MLN4924 Treatment Diminishes Excessive Lipid Storage in High-Fat Diet-Induced Non-Alcoholic Fatty Liver Disease (NAFLD) by Stimulating Hepatic Mitochondrial Fatty Acid Oxidation and Lipid Metabolites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Cell Culture and Lipid Droplet Induction
2.3. Western Blotting
2.4. Mitochondria, Bodipy and DAPI Staining
2.5. Isolation of Mouse Primary Hepatocyte
2.6. Liver Triglyceride Extraction
2.7. Small Animal NMR Analyzer and Metabolic Cages Measurements
2.8. Fatty Acid Oxidase Testing
2.9. Statistics
3. Results
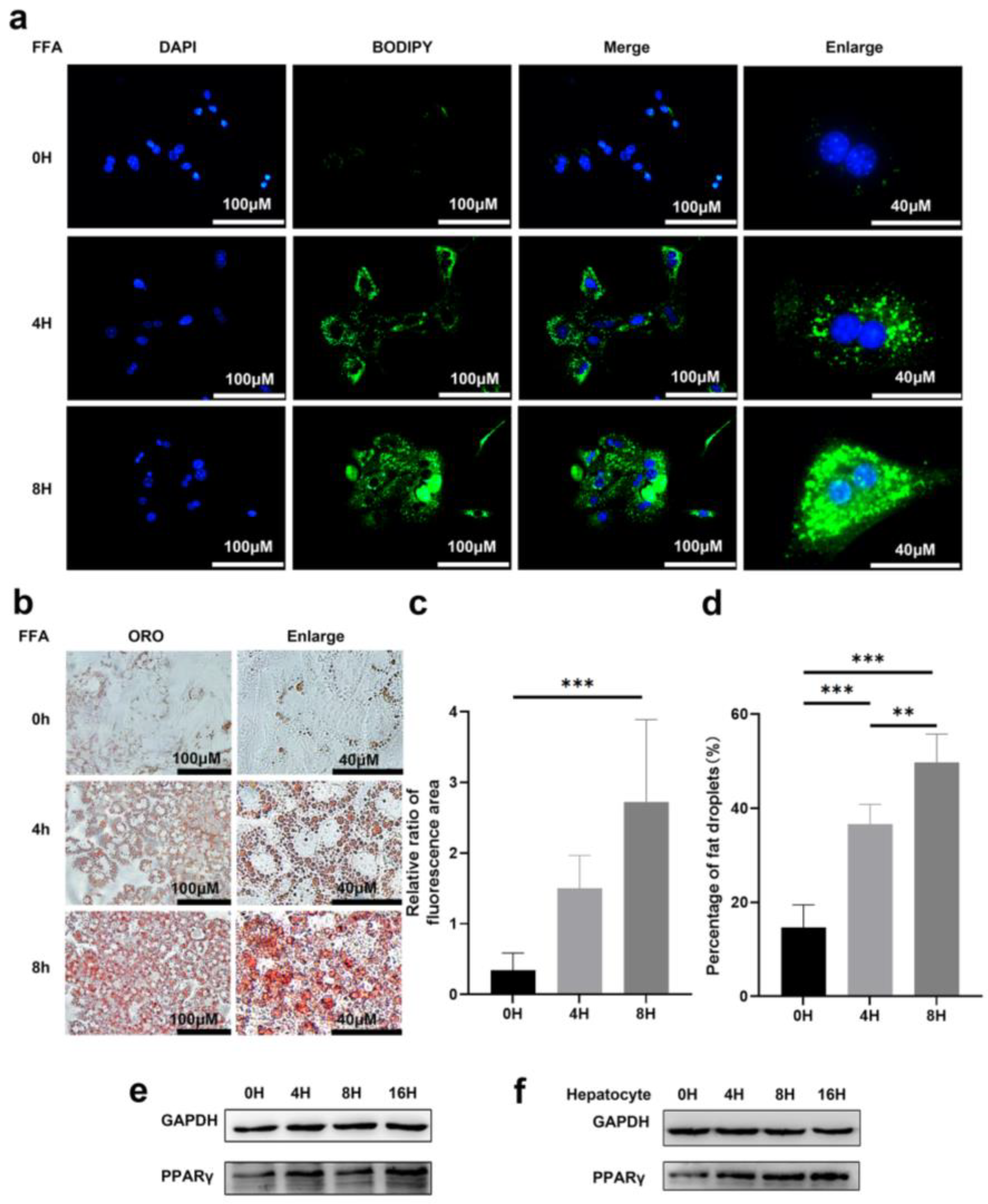
3.1. Prolonged Exposure to Free Fatty Acid Can Induce Lipid Accumulation in Primary Hepatocytes
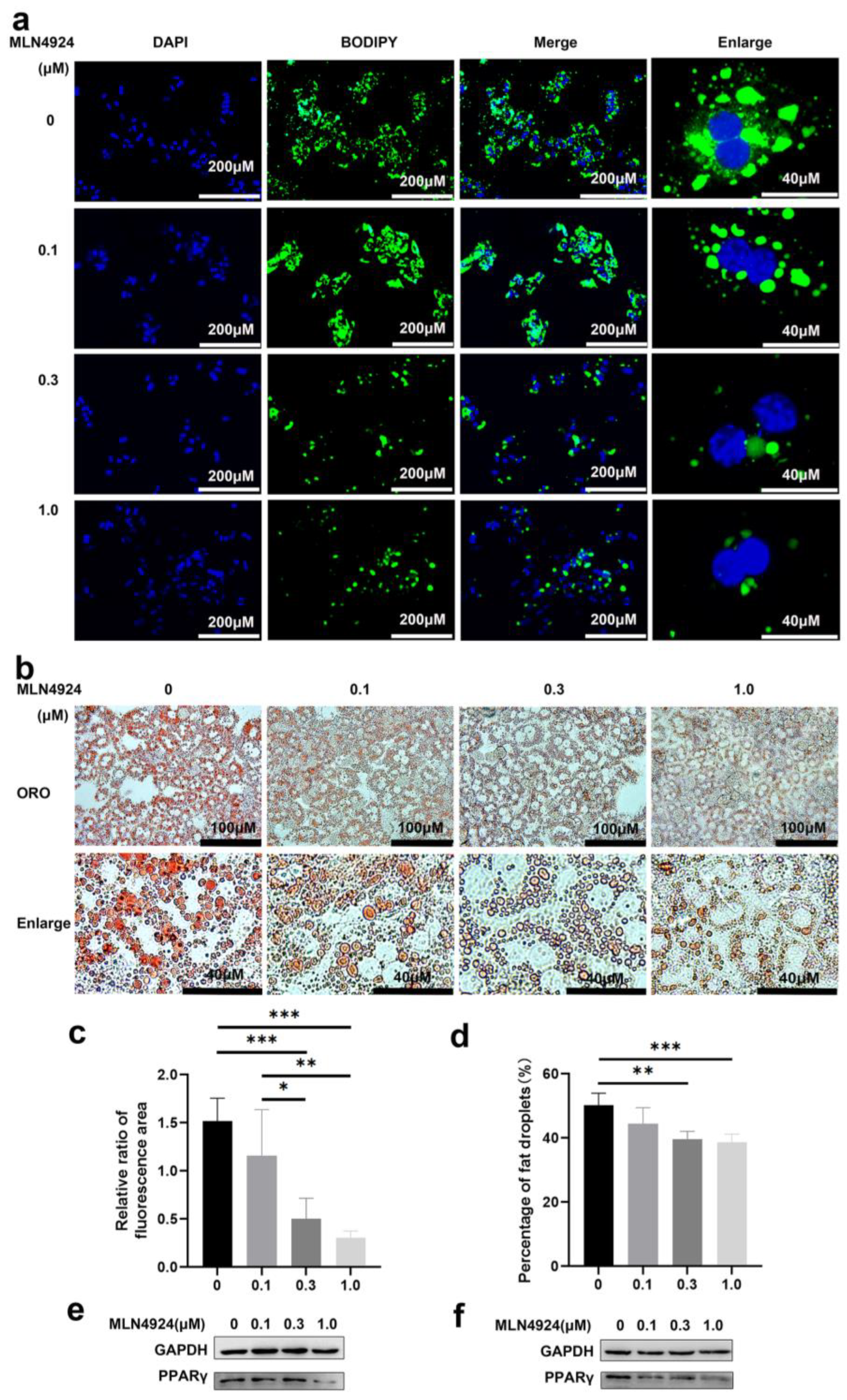
3.2. Neddylation Inhibitor MLN4924 Can Prevent Lipid Accumulation Induced by FFA in Hepatocytes
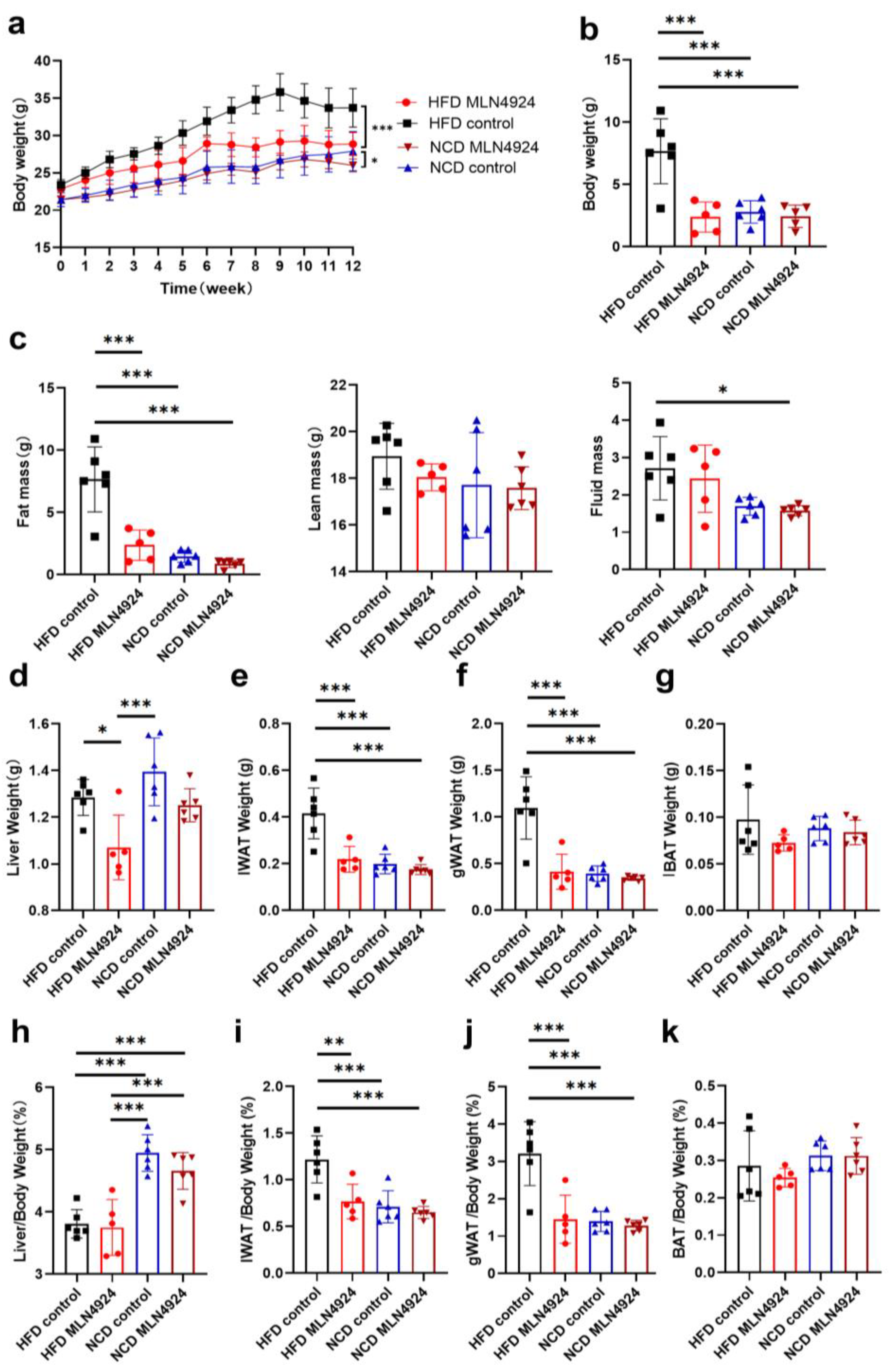
3.3. MLN4924 Treatment Protected HFD-Fed Mice from Developmenting of NAFLD and Obesity
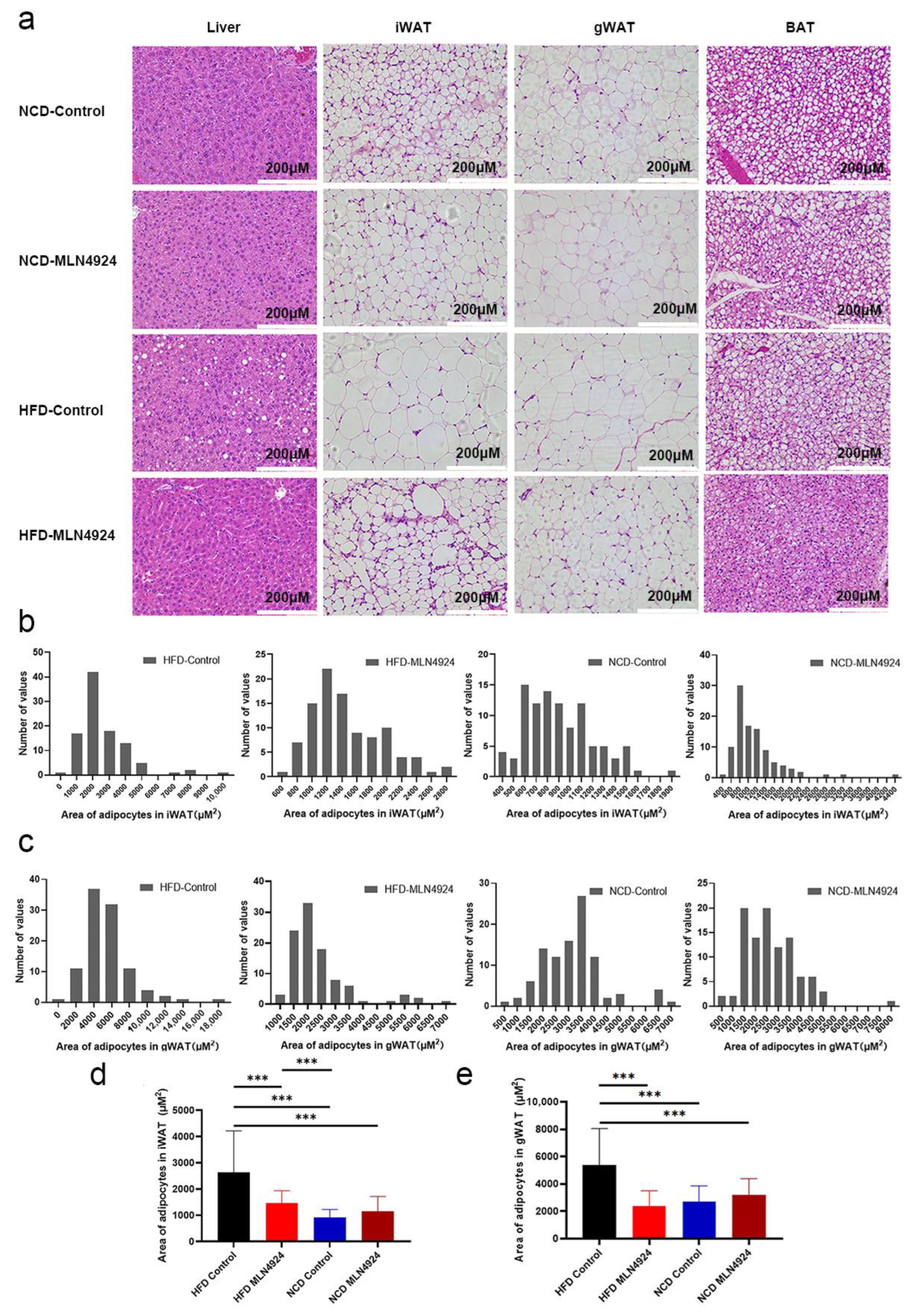
3.4. MLN4924 Limits Lipid Accumulation and the Size of Lipid Droplets in Metabolic Tissues
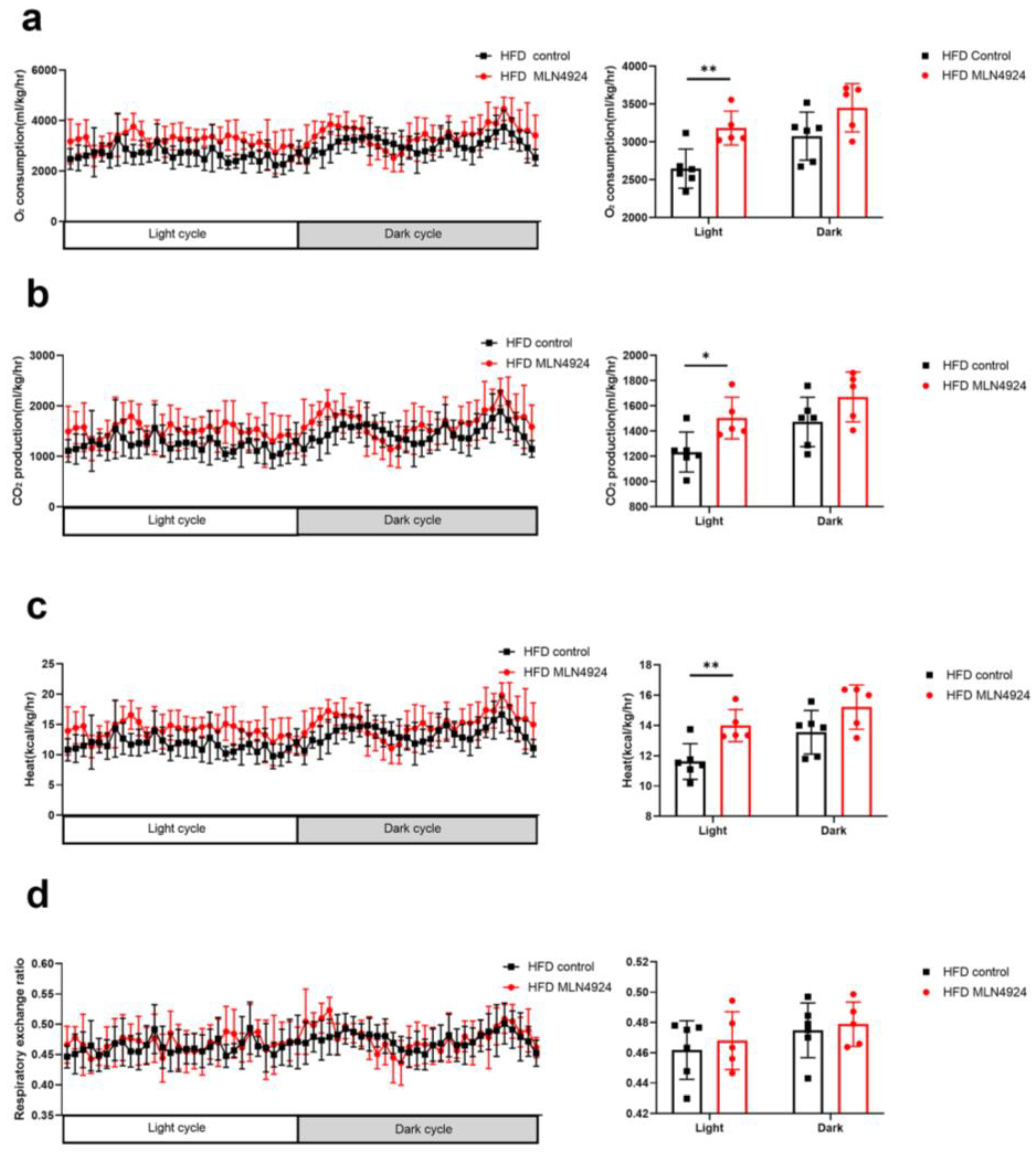
