Phospholipid-Membrane-Based Nanovesicles Acting as Vaccines for Tumor Immunotherapy: Classification, Mechanisms and Applications
Abstract
:1. Introduction
2. Different Origins of Membrane-Based Nanovesicles Are Likely to Act as Tumor Vaccines
2.1. Liposomes
2.2. Bacterial Membrane Vesicles
2.3. Tumor-Cell-Derived EVs
2.4. Dendritic-Cell-Derived EVs
3. Membrane Vesicles Work to Bring a Stone to the Building of Tumor Immunotherapy from the Perspective of Basic Mechanisms
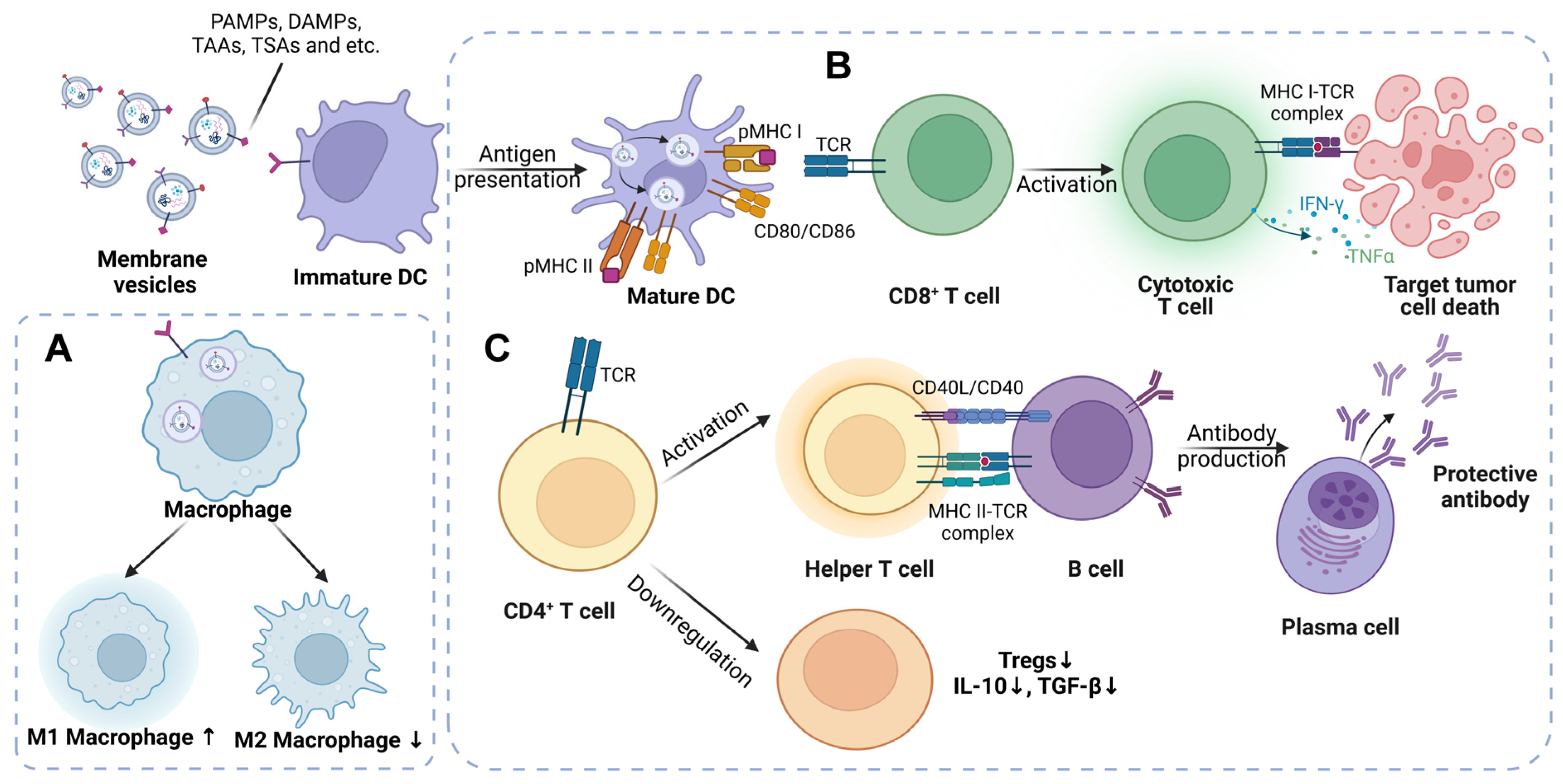
3.1. The Mechanisms of Liposomes as a Tumor Vaccine
3.2. The Immunologic Mechanisms Triggered by Bacterial Membrane Vesicles
3.3. The Mechanisms of Tumor-Cell-Derived EVs Designed to Be Tumor Vaccines
3.4. The Potential Mechanisms of Dendritic-Cell-Derived EVs Acting as a Tumor Vaccine
4. The Current Advances of Representative Membrane Vesicles as Tumor Vaccines in Preclinical Studies
4.1. Synthetic Liposomes Used as a Tumor Vaccine
4.2. The Design and Engineering Modification of Bacterial Membrane Vesicles
| Parent Bacteria | Modification Strategy | Targeting Tumor Types | Mechanisms and Outcomes | Year, Reference |
|---|---|---|---|---|
| Escherichia coli (E. coli) | Bacteria and liposome biohybrid vaccine combined with tumor antigen and adjuvant | Colorectal cancer | Increased expression of CD40, CD80 and CD86 on BMDCs and enhanced infiltration of CD8+ T cell | 2021, [103] |
| E. coli | Genetically engineered OMVs binding with L7Ae (RNA binding protein) and listeriolysin O (lysosomal escape protein) | Melanoma; colon cancer | Listeriolysin O-mediated endosomal escape contributes to cross-presentation of DCs; induction of a long-term immune memory. | 2022, [104] |
| E. coli | OMVs fused with thylakoid membranes from spinach | Colon cancer; breast cancer | Photodynamic effects from thylakoid cause tumor destruction, resulting in release of TAAs and DAMPs presented by DCs and inducing tumor-specific CD8+ T cell responses. | 2022, [105] |
| E. coli | OMVs fused with protein cytolysin A | Pulmonary metastatic melanoma; colon cancer | The antigen-bearing OMVs stimulate DCs maturation and protect animals against tumorous rechallenge. | 2022, [106] |
| E. coli | Conjunctive products of OMVs, Mal and 1-MT (IDO inhibitor) | Colon cancer | The nanoparticles bind to tumor antigens and overcome the immune inhibition of IDO on effector T cells. | 2022, [107] |
| E. coli | OMVs fused with ClyA protein and decorated with tag/catcher protein pairs | Lung melanoma metastasis; colorectal cancer | The vaccine platform “Plug-and-Display” technology displays the tumor antigens and induces innate and specific T-cell-mediated immune responses. | 2021, [108] |
| E. coli | Synthetic OMVs combined with TEVs | Melanoma | Synthetic OMVs have barely any systemic proinflammatory responses; The combined membrane vesicles activate BMDCs, Th-1 T cells and balance antibody production; efficacy of antiPD-1 inhibitor is improved. | 2021, [61] |
| Salmonella Typhimurium | A eukaryotic–prokaryotic vesicle (EPV) nanoplatform containing TEVs and OMVs | Melanoma | It is verified to be a prevention vaccine to trigger antitumor memory immune responses; photothermal effects are motivated by combination with EPV through DCs maturation and production of TNF-α and IL-12. | 2020, [109] |
| E. coli | OMVs modified by insertion of the ectodomain of PD1 | Colon cancer; melanoma | OMVs bind to PD-L1 on the tumor cell surface and thus protect T cells from PD1/PD-L1 axis; OMVs induce the accumulation of effector T cells in TME. | 2020, [110] |
| Salmonella Typhimurium | OMVs from Salmonella Typhimurium | Colorectal carcinoma; hepatocellular carcinoma; breast cancer | OMVs enhance recruitment of NK cells through upregulation of caspase-3, Beclin-1 and CD49b. | 2021, [111] |
4.3. The Proper and Multiple Strategies for Producing Tumor Vaccines from Tumor-Cell-Derived EVs
4.4. Transforming Dendritic-Cell-Derived EVs into an Ideal Tumor Vaccine
5. The Clinical Applications Relevant to Tumor Vaccines of Biomembrane-Based Nanovesicles Are Developing
6. Outlooks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Wang, J.; Zhu, M.; Nie, G. Biomembrane-Based Nanostructures for Cancer Targeting and Therapy: From Synthetic Liposomes to Natural Biomembranes and Membrane-Vesicles. Adv. Drug Deliv. Rev. 2021, 178, 113974. [Google Scholar] [CrossRef] [PubMed]
- Pick, H.; Alves, A.C.; Vogel, H. Single-Vesicle Assays Using Liposomes and Cell-Derived Vesicles: From Modeling Complex Membrane Processes to Synthetic Biology and Biomedical Applications. Chem. Rev. 2018, 118, 8598–8654. [Google Scholar] [CrossRef] [PubMed]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal Information for Studies of Extracellular Vesicles 2018 (MISEV2018): A Position Statement of the International Society for Extracellular Vesicles and Update of the MISEV2014 Guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.E. Extracellular Vesicles in Cancer Therapy. Semin. Cancer Biol. 2022, 86, 296–309. [Google Scholar] [CrossRef] [PubMed]
- Toyofuku, M.; Nomura, N.; Eberl, L. Types and Origins of Bacterial Membrane Vesicles. Nat. Rev. Microbiol. 2019, 17, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Brahmbhatt, H.; MacDiarmid, J.A. Bacterial Minicells to the Rescue: Cyto-Immunotherapy for the Treatment of Late Stage Cancers with Minimal to No Toxicity. Microb. Biotechnol. 2022, 15, 91–94. [Google Scholar] [CrossRef]
- Kim, S.-H.; Kim, K.-S.; Lee, S.-R.; Kim, E.; Kim, M.-S.; Lee, E.-Y.; Gho, Y.S.; Kim, J.-W.; Bishop, R.E.; Chang, K.-T. Structural Modifications of Outer Membrane Vesicles to Refine Them as Vaccine Delivery Vehicles. Biochim. Biophys. Acta 2009, 1788, 2150–2159. [Google Scholar] [CrossRef] [Green Version]
- Luo, P.; Mao, K.; Xu, J.; Wu, F.; Wang, X.; Wang, S.; Zhou, M.; Duan, L.; Tan, Q.; Ma, G.; et al. Metabolic Characteristics of Large and Small Extracellular Vesicles from Pleural Effusion Reveal Biomarker Candidates for the Diagnosis of Tuberculosis and Malignancy. J. Extracell. Vesicles 2020, 9, 1790158. [Google Scholar] [CrossRef]
- Cheng, L.; Hill, A.F. Therapeutically Harnessing Extracellular Vesicles. Nat. Rev. Drug Discov. 2022, 21, 379–399. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, H.; Gu, J.; Zhang, J.; Shi, H.; Qian, H.; Wang, D.; Xu, W.; Pan, J.; Santos, H.A. Engineered Extracellular Vesicles for Cancer Therapy. Adv. Mater. 2021, 33, 2005709. [Google Scholar] [CrossRef]
- Kalluri, R.; LeBleu, V.S. The Biology, Function, and Biomedical Applications of Exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef] [PubMed]
- Kita-Tokarczyk, K.; Itel, F.; Grzelakowski, M.; Egli, S.; Rossbach, P.; Meier, W. Monolayer Interactions between Lipids and Amphiphilic Block Copolymers. Langmuir ACS J. Surf. Colloids 2009, 25, 9847–9856. [Google Scholar] [CrossRef]
