, very few blood vessels were seen in the xenograft model, indicating anti-angiogenesis activity and in the TQN-P-HA NP-treated xenograft embryos models, very few human cells were metastasize to the lungs and liver of the embryos, signifying an anti-metastatic effect. | [175] |
| Doxorubicin (DXN), | Chitosan | Lecithin | Folate receptor-conjugated doxorubicin-loaded lecithin-polysorbate 80-chitosan-coated lipid core nanocapsules (FA-DXN-LPC-L-NCs) | In vitro | MDA-MB-231 cell line | - | Results obtained after different in vitro studies including cellular uptake assay, oxidative stress assay, gene expression evaluation, and migration assay revealed the promising activity of FA-DXN-LPC-L-NCs against TNBC. | [176] |
| Doxorubicin (DXN) and anti-CD73 antibody | PEG | Rose Bengal, Thioketal | lanthanide-doped up-conversion nanoparticles (LUCVNPs) | In vitro and in vivo | Murine 4T1 cell line | Balb/C mice | Novel bio-mimicking multi-functional fusion of LUCVNPs with anti-CD73 antibodies could prove to be a promising regimen for targeting and treating metastatic TNBC | [177] |
| Resiquimod (R848) | poly-L-histidine (PL-Hist) | Triethylenetetramine-bis (dithiocarbamate) (TETA-DTC), RGD (Arg-Gly-Asp) peptide | Resiquimod loaded-(TETA-DTC), RGD-(PL-Hist) nanoparticles | In vitro and in vivo | Human MDA-MB-231, Murine 4T1, MCF-7 breast cancer cell lines and BEAS-2B (normal lung epithelial cell line) | Female Balb/C mice | Remarkable tumor growth suppression and anti-metastasis activity via Cu deficiency-induced anti-angiogenesis and R848 activated immune response | [178] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fatima, M.; Sheikh, A.; Abourehab, M.A.S.; Kesharwani, P. Advancements in Polymeric Nanocarriers to Mediate Targeted Therapy against Triple-Negative Breast Cancer. Pharmaceutics 2022, 14, 2432. https://doi.org/10.3390/pharmaceutics14112432
Fatima M, Sheikh A, Abourehab MAS, Kesharwani P. Advancements in Polymeric Nanocarriers to Mediate Targeted Therapy against Triple-Negative Breast Cancer. Pharmaceutics. 2022; 14(11):2432. https://doi.org/10.3390/pharmaceutics14112432
Chicago/Turabian StyleFatima, Mahak, Afsana Sheikh, Mohammed A. S. Abourehab, and Prashant Kesharwani. 2022. "Advancements in Polymeric Nanocarriers to Mediate Targeted Therapy against Triple-Negative Breast Cancer" Pharmaceutics 14, no. 11: 2432. https://doi.org/10.3390/pharmaceutics14112432
APA StyleFatima, M., Sheikh, A., Abourehab, M. A. S., & Kesharwani, P. (2022). Advancements in Polymeric Nanocarriers to Mediate Targeted Therapy against Triple-Negative Breast Cancer. Pharmaceutics, 14(11), 2432. https://doi.org/10.3390/pharmaceutics14112432






