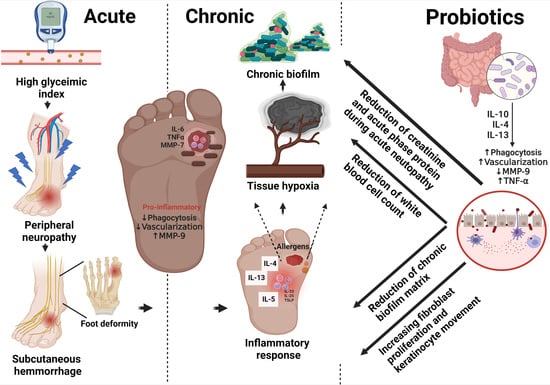
A Review of Immunomodulatory Reprogramming by Probiotics in Combating Chronic and Acute Diabetic Foot Ulcers (DFUs)
Abstract
:1. Introduction
2. Method
3. Diabetes: A Constant Fluctuation in Blood Glucose Levels
4. The Diabetic Foot Ulcer: A Long-Lasting Foot Deformity
4.1. Evaluation and Classification of DFU Extent
4.1.1. Meggitt–Wagner (MW) Classification System
4.1.2. University of Texas (UT) Classification
4.1.3. Perfusion, Extent, Depth, Infection, and Sensation (PEDIS)
4.1.4. Saint Elian Wound Score System (SEWSS)
4.1.5. Site, Ischemia, Neuropathy, Bacterial Infection, Area, and Depth (SINBAD)
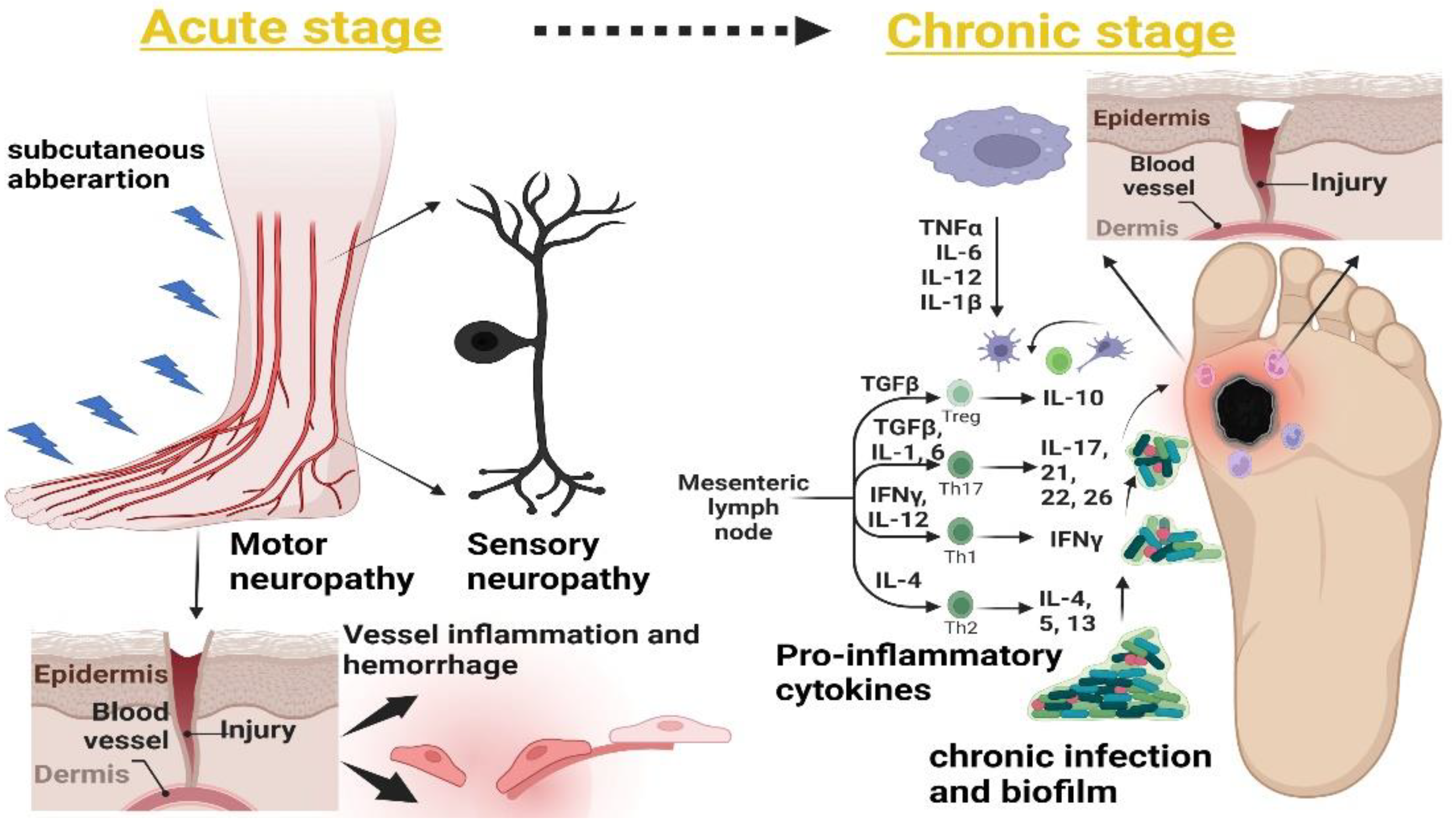
5. Timeline and Stages of DFUs
5.1. Sensory and Motor Neuropathy
5.2. Subcutaneous Hemorrhage
5.3. Foot Deformity
5.4. Acute Inflammatory Responses (Neutrophils and Macrophages) and Accumulation of Extracellular Matrix (ECM)
5.5. Abnormal Matrix Metalloproteinase (MMP) Expression
5.6. Bacterial Invasion and Persistent Infection
6. Microbial Bioburden in DFUs
7. Innate Immune and Proinflammatory Mediators in Non-Healing DFUs
7.1. Innate Immune Cells
7.2. Proinflammatory Cytokines
7.3. Matrix Metalloproteinase
7.4. Toll-Like Receptors (TLRs)
8. Novel Therapies Targeting Inflammatory Modulators during Non-Healing DFUs
8.1. Fibroblast Growth Factor (FGF)-Associated Therapy
8.2. Macrophage-Regulating Therapy
8.3. Stem Cell Therapy
8.4. Neuropeptides
8.5. Matrix Metalloproteinase (MMP) Inhibitors
8.6. Monoclonal Antibodies (mAbs)
8.7. Bioactive Molecules
9. Everything Starts in the Gut: Gut–Skin Axis in Non-Healing Wounds
9.1. Staphylococcus epidermidis and Propionibacterium spp.: Potential Skin Commensal Probiotics
9.2. Lactobacillus Species: A Superior Microbiota Regulating Gut–Skin Homeostasis
10. Probiotics: Current Value in Host–Microbe Interactions
10.1. Probiotics: A Primitive Class of Microbes
10.2. Basic Immunomodulatory Action of Probiotics
11. Probiotic Effect in Acute and Chronic Biomarkers of Non-Healing DFUs
11.1. C-Reactive Proteins (CRPs)
11.2. Procalcitonin
11.3. White Blood Cell (WBC) Count
11.4. Growth Factors
11.5. Chronic Biofilm Formation
11.6. Extracellular Matrix (ECM)
11.7. Matrix Metalloproteinase (MMP)
11.8. Proinflammatory Cytokine Response
12. Results
13. Novel Prospects of Probiotic-Related Therapies against DFUs
13.1. Probiotic Encapsulation
13.2. Prebiotics and Synbiotics: Nanoformulations
13.3. Probiotic-Derived Biogenic Nanoparticles (NPs)
13.4. Probiotic-Derived Extracellular Vesicles (EVs)
14. Management of Acute and Chronic DFU Conditions via Probiotic Remodeling
15. Limitations
16. Conclusions
16.1. Current Progress
16.2. Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Feldman, E.L.; Callaghan, B.C.; Pop-Busui, R.; Zochodne, D.W.; Wright, D.E.; Bennett, D.L.; Bril, V.; Russell, J.W.; Viswanathan, V. Diabetic neuropathy. Nat. Rev. Dis. Primers 2019, 5, 41. [Google Scholar] [CrossRef] [PubMed]
- Ince, P.; Abbas, Z.G.; Lutale, J.K.; Basit, A.; Ali, S.M.; Chohan, F.; Morbach, S.; Mollenberg, J.; Game, F.L.; Jeffcoate, W.J. Use of the SINBAD classification system and score in comparing outcome of foot ulcer management on three continents. Diabetes Care 2008, 31, 964–967. [Google Scholar] [CrossRef] [Green Version]
- Shi, L.; Wei, H.; Zhang, T.; Li, Z.; Chi, X.; Liu, D.; Chang, D.; Zhang, Y.; Wang, X.; Zhao, Q. A potent weighted risk model for evaluating the occurrence and severity of diabetic foot ulcers. Diabetol. Metab. Syndr 2021, 13, 92. [Google Scholar] [CrossRef]
- Karri, V.V.; Kuppusamy, G.; Talluri, S.V.; Yamjala, K.; Mannemala, S.S.; Malayandi, R. Current and emerging therapies in the management of diabetic foot ulcers. Curr. Med. Res. Opin. 2016, 32, 519–542. [Google Scholar] [CrossRef]
- Jodheea-Jutton, A.; Hindocha, S.; Bhaw-Luximon, A. Health economics of diabetic foot ulcer and recent trends to accelerate treatment. Foot 2022, 52, 101909. [Google Scholar] [CrossRef] [PubMed]
- Coates, M.; Lee, M.J.; Norton, D.; MacLeod, A.S. The Skin and Intestinal Microbiota and Their Specific Innate Immune Systems. Front. Immunol. 2019, 10, 2950. [Google Scholar] [CrossRef]
- Kawai, K.; Kamochi, R.; Oiki, S.; Murata, K.; Hashimoto, W. Probiotics in human gut microbiota can degrade host glycosaminoglycans. Sci. Rep. 2018, 8, 10674. [Google Scholar] [CrossRef] [Green Version]
- Zuo, F.; Marcotte, H. Advancing mechanistic understanding and bioengineering of probiotic lactobacilli and bifidobacteria by genome editing. Curr. Opin. Biotechnol. 2021, 70, 75–82. [Google Scholar] [CrossRef]
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.N.; Mbanya, J.C.; et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pr. 2022, 183, 109119. [Google Scholar] [CrossRef]
- Magliano, D.J.; Boyko, E.J. IDF Diabetes Atlas, 10th ed.; International Diabetes Federation: Brussels, Belgium, 2021; Available online: https://www.idf.org/e-library/epidemiology-research/diabetes-atlas.html (accessed on 18 October 2022).
- Sato, J.; Kanazawa, A.; Ikeda, F.; Yoshihara, T.; Goto, H.; Abe, H.; Komiya, K.; Kawaguchi, M.; Shimizu, T.; Ogihara, T.; et al. Gut dysbiosis and detection of “live gut bacteria” in blood of Japanese patients with type 2 diabetes. Diabetes Care 2014, 37, 2343–2350. [Google Scholar] [CrossRef] [PubMed]
- Gerard, C.; Vidal, H. Impact of Gut Microbiota on Host Glycemic Control. Front. Endocrinol. 2019, 10, 29. [Google Scholar] [CrossRef]
- He, X.; Sun, J.; Liu, C.; Yu, X.; Li, H.; Zhang, W.; Li, Y.; Geng, Y.; Wang, Z. Compositional Alterations of Gut Microbiota in Patients with Diabetic Kidney Disease and Type 2 Diabetes Mellitus. Diabetes Metab. Syndr. Obes. 2022, 15, 755–765. [Google Scholar] [CrossRef] [PubMed]
- Global Report on Diabetes; World Health Organization: Geneva, Switzerland, 2016.
- Pourkazemi, A.; Ghanbari, A.; Khojamli, M.; Balo, H.; Hemmati, H.; Jafaryparvar, Z.; Motamed, B. Diabetic foot care: Knowledge and practice. BMC Endocr. Disord. 2020, 20, 40. [Google Scholar] [CrossRef]
- Jiang, M.; Gan, F.; Gan, M.; Deng, H.; Chen, X.; Yuan, X.; Huang, D.; Liu, S.; Qin, B.; Wei, Y.; et al. Predicting the Risk of Diabetic Foot Ulcers From Diabetics With Dysmetabolism: A Retrospective Clinical Trial. Front Endocrinol. 2022, 13, 929864. [Google Scholar] [CrossRef]
- Armstrong, D.G.; Boulton, A.J.M.; Bus, S.A. Diabetic Foot Ulcers and Their Recurrence. N. Engl. J. Med. 2017, 376, 2367–2375. [Google Scholar] [CrossRef] [PubMed]
- Okonkwo, U.A.; Chen, L.; Ma, D.; Haywood, V.A.; Barakat, M.; Urao, N.; DiPietro, L.A. Compromised angiogenesis and vascular Integrity in impaired diabetic wound healing. PLoS ONE 2020, 15, e0231962. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morbach, S.; Furchert, H.; Groblinghoff, U.; Hoffmeier, H.; Kersten, K.; Klauke, G.T.; Klemp, U.; Roden, T.; Icks, A.; Haastert, B.; et al. Long-term prognosis of diabetic foot patients and their limbs: Amputation and death over the course of a decade. Diabetes Care 2012, 35, 2021–2027. [Google Scholar] [CrossRef] [Green Version]
- Ndosi, M.; Wright-Hughes, A.; Brown, S.; Backhouse, M.; Lipsky, B.A.; Bhogal, M.; Reynolds, C.; Vowden, P.; Jude, E.B.; Nixon, J.; et al. Prognosis of the infected diabetic foot ulcer: A 12-month prospective observational study. Diabet. Med. 2018, 35, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Song, J.F.; Zhang, L.; Li, X. Analysis of risk factors for multidrug-resistant organisms in diabetic foot infection. BMC Endocr. Disord. 2022, 22, 46. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, A.; Berkache, M.; Morency-Potvin, P.; Juneau, D.; Koenig, M.; Bourduas, K.; Freire, V. Diabetic foot infections: How to investigate more efficiently? A retrospective study in a quaternary university center. Insights Imaging 2022, 13, 88. [Google Scholar] [CrossRef] [PubMed]
- Game, F. Classification of diabetic foot ulcers. Diabetes Metab. Res. Rev. 2016, 32 (Suppl. 1), 186–194. [Google Scholar] [CrossRef]
- Santema, T.B.; Lenselink, E.A.; Balm, R.; Ubbink, D.T. Comparing the Meggitt-Wagner and the University of Texas wound classification systems for diabetic foot ulcers: Inter-observer analyses. Int. Wound J. 2016, 13, 1137–1141. [Google Scholar] [CrossRef] [PubMed]
- Karthikesalingam, A.; Holt, P.J.; Moxey, P.; Jones, K.G.; Thompson, M.M.; Hinchliffe, R.J. A systematic review of scoring systems for diabetic foot ulcers. Diabet. Med. 2010, 27, 544–549. [Google Scholar] [CrossRef] [PubMed]
- Oyibo, S.O.; Jude, E.B.; Tarawneh, I.; Nguyen, H.C.; Armstrong, D.G.; Harkless, L.B.; Boulton, A.J.M. The effects of ulcer size and site, patient’s age, sex and type and duration of diabetes on the outcome of diabetic foot ulcers. Diabet. Med. 2001, 18, 8. [Google Scholar] [CrossRef] [PubMed]
- Bravo-Molina, A.; Linares-Palomino, J.P.; Vera-Arroyo, B.; Salmeron-Febres, L.M.; Ros-Die, E. Inter-observer agreement of the Wagner, University of Texas and PEDIS classification systems for the diabetic foot syndrome. Foot Ankle Surg. 2018, 24, 60–64. [Google Scholar] [CrossRef]
- Forsythe, R.O.; Ozdemir, B.A.; Chemla, E.S.; Jones, K.G.; Hinchliffe, R.J. Interobserver Reliability of Three Validated Scoring Systems in the Assessment of Diabetic Foot Ulcers. Int. J. Low Extrem. Wounds 2016, 15, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Xie, T.; Cao, Y.; Wu, M.; Yu, L.; Lu, S.; Xu, G.; Hu, J.; Ruan, H. Comparison of two classification systems in predicting the outcome of diabetic foot ulcers: The Wagner grade and the Saint Elian Wound score systems. Wound Repair Regen 2015, 23, 379–385. [Google Scholar] [CrossRef]
- Monteiro-Soares, M.; Russell, D.; Boyko, E.J.; Jeffcoate, W.J.; Mills, J.L.; Morbach, S.; Game, F. IWGDF Guidelines on the Classification of Diabetic Foot Ulcers. Diabetes Metab. Res. Rev. 2019, 36 (Suppl. 1), e3273. [Google Scholar]
- Pastar, I.; Stojadinovic, O.; Yin, N.C.; Ramirez, H.; Nusbaum, A.G.; Sawaya, A.; Patel, S.B.; Khalid, L.; Isseroff, R.R.; Tomic-Canic, M. Epithelialization in Wound Healing: A Comprehensive Review. Adv. Wound Care 2014, 3, 445–464. [Google Scholar] [CrossRef] [Green Version]
- Volmer-Thole, M.; Lobmann, R. Neuropathy and Diabetic Foot Syndrome. Int. J. Mol. Sci. 2016, 17, 917. [Google Scholar] [CrossRef] [Green Version]
- Amin, N.; Doupis, J. Diabetic foot disease: From the evaluation of the "foot at risk" to the novel diabetic ulcer treatment modalities. World J. Diabetes 2016, 7, 153–164. [Google Scholar] [CrossRef]
- Nishide, K.; Nagase, T.; Oba, M.; Oe, M.; Ohashi, Y.; Iizaka, S.; Nakagami, G.; Kadowaki, T.; Sanada, H. Ultrasonographic and thermographic screening for latent inflammation in diabetic foot callus. Diabetes Res. Clin. Pr. 2009, 85, 304–309. [Google Scholar] [CrossRef]
- Cheng, Y.; Zu, P.; Zhao, J.; Shi, L.; Shi, H.; Zhang, M.; Wang, A. Differences in initial versus recurrent diabetic foot ulcers at a specialized tertiary diabetic foot care center in China. J. Int. Med. Res. 2021, 49, 300060520987398. [Google Scholar] [CrossRef] [PubMed]
- Petrova, N.L.; Edmonds, M.E. Acute Charcot neuro-osteoarthropathy. Diabetes Metab. Res. Rev. 2016, 32 (Suppl. 1), 281–286. [Google Scholar] [CrossRef] [Green Version]
- Jansen, R.B.; Jorgensen, B.; Holstein, P.E.; Moller, K.K.; Svendsen, O.L. Mortality and complications after treatment of acute diabetic Charcot foot. J. Diabetes Complicat. 2018, 32, 1141–1147. [Google Scholar] [CrossRef] [PubMed]
- Weigelt, C.; Rose, B.; Poschen, U.; Ziegler, D.; Friese, G.; Kempf, K.; Koenig, W.; Martin, S.; Herder, C. Immune mediators in patients with acute diabetic foot syndrome. Diabetes Care 2009, 32, 1491–1496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Korkmaz, P.; Kocak, H.; Onbasi, K.; Bicici, P.; Ozmen, A.; Uyar, C.; Ozatag, D.M. The Role of Serum Procalcitonin, Interleukin-6, and Fibrinogen Levels in Differential Diagnosis of Diabetic Foot Ulcer Infection. J. Diabetes Res. 2018, 2018, 7104352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muller, S.; Martin, S.; Koenig, W.; Hanifi-Moghaddam, P.; Rathmann, W.; Haastert, B.; Giani, G.; Illig, T.; Thorand, B.; Kolb, H. Impaired glucose tolerance is associated with increased serum concentrations of interleukin 6 and co-regulated acute-phase proteins but not TNF-alpha or its receptors. Diabetologia 2002, 45, 805–812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holl, J.; Kowalewski, C.; Zimek, Z.; Fiedor, P.; Kaminski, A.; Oldak, T.; Moniuszko, M.; Eljaszewicz, A. Chronic Diabetic Wounds and Their Treatment with Skin Substitutes. Cells 2021, 10, 655. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Y.; Deng, J.; Li, W.; Nie, X. Fibroblast Growth Factor in Diabetic Foot Ulcer: Progress and Therapeutic Prospects. Front Endocrinol. 2021, 12, 744868. [Google Scholar] [CrossRef]
- Ayuk, S.M.; Abrahamse, H.; Houreld, N.N. The Role of Matrix Metalloproteinases in Diabetic Wound Healing in relation to Photobiomodulation. J. Diabetes Res. 2016, 2016, 2897656. [Google Scholar] [CrossRef] [PubMed]
- Jouhar, L.; Jaafar, R.F.; Nasreddine, R.; Itani, O.; Haddad, F.; Rizk, N.; Hoballah, J.J. Microbiological profile and antimicrobial resistance among diabetic foot infections in Lebanon. Int. Wound J. 2020, 17, 1764–1773. [Google Scholar] [CrossRef]
- Baig, M.S.; Banu, A.; Zehravi, M.; Rana, R.; Burle, S.S.; Khan, S.L.; Islam, F.; Siddiqui, F.A.; Massoud, E.E.S.; Rahman, M.H.; et al. An Overview of Diabetic Foot Ulcers and Associated Problems with Special Emphasis on Treatments with Antimicrobials. Life 2022, 12, 1054. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.; Wang, C.; Zhang, J.; Wang, P.; Xu, J.; Ding, M.; Li, X.; Hou, X.; Feng, S.; Li, X. Can We Stop Antibiotic Therapy When Signs and Symptoms Have Resolved in Diabetic Foot Infection Patients? Int. J. Low Extrem. Wounds 2015, 14, 277–283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aragon-Sanchez, J.; Lazaro-Martinez, J.L.; Pulido-Duque, J.; Maynar, M. From the diabetic foot ulcer and beyond: How do foot infections spread in patients with diabetes? Diabet. Foot Ankle 2012, 3, 18693. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, X.; Chen, Y.; Wang, G.; Ding, P.; Zhao, Z.; Bi, H. Clinical study on orthopaedic treatment of chronic osteomyelitis with soft tissue defect in adults. Int. Wound J. 2022, 19, 1349–1356. [Google Scholar] [CrossRef]
- Sadeghpour Heravi, F.; Zakrzewski, M.; Vickery, K.D.; Armstrong, D.; Hu, H. Bacterial Diversity of Diabetic Foot Ulcers: Current Status and Future Prospectives. J. Clin. Med. 2019, 8, 1935. [Google Scholar] [CrossRef] [Green Version]
- Buch, P.J.; Chai, Y.; Goluch, E.D. Bacterial chatter in chronic wound infections. Wound Repair Regen. 2021, 29, 106–116. [Google Scholar] [CrossRef]
- Buch, P.J.; Chai, Y.; Goluch, E.D. Treating Polymicrobial Infections in Chronic Diabetic Wounds. Clin. Microbiol. Rev. 2019, 32, e00091-18. [Google Scholar] [CrossRef] [Green Version]
- Percival, S.L.; Malone, M.; Mayer, D.; Salisbury, A.M.; Schultz, G. Role of anaerobes in polymicrobial communities and biofilms complicating diabetic foot ulcers. Int. Wound J. 2018, 15, 776–782. [Google Scholar] [CrossRef]
- Nussbaum, S.R.; Carter, M.J.; Fife, C.E.; DaVanzo, J.; Haught, R.; Nusgart, M.; Cartwright, D. An Economic Evaluation of the Impact, Cost, and Medicare Policy Implications of Chronic Nonhealing Wounds. Value Health 2018, 21, 27–32. [Google Scholar] [CrossRef]
- Srivastava, P.; Sivashanmugam, K. Combinatorial Drug Therapy for Controlling Pseudomonas aeruginosa and Its Association with Chronic Condition of Diabetic Foot Ulcer. Int. J. Low Extrem. Wounds 2020, 19, 7–20. [Google Scholar] [CrossRef] [PubMed]
- Johani, K.; Malone, M.; Jensen, S.; Gosbell, I.; Dickson, H.; Hu, H.; Vickery, K. Microscopy visualisation confirms multi-species biofilms are ubiquitous in diabetic foot ulcers. Int. Wound J. 2017, 14, 1160–1169. [Google Scholar] [CrossRef] [PubMed]
- Dunnill, C.; Patton, T.; Brennan, J.; Barrett, J.; Dryden, M.; Cooke, J.; Leaper, D.; Georgopoulos, N.T. Reactive oxygen species (ROS) and wound healing: The functional role of ROS and emerging ROS-modulating technologies for augmentation of the healing process. Int. Wound J. 2017, 14, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Srivastava, S.; Singh, M.R.; Singh, D. Mechanistic insight into diabetic wounds: Pathogenesis, molecular targets and treatment strategies to pace wound healing. Biomed. Pharm. 2019, 112, 108615. [Google Scholar] [CrossRef] [PubMed]
- Mouritzen, M.V.; Petkovic, M.; Qvist, K.; Poulsen, S.S.; Alarico, S.; Leal, E.C.; Dalgaard, L.T.; Empadinhas, N.; Carvalho, E.; Jenssen, H. Improved diabetic wound healing by LFcinB is associated with relevant changes in the skin immune response and microbiota. Mol. Methods Clin. Dev. 2021, 20, 726–739. [Google Scholar] [CrossRef] [PubMed]
- Suh, W.; Kim, K.L.; Kim, J.M.; Shin, I.S.; Lee, Y.S.; Lee, J.Y.; Jang, H.S.; Lee, J.S.; Byun, J.; Choi, J.H.; et al. Transplantation of endothelial progenitor cells accelerates dermal wound healing with increased recruitment of monocytes/macrophages and neovascularization. Stem Cells 2005, 23, 1571–1578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bjarnsholt, T.; Kirketerp-Moller, K.; Jensen, P.O.; Madsen, K.G.; Phipps, R.; Krogfelt, K.; Hoiby, N.; Givskov, M. Why chronic wounds will not heal: A novel hypothesis. Wound Repair. Regen 2008, 16, 2–10. [Google Scholar] [CrossRef]
- Li, M.; Hou, Q.; Zhong, L.; Zhao, Y.; Fu, X. Macrophage Related Chronic Inflammation in Non-Healing Wounds. Front. Immunol. 2021, 12, 681710. [Google Scholar] [CrossRef]
- Mirza, R.E.; Fang, M.M.; Ennis, W.J.; Koh, T.J. Blocking interleukin-1beta induces a healing-associated wound macrophage phenotype and improves healing in type 2 diabetes. Diabetes 2013, 62, 2579–2587. [Google Scholar] [CrossRef] [Green Version]
- Pang, J.; Maienschein-Cline, M.; Koh, T.J. Enhanced Proliferation of Ly6C(+) Monocytes/Macrophages Contributes to Chronic Inflammation in Skin Wounds of Diabetic Mice. J. Immunol. 2021, 206, 621–630. [Google Scholar] [CrossRef]
- Kolumam, G.; Wu, X.; Lee, W.P.; Hackney, J.A.; Zavala-Solorio, J.; Gandham, V.; Danilenko, D.M.; Arora, P.; Wang, X.; Ouyang, W. IL-22R Ligands IL-20, IL-22, and IL-24 Promote Wound Healing in Diabetic db/db Mice. PLoS ONE 2017, 12, e0170639. [Google Scholar] [CrossRef] [PubMed]
- Lampropoulou, I.T.; Stangou, M.; Papagianni, A.; Didangelos, T.; Iliadis, F.; Efstratiadis, G. TNF-alpha and microalbuminuria in patients with type 2 diabetes mellitus. J. Diabetes Res. 2014, 2014, 394206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trengove, N.J.; Bielefeldt-Ohmann, H.; Stacey, M.C. Mitogenic activity and cytokine levels in non-healing and healing chronic leg ulcers. Wound Repair. Regen 2000, 8, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Medzhitov, R. TOLL-LIKE RECEPTORS AND INNATE IMMUNITY. Nat. Rev. Neurosci. 2001, 1, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Pukstad, B.S.; Ryan, L.; Flo, T.H.; Stenvik, J.; Moseley, R.; Harding, K.; Thomas, D.W.; Espevik, T. Non-healing is associated with persistent stimulation of the innate immune response in chronic venous leg ulcers. J. Derm. Sci. 2010, 59, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Shen, J.; Chai, Y.; Chen, H. IL-1beta Impaired Diabetic Wound Healing by Regulating MMP-2 and MMP-9 through the p38 Pathway. Mediat. Inflamm. 2021, 2021, 6645766. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Yang, C.; Zhang, S.; Ke, Z.X.; Chen, D.X.; Li, Y.Q.; Li, Q. The Imbalance of MMP-2/TIMP-2 and MMP-9/TIMP-1 Contributes to Collagen Deposition Disorder in Diabetic Non-Injured Skin. Front. Endocrinol. 2021, 12, 734485. [Google Scholar] [CrossRef]
- Lobmann, R.; Ambrosch, A.; Schultz, G.; Waldmann, K.; Schiweck, S.; Lehnert, H. Expression of matrix-metalloproteinases and their inhibitors in the wounds of diabetic and non-diabetic patients. Diabetologia 2002, 45, 1011–1016. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Akira, S. The roles of TLRs, RLRs and NLRs in pathogen recognition. Int. Immunol. 2009, 21, 317–337. [Google Scholar] [CrossRef] [Green Version]
- Sepehri, Z.; Kiani, Z.; Nasiri, A.A.; Kohan, F. Toll-like receptor 2 and type 2 diabetes. Cell Mol. Biol. Lett. 2016, 21, 2. [Google Scholar] [CrossRef]
- Dasu, M.R.; Thangappan, R.K.; Bourgette, A.; DiPietro, L.A.; Isseroff, R.; Jialal, I. TLR2 expression and signaling-dependent inflammation impair wound healing in diabetic mice. Lab Investig. 2010, 90, 1628–1636. [Google Scholar] [CrossRef] [Green Version]
- Creely, S.J.; McTernan, P.G.; Kusminski, C.M.; Fisher, F.M.; Da Silva, N.F.; Khanolkar, M.; Evans, M.; Harte, A.L.; Kumar, S. Lipopolysaccharide activates an innate immune system response in human adipose tissue in obesity and type 2 diabetes. Am. J. Physiol. Endocrinol. Metab. 2007, 292, E740–E747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, K.T.; Seth, A.K.; Hong, S.J.; Geringer, M.R.; Xie, P.; Leung, K.P.; Mustoe, T.A.; Galiano, R.D. Deficient cytokine expression and neutrophil oxidative burst contribute to impaired cutaneous wound healing in diabetic, biofilm-containing chronic wounds. Wound Repair. Regen. 2013, 21, 833–841. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.; Agrawal, N.K.; Gupta, S.K.; Mohan, G.; Chaturvedi, S.; Singh, K. Genetic and epigenetic alterations in Toll like receptor 2 and wound healing impairment in type 2 diabetes patients. J. Diabetes Complicat. 2015, 29, 222–229. [Google Scholar] [CrossRef]
- Blakely, M. The Use of Best Practice in the Treatment of a Complex Diabetic Foot Ulcer: A Case Report. Healthcare 2016, 4, 18. [Google Scholar] [CrossRef] [Green Version]
- Tettelbach, W.H.; Cazzell, S.M.; Hubbs, B.; Jong, J.L.D.; Forsyth, R.A.; Reyzelman, A.M. The influence of adequate debridement and placental-derived allografts on diabetic foot ulcers. J. Wound Care 2022, 31, S16–S29. [Google Scholar] [CrossRef] [PubMed]
- Brouwer, R.J.; Lalieu, R.C.; Hoencamp, R.; van Hulst, R.A.; Ubbink, D.T. A systematic review and meta-analysis of hyperbaric oxygen therapy for diabetic foot ulcers with arterial insufficiency. J. Vasc. Surg. 2020, 71, 682–692.e681. [Google Scholar] [CrossRef] [PubMed]
- Snyder, R.; Galiano, R.; Mayer, P.; Rogers, L.C.; Alvarez, O.; Sanuwave Trial Investigators. Diabetic foot ulcer treatment with focused shockwave therapy: Two multicentre, prospective, controlled, double-blinded, randomised phase III clinical trials. J. Wound Care 2018, 27, 822–836. [Google Scholar] [CrossRef] [PubMed]
- Racaru, S.; Bolton Saghdaoui, L.; Roy Choudhury, J.; Wells, M.; Davies, A.H. Offloading treatment in people with diabetic foot disease: A systematic scoping review on adherence to foot offloading. Diabetes Metab. Syndr. 2022, 16, 102493. [Google Scholar] [CrossRef]
- Siavash, M.; Najjarnezhad, A.; Mohseni, N.; Abtahi, S.M.; Karimy, A.; Sabzevari, M.H. Efficacy of Maggot Debridement Therapy on Refractory Atypical Diabetic Foot Ulcers: An Open-Label Study. Int. J. Low Extrem. Wounds 2021, 20, 315–320. [Google Scholar] [CrossRef]
- Huang, J.; Chen, J.; Xiong, S.; Huang, J.; Liu, Z. The effect of low-level laser therapy on diabetic foot ulcers: A meta-analysis of randomised controlled trials. Int. Wound J. 2021, 18, 763–776. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.; Nguyen, T.T. Strategy for Treatment of Infected Diabetic Foot Ulcers. Acc. Chem. Res. 2021, 54, 1080–1093. [Google Scholar] [CrossRef] [PubMed]
- Game, F. The advantages and disadvantages of non-surgical management of the diabetic foot. Diabetes Metab. Res. Rev. 2008, 24 (Suppl. 1), S72–S75. [Google Scholar] [CrossRef]
- Oley, M.H.; Oley, M.C.; Tjandra, D.E.; Sedu, S.W.; Sumarauw, E.R.N.; Aling, D.M.R.; Kalangi, J.A.; Islam, A.A.; Hatta, M.; Faruk, M. Hyperbaric oxygen therapy in the healing process of foot ulcers in diabetic type 2 patients marked by interleukin 6, vascular endothelial growth factor, and PEDIS score: A randomized controlled trial study. Int. J. Surg. Open 2020, 27, 154–161. [Google Scholar] [CrossRef]
- Jebril, W.; Nowak, M.; Palin, L.; Nordgren, M.; Bachar-Wikstrom, E.; Wikstrom, J.D. Topical oxygen treatment relieves pain from hard-to-heal leg ulcers and improves healing: A case series. J. Wound Care 2022, 31, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Shendy, W.S.; Elsoghier, O.M.; El Semary, M.M.; Ahmed, A.A.; Ali, A.F.; Saber-Khalaf, M. Effect of low-intensity extracorporeal shock wave therapy on diabetic erectile dysfunction: Randomised control trial. Andrologia 2021, 53, e13997. [Google Scholar] [CrossRef]
- Lazzarini, P.A.; Jarl, G.; Gooday, C.; Viswanathan, V.; Caravaggi, C.F.; Armstrong, D.G.; Bus, S.A. Effectiveness of offloading interventions to heal foot ulcers in persons with diabetes: A systematic review. Diabetes Metab. Res. Rev. 2020, 36 (Suppl. 1), e3275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bazalinski, D.; Kozka, M.; Karnas, M.; Wiech, P. Effectiveness of Chronic Wound Debridement with the Use of Larvae of Lucilia Sericata. J. Clin. Med. 2019, 8, 1845. [Google Scholar] [CrossRef] [Green Version]
- Ramirez-Acuna, J.M.; Cardenas-Cadena, S.A.; Marquez-Salas, P.A.; Garza-Veloz, I.; Perez-Favila, A.; Cid-Baez, M.A.; Flores-Morales, V.; Martinez-Fierro, M.L. Diabetic Foot Ulcers: Current Advances in Antimicrobial Therapies and Emerging Treatments. Antibiotics 2019, 8, 193. [Google Scholar] [CrossRef] [Green Version]
- Shaikh-Kader, A.; Houreld, N.N.; Rajendran, N.K.; Abrahamse, H. The link between advanced glycation end products and apoptosis in delayed wound healing. Cell Biochem. Funct. 2019, 37, 432–442. [Google Scholar] [CrossRef]
- Futrega, K.; King, M.; Lott, W.B.; Doran, M.R. Treating the whole not the hole: Necessary coupling of technologies for diabetic foot ulcer treatment. Trends Mol. Med. 2014, 20, 137–142. [Google Scholar] [CrossRef]
- Sica, A.; Erreni, M.; Allavena, P.; Porta, C. Macrophage polarization in pathology. Cell Mol. Life Sci. 2015, 72, 4111–4126. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-W.; Hung, C.-M.; Chen, W.-J.; Chen, J.-C.; Huang, W.-Y.; Lu, C.-S.; Kuo, M.-L.; Chen, S.-G. New Horizons of Macrophage Immunomodulation in the Healing of Diabetic Foot Ulcers. Pharmaceutics 2022, 14, 2065. [Google Scholar] [CrossRef]
- Leu, W.J.; Chen, J.C.; Guh, J.H. Extract From Plectranthus amboinicus Inhibit Maturation and Release of Interleukin 1beta Through Inhibition of NF-kappaB Nuclear Translocation and NLRP3 Inflammasome Activation. Front. Pharm. 2019, 10, 573. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.W.; Chen, C.C.; Huang, W.Y.; Chen, Y.Y.; Chen, S.T.; Chou, H.W.; Hung, C.M.; Chen, W.J.; Lu, C.S.; Nian, S.X.; et al. Restoring Prohealing/Remodeling-Associated M2a/c Macrophages Using ON101 Accelerates Diabetic Wound Healing. JID Innov. 2022, 2, 100138. [Google Scholar] [CrossRef] [PubMed]
- Chiang, K.J.; Chiu, L.C.; Kang, Y.N.; Chen, C. Autologous Stem Cell Therapy for Chronic Lower Extremity Wounds: A Meta-Analysis of Randomized Controlled Trials. Cells 2021, 10, 3307. [Google Scholar] [CrossRef] [PubMed]
- Xie, F.; Teng, L.; Lu, J.; Xu, J.; Zhang, C.; Yang, L.; Ma, X.; Zhao, M. Interleukin-10-Modified Adipose-Derived Mesenchymal Stem Cells Prevent Hypertrophic Scar Formation via Regulating the Biological Characteristics of Fibroblasts and Inflammation. Mediat. Inflamm. 2022, 2022, 6368311. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, L.; Nabzdyk, C.; Andersen, N.D.; LoGerfo, F.W.; Veves, A. Inflammation and neuropeptides: The connection in diabetic wound healing. Expert. Rev. Mol. Med. 2009, 11, e2. [Google Scholar] [CrossRef] [Green Version]
- Dallos, A.; Kiss, M.; Polyanka, H.; Dobozy, A.; Kemeny, L.; Husz, S. Effects of the neuropeptides substance P, calcitonin gene-related peptide, vasoactive intestinal polypeptide and galanin on the production of nerve growth factor and inflammatory cytokines in cultured human keratinocytes. Neuropeptides 2006, 40, 251–263. [Google Scholar] [CrossRef] [PubMed]
- Gajendrareddy, P.K.; Engeland, C.G.; Junges, R.; Horan, M.P.; Rojas, I.G.; Marucha, P.T. MMP-8 overexpression and persistence of neutrophils relate to stress-impaired healing and poor collagen architecture in mice. Brain Behav. Immun. 2013, 28, 44–48. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Fernandez, A.; Inada, M.; Balbin, M.; Fueyo, A.; Pitiot, A.S.; Astudillo, A.; Hirose, K.; Hirata, M.; Shapiro, S.D.; Noel, A.; et al. Increased inflammation delays wound healing in mice deficient in collagenase-2 (MMP-8). FASEB J. 2007, 21, 2580–2591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, T.T.; Ding, D.; Wolter, W.R.; Perez, R.L.; Champion, M.M.; Mahasenan, K.V.; Hesek, D.; Lee, M.; Schroeder, V.A.; Jones, J.I.; et al. Validation of Matrix Metalloproteinase-9 (MMP-9) as a Novel Target for Treatment of Diabetic Foot Ulcers in Humans and Discovery of a Potent and Selective Small-Molecule MMP-9 Inhibitor That Accelerates Healing. J. Med. Chem. 2018, 61, 8825–8837. [Google Scholar] [CrossRef] [PubMed]
- Gooyit, M.; Lee, M.; Schroeder, V.A.; Ikejiri, M.; Suckow, M.A.; Mobashery, S.; Chang, M. Selective water-soluble gelatinase inhibitor prodrugs. J. Med. Chem. 2011, 54, 6676–6690. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Yang, C.; Wang, X.Y.; Zhou, L.Y.; Lao, G.J.; Liu, D.; Wang, C.; Hu, M.D.; Zeng, T.T.; Yan, L.; et al. MicroRNA-129 and -335 Promote Diabetic Wound Healing by Inhibiting Sp1-Mediated MMP-9 Expression. Diabetes 2018, 67, 1627–1638. [Google Scholar] [CrossRef] [Green Version]
- Motley, M.P.; Banerjee, K.; Fries, B.C. Monoclonal antibody-based therapies for bacterial infections. Curr. Opin. Infect. Dis. 2019, 32, 210–216. [Google Scholar] [CrossRef]
- Tkaczyk, C.; Jones-Nelson, O.; Shi, Y.Y.; Tabor, D.E.; Cheng, L.; Zhang, T.; Sellman, B.R. Neutralizing Staphylococcus aureus Virulence with AZD6389, a Three mAb Combination, Accelerates Closure of a Diabetic Polymicrobial Wound. mSphere 2022, 7, e00130-00122. [Google Scholar] [CrossRef]
- Choi, M.H.; Jang, T.S.; Kim, H.; Ku, I.; Lee, J.; Jeong, J.G.; Kim, S.; Park, J.U. An Agonistic Monoclonal Antibody Targeting cMet Attenuates Inflammation and Up-Regulates Collagen Synthesis and Angiogenesis in Type 2 Diabetic Mice Wounds. Plast Reconstr. Surg. 2022, 150, 572e–583e. [Google Scholar] [CrossRef]
- Artem Ataide, J.; Caramori Cefali, L.; Machado Croisfelt, F.; Arruda Martins Shimojo, A.; Oliveira-Nascimento, L.; Gava Mazzola, P. Natural actives for wound healing: A review. Phytother Res. 2018, 32, 1664–1674. [Google Scholar] [CrossRef]
- Hozzein, W.N.; Badr, G.; Al Ghamdi, A.A.; Sayed, A.; Al-Waili, N.S.; Garraud, O. Topical application of propolis enhances cutaneous wound healing by promoting TGF-beta/Smad-mediated collagen production in a streptozotocin-induced type I diabetic mouse model. Cell Physiol. Biochem. 2015, 37, 940–954. [Google Scholar] [CrossRef]
- Powell, L.C.; Cullen, J.K.; Boyle, G.M.; De Ridder, T.; Yap, P.-Y.; Xue, W.; Pierce, C.J.; Pritchard, M.F.; Menzies, G.E.; Abdulkarim, M.; et al. Topical, immunomodulatory epoxy-tiglianes induce biofilm disruption and healing in acute and chronic skin wounds. Sci. Transl. Med. 2022, 14, eabn3758. [Google Scholar] [CrossRef] [PubMed]
- Game, F.; Jeffcoate, W.; Tarnow, L.; Jacobsen, J.L.; Whitham, D.J.; Harrison, E.F.; Ellender, S.J.; Fitzsimmons, D.; Löndahl, M.; Dhatariya, K.; et al. LeucoPatch system for the management of hard-to-heal diabetic foot ulcers in the UK, Denmark, and Sweden: An observer-masked, randomised controlled trial. Lancet Diabetes Endocrinol. 2018, 6, 870–878. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.Y.; Chen, C.C.; Chang, S.C.; Yeh, J.T.; Huang, H.F.; Lin, H.C.; Lin, S.H.; Lin, Y.H.; Wei, L.G.; Liu, T.J.; et al. ENERGI-F703 gel, as a new topical treatment for diabetic foot and leg ulcers: A multicenter, randomized, double-blind, phase II trial. EClinicalMedicine 2022, 51, 101497. [Google Scholar] [CrossRef] [PubMed]
- Luqman, A.; Gotz, F. The Ambivalent Role of Skin Microbiota and Adrenaline in Wound Healing and the Interplay between Them. Int. J. Mol. Sci. 2021, 22, 4996. [Google Scholar] [CrossRef]
- Diotallevi, F.; Campanati, A.; Martina, E.; Radi, G.; Paolinelli, M.; Marani, A.; Molinelli, E.; Candelora, M.; Taus, M.; Galeazzi, T.; et al. The Role of Nutrition in Immune-Mediated, Inflammatory Skin Disease: A Narrative Review. Nutrients 2022, 14, 591. [Google Scholar] [CrossRef]
- Thye, A.Y.; Bah, Y.R.; Law, J.W.; Tan, L.T.; He, Y.W.; Wong, S.H.; Thurairajasingam, S.; Chan, K.G.; Lee, L.H.; Letchumanan, V. Gut-Skin Axis: Unravelling the Connection between the Gut Microbiome and Psoriasis. Biomedicines 2022, 10, 1037. [Google Scholar] [CrossRef]
- Salem, I.; Ramser, A.; Isham, N.; Ghannoum, M.A. The Gut Microbiome as a Major Regulator of the Gut-Skin Axis. Front. Microbiol. 2018, 9, 1459. [Google Scholar] [CrossRef] [Green Version]
- Szabo, K.; Erdei, L.; Bolla, B.S.; Tax, G.; Biro, T.; Kemeny, L. Factors shaping the composition of the cutaneous microbiota. Br. J. Derm. 2017, 176, 344–351. [Google Scholar] [CrossRef] [Green Version]
- Naik, S.; Bouladoux, N.; Linehan, J.L.; Han, S.J.; Harrison, O.J.; Wilhelm, C.; Conlan, S.; Himmelfarb, S.; Byrd, A.L.; Deming, C.; et al. Commensal-dendritic-cell interaction specifies a unique protective skin immune signature. Nature 2015, 520, 104–108. [Google Scholar] [CrossRef] [Green Version]
- Naik, S.; Bouladoux, N.; Wilhelm, C.; Molloy, M.J.; Salcedo, R.; Kastenmuller, W.; Deming, C.; Quinones, M.; Koo, L.; Conlan, S.; et al. Compartmentalized control of skin immunity by resident commensals. Sci. Transl. Med. 2012, 337, 1115–1119. [Google Scholar] [CrossRef] [Green Version]
- Kao, M.S.; Huang, S.; Chang, W.L.; Hsieh, M.F.; Huang, C.J.; Gallo, R.L.; Huang, C.M. Microbiome precision editing: Using PEG as a selective fermentation initiator against methicillin-resistant Staphylococcus aureus. Biotechnol. J. 2017, 12, 1600399. [Google Scholar] [CrossRef]
- Argenta, A.; Satish, L.; Gallo, P.; Liu, F.; Kathju, S. Local Application of Probiotic Bacteria Prophylaxes against Sepsis and Death Resulting from Burn Wound Infection. PLoS ONE 2016, 11, e0165294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peral, M.C.; Huaman Martinez, M.A.; Valdez, J.C. Bacteriotherapy with Lactobacillus plantarum in burns. Int. Wound J. 2009, 6, 73–81. [Google Scholar] [CrossRef]
- Poutahidis, T.; Kearney, S.M.; Levkovich, T.; Qi, P.; Varian, B.J.; Lakritz, J.R.; Ibrahim, Y.M.; Chatzigiagkos, A.; Alm, E.J.; Erdman, S.E. Microbial symbionts accelerate wound healing via the neuropeptide hormone oxytocin. PLoS ONE 2013, 8, e78898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tegegne, B.A.; Kebede, B. Probiotics, their prophylactic and therapeutic applications in human health development: A review of the literature. Heliyon 2022, 8, e09725. [Google Scholar] [CrossRef]
- Gurung, M.; Li, Z.; You, H.; Rodrigues, R.; Jump, D.B.; Morgun, A.; Shulzhenko, N. Role of gut microbiota in type 2 diabetes pathophysiology. EBioMedicine 2020, 51, 102590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dahiya, D.; Nigam, P.S. Probiotics, Prebiotics, Synbiotics, and Fermented Foods as Potential Biotics in Nutrition Improving Health via Microbiome-Gut-Brain Axis. Fermentation 2022, 8, 303. [Google Scholar] [CrossRef]
- McLoughlin, I.J.; Wright, E.M.; Tagg, J.R.; Jain, R.; Hale, J.D.F. Skin Microbiome-The Next Frontier for Probiotic Intervention. Probiotics Antimicrob. Proteins 2022, 14, 630–647. [Google Scholar] [CrossRef] [PubMed]