- Dimova, R. Recent Developments in the Field of Bending Rigidity Measurements on Membranes. Adv. Colloid Interface Sci. 2014, 208, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Bayer, A.M.; Alam, S.; Mattern-Schain, S.I.; Best, M.D. Triggered Liposomal Release through a Synthetic Phosphatidylcholine Analogue Bearing a Photocleavable Moiety Embedded within the Sn-2 Acyl Chain. Chem. Weinh. Bergstr. Ger. 2014, 20, 3350–3357. [Google Scholar] [CrossRef]
- Immordino, M.L.; Dosio, F.; Cattel, L. Stealth Liposomes: Review of the Basic Science, Rationale, and Clinical Applications, Existing and Potential. Int. J. Nanomed. 2006, 1, 297–315. [Google Scholar]
- Mader, C.; Küpcü, S.; Sleytr, U.B.; Sára, M. S-Layer-Coated Liposomes as a Versatile System for Entrapping and Binding Target Molecules. Biochim. Biophys. Acta 2000, 1463, 142–150. [Google Scholar] [CrossRef] [Green Version]
- Ho, N.T.; Siggel, M.; Camacho, K.V.; Bhaskara, R.M.; Hicks, J.M.; Yao, Y.-C.; Zhang, Y.; Köfinger, J.; Hummer, G.; Noy, A. Membrane Fusion and Drug Delivery with Carbon Nanotube Porins. Proc. Natl. Acad. Sci. USA 2021, 118, e2016974118. [Google Scholar] [CrossRef]
- Lian, S.; Xie, R.; Ye, Y.; Xie, X.; Li, S.; Lu, Y.; Li, B.; Cheng, Y.; Katanaev, V.L.; Jia, L. Simultaneous Blocking of CD47 and PD-L1 Increases Innate and Adaptive Cancer Immune Responses and Cytokine Release. EBioMedicine 2019, 42, 281–295. [Google Scholar] [CrossRef] [Green Version]
- Krishnan, N.; Kubiatowicz, L.J.; Holay, M.; Zhou, J.; Fang, R.H.; Zhang, L. Bacterial Membrane Vesicles for Vaccine Applications. Adv. Drug Deliv. Rev. 2022, 185, 114294. [Google Scholar] [CrossRef]
- Silhavy, T.J.; Kahne, D.; Walker, S. The Bacterial Cell Envelope. Cold Spring Harb. Perspect. Biol. 2010, 2, a000414. [Google Scholar] [CrossRef]
- Gerritzen, M.J.H.; Martens, D.E.; Wijffels, R.H.; van der Pol, L.; Stork, M. Bioengineering Bacterial Outer Membrane Vesicles as Vaccine Platform. Biotechnol. Adv. 2017, 35, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Mehanny, M.; Lehr, C.-M.; Fuhrmann, G. Extracellular Vesicles as Antigen Carriers for Novel Vaccination Avenues. Adv. Drug Deliv. Rev. 2021, 173, 164–180. [Google Scholar] [CrossRef] [PubMed]
- Jan, A.T. Outer Membrane Vesicles (OMVs) of Gram-Negative Bacteria: A Perspective Update. Front. Microbiol. 2017, 8, 1053. [Google Scholar] [CrossRef] [PubMed]
- Gnopo, Y.M.D.; Watkins, H.C.; Stevenson, T.C.; DeLisa, M.P.; Putnam, D. Designer Outer Membrane Vesicles as Immunomodulatory Systems—Reprogramming Bacteria for Vaccine Delivery. Adv. Drug Deliv. Rev. 2017, 114, 132–142. [Google Scholar] [CrossRef]
- Kaparakis-Liaskos, M.; Ferrero, R.L. Immune Modulation by Bacterial Outer Membrane Vesicles. Nat. Rev. Immunol. 2015, 15, 375–387. [Google Scholar] [CrossRef]
- Bachmann, M.F.; Jennings, G.T. Vaccine Delivery: A Matter of Size, Geometry, Kinetics and Molecular Patterns. Nat. Rev. Immunol. 2010, 10, 787–796. [Google Scholar] [CrossRef]
- Brown, L.; Wolf, J.M.; Prados-Rosales, R.; Casadevall, A. Through the Wall: Extracellular Vesicles in Gram-Positive Bacteria, Mycobacteria and Fungi. Nat. Rev. Microbiol. 2015, 13, 620–630. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Thompson, C.D.; Weidenmaier, C.; Lee, J.C. Release of Staphylococcus Aureus Extracellular Vesicles and Their Application as a Vaccine Platform. Nat. Commun. 2018, 9, 1379. [Google Scholar] [CrossRef] [Green Version]
- Hoshino, A.; Costa-Silva, B.; Shen, T.-L.; Rodrigues, G.; Hashimoto, A.; Tesic Mark, M.; Molina, H.; Kohsaka, S.; Di Giannatale, A.; Ceder, S.; et al. Tumour Exosome Integrins Determine Organotropic Metastasis. Nature 2015, 527, 329–335. [Google Scholar] [CrossRef] [Green Version]
- Willms, E.; Cabañas, C.; Mäger, I.; Wood, M.J.A.; Vader, P. Extracellular Vesicle Heterogeneity: Subpopulations, Isolation Techniques, and Diverse Functions in Cancer Progression. Front. Immunol. 2018, 9, 738. [Google Scholar] [CrossRef] [Green Version]
- Raulf, N.; Lucarelli, P.; Thavaraj, S.; Brown, S.; Vicencio, J.M.; Sauter, T.; Tavassoli, M. Annexin A1 Regulates EGFR Activity and Alters EGFR-Containing Tumour-Derived Exosomes in Head and Neck Cancers. Eur. J. Cancer Oxf. Engl. 1990 2018, 102, 52–68. [Google Scholar] [CrossRef] [PubMed]
- Demory Beckler, M.; Higginbotham, J.N.; Franklin, J.L.; Ham, A.-J.; Halvey, P.J.; Imasuen, I.E.; Whitwell, C.; Li, M.; Liebler, D.C.; Coffey, R.J. Proteomic Analysis of Exosomes from Mutant KRAS Colon Cancer Cells Identifies Intercellular Transfer of Mutant KRAS. Mol. Cell. Proteom. MCP 2013, 12, 343–355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, J.A.; Park, H.; Lim, E.H.; Lee, K.W. Exosomes from Breast Cancer Cells Can Convert Adipose Tissue-Derived Mesenchymal Stem Cells into Myofibroblast-like Cells. Int. J. Oncol. 2012, 40, 130–138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Nedawi, K.; Meehan, B.; Kerbel, R.S.; Allison, A.C.; Rak, J. Endothelial Expression of Autocrine VEGF upon the Uptake of Tumor-Derived Microvesicles Containing Oncogenic EGFR. Proc. Natl. Acad. Sci. USA 2009, 106, 3794–3799. [Google Scholar] [CrossRef] [Green Version]
- Tkach, M.; Kowal, J.; Théry, C. Why the Need and How to Approach the Functional Diversity of Extracellular Vesicles. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2018, 373, 20160479. [Google Scholar] [CrossRef]
- Herrmann, I.K.; Wood, M.J.A.; Fuhrmann, G. Extracellular Vesicles as a Next-Generation Drug Delivery Platform. Nat. Nanotechnol. 2021, 16, 748–759. [Google Scholar] [CrossRef]
- Robbins, P.D.; Morelli, A.E. Regulation of Immune Responses by Extracellular Vesicles. Nat. Rev. Immunol. 2014, 14, 195–208. [Google Scholar] [CrossRef] [Green Version]
- Villa, A.; Garofalo, M.; Crescenti, D.; Rizzi, N.; Brunialti, E.; Vingiani, A.; Belotti, P.; Sposito, C.; Franzè, S.; Cilurzo, F.; et al. Transplantation of Autologous Extracellular Vesicles for Cancer-Specific Targeting. Theranostics 2021, 11, 2034–2047. [Google Scholar] [CrossRef]
- Pelissier Vatter, F.A.; Cioffi, M.; Hanna, S.J.; Castarede, I.; Caielli, S.; Pascual, V.; Matei, I.; Lyden, D. Extracellular Vesicle- and Particle-Mediated Communication Shapes Innate and Adaptive Immune Responses. J. Exp. Med. 2021, 218, e20202579. [Google Scholar] [CrossRef]
- Okoye, I.S.; Coomes, S.M.; Pelly, V.S.; Czieso, S.; Papayannopoulos, V.; Tolmachova, T.; Seabra, M.C.; Wilson, M.S. MicroRNA-Containing T-Regulatory-Cell-Derived Exosomes Suppress Pathogenic T Helper 1 Cells. Immunity 2014, 41, 89–103. [Google Scholar] [CrossRef] [Green Version]
- Banchereau, J.; Briere, F.; Caux, C.; Davoust, J.; Lebecque, S.; Liu, Y.J.; Pulendran, B.; Palucka, K. Immunobiology of Dendritic Cells. Annu. Rev. Immunol. 2000, 18, 767–811. [Google Scholar] [CrossRef] [PubMed]
- Kambayashi, T.; Laufer, T.M. Atypical MHC Class II-Expressing Antigen-Presenting Cells: Can Anything Replace a Dendritic Cell? Nat. Rev. Immunol. 2014, 14, 719–730. [Google Scholar] [CrossRef] [PubMed]
- Wculek, S.K.; Cueto, F.J.; Mujal, A.M.; Melero, I.; Krummel, M.F.; Sancho, D. Dendritic Cells in Cancer Immunology and Immunotherapy. Nat. Rev. Immunol. 2020, 20, 7–24. [Google Scholar] [CrossRef] [PubMed]
- Sheng, K.-C.; Day, S.; Wright, M.D.; Stojanovska, L.; Apostolopoulos, V. Enhanced Dendritic Cell-Mediated Antigen-Specific CD4+ T Cell Responses: IFN-Gamma Aids TLR Stimulation. J. Drug Deliv. 2013, 2013, 516749. [Google Scholar] [CrossRef]
- Segura, E.; Nicco, C.; Lombard, B.; Véron, P.; Raposo, G.; Batteux, F.; Amigorena, S.; Théry, C. ICAM-1 on Exosomes from Mature Dendritic Cells Is Critical for Efficient Naive T-Cell Priming. Blood 2005, 106, 216–223. [Google Scholar] [CrossRef] [Green Version]
- Leone, D.A.; Rees, A.J.; Kain, R. Dendritic Cells and Routing Cargo into Exosomes. Immunol. Cell Biol. 2018, 96, 683–693. [Google Scholar] [CrossRef] [Green Version]
- Mardpour, S.; Hamidieh, A.A.; Taleahmad, S.; Sharifzad, F.; Taghikhani, A.; Baharvand, H. Interaction between Mesenchymal Stromal Cell-Derived Extracellular Vesicles and Immune Cells by Distinct Protein Content. J. Cell. Physiol. 2019, 234, 8249–8258. [Google Scholar] [CrossRef]
- Sprent, J. Direct Stimulation of Naïve T Cells by Antigen-Presenting Cell Vesicles. Blood Cells. Mol. Dis. 2005, 35, 17–20. [Google Scholar] [CrossRef]
- Damo, M.; Wilson, D.S.; Simeoni, E.; Hubbell, J.A. TLR-3 Stimulation Improves Anti-Tumor Immunity Elicited by Dendritic Cell Exosome-Based Vaccines in a Murine Model of Melanoma. Sci. Rep. 2015, 5, 17622. [Google Scholar] [CrossRef] [Green Version]
- Kiaie, S.H.; Mojarad-Jabali, S.; Khaleseh, F.; Allahyari, S.; Taheri, E.; Zakeri-Milani, P.; Valizadeh, H. Axial Pharmaceutical Properties of Liposome in Cancer Therapy: Recent Advances and Perspectives. Int. J. Pharm. 2020, 581, 119269. [Google Scholar] [CrossRef]
- Ye, T.; Li, F.; Ma, G.; Wei, W. Enhancing Therapeutic Performance of Personalized Cancer Vaccine via Delivery Vectors. Adv. Drug Deliv. Rev. 2021, 177, 113927. [Google Scholar] [CrossRef] [PubMed]
- Arbelaez, C.A.; Estrada, J.; Gessner, M.A.; Glaus, C.; Morales, A.B.; Mohn, D.; Phee, H.; Lipford, J.R.; Johnston, J.A. A Nanoparticle Vaccine That Targets Neoantigen Peptides to Lymphoid Tissues Elicits Robust Antitumor T Cell Responses. Npj Vaccines 2020, 5, 106. [Google Scholar] [CrossRef] [PubMed]
- Tu, K.; Yu, Y.; Wang, Y.; Yang, T.; Hu, Q.; Qin, X.; Tu, J.; Yang, C.; Kong, L.; Zhang, Z. Combination of Chidamide-Mediated Epigenetic Modulation with Immunotherapy: Boosting Tumor Immunogenicity and Response to PD-1/PD-L1 Blockade. ACS Appl. Mater. Interfaces 2021, 13, 39003–39017. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Tang, L.; Tian, Y.; Ji, X.; Hu, Q.; Zhou, B.; Ding, Z.; Xu, H.; Yang, L. DP7-C-Modified Liposomes Enhance Immune Responses and the Antitumor Effect of a Neoantigen-Based MRNA Vaccine. J. Control. Release Off. J. Control. Release Soc. 2020, 328, 210–221. [Google Scholar] [CrossRef] [PubMed]
- Liang, R.; Xie, J.; Li, J.; Wang, K.; Liu, L.; Gao, Y.; Hussain, M.; Shen, G.; Zhu, J.; Tao, J. Liposomes-Coated Gold Nanocages with Antigens and Adjuvants Targeted Delivery to Dendritic Cells for Enhancing Antitumor Immune Response. Biomaterials 2017, 149, 41–50. [Google Scholar] [CrossRef]
- Long, Q.; Zheng, P.; Zheng, X.; Li, W.; Hua, L.; Yang, Z.; Huang, W.; Ma, Y. Engineered Bacterial Membrane Vesicles Are Promising Carriers for Vaccine Design and Tumor Immunotherapy. Adv. Drug Deliv. Rev. 2022, 186, 114321. [Google Scholar] [CrossRef]
- Allison, C.C.; Kufer, T.A.; Kremmer, E.; Kaparakis, M.; Ferrero, R.L. Helicobacter Pylori Induces MAPK Phosphorylation and AP-1 Activation via a NOD1-Dependent Mechanism. J. Immunol. Baltim. Md. 1950 2009, 183, 8099–8109. [Google Scholar] [CrossRef] [Green Version]
- Söderblom, T.; Oxhamre, C.; Wai, S.N.; Uhlén, P.; Aperia, A.; Uhlin, B.E.; Richter-Dahlfors, A. Effects of the Escherichia Coli Toxin Cytolysin A on Mucosal Immunostimulation via Epithelial Ca2+ Signalling and Toll-like Receptor 4. Cell. Microbiol. 2005, 7, 779–788. [Google Scholar] [CrossRef]
- Kaparakis, M.; Turnbull, L.; Carneiro, L.; Firth, S.; Coleman, H.A.; Parkington, H.C.; Le Bourhis, L.; Karrar, A.; Viala, J.; Mak, J.; et al. Bacterial Membrane Vesicles Deliver Peptidoglycan to NOD1 in Epithelial Cells. Cell. Microbiol. 2010, 12, 372–385. [Google Scholar] [CrossRef] [Green Version]
- Hua, L.; Yang, Z.; Li, W.; Zhang, Q.; Ren, Z.; Ye, C.; Zheng, X.; Li, D.; Long, Q.; Bai, H.; et al. A Novel Immunomodulator Delivery Platform Based on Bacterial Biomimetic Vesicles for Enhanced Antitumor Immunity. Adv. Mater. Deerfield Beach Fla. 2021, 33, e2103923. [Google Scholar] [CrossRef]
- Park, K.; Svennerholm, K.; Crescitelli, R.; Lässer, C.; Gribonika, I.; Lötvall, J. Synthetic Bacterial Vesicles Combined with Tumour Extracellular Vesicles as Cancer Immunotherapy. J. Extracell. Vesicles 2021, 10, e12120. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Lee, J.; Park, J.; Gho, Y.S. Gram-Negative and Gram-Positive Bacterial Extracellular Vesicles. Semin. Cell Dev. Biol. 2015, 40, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Liu, Q.; Yi, J.; Liang, K.; Liu, T.; Roland, K.L.; Jiang, Y.; Kong, Q. Outer Membrane Vesicles Derived from Salmonella Typhimurium Mutants with Truncated LPS Induce Cross-Protective Immune Responses against Infection of Salmonella Enterica Serovars in the Mouse Model. Int. J. Med. Microbiol. 2016, 306, 697–706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, O.Y.; Park, H.T.; Dinh, N.T.H.; Choi, S.J.; Lee, J.; Kim, J.H.; Lee, S.-W.; Gho, Y.S. Bacterial Outer Membrane Vesicles Suppress Tumor by Interferon-γ-Mediated Antitumor Response. Nat. Commun. 2017, 8, 626. [Google Scholar] [CrossRef] [Green Version]
- Bardi, G.T.; Smith, M.A.; Hood, J.L. Melanoma Exosomes Promote Mixed M1 and M2 Macrophage Polarization. Cytokine 2018, 105, 63–72. [Google Scholar] [CrossRef]
- Liu, C.; Yu, S.; Zinn, K.; Wang, J.; Zhang, L.; Jia, Y.; Kappes, J.C.; Barnes, S.; Kimberly, R.P.; Grizzle, W.E.; et al. Murine Mammary Carcinoma Exosomes Promote Tumor Growth by Suppression of NK Cell Function. J. Immunol. Baltim. Md. 1950 2006, 176, 1375–1385. [Google Scholar] [CrossRef] [Green Version]
- Chen, G.; Huang, A.C.; Zhang, W.; Zhang, G.; Wu, M.; Xu, W.; Yu, Z.; Yang, J.; Wang, B.; Sun, H.; et al. Exosomal PD-L1 Contributes to Immunosuppression and Is Associated with Anti-PD-1 Response. Nature 2018, 560, 382–386. [Google Scholar] [CrossRef]
- Chen, Z.; You, L.; Wang, L.; Huang, X.; Liu, H.; Wei, J.Y.; Zhu, L.; Qian, W. Dual Effect of DLBCL-Derived EXOs in Lymphoma to Improve DC Vaccine Efficacy in Vitro While Favor Tumorgenesis in Vivo. J. Exp. Clin. Cancer Res. CR 2018, 37, 190. [Google Scholar] [CrossRef] [Green Version]
- Wan, C.; Sun, Y.; Tian, Y.; Lu, L.; Dai, X.; Meng, J.; Huang, J.; He, Q.; Wu, B.; Zhang, Z.; et al. Irradiated Tumor Cell–Derived Microparticles Mediate Tumor Eradication via Cell Killing and Immune Reprogramming. Sci. Adv. 2020, 6, eaay9789. [Google Scholar] [CrossRef] [Green Version]
- Veerman, R.E.; Güçlüler Akpinar, G.; Eldh, M.; Gabrielsson, S. Immune Cell-Derived Extracellular Vesicles—Functions and Therapeutic Applications. Trends Mol. Med. 2019, 25, 382–394. [Google Scholar] [CrossRef]
- Ma, J.; Wei, K.; Zhang, H.; Tang, K.; Li, F.; Zhang, T.; Liu, J.; Xu, P.; Yu, Y.; Sun, W.; et al. Mechanisms by Which Dendritic Cells Present Tumor Microparticle Antigens to CD8(+) T Cells. Cancer Immunol. Res. 2018, 6, 1057–1068. [Google Scholar] [CrossRef] [PubMed]
- Elsner, L.; Muppala, V.; Gehrmann, M.; Lozano, J.; Malzahn, D.; Bickeböller, H.; Brunner, E.; Zientkowska, M.; Herrmann, T.; Walter, L.; et al. The Heat Shock Protein HSP70 Promotes Mouse NK Cell Activity against Tumors That Express Inducible NKG2D Ligands. J. Immunol. Baltim. Md. 1950 2007, 179, 5523–5533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taha, E.A.; Ono, K.; Eguchi, T. Roles of Extracellular HSPs as Biomarkers in Immune Surveillance and Immune Evasion. Int. J. Mol. Sci. 2019, 20, 4588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gougousis, S.; Petanidis, S.; Poutoglidis, A.; Tsetsos, N.; Vrochidis, P.; Skoumpas, I.; Argyriou, N.; Katopodi, T.; Domvri, K. Epigenetic Editing and Tumor-dependent Immunosuppressive Signaling in Head and Neck Malignancies (Review). Oncol. Lett. 2022, 23, 196. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Liu, B.; Cao, Y.; Yao, S.; Liu, Y.; Jin, G.; Qin, Y.; Chen, Y.; Cui, K.; Zhou, L.; et al. Colorectal Cancer-Derived Small Extracellular Vesicles Promote Tumor Immune Evasion by Upregulating PD-L1 Expression in Tumor-Associated Macrophages. Adv. Sci. Weinh. Baden-Wurtt. Ger. 2022, 9, 2102620. [Google Scholar] [CrossRef] [PubMed]
- Mannavola, F.; D’Oronzo, S.; Cives, M.; Stucci, L.S.; Ranieri, G.; Silvestris, F.; Tucci, M. Extracellular Vesicles and Epigenetic Modifications Are Hallmarks of Melanoma Progression. Int. J. Mol. Sci. 2019, 21, 52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jang, S.C.; Economides, K.D.; Moniz, R.J.; Sia, C.L.; Lewis, N.; McCoy, C.; Zi, T.; Zhang, K.; Harrison, R.A.; Lim, J.; et al. ExoSTING, an Extracellular Vesicle Loaded with STING Agonists, Promotes Tumor Immune Surveillance. Commun. Biol. 2021, 4, 497. [Google Scholar] [CrossRef]
- Zhang, C.-X.; Ye, S.-B.; Ni, J.-J.; Cai, T.-T.; Liu, Y.-N.; Huang, D.-J.; Mai, H.-Q.; Chen, Q.-Y.; He, J.; Zhang, X.-S.; et al. STING Signaling Remodels the Tumor Microenvironment by Antagonizing Myeloid-Derived Suppressor Cell Expansion. Cell Death Differ. 2019, 26, 2314–2328. [Google Scholar] [CrossRef]
- Kitai, Y.; Kawasaki, T.; Sueyoshi, T.; Kobiyama, K.; Ishii, K.J.; Zou, J.; Akira, S.; Matsuda, T.; Kawai, T. DNA-Containing Exosomes Derived from Cancer Cells Treated with Topotecan Activate a STING-Dependent Pathway and Reinforce Antitumor Immunity. J. Immunol. Baltim. Md 1950 2017, 198, 1649–1659. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.; Tan, Q.; Guo, M.; Liao, T.; Li, Y.; Yin, Z.; Zhou, E.; Deng, J.; Li, M.; Yang, Z.; et al. Tumor Cell-Derived Microparticles Packaging Monocarboxylate Transporter4 Inhibitor Fluvastatin Suppress Lung Adenocarcinoma via Tumor Microenvironment Remodeling and Improve Chemotherapy. Chem. Eng. J. 2023, 451, 138972. [Google Scholar] [CrossRef]
- Zuo, B.; Zhang, Y.; Zhao, K.; Wu, L.; Qi, H.; Yang, R.; Gao, X.; Geng, M.; Wu, Y.; Jing, R.; et al. Universal Immunotherapeutic Strategy for Hepatocellular Carcinoma with Exosome Vaccines That Engage Adaptive and Innate Immune Responses. J. Hematol. Oncol. 2022, 15, 46. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Zhu, M.; Tian, Y.; Ramm, G.A.; Zhao, Y.; Nie, G. A Membrane Vesicle-Based Dual Vaccine against Melanoma and Lewis Lung Carcinoma. Biomaterials 2012, 33, 6147–6154. [Google Scholar] [CrossRef] [PubMed]
- Zitvogel, L.; Regnault, A.; Lozier, A.; Wolfers, J.; Flament, C.; Tenza, D.; Ricciardi-Castagnoli, P.; Raposo, G.; Amigorena, S. Eradication of Established Murine Tumors Using a Novel Cell-Free Vaccine: Dendritic Cell-Derived Exosomes. Nat. Med. 1998, 4, 594–600. [Google Scholar] [CrossRef] [PubMed]
- Théry, C.; Duban, L.; Segura, E.; Véron, P.; Lantz, O.; Amigorena, S. Indirect Activation of Naïve CD4+ T Cells by Dendritic Cell-Derived Exosomes. Nat. Immunol. 2002, 3, 1156–1162. [Google Scholar] [CrossRef] [PubMed]
- Wahlund, C.J.E.; Güclüler, G.; Hiltbrunner, S.; Veerman, R.E.; Näslund, T.I.; Gabrielsson, S. Exosomes from Antigen-Pulsed Dendritic Cells Induce Stronger Antigen-Specific Immune Responses than Microvesicles in Vivo. Sci. Rep. 2017, 7, 17095. [Google Scholar] [CrossRef] [Green Version]
- Admyre, C.; Johansson, S.M.; Paulie, S.; Gabrielsson, S. Direct Exosome Stimulation of Peripheral Human T Cells Detected by ELISPOT. Eur. J. Immunol. 2006, 36, 1772–1781. [Google Scholar] [CrossRef]
- Mai, Y.; Guo, J.; Zhao, Y.; Ma, S.; Hou, Y.; Yang, J. Intranasal Delivery of Cationic Liposome-Protamine Complex MRNA Vaccine Elicits Effective Anti-Tumor Immunity. Cell. Immunol. 2020, 354, 104143. [Google Scholar] [CrossRef] [PubMed]
- Wallis, J.; Katti, P.; Martin, A.M.; Hills, T.; Seymour, L.W.; Shenton, D.P.; Carlisle, R.C. A Liposome-Based Cancer Vaccine for a Rapid and High-Titre Anti-ErbB-2 Antibody Response. Eur. J. Pharm. Sci. Off. J. Eur. Fed. Pharm. Sci. 2020, 152, 105456. [Google Scholar] [CrossRef]
- Affandi, A.J.; Grabowska, J.; Olesek, K.; Lopez Venegas, M.; Barbaria, A.; Rodríguez, E.; Mulder, P.P.G.; Pijffers, H.J.; Ambrosini, M.; Kalay, H.; et al. Selective Tumor Antigen Vaccine Delivery to Human CD169(+) Antigen-Presenting Cells Using Ganglioside-Liposomes. Proc. Natl. Acad. Sci. USA 2020, 117, 27528–27539. [Google Scholar] [CrossRef]
- Okazaki, S.; Iwasaki, T.; Yuba, E.; Watarai, S. Evaluation of PH-Sensitive Fusogenic Polymer-Modified Liposomes Co-Loaded with Antigen and α-Galactosylceramide as an Anti-Tumor Vaccine. J. Vet. Med. Sci. 2018, 80, 197–204. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Wang, H.; Yang, Y.; Jia, W.; Su, T.; Che, Y.; Feng, Y.; Yuan, X.; Wang, X. Mannose-Modified Liposome Co-Delivery of Human Papillomavirus Type 16 E7 Peptide and CpG Oligodeoxynucleotide Adjuvant Enhances Antitumor Activity Against Established Large TC-1 Grafted Tumors in Mice. Int. J. Nanomed. 2020, 15, 9571–9586. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Wang, Z.; Yuan, Y.; Qian, T.; Zhou, Q. CRGD Target Liposome Delivery System Promoted Immunogenic Cell Death through Enhanced Anticancer Potency of a Thymidine Conjugate under UVA Activation as a Cancer Vaccine. Eur. J. Med. Chem. 2019, 167, 499–509. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Zhou, S.; Quinn, B.; Jahagirdar, D.; Ortega, J.; Abrams, S.I.; Lovell, J.F. HPV-Associated Tumor Eradication by Vaccination with Synthetic Short Peptides and Particle-Forming Liposomes. Small Weinh. Bergstr. Ger. 2021, 17, e2007165. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Zhou, S.; Huang, W.-C.; Seffouh, A.; Mabrouk, M.T.; Morgan, M.T.; Ortega, J.; Abrams, S.I.; Lovell, J.F. A Potent Cancer Vaccine Adjuvant System for Particleization of Short, Synthetic CD8(+) T Cell Epitopes. ACS Nano 2021, 15, 4357–4371. [Google Scholar] [CrossRef]
- Liu, X.; Liu, Y.; Li, X.; Huang, J.; Guo, X.; Zhang, J.; Luo, Z.; Shi, Y.; Jiang, M.; Qin, B.; et al. ER-Targeting PDT Converts Tumors into In Situ Therapeutic Tumor Vaccines. ACS Nano 2022, 16, 9240–9253. [Google Scholar] [CrossRef]
- Grippin, A.J.; Wummer, B.; Wildes, T.; Dyson, K.; Trivedi, V.; Yang, C.; Sebastian, M.; Mendez-Gomez, H.R.; Padala, S.; Grubb, M.; et al. Dendritic Cell-Activating Magnetic Nanoparticles Enable Early Prediction of Antitumor Response with Magnetic Resonance Imaging. ACS Nano 2019, 13, 13884–13898. [Google Scholar] [CrossRef]
- Verbeke, R.; Lentacker, I.; Breckpot, K.; Janssens, J.; Van Calenbergh, S.; De Smedt, S.C.; Dewitte, H. Broadening the Message: A Nanovaccine Co-Loaded with Messenger RNA and α-GalCer Induces Antitumor Immunity through Conventional and Natural Killer T Cells. ACS Nano 2019, 13, 1655–1669. [Google Scholar] [CrossRef]
- Liu, F.; Dai, Z.; Cheng, Q.; Xu, L.; Huang, L.; Liu, Z.; Li, X.; Wang, N.; Wang, G.; Wang, L.; et al. LncRNA-Targeting Bio-Scaffold Mediates Triple Immune Effects for Postoperative Colorectal Cancer Immunotherapy. Biomaterials 2022, 284, 121485. [Google Scholar] [CrossRef]
- Sayour, E.J.; Grippin, A.; De Leon, G.; Stover, B.; Rahman, M.; Karachi, A.; Wummer, B.; Moore, G.; Castillo-Caro, P.; Fredenburg, K.; et al. Personalized Tumor RNA Loaded Lipid-Nanoparticles Prime the Systemic and Intratumoral Milieu for Response to Cancer Immunotherapy. Nano Lett. 2018, 18, 6195–6206. [Google Scholar] [CrossRef]
- Deng, C.; Zhang, Q.; Jia, M.; Zhao, J.; Sun, X.; Gong, T.; Zhang, Z. Tumors and Their Microenvironment Dual-Targeting Chemotherapy with Local Immune Adjuvant Therapy for Effective Antitumor Immunity against Breast Cancer. Adv. Sci. 2019, 6, 1801868. [Google Scholar] [CrossRef] [Green Version]
- Kim, O.Y.; Choi, S.J.; Jang, S.C.; Park, K.-S.; Kim, S.R.; Choi, J.P.; Lim, J.H.; Lee, S.-W.; Park, J.; Di Vizio, D.; et al. Bacterial Protoplast-Derived Nanovesicles as Vaccine Delivery System against Bacterial Infection. Nano Lett. 2015, 15, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Klimentová, J.; Stulík, J. Methods of Isolation and Purification of Outer Membrane Vesicles from Gram-Negative Bacteria. Microbiol. Res. 2015, 170, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Naciute, M.; Kiwitt, T.; Kemp, R.A.; Hook, S. Bacteria Biohybrid Oral Vaccines for Colorectal Cancer Treatment Reduce Tumor Growth and Increase Immune Infiltration. Vaccine 2021, 39, 5589–5599. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ma, X.; Yue, Y.; Zhang, K.; Cheng, K.; Feng, Q.; Ma, N.; Liang, J.; Zhang, T.; Zhang, L.; et al. Rapid Surface Display of MRNA Antigens by Bacteria-Derived Outer Membrane Vesicles for a Personalized Tumor Vaccine. Adv. Mater. Deerfield Beach Fla. 2022, 34, e2109984. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, W.-R.; Wang, Y.; Lei, Y.; Zuo, L.; Jiang, A.; Wu, G.; Nie, W.; Huang, L.-L.; Xie, H.-Y. Phytochemical Engineered Bacterial Outer Membrane Vesicles for Photodynamic Effects Promoted Immunotherapy. Nano Lett. 2022, 22, 4491–4500. [Google Scholar] [CrossRef] [PubMed]
- Yue, Y.; Xu, J.; Li, Y.; Cheng, K.; Feng, Q.; Ma, X.; Ma, N.; Zhang, T.; Wang, X.; Zhao, X.; et al. Antigen-Bearing Outer Membrane Vesicles as Tumour Vaccines Produced in Situ by Ingested Genetically Engineered Bacteria. Nat. Biomed. Eng. 2022, 6, 898–909. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, K.; Wu, Y.; Yue, Y.; Cheng, K.; Feng, Q.; Ma, X.; Liang, J.; Ma, N.; Liu, G.; et al. Antigen Capture and Immune Modulation by Bacterial Outer Membrane Vesicles as In Situ Vaccine for Cancer Immunotherapy Post-Photothermal Therapy. Small Weinh. Bergstr. Ger. 2022, 18, e2107461. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.; Zhao, R.; Li, Y.; Qi, Y.; Wang, Y.; Zhang, Y.; Qin, H.; Qin, Y.; Chen, L.; Li, C.; et al. Bioengineered Bacteria-Derived Outer Membrane Vesicles as a Versatile Antigen Display Platform for Tumor Vaccination via Plug-and-Display Technology. Nat. Commun. 2021, 12, 2041. [Google Scholar] [CrossRef]
- Chen, Q.; Huang, G.; Wu, W.; Wang, J.; Hu, J.; Mao, J.; Chu, P.K.; Bai, H.; Tang, G. A Hybrid Eukaryotic-Prokaryotic Nanoplatform with Photothermal Modality for Enhanced Antitumor Vaccination. Adv. Mater. Deerfield Beach Fla. 2020, 32, e1908185. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, R.; Cheng, K.; Zhang, K.; Wang, Y.; Zhang, Y.; Li, Y.; Liu, G.; Xu, J.; Xu, J.; et al. Bacterial Outer Membrane Vesicles Presenting Programmed Death 1 for Improved Cancer Immunotherapy via Immune Activation and Checkpoint Inhibition. ACS Nano 2020, 14, 16698–16711. [Google Scholar] [CrossRef]
- Aly, R.G.; El-Enbaawy, M.I.; Abd El-Rahman, S.S.; Ata, N.S. Antineoplastic Activity of Salmonella Typhimurium Outer Membrane Nanovesicles. Exp. Cell Res. 2021, 399, 112423. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Zhao, B.; Wu, L.; Xiao, H.; Ding, K.; Zheng, C.; Song, Q.; Sun, L.; Wang, L.; Zhang, Z. Amplified Cancer Immunotherapy of a Surface-Engineered Antigenic Microparticle Vaccine by Synergistically Modulating Tumor Microenvironment. ACS Nano 2019, 13, 12553–12566. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Xu, Y.; Chen, X.; Liu, J.; Weng, Y.; Zhuang, Q.; Lin, F.; Huang, Z.; Wu, S.; Ding, J.; et al. Radiation-Induced Small Extracellular Vesicles as “Carriages” Promote Tumor Antigen Release and Trigger Antitumor Immunity. Theranostics 2020, 10, 4871–4884. [Google Scholar] [CrossRef] [PubMed]
- Dusoswa, S.A.; Horrevorts, S.K.; Ambrosini, M.; Kalay, H.; Paauw, N.J.; Nieuwland, R.; Pegtel, M.D.; Würdinger, T.; Van Kooyk, Y.; Garcia-Vallejo, J.J. Glycan Modification of Glioblastoma-Derived Extracellular Vesicles Enhances Receptor-Mediated Targeting of Dendritic Cells. J. Extracell. Vesicles 2019, 8, 1648995. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, L.; Rong, Y.; Tang, X.; Yi, K.; Qi, P.; Hou, J.; Liu, W.; He, Y.; Gao, X.; Yuan, C.; et al. Engineered Exosomes as an in Situ DC-Primed Vaccine to Boost Antitumor Immunity in Breast Cancer. Mol. Cancer 2022, 21, 45. [Google Scholar] [CrossRef]
- Matsumoto, A.; Takahashi, Y.; Ariizumi, R.; Nishikawa, M.; Takakura, Y. Development of DNA-Anchored Assembly of Small Extracellular Vesicle for Efficient Antigen Delivery to Antigen Presenting Cells. Biomaterials 2019, 225, 119518. [Google Scholar] [CrossRef]
- Zhou, W.; Chen, X.; Zhou, Y.; Shi, S.; Liang, C.; Yu, X.; Chen, H.; Guo, Q.; Zhang, Y.; Liu, P.; et al. Exosomes Derived from Immunogenically Dying Tumor Cells as a Versatile Tool for Vaccination against Pancreatic Cancer. Biomaterials 2022, 280, 121306. [Google Scholar] [CrossRef]
- Pineda, B.; Sánchez García, F.J.; Olascoaga, N.K.; Pérez de la Cruz, V.; Salazar, A.; Moreno-Jiménez, S.; Hernández Pedro, N.; Márquez-Navarro, A.; Ortiz Plata, A.; Sotelo, J. Malignant Glioma Therapy by Vaccination with Irradiated C6 Cell-Derived Microvesicles Promotes an Antitumoral Immune Response. Mol. Ther. J. Am. Soc. Gene Ther. 2019, 27, 1612–1620. [Google Scholar] [CrossRef] [Green Version]
- Diamond, J.M.; Vanpouille-Box, C.; Spada, S.; Rudqvist, N.-P.; Chapman, J.R.; Ueberheide, B.M.; Pilones, K.A.; Sarfraz, Y.; Formenti, S.C.; Demaria, S. Exosomes Shuttle TREX1-Sensitive IFN-Stimulatory DsDNA from Irradiated Cancer Cells to DCs. Cancer Immunol. Res. 2018, 6, 910–920. [Google Scholar] [CrossRef] [Green Version]
- Zuo, B.; Qi, H.; Lu, Z.; Chen, L.; Sun, B.; Yang, R.; Zhang, Y.; Liu, Z.; Gao, X.; You, A.; et al. Alarmin-Painted Exosomes Elicit Persistent Antitumor Immunity in Large Established Tumors in Mice. Nat. Commun. 2020, 11, 1790. [Google Scholar] [CrossRef] [Green Version]
- Taghikhani, A.; Hassan, Z.M.; Ebrahimi, M.; Moazzeni, S.-M. MicroRNA Modified Tumor-Derived Exosomes as Novel Tools for Maturation of Dendritic Cells. J. Cell. Physiol. 2019, 234, 9417–9427. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Li, Z.; Zhang, W.; Li, J.; Hao, S. Enhancing the Anti-Leukemia Immunity of Acute Lymphocytic Leukemia-Derived Exosome-Based Vaccine by Downregulation of PD-L1 Expression. Cancer Immunol. Immunother. CII 2022, 71, 2197–2212. [Google Scholar] [CrossRef] [PubMed]
- Viaud, S.; Théry, C.; Ploix, S.; Tursz, T.; Lapierre, V.; Lantz, O.; Zitvogel, L.; Chaput, N. Dendritic Cell-Derived Exosomes for Cancer Immunotherapy: What’s Next? Cancer Res. 2010, 70, 1281–1285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, H.; Gao, H.; Wang, L.; Cheng, Y.; Wu, X.; Shen, X.; Wang, H.; Wang, Z.; Zhan, P.; Liu, J.; et al. Biosynthetic Dendritic Cell-Exocytosed Aggregation-Induced Emission Nanoparticles for Synergistic Photodynamic Immunotherapy. ACS Nano 2022, 16, 13992–14006. [Google Scholar] [CrossRef]
- Xiong, X.; Ke, X.; Wang, L.; Lin, Y.; Wang, S.; Yao, Z.; Li, K.; Luo, Y.; Liu, F.; Pan, Y.; et al. Neoantigen-Based Cancer Vaccination Using Chimeric RNA-Loaded Dendritic Cell-Derived Extracellular Vesicles. J. Extracell. Vesicles 2022, 11, e12243. [Google Scholar] [CrossRef]
- Lu, Z.; Zuo, B.; Jing, R.; Gao, X.; Rao, Q.; Liu, Z.; Qi, H.; Guo, H.; Yin, H. Dendritic Cell-Derived Exosomes Elicit Tumor Regression in Autochthonous Hepatocellular Carcinoma Mouse Models. J. Hepatol. 2017, 67, 739–748. [Google Scholar] [CrossRef]
- Zhu, H.; Wang, K.; Wang, Z.; Wang, D.; Yin, X.; Liu, Y.; Yu, F.; Zhao, W. An Efficient and Safe MUC1-Dendritic Cell-Derived Exosome Conjugate Vaccine Elicits Potent Cellular and Humoral Immunity and Tumor Inhibition in Vivo. Acta Biomater. 2022, 138, 491–504. [Google Scholar] [CrossRef]
- Phung, C.D.; Pham, T.T.; Nguyen, H.T.; Nguyen, T.T.; Ou, W.; Jeong, J.-H.; Choi, H.-G.; Ku, S.K.; Yong, C.S.; Kim, J.O. Anti-CTLA-4 Antibody-Functionalized Dendritic Cell-Derived Exosomes Targeting Tumor-Draining Lymph Nodes for Effective Induction of Antitumor T-Cell Responses. Acta Biomater. 2020, 115, 371–382. [Google Scholar] [CrossRef]
- Chen, S.; Lv, M.; Fang, S.; Ye, W.; Gao, Y.; Xu, Y. Poly(I:C) Enhanced Anti-Cervical Cancer Immunities Induced by Dendritic Cells-Derived Exosomes. Int. J. Biol. Macromol. 2018, 113, 1182–1187. [Google Scholar] [CrossRef]
- Than, U.T.T.; Le, H.T.; Hoang, D.H.; Nguyen, X.-H.; Pham, C.T.; Bui, K.T.V.; Bui, H.T.H.; Nguyen, P.V.; Nguyen, T.D.; Do, T.T.H.; et al. Induction of Antitumor Immunity by Exosomes Isolated from Cryopreserved Cord Blood Monocyte-Derived Dendritic Cells. Int. J. Mol. Sci. 2020, 21, 1834. [Google Scholar] [CrossRef] [Green Version]
- Wadman, M. Public Needs to Prep for Vaccine Side Effects. Science 2020, 370, 1022. [Google Scholar] [CrossRef] [PubMed]
- Papachristofilou, A.; Hipp, M.M.; Klinkhardt, U.; Früh, M.; Sebastian, M.; Weiss, C.; Pless, M.; Cathomas, R.; Hilbe, W.; Pall, G.; et al. Phase Ib Evaluation of a Self-Adjuvanted Protamine Formulated MRNA-Based Active Cancer Immunotherapy, BI1361849 (CV9202), Combined with Local Radiation Treatment in Patients with Stage IV Non-Small Cell Lung Cancer. J. Immunother. Cancer 2019, 7, 38. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Peng, L.; Han, Y.; Wang, D.; He, X.; Wang, J.; Ou, C. Lipid Nanoparticle-Based MRNA Vaccines in Cancers: Current Advances and Future Prospects. Front. Immunol. 2022, 13, 922301. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Li, Q.; Haesebrouck, F.; Van Hoecke, L.; Vandenbroucke, R.E. The Tremendous Biomedical Potential of Bacterial Extracellular Vesicles. Trends Biotechnol. 2022, 40, 1173–1194. [Google Scholar] [CrossRef] [PubMed]
- Johnson, P.V.; Blair, B.M.; Zeller, S.; Kotton, C.N.; Hohmann, E.L. Attenuated Listeria Monocytogenes Vaccine Vectors Expressing Influenza A Nucleoprotein: Preclinical Evaluation and Oral Inoculation of Volunteers. Microbiol. Immunol. 2011, 55, 304–317. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Zhou, H.; Yang, C.; Wu, Y.; Zhou, X.; Liu, H.; Wang, Y. Bacterial Outer Membrane Vesicles as a Platform for Biomedical Applications: An Update. J. Control. Release Off. J. Control. Release Soc. 2020, 323, 253–268. [Google Scholar] [CrossRef]
- Cheng, K.; Kang, Q.; Zhao, X. Biogenic Nanoparticles as Immunomodulator for Tumor Treatment. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2020, 12, e1646. [Google Scholar] [CrossRef]
- Guo, M.; Wu, F.; Hu, G.; Chen, L.; Xu, J.; Xu, P.; Wang, X.; Li, Y.; Liu, S.; Zhang, S.; et al. Autologous Tumor Cell–Derived Microparticle-Based Targeted Chemotherapy in Lung Cancer Patients with Malignant Pleural Effusion. Sci. Transl. Med. 2019, 11, eaat5690. [Google Scholar] [CrossRef]
- Kantoff, P.W.; Higano, C.S.; Shore, N.D.; Berger, E.R.; Small, E.J.; Penson, D.F.; Redfern, C.H.; Ferrari, A.C.; Dreicer, R.; Sims, R.B.; et al. Sipuleucel-T Immunotherapy for Castration-Resistant Prostate Cancer. N. Engl. J. Med. 2010, 363, 411–422. [Google Scholar] [CrossRef] [Green Version]
- Narita, M.; Kanda, T.; Abe, T.; Uchiyama, T.; Iwafuchi, M.; Zheng, Z.; Liu, A.; Kaifu, T.; Kosugi, S.; Minagawa, M.; et al. Immune Responses in Patients with Esophageal Cancer Treated with SART1 Peptide-Pulsed Dendritic Cell Vaccine. Int. J. Oncol. 2015, 46, 1699–1709. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.; Kroll, A.V.; Holay, M.; Fang, R.H.; Zhang, L. Biomimetic Nanotechnology toward Personalized Vaccines. Adv. Mater. Deerfield Beach Fla. 2020, 32, e1901255. [Google Scholar] [CrossRef] [PubMed]
- Cao, M.; Isaac, R.; Yan, W.; Ruan, X.; Jiang, L.; Wan, Y.; Wang, J.; Wang, E.; Caron, C.; Neben, S.; et al. Cancer-Cell-Secreted Extracellular Vesicles Suppress Insulin Secretion through MiR-122 to Impair Systemic Glucose Homeostasis and Contribute to Tumour Growth. Nat. Cell Biol. 2022, 24, 954–967. [Google Scholar] [CrossRef] [PubMed]
- Görgens, A.; Corso, G.; Hagey, D.W.; Jawad Wiklander, R.; Gustafsson, M.O.; Felldin, U.; Lee, Y.; Bostancioglu, R.B.; Sork, H.; Liang, X.; et al. Identification of Storage Conditions Stabilizing Extracellular Vesicles Preparations. J. Extracell. Vesicles 2022, 11, e12238. [Google Scholar] [CrossRef]
- Kudo, K.; Miki, Y.; Carreras, J.; Nakayama, S.; Nakamoto, Y.; Ito, M.; Nagashima, E.; Yamamoto, K.; Higuchi, H.; Morita, S.-Y.; et al. Secreted Phospholipase A(2) Modifies Extracellular Vesicles and Accelerates B Cell Lymphoma. Cell Metab. 2022, 34, 615–633.e8. [Google Scholar] [CrossRef]
- Xiao, Y.; Tian, J.; Wu, W.-C.; Gao, Y.-H.; Guo, Y.-X.; Song, S.-J.; Gao, R.; Wang, L.-B.; Wu, X.-Y.; Zhang, Y.; et al. Targeting Central Nervous System Extracellular Vesicles Enhanced Triiodothyronine Remyelination Effect on Experimental Autoimmune Encephalomyelitis. Bioact. Mater. 2022, 9, 373–384. [Google Scholar] [CrossRef] [PubMed]

| Modification Strategy | Targeting Tumor Types | Mechanisms and Outcomes | Year, Reference |
|---|---|---|---|
| Cationic liposome encapsulated with mRNA encoding cytokeratin 19 | Lung caner | DC maturation (CD86 ↑; MHCII ↑); cytokine elevation (IL-12 ↑, TNF-α ↑, IL-2 ↑, IL-4 ↑). Induction of an antitumor immune response. | 2020, [87] |
| Liposomes enveloped with ErbB-2 and OVA peptide | Lung carcinoma cells; breast cancer | ErbB-2 (known as Her-2) activates B cells to generate antibodies targeted by Pertuzumab; OVA provides T cell support. | 2020, [88] |
| Liposomes carrying tumor antigens Gangliosides | Pancreatic cancer | Ganglioside liposomes bind to CD169 and are internalized by CD169+ DCs and macrophages causing cytokine production, robust cross-presentation and specific activation of CD8+ T cells. | 2020, [89] |
| pH-sensitive liposomes containing OVA and α-GalCer | T lymphoma | Induction of OVA-specific IgG1 and IgG2b antibody responses; increased production of IFN-γ and IL-4; prophylactic vaccination efficacy. | 2018, [90] |
| Liposomes containing HPV16 E7 peptide and CpG oligodeoxynucleotides and modified with DC-targeting mannose | Cervical cancer | Increased proportions of CD4+ and CD8+ T cells and CTL; reducing numbers of inhibitory immune cells such as MDSCs. | 2020, [91] |
| Liposomes conjugated with adjuvant cRGD | Lung cancer; melanoma; breast cancer; liver cancer | cRGD promotes immunogenic cell death; cRGD-liposomes increase cellular accumulation of thymidine conjugate and enhance cytotoxicity following UVA activation. | 2019, [92] |
| Liposomes admixed with HPV-16 E7 epitope | Cervical cancer | Induction of antigen-specific CD8+ T cells and production of relevant cytokines (TNF-α and IL-2); increased percentages of central and effector memory T cells. | 2021, [93] |
| Liposomes modified with the adjuvant system including CoPoP, PHAD and immunostimulatory molecules QS-21 | Colon cancer | CoPoP induces particle formation of peptides; particle-based peptides are better taken up by APCs and are represented on an MHC-I surface; generation of antigen-specific CD8+ T cells. | 2021, [94] |
| Liposomes modified with ICG and pardaxin peptide | Melanoma | Under NIR, the liposomes induce the release of DAMPs and TAAs with high immunogenicity. | 2022, [95] |
| RNA-loaded magnetic liposomes | Glioblastoma | Iron oxide enhances DCs transfection and enables tracking of DCs migration with MRI, thus predicting individual treatment effects | 2019, [96] |
| Liposomal nanoparticles composed of mRNA (containing Ψ and 5meC) and α-GalCer | Melanoma; lymphoma | The nanosystem leads to the activation of iNKT after presented by APCs, and then cytokines (IFN-γ, IL-4, etc.) secreted by iNKT activate DCs and CTL. | 2019, [97] |
| Liposome-decorated cancer cell membrane enveloping a plasmid encoding shRNA against Pvt1 | Colorectal cancer | The biolipid nanoparticles strengthen Oxa-induced ICD; activation of DCs; inhibition of MDSCs; generation of immune memory responses for tumor ectopic rechallenging and metastasis. | 2022, [98] |
| Cationic liposome encapsulated with tumor-derived mRNA | Melanoma; lung cancer | Increased coexpression of CD11c and PD-L1 in host-myeloid cells sensitize immunologically “cold” tumor; PD-L1+ APCs elicit IFN-γ production causing expansion of specific CD8+ T cells; combination with ICIs enhances T cell activity and synergistic antitumor efficacy. | 2018, [99] |
| MMP2 responsive folate-modified liposome carrying doxorubicin | Breast cancer | Elimination of M2-TAMs resulting in a decrease in immunosuppressive cytokines and Treg cells, ensuring antitumor effector T cells; promotion of DCs maturation and immunostimulatory cytokines secretion. | 2019, [100] |
| Modification Strategy | Targeting Tumor Types | Mechanisms and Outcomes | Year, Reference |
|---|---|---|---|
| Tumor-derived antigenic microparticles (T-MPs) carrying nanoFe3O4 and adjuvant CpG | Melanoma; colon cancer | Nanomaterials absorbed by APCs elicit antigen-specific host immune responses; reversion of tumor-associated macrophages into a tumor-suppressive M1 phenotype; increased infiltration of CTL. | 2019, [112] |
| Irradiated tumor-cell-derived EVs | Hepatoma; breast cancer | Radiation endows TEVs with tumor antigens (for example, CDCP1) and HSP; enhanced infiltration of CD8+ and CD4+ T cells and activation of CTL. | 2020, [113] |
| TEV surface modification with glycocalyx and removal of sialic acids | Glioblastoma | Increased internalization by DCs via receptor-mediated glycan-depending targeting to DCs. | 2019, [114] |
| α-LA-engineered cancer exosomes loaded with ICD stimulators (ELANE and TLR3 agonist Hiltonol) | Breast cancer | Homing to the tumor sites and induction of ICD in cancer cells; activation of cDC1s and tumor-reactive CD8+ T cells. | 2022, [115] |
| TEVs mixed with an oligonucleotide duplex and assembled with CpG-DNA | Melanoma | TEVs prolong residence in tumor tissue and activate DCs more efficiently than tumor or fibroblast cells. | 2019, [116] |
| Exosomes derived from immunogenically dying tumor cells and modified with MART-1 and CCL22 siRNA | Pancreatic cancer | MART-1 peptide can expand T-cell-related responses; CCL22 siRNA inhibits the communication between DCs and Tregs via the CCR4/CCL22 axis. | 2022, [117] |
| irradiated C6 (malignant glioma-cell-derived EVs | Glioblastoma | Increased percentages of apoptotic tumor cells and helper, cytotoxic and regulatory T cells. | 2019, [118] |
| TEVs derived from irradiated cancer cells | Breast cancer | TEVs transfer dsDNA to promote production of IFN-γ via cGAS/STING pathway; TEVs evoke specific antitumor responses of CD8+ T cells and perform prophylactic vaccination. | 2018, [119] |
| TEVs carrying adjuvant HMGN1 | Hepatocellular carcinoma | TEVs potentiate immunogenicity and activate DCs; TEVs promote DCs homing to lymphoid tissues and augment memory lymphocytes. | 2020, [120] |
| TEVs modified with microRNA (miR-155, miR-142 and let-7i) | Breast cancer | Induction of DCs maturation by detecting expression of MHCII, CD80 and CD40; microRNA-targeting genes (IL-6, TGFβ, IFN-γ, TLR4, SOCS1, etc.) are confirmed to mature DCs. | 2019, [121] |
| TEVs derived from leukemia cells whose PD-L1 have been downregulated by PD-L1 shRNA | Leukemia | Modified TEVs evoke DCs maturation, T-cell activation and release of Th1 cytokine. | 2022, [122] |
| Modification Strategy | Targeting Tumor Types | Mechanisms and Outcomes | Year, Reference |
|---|---|---|---|
| DEVs loaded with MBPN-TCyP (an AIE-photosensitizer) | Breast cancer; colon cancer | The modified DEVs induce ICD and immune-modulation function like parental DCs; DEVs synergize photodynamic immunotherapy. | 2022, [124] |
| DEVs derived from A-Pas chiRNA-transfected DCs | Esophagus cancer | DEXs induce DC maturation (upregulation of CD83, CD86, MHC-I and MHC-II) and CD8+ T-cell-mediated antitumor responses. | 2022, [125] |
| DEVs assembled with tumor peptide P47-P, AFP and immunomodulators N1ND-N | Hepatocellular carcinoma | DEVs promote DCs recruitment, activation, cross-presentation of antigens; DEVs induce antitumor responses by increasing IFN-γ+CD8+ effector T cells. | 2022, [81] |
| Exosomes derived from AFP-expressing DCs | Hepatocellular carcinoma | DEVs remodel TME by increasing IFN-γ+CD8+ T cells and cytokines (IFN-γ and IL-2) and by decreasing CD25+Foxp3+ Treg and cytokines (IL-2 and TGF-β). | 2017, [126] |
| DEVs conjugated with MUC1 glycopeptide antigen | Melanoma | Induction of MUC1-specific IgG antibody; activation of CTL against MUC1-positive tumor cells. | 2022, [127] |
| DEVs derived from OVA-pulsed and activated dendritic cells modified with antiCTLA-4 antibody | Melanoma | DEVs target to T cells and activate tumor-specific T-cell responses; CTLA-4 in DEVs block inhibitory immunity and enhance the specific responses by T cells. | 2020, [128] |
| DEVs loaded with antigen E749-57 peptide and inducer poly(I:C) | Cervical cancer | Activation of CTL; Promoted immunity of vaccinated mice splenocytes. | 2018, [129] |
| DEVs derived from tumor cell lysate-pulsed DCs | Lung cancer | Induced proliferation of allogeneic T cell, including the subpopulation of CD3+Vγ9 T and CD8+ T cells; Activated cytotoxicity of alloPBMCs against tumor cells. | 2020, [130] |
| Trial ID | Phase | Status | Intervention | Applied Conditions |
|---|---|---|---|---|
| NCT04163094 | Phase 1 | Active, not recruiting | A liposome-based mRNA vaccine combined with chemotherapy. | Ovarian cancer |
| NCT01915524 | Phase 1 | Terminated | RNActive®-derived cancer vaccine coding for tumor antigens. | Nonsmall cell lung carcinoma |
| NCT01052142 | Phase 1 | Completed | A liposomal vaccine. | Melanoma |
| NCT01095848 | Phase 1 | Completed | DPX-0907 consists of seven tumor-specific HLA-A2-restricted peptides, a universal T-Helper peptide, a polynucleotide adjuvant, a liposome, etc. | Ovarian, breast and prostatic neoplasms |
| NCT00623831 | Phase 1 | Completed; has Results | Mixed bacterial vaccine. | Melanoma, sarcoma, gastrointestinal stromal tumor, etc. |
| NCT02010203 | Phase 1/2 | Terminated; has Results | HS-410: a vaccine derived from irradiated cancer cells genetically engineered to continually secrete gp96; BCG: a vaccine derived from a live bacterium. | Bladder cancer |
| NCT03762291 | Phase 1 | Recruiting | CVD908ssb-TXSV: Salmonella-based survivin vaccine. | Multiple myeloma |
| NCT02657460 | Phase 2 | Unknown | Tumor-derived microparticles packaging chemotherapy drugs. | Malignant pleural effusion |
| NCT01550523 | Phase 1 | Completed | Exosomes from autologous glioma cells combined with an antisense molecule. | Malignant glioma of brain |
| NCT00020462 | Phase 1 | Completed | Autologous tumor cell vaccine plus interleukin-2. | Lymphoma |
| NCT05559177 | Early Phase 1 | Recruiting | Personalized chimeric exosome tumor vaccines. | Recurrent or metastatic bladder cancer |
| NCT00065442 | Phase 3 | Completed; has Results | Sipuleucel-T: Autologous antigen presenting cells loading with PA2024. | Prostate Cancer |
| NCT02693236 | Phase1/2 | Unknown | Monocyte-derived dendritic cells (moDCs) combined with cytokine-induced killer cells. | Squamous cell carcinoma of esophagus |
| NCT01159288 | Phase 2 | Completed | Dex2: tumor antigen-loaded dendritic-cell-derived exosomes. | Nonsmall cell lung carcinoma |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, W.; Wu, Y.; Deng, J.; Yang, Z.; Chen, J.; Tan, Q.; Guo, M.; Jin, Y. Phospholipid-Membrane-Based Nanovesicles Acting as Vaccines for Tumor Immunotherapy: Classification, Mechanisms and Applications. Pharmaceutics 2022, 14, 2446. https://doi.org/10.3390/pharmaceutics14112446
Chen W, Wu Y, Deng J, Yang Z, Chen J, Tan Q, Guo M, Jin Y. Phospholipid-Membrane-Based Nanovesicles Acting as Vaccines for Tumor Immunotherapy: Classification, Mechanisms and Applications. Pharmaceutics. 2022; 14(11):2446. https://doi.org/10.3390/pharmaceutics14112446
Chicago/Turabian StyleChen, Wenjuan, Yali Wu, Jingjing Deng, Zimo Yang, Jiangbin Chen, Qi Tan, Mengfei Guo, and Yang Jin. 2022. "Phospholipid-Membrane-Based Nanovesicles Acting as Vaccines for Tumor Immunotherapy: Classification, Mechanisms and Applications" Pharmaceutics 14, no. 11: 2446. https://doi.org/10.3390/pharmaceutics14112446
APA StyleChen, W., Wu, Y., Deng, J., Yang, Z., Chen, J., Tan, Q., Guo, M., & Jin, Y. (2022). Phospholipid-Membrane-Based Nanovesicles Acting as Vaccines for Tumor Immunotherapy: Classification, Mechanisms and Applications. Pharmaceutics, 14(11), 2446. https://doi.org/10.3390/pharmaceutics14112446