3.5. MLN4924 Promotes O2 Consumption, CO2 Production and Thermogenesis in HFD-Induced NAFLD Mice Model
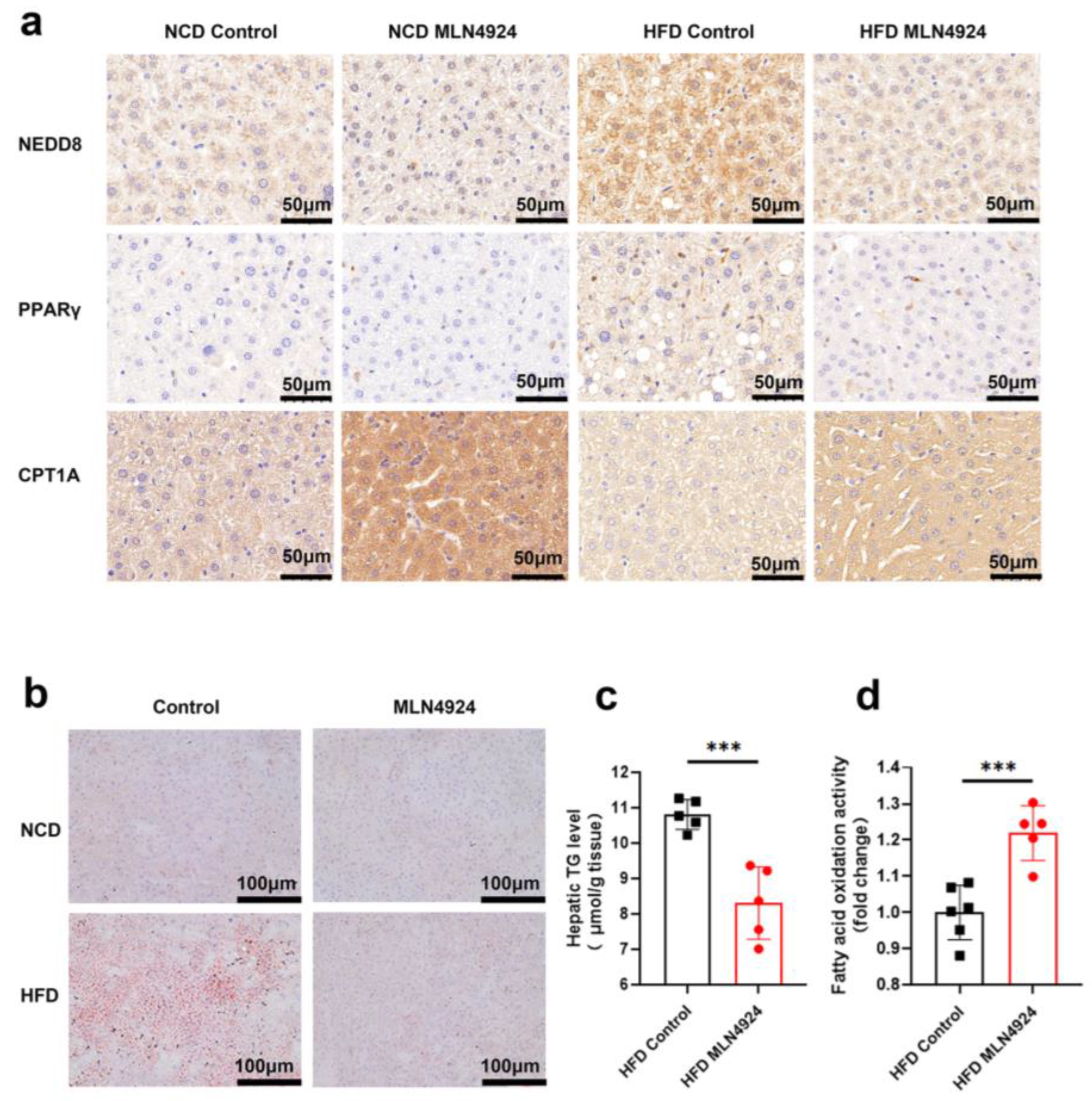
3.6. MLN4924 Inhibits Neddylation, Elevates Hepatic Fatty Acid Oxidase Levels, and Reduces Hepatic Triglyceride Levels in HFD-Induced NAFLD Mice Model
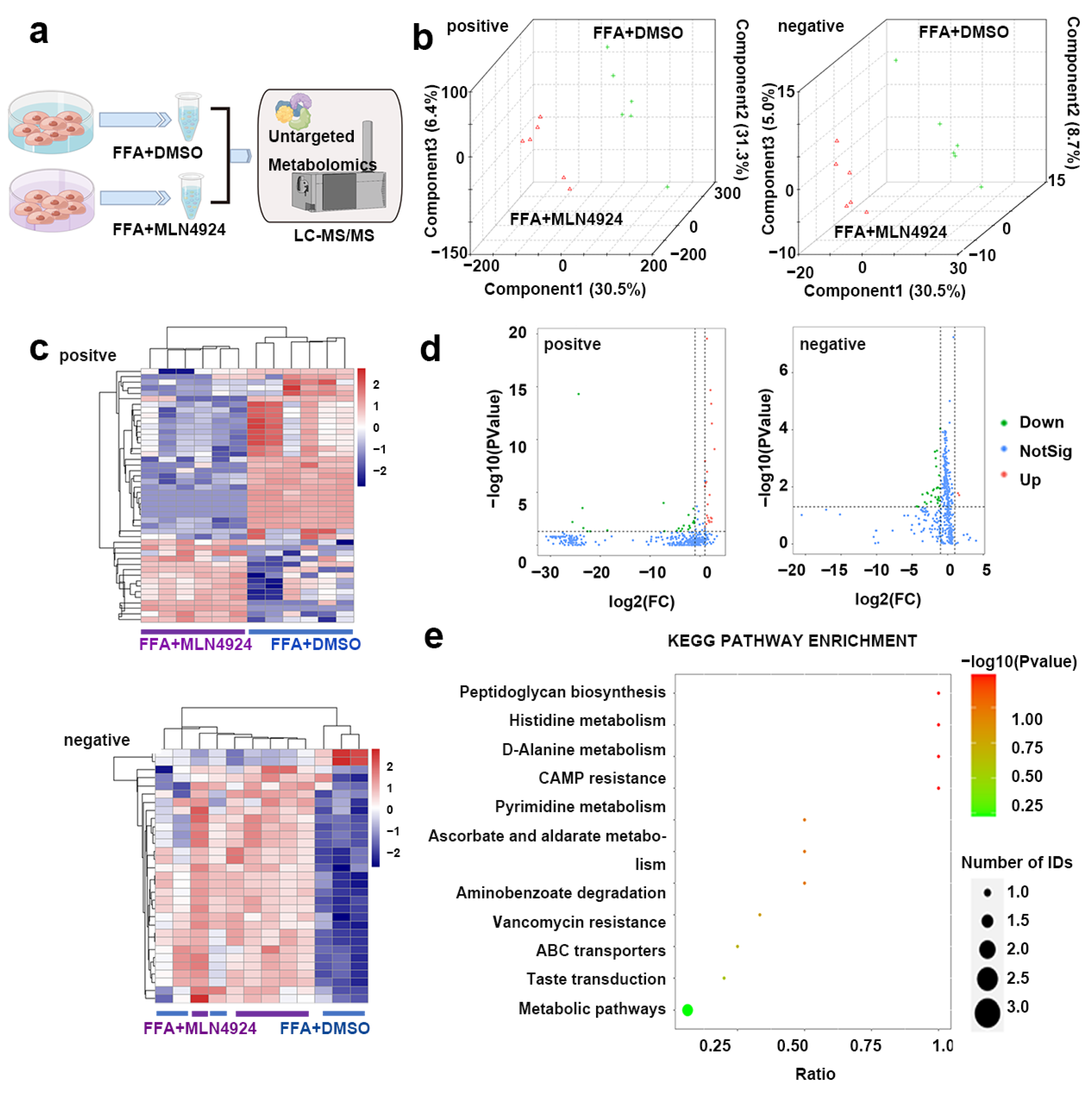
3.7. Lipidomics Analysis Uncovered Differences in Lipid Metabolites from Liver Cells Treated with MLN4924
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ipsen, D.H.; Lykkesfeldt, J.; Tveden-Nyborg, P. Molecular mechanisms of hepatic lipid