- de Sire, A.; de Sire, R.; Curci, C.; Castiglione, F.; Wahli, W. Role of Dietary Supplements and Probiotics in Modulating Microbiota and Bone Health: The Gut-Bone Axis. Cells 2022, 11, 743. [Google Scholar] [CrossRef] [PubMed]
- Santos, R.; Veiga, A.S.; Tavares, L.; Castanho, M.; Oliveira, M. Bacterial Biofilms in Diabetic Foot Ulcers: Potential Alternative Therapeutics. In Microbial Biofilms—Importance and Applications; IntechOpen: Rijeka, Croatia, 2016; pp. 251–269. [Google Scholar]
- Klaenhammer, T.R.; Kleerebezem, M.; Kopp, M.V.; Rescigno, M. The impact of probiotics and prebiotics on the immune system. Nat. Rev. Immunol. 2012, 12, 728–734. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.M.; Jeong, J.J.; Woo, K.H.; Han, M.J.; Kim, D.H. Lactobacillus sakei OK67 ameliorates high-fat diet-induced blood glucose intolerance and obesity in mice by inhibiting gut microbiota lipopolysaccharide production and inducing colon tight junction protein expression. Nutr. Res. 2016, 36, 337–348. [Google Scholar] [CrossRef]
- Balakumar, M.; Prabhu, D.; Sathishkumar, C.; Prabu, P.; Rokana, N.; Kumar, R.; Raghavan, S.; Soundarajan, A.; Grover, S.; Batish, V.K.; et al. Improvement in glucose tolerance and insulin sensitivity by probiotic strains of Indian gut origin in high-fat diet-fed C57BL/6J mice. Eur. J. Nutr. 2018, 57, 279–295. [Google Scholar] [CrossRef]
- Maldonado Galdeano, C.; Cazorla, S.I.; Lemme Dumit, J.M.; Velez, E.; Perdigon, G. Beneficial Effects of Probiotic Consumption on the Immune System. Ann. Nutr. Metab. 2019, 74, 115–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Baarlen, P.; Troost, F.J.; van Hemert, S.; van der Meer, C.; de Vos, W.M.; de Groot, P.J.; Hooiveld, G.J.; Brummer, R.J.M.; Kleerebezem, M. Differential NF-κB pathways induction by Lactobacillus plantarum in the duodenum of healthy humans correlating with immune tolerance. Proc. Natl. Acad. Sci. USA 2009, 106, 2371–2376. [Google Scholar] [CrossRef] [Green Version]
- van Baarlen, P.; Troost, F.; van der Meer, C.; Hooiveld, G.; Boekschoten, M.; Brummer, R.J.; Kleerebezem, M. Human mucosal in vivo transcriptome responses to three lactobacilli indicate how probiotics may modulate human cellular pathways. Proc. Natl. Acad. Sci. USA 2011, 108 (Suppl. 1), 4562–4569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, V.; Naik, B.; Kumar, A.; Khanduri, N.; Rustagi, S.; Kumar, S. Probiotics media: Significance, challenges, and future perspective—A mini review. Food Prod. Processing Nutr. 2022, 4, 17. [Google Scholar] [CrossRef]
- Han, H.; Li, Y.; Fang, J.; Liu, G.; Yin, J.; Li, T.; Yin, Y. Gut Microbiota and Type 1 Diabetes. Int. J. Mol. Sci. 2018, 19, 995. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.-H.; Yen, H.-R.; Lu, W.-L.; Ho, H.-H.; Lin, W.-Y.; Kuo, Y.-W.; Huang, Y.-Y.; Tsai, S.-Y.; Lin, H.-C. Adjuvant Probiotics of Lactobacillus salivarius subsp. salicinius AP-32, L. johnsonii MH-68, and Bifidobacterium animalis subsp. lactis CP-9 Attenuate Glycemic Levels and Inflammatory Cytokines in Patients With Type 1 Diabetes Mellitus. Front. Endocrinol. 2022, 13, 754401. [Google Scholar] [CrossRef]
- Madempudi, R.S.; Ahire, J.J.; Neelamraju, J.; Tripathi, A.; Nanal, S. Efficacy of UB0316, a multi-strain probiotic formulation in patients with type 2 diabetes mellitus: A double blind, randomized, placebo controlled study. PLoS ONE 2019, 14, e0225168. [Google Scholar] [CrossRef]
- Zamora-Pineda, J.; Kalinina, O.; Osborne, B.A.; Knight, K.L. Probiotic Molecules That Inhibit Inflammatory Diseases. Appl. Sci. 2022, 12, 1147. [Google Scholar] [CrossRef]
- Mantis, N.J.; Rol, N.; Corthesy, B. Secretory IgA’s complex roles in immunity and mucosal homeostasis in the gut. Mucosal. Immunol. 2011, 4, 603–611. [Google Scholar] [CrossRef] [PubMed]
- Azad, M.A.K.; Sarker, M.; Wan, D. Immunomodulatory Effects of Probiotics on Cytokine Profiles. Biomed. Res. Int. 2018, 2018, 8063647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hickey, A.; Stamou, P.; Udayan, S.; Ramon-Vazquez, A.; Esteban-Torres, M.; Bottacini, F.; Woznicki, J.A.; Hughes, O.; Melgar, S.; Ventura, M.; et al. Bifidobacterium breve Exopolysaccharide Blocks Dendritic Cell Maturation and Activation of CD4(+) T Cells. Front. Microbiol. 2021, 12, 653587. [Google Scholar] [CrossRef] [PubMed]
- Schlee, M.; Wehkamp, J.; Altenhoefer, A.; Oelschlaeger, T.A.; Stange, E.F.; Fellermann, K. Induction of human beta-defensin 2 by the probiotic Escherichia coli Nissle 1917 is mediated through flagellin. Infect. Immun. 2007, 75, 2399–2407. [Google Scholar] [CrossRef] [Green Version]
- Yan, S.; Yang, B.; Zhao, J.; Zhao, J.; Stanton, C.; Ross, R.P.; Zhang, H.; Chen, W. A ropy exopolysaccharide producing strain Bifidobacterium longum subsp. longum YS108R alleviates DSS-induced colitis by maintenance of the mucosal barrier and gut microbiota modulation. Food Funct. 2019, 10, 1595–1608. [Google Scholar] [CrossRef] [PubMed]
- Sproston, N.R.; Ashworth, J.J. Role of C-Reactive Protein at Sites of Inflammation and Infection. Front. Immunol. 2018, 9, 754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazruei Arani, N.; Emam-Djomeh, Z.; Tavakolipour, H.; Sharafati-Chaleshtori, R.; Soleimani, A.; Asemi, Z. The Effects of Probiotic Honey Consumption on Metabolic Status in Patients with Diabetic Nephropathy: A Randomized, Double-Blind, Controlled Trial. Probiotics Antimicrob. Proteins 2019, 11, 1195–1201. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, S.N.; Saboori, S.; Asbaghi, O. Effect of daily probiotic yogurt consumption on inflammation: A systematic review and meta-analysis of randomized Controlled Clinical trials. Obes. Med. 2020, 18, 100221. [Google Scholar] [CrossRef]
- Mafi, A.; Namazi, G.; Soleimani, A.; Bahmani, F.; Aghadavod, E.; Asemi, Z. Metabolic and genetic response to probiotics supplementation in patients with diabetic nephropathy: A randomized, double-blind, placebo-controlled trial. Food Funct. 2018, 9, 4763–4770. [Google Scholar] [CrossRef] [PubMed]
- Sharma, H.; Sharma, S.; Krishnan, A.; Yuan, D.; Vangaveti, V.N.; Malabu, U.H.; Haleagrahara, N. The efficacy of inflammatory markers in diagnosing infected diabetic foot ulcers and diabetic foot osteomyelitis: Systematic review and meta-analysis. PLoS ONE 2022, 17, e0267412. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Suh, D.H.; Kim, H.J.; Lee, Y.I.; Kwak, I.H.; Choi, G.W. Role of procalcitonin in infected diabetic foot ulcer. Diabetes Res. Clin. Pr. 2017, 128, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Sanaie, S.; Ebrahimi-Mameghani, M.; Hamishehkar, H.; Mojtahedzadeh, M.; Mahmoodpoor, A. Effect of a multispecies probiotic on inflammatory markers in critically ill patients: A randomized, double-blind, placebo-controlled trial. J. Res. Med. Sci. 2014, 9, 827. [Google Scholar]
- Yunir, E.; Tahapary, D.L.; Tarigan, T.J.E.; Harbuwono, D.S.; Oktavianda, Y.D.; Kristanti, M.; Iswati, E.; Sarumpaet, A.; Soewondo, P. Non-vascular contributing factors of diabetic foot ulcer severity in national referral hospital of Indonesia. J. Diabetes Metab. Disord. 2021, 20, 805–813. [Google Scholar] [CrossRef] [PubMed]
- Wilmink, J.M.; Ladefoged, S.; Jongbloets, A.; Vernooij, J.C.M. The evaluation of the effect of probiotics on the healing of equine distal limb wounds. PLoS ONE 2020, 15, e0236761. [Google Scholar] [CrossRef] [PubMed]
- Stene, C.; Rome, A.; Palmquist, I.; Linninge, C.; Molin, G.; Ahrne, S.; Johnson, L.B.; Jeppsson, B. Administration of probiotics to healthy volunteers: Effects on reactivity of intestinal mucosa and systemic leukocytes. BMC Gastroenterol. 2022, 22, 100. [Google Scholar] [CrossRef] [PubMed]
- Maione, A.G.; Smith, A.; Kashpur, O.; Yanez, V.; Knight, E.; Mooney, D.J.; Veves, A.; Tomic-Canic, M.; Garlick, J.A. Altered ECM deposition by diabetic foot ulcer-derived fibroblasts implicates fibronectin in chronic wound repair. Wound Repair Regen. 2016, 24, 630–643. [Google Scholar] [CrossRef] [Green Version]
- Woo, Y.M.; Kim, O.J.; Jo, E.S.; Jo, M.Y.; Ahn, M.Y.; Lee, Y.-H.; Li, C.-r.; Lee, S.-H.; Choi, J.-S.; Ha, J.M.; et al. The effect of Lactobacillus plantarum hydrolysates promoting VEGF production on vascular growth and hair growth of C57BL/6 mice. J. Anal. Sci. Technol. 2019, 10, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Ridiandries, A.; Tan, J.T.M.; Bursill, C.A. The Role of Chemokines in Wound Healing. Int. J. Mol. Sci. 2018, 19, 3217. [Google Scholar] [CrossRef] [Green Version]
- Watters, C.; DeLeon, K.; Trivedi, U.; Griswold, J.A.; Lyte, M.; Hampel, K.J.; Wargo, M.J.; Rumbaugh, K.P. Pseudomonas aeruginosa biofilms perturb wound resolution and antibiotic tolerance in diabetic mice. Med. Microbiol. Immunol. 2013, 202, 131–141. [Google Scholar] [CrossRef] [Green Version]
- Algburi, A.; Al-Hasani, H.M.; Ismael, T.K.; Abdelhameed, A.; Weeks, R.; Ermakov, A.M.; Chikindas, M.L. Antimicrobial Activity of Bacillus subtilis KATMIRA1933 and Bacillus amyloliquefaciens B-1895 Against Staphylococcus aureus Biofilms Isolated from Wound Infection. Probiotics Antimicrob. Proteins 2021, 13, 125–134. [Google Scholar] [CrossRef]
- Varma, P.; Nisha, N.; Dinesh, K.R.; Kumar, A.V.; Biswas, R. Anti-infective properties of Lactobacillus fermentum against Staphylococcus aureus and Pseudomonas aeruginosa. J. Mol. Microbiol. Biotechnol. 2011, 20, 137–143. [Google Scholar] [CrossRef]
- Huang, Y.; Kyriakides, T.R. The role of extracellular matrix in the pathophysiology of diabetic wounds. Matrix Biol. Plus 2020, 6–7, 100037. [Google Scholar] [CrossRef] [PubMed]
- Lorca, G.; Torino, M.I.; Font de Valdez, G.; Ljungh, Å. Lactobacilli express cell surface proteins which mediate binding of immobilized collagen and fibronectin. FEMS Microbiol. Lett. 2002, 206, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Styriak, I.; Nemcova, R.; Chang, Y.H.; Ljungh, A. Binding of extracellular matrix molecules by probiotic bacteria. Lett. Appl. Microbiol. 2003, 37, 329–333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCarty, S.M.; Percival, S.L. Proteases and Delayed Wound Healing. Adv. Wound Care 2013, 2, 438–447. [Google Scholar] [CrossRef]
- Maghsood, F.; Mirshafiey, A.; Farahani, M.M.; Modarressi, M.H.; Jafari, P.; Motevaseli, E. Dual Effects of Cell Free Supernatants from Lactobacillus acidophilus and Lactobacillus rhamnosus GG in Regulation of MMP-9 by Up-Regulating TIMP-1 and Down-Regulating CD147 in PMADifferentiated THP-1 Cells. Cell J. 2018, 19, 559–568. [Google Scholar] [CrossRef] [PubMed]
- Chuang, Y.C.; Cheng, M.C.; Lee, C.C.; Chiou, T.Y.; Tsai, T.Y. Effect of ethanol extract from Lactobacillus plantarum TWK10-fermented soymilk on wound healing in streptozotocin-induced diabetic rat. AMB Express 2019, 9, 163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maruyama, K.; Asai, J.; Ii, M.; Thorne, T.; Losordo, D.W.; D’Amore, P.A. Decreased macrophage number and activation lead to reduced lymphatic vessel formation and contribute to impaired diabetic wound healing. Am. J. Pathol. 2007, 170, 1178–1191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, N.; Jia, L.; Su, Y.; Du, J.; Guo, L.; Luo, Z.; Liu, Y. Lactobacillus reuteri extracts promoted wound healing via PI3K/AKT/beta-catenin/TGFbeta1 pathway. Stem Cell Res. 2019, 10, 243. [Google Scholar] [CrossRef] [Green Version]
- Arganaraz Aybar, J.N.; Ortiz Mayor, S.; Olea, L.; Garcia, J.J.; Nisoria, S.; Kolling, Y.; Melian, C.; Rachid, M.; Torres Dimani, R.; Werenitzky, C.; et al. Topical Administration of Lactiplantibacillus plantarum Accelerates the Healing of Chronic Diabetic Foot Ulcers through Modifications of Infection, Angiogenesis, Macrophage Phenotype and Neutrophil Response. Microorganisms 2022, 10, 634. [Google Scholar] [CrossRef] [PubMed]
- Mohammedsaeed, W.; Cruickshank, S.; McBain, A.J.; O’Neill, C.A. Lactobacillus rhamnosus GG Lysate Increases Re-Epithelialization of Keratinocyte Scratch Assays by Promoting Migration. Sci. Rep. 2015, 5, 16147. [Google Scholar] [CrossRef] [Green Version]
- Mohseni, S.; Bayani, M.; Bahmani, F.; Tajabadi-Ebrahimi, M.; Bayani, M.A.; Jafari, P.; Asemi, Z. The beneficial effects of probiotic administration on wound healing and metabolic status in patients with diabetic foot ulcer: A randomized, double-blind, placebo-controlled trial. Diabetes Metab. Res. Rev. 2018, 34, 2970. [Google Scholar] [CrossRef] [PubMed]
- Vagesjo, E.; Ohnstedt, E.; Mortier, A.; Lofton, H.; Huss, F.; Proost, P.; Roos, S.; Phillipson, M. Accelerated wound healing in mice by on-site production and delivery of CXCL12 by transformed lactic acid bacteria. Proc. Natl. Acad. Sci. USA 2018, 115, 1895–1900. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campos, L.F.; Tagliari, E.; Casagrande, T.A.C.; Noronha, L.; Campos, A.C.L.; Matias, J.E.F. Effects of Probiotics Supplementation on Skin Wound Healing in Diabetic Rats. Arq Bras. Cir. Dig. 2020, 33, e1498. [Google Scholar] [CrossRef]
- Mohtashami, M.; Mohamadi, M.; Azimi-Nezhad, M.; Saeidi, J.; Nia, F.F.; Ghasemi, A. Lactobacillus bulgaricus and Lactobacillus plantarum improve diabetic wound healing through modulating inflammatory factors. Biotechnol. Appl. Biochem. 2021, 68, 1421–1431. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, Z.; Bai, L.; Deng, J.; Zhou, Q. Biomaterial-based encapsulated probiotics for biomedical applications: Current status and future perspectives. Mater. Des. 2021, 210, 110018. [Google Scholar] [CrossRef]
- Li, Z.; Behrens, A.M.; Ginat, N.; Tzeng, S.Y.; Lu, X.; Sivan, S.; Langer, R.; Jaklenec, A. Biofilm-Inspired Encapsulation of Probiotics for the Treatment of Complex Infections. Adv. Mater. 2018, 30, e1803925. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhang, G.; Yang, X.; Peng, M.; Ge, S.; Tan, S.; Wen, Z.; Wang, Y.; Wu, S.; Liang, Y.; et al. An oral “Super probiotics” with versatile self-assembly adventitia for enhanced intestinal colonization by autonomous regulating the pathological microenvironment. Chem. Eng. J. 2022, 446, 137204. [Google Scholar] [CrossRef]
- Peng, Y.; He, D.; Ge, X.; Lu, Y.; Chai, Y.; Zhang, Y.; Mao, Z.; Luo, G.; Deng, J.; Zhang, Y. Construction of heparin-based hydrogel incorporated with Cu5.4O ultrasmall nanozymes for wound healing and inflammation inhibition. Bioact. Mater. 2021, 6, 3109–3124. [Google Scholar] [CrossRef]
- Durazzo, A.; Nazhand, A.; Lucarini, M.; Atanasov, A.G.; Souto, E.B.; Novellino, E.; Capasso, R.; Santini, A. An Updated Overview on Nanonutraceuticals: Focus on Nanoprebiotics and Nanoprobiotics. Int. J. Mol. Sci. 2020, 21, 2285. [Google Scholar] [CrossRef] [Green Version]
- Zhu, W.; Zhou, S.; Liu, J.; McLean, R.J.C.; Chu, W. Prebiotic, immuno-stimulating and gut microbiota-modulating effects of Lycium barbarum polysaccharide. Biomed. Pharm. 2020, 121, 109591. [Google Scholar] [CrossRef]
- Ghafouri, A.; Zarrati, M.; Shidfar, F.; Heydari, I.; Shokouhi Shoormasti, R.; Eslami, O. Effect of synbiotic bread containing lactic acid on glycemic indicators, biomarkers of antioxidant status and inflammation in patients with type 2 diabetes: A randomized controlled trial. Diabetol. Metab. Syndr. 2019, 11, 103. [Google Scholar] [CrossRef]
- Pistol, G.C.; Marin, D.E.; Dragomir, C.; Taranu, I. Synbiotic combination of prebiotic grape pomace extract and probiotic Lactobacillus sp. reduced important intestinal inflammatory markers and in-depth signalling mediators in lipopolysaccharide-treated Caco-2 cells. Br. J. Nutr. 2019, 121, 291–305. [Google Scholar] [CrossRef] [PubMed]
- Hong, L.; Kim, W.S.; Lee, S.M.; Kang, S.K.; Choi, Y.J.; Cho, C.S. Pullulan Nanoparticles as Prebiotics Enhance the Antibacterial Properties of Lactobacillus plantarum Through the Induction of Mild Stress in Probiotics. Front. Microbiol. 2019, 10, 142. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, S.; Ahmad, R.; Banerjee, K.; AlAjmi, M.F.; Rahman, S. Mechanistic Aspects of Microbe-Mediated Nanoparticle Synthesis. Front. Microbiol. 2021, 12, 638068. [Google Scholar] [CrossRef]
- Sharma, S.; Sharma, N.; Kaushal, N. Comparative Account of Biogenic Synthesis of Silver Nanoparticles Using Probiotics and Their Antimicrobial Activity Against Challenging Pathogens. BioNanoScience 2022, 12, 833–840. [Google Scholar] [CrossRef]
- Joshi, B.S.; Ortiz, D.; Zuhorn, I.S. Converting extracellular vesicles into nanomedicine: Loading and unloading of cargo. Mater. Today Nano 2021, 16, 100148. [Google Scholar] [CrossRef]
- Rodovalho, V.R.; da Luz, B.S.R.; Rabah, H.; do Carmo, F.L.R.; Folador, E.L.; Nicolas, A.; Jardin, J.; Briard-Bion, V.; Blottiere, H.; Lapaque, N.; et al. Extracellular Vesicles Produced by the Probiotic Propionibacterium freudenreichii CIRM-BIA 129 Mitigate Inflammation by Modulating the NF-kappaB Pathway. Front. Microbiol. 2020, 11, 1544. [Google Scholar] [CrossRef]
- Morishita, M.; Horita, M.; Higuchi, A.; Marui, M.; Katsumi, H.; Yamamoto, A. Characterizing Different Probiotic-Derived Extracellular Vesicles as a Novel Adjuvant for Immunotherapy. Mol. Pharm. 2021, 18, 1080–1092. [Google Scholar] [CrossRef] [PubMed]
- Bauerl, C.; Coll-Marques, J.M.; Tarazona-Gonzalez, C.; Perez-Martinez, G. Lactobacillus casei extracellular vesicles stimulate EGFR pathway likely due to the presence of proteins P40 and P75 bound to their surface. Sci. Rep. 2020, 10, 19237. [Google Scholar] [CrossRef]
- Yazdanpanah, L.; Nasiri, M.; Adarvishi, S. Literature review on the management of diabetic foot ulcer. World J. Diabetes 2015, 6, 37–53. [Google Scholar] [CrossRef] [PubMed]
- Goyal, M.; Reeves, N.D.; Rajbhandari, S.; Ahmad, N.; Wang, C.; Yap, M.H. Recognition of ischaemia and infection in diabetic foot ulcers: Dataset and techniques. Comput. Biol. Med. 2020, 117, 103616. [Google Scholar] [CrossRef] [PubMed]
- Abdelhamid, A.G.; El-Masry, S.S.; El-Dougdoug, N.K. Probiotic Lactobacillus and Bifidobacterium strains possess safety characteristics, antiviral activities and host adherence factors revealed by genome mining. EPMA J. 2019, 10, 337–350. [Google Scholar] [CrossRef] [PubMed]
- Martin Manuel, P.; Elena, B.; Carolina, M.G.; Gabriela, P. Oral probiotics supplementation can stimulate the immune system in a stress process. J. Nutr. Intermed. Metab. 2017, 8, 29–40. [Google Scholar] [CrossRef]
- Kilic Yildirim, G.; Dinleyici, M.; Vandenplas, Y.; Dinleyici, E.C. Effects of Multispecies Synbiotic Supplementation on Anthropometric Measurements, Glucose and Lipid Parameters in Children With Exogenous Obesity: A Randomized, Double Blind, Placebo-Controlled Clinical Trial (Probesity-2 Trial). Front. Nutr. 2022, 9, 898037. [Google Scholar] [CrossRef]
- Nataraj, B.H.; Ali, S.A.; Behare, P.V.; Yadav, H. Postbiotics-parabiotics: The new horizons in microbial biotherapy and functional foods. Microb. Cell Fact. 2020, 19, 168. [Google Scholar] [CrossRef]



| Therapy | Advantages | Disadvantages | Reference |
|---|---|---|---|
| Debridement |
|
| [86] |
| Hyperbaric oxygen therapy |
|
| [87,88] |
| Shock wave therapy |
|
| [89] |
| Offloading therapy |
|
| [90] |
| Larval therapy |
|
| [91] |
| Antibiotic treatment |
|
| [92] |
| Author | Acute/Chronic Markers | Study Description | Probiotic Source | Level of Proteins or Acute and Chronic Marker Response | Microbial Burden | Inflammatory Mediators |
|---|---|---|---|---|---|---|
| Mousavi et al. [151] | CRP | Systematic meta-analysis | Probiotic yogurt | <3 mg·dL−1 | NA | IL-6 |
| Mafi et al. [152] | Randomized placebo trial | Bifidobacterium, L. acidophilus, L. fermentum, and L. reuteri | 3.8 ± 1.9 (mg·L−1) | NA | IL-1, TNF- α, and TGF-β | |
| Sanaie et al. [155] | Procalcitonin | Randomized placebo trial | Bifidobacterium, Lactobacillus, and Streptococcus | 1.67 ± 1.27 (μg·mL−1) ~0.47 ± 0.41 (μg·mL−1) | NA | IL-6 and acute phase proteins |
| Stene et al. [158] | WBC | Clinical trial | Bifidobacterium infantis and L. plantarum | 109 circulating leukocytes | NA | IL-6 and IL-10 |
| Varma et al. [164] | EPS | Observational research analysis | L. fermentum | 2.5 and 5 μg of culture supernatant: antibiofilm activity | Pseudomonas and Staphylococcus biofilm | NA |
| Lorca et al. [166] | Extracellular matrix, collagen, fibronectin | Observational research analysis | L. acidophilus | Improved collagen and fibronectin binding (4.6- to 6.3-fold) | NA | NA |
| Chuang et al. [170] | MMP-9 | Observational research analysis | L. plantarum | Low MMP-9 | NA | Low levels of IL-6 and TNF-α |
| Han et al. [172] | Proinflammatory cytokines | Observational research analysis | L. reuteri | Mesenchymal stem cell migration | NA | Enhanced MMP proteinase, TGF-1 |
| Arganaraz et al. [173] | Innate immune response | Observational research analysis | Subcutaneous debridement plus Lactobacillus (topical) | Enhanced phagocytosis and macrophage maturation | Lower bioburden | M1 and M2 macrophages |
| Source | Response against DFU Markers | Reference |
|---|---|---|
| L. rhamnosus GG lysate | Chemokine movement (CXCL2 and CXCR2) induced re-epithelization and keratinocyte movement during non-healing wound | [174] |
| Bifidobacterium bifidum, L. acidophilus, L. casei, and L. fermentum | Decrease in total cholesterol level and high sensitivity CRPs in DFU patients after 12 weeks of continuous supplementation of probiotics | [175] |
| Genetically modified L. reuteri with a plasmid-encoding CXCL2 chemokine | Rapid wound closure with persistent proliferation of dermal cells and prolonged bioavailability of immune cells such as macrophages | [176] |
| Ethanol extract from L. plantarum TWK10 | Enhance wound-healing properties with reduced expression of proinflammatory markers (TNF-α, IL-6, and MMP-9) | [170] |
| Bifidobacterium lactis, L. acidophilus, L. paracasei, and L. rhamnosus | Higher neovascular formation and reduced expression of proinflammatory markers | [177] |
| L. bulgaricus and L. plantarum | Decreased expression of proinflammatory markers (IL-1β and TNF-α); increased expression of anti-inflammatory markers (IL-10 and TGF-β) | [178] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Srivastava, P.; Sondak, T.; Sivashanmugam, K.; Kim, K.-s. A Review of Immunomodulatory Reprogramming by Probiotics in Combating Chronic and Acute Diabetic Foot Ulcers (DFUs). Pharmaceutics 2022, 14, 2436. https://doi.org/10.3390/pharmaceutics14112436
Srivastava P, Sondak T, Sivashanmugam K, Kim K-s. A Review of Immunomodulatory Reprogramming by Probiotics in Combating Chronic and Acute Diabetic Foot Ulcers (DFUs). Pharmaceutics. 2022; 14(11):2436. https://doi.org/10.3390/pharmaceutics14112436
Chicago/Turabian StyleSrivastava, Prakhar, Tesalonika Sondak, Karthikeyan Sivashanmugam, and Kwang-sun Kim. 2022. "A Review of Immunomodulatory Reprogramming by Probiotics in Combating Chronic and Acute Diabetic Foot Ulcers (DFUs)" Pharmaceutics 14, no. 11: 2436. https://doi.org/10.3390/pharmaceutics14112436