accumulation in non-alcoholic fatty liver disease. Cell. Mol. Life Sci. 2018, 75, 3313–3327. [Google Scholar] [PubMed] [Green Version]
- Powell, E.E.; Wong, V.W.-S.; Rinella, M. Non-alcoholic fatty liver disease. Lancet 2021, 397, 2212–2224. [Google Scholar] [CrossRef]
- Stefan, N.; Häring, H.-U.; Cusi, K. Non-alcoholic fatty liver disease: Causes, diagnosis, cardiometabolic consequences, and treatment strategies. Lancet Diabetes Endocrinol. 2019, 7, 313–324. [Google Scholar] [CrossRef]
- Suzuki, A.; Diehl, A.M. Nonalcoholic Steatohepatitis. Annu. Rev. Med. 2017, 68, 85–98. [Google Scholar] [CrossRef]
- Ding, M.; Ma, Y.J.; Du, R.Q.; Zhou, W.Y.; Dou, X.; Yang, Q.Q.; Tang, Y.; Qian, S.W.; Liu, Y.; Pan, D.N.; et al. CHCHD10 Modulates Thermogenesis of Adipocytes by Regulating Lipolysis. Diabetes 2022, 71, 1862–1879. [Google Scholar] [CrossRef]
- Townsend, K.L.; Pritchard, E.; Coburn, J.M.; Kwon, Y.M.; Blaszkiewicz, M.; Lynes, M.D.; Kaplan, D.L.; Tseng, Y.H. Silk Hydrogel-Mediated Delivery of Bone Morphogenetic Protein 7 Directly to Subcutaneous White Adipose Tissue Increases Browning and Energy Expenditure. Front. Bioeng. Biotechnol. 2022, 10, 884601. [Google Scholar] [CrossRef]
- Franczyk, M.P.; He, M.; Yoshino, J. Removal of Epididymal Visceral Adipose Tissue Prevents Obesity-Induced Multi-organ Insulin Resistance in Male Mice. J. Endocr. Soc. 2021, 5, bvab024. [Google Scholar] [CrossRef]
- Wibmer, A.G.; Becher, T.; Eljalby, M.; Crane, A.; Andrieu, P.C.; Jiang, C.S.; Vaughan, R.; Schoder, H.; Cohen, P. Brown adipose tissue is associated with healthier body fat distribution and metabolic benefits independent of regional adiposity. Cell Rep. Med. 2021, 2, 100332. [Google Scholar] [CrossRef]
- Liu, Y.; Dou, X.; Zhou, W.Y.; Ding, M.; Liu, L.; Du, R.Q.; Guo, L.; Qian, S.W.; Tang, Y.; Yang, Q.Q.; et al. Hepatic Small Ubiquitin-Related Modifier (SUMO)-Specific Protease 2 Controls Systemic Metabolism Through SUMOylation-Dependent Regulation of Liver-Adipose Tissue Crosstalk. Hepatology 2021, 74, 1864–1883. [Google Scholar] [CrossRef]
- Song, T.; Yang, Y.; Wei, H.; Xie, X.; Lu, J.; Zeng, Q.; Peng, J.; Zhou, Y.; Jiang, S.; Peng, J. Zfp217 mediates m6A mRNA methylation to orchestrate transcriptional and post-transcriptional regulation to promote adipogenic differentiation. Nucleic Acids Res. 2019, 47, 6130–6144. [Google Scholar] [CrossRef]
- Kumar, D.; Das, M.; Sauceda, C.; Ellies, L.G.; Kuo, K.; Parwal, P.; Kaur, M.; Jih, L.; Bandyopadhyay, G.K.; Burton, D.; et al. Degradation of splicing factor SRSF3 contributes to progressive liver disease. J. Clin. Investig. 2019, 129, 4477–4491. [Google Scholar] [CrossRef] [PubMed]
- Zubiete-Franco, I.; Fernández-Tussy, P.; Barbier-Torres, L.; Simon, J.; Fernández-Ramos, D.; Lopitz-Otsoa, F.; Gutiérrez-de Juan, V.; de Davalillo, S.L.; Duce, A.M.; Iruzubieta, P.; et al. Deregulated Neddylation in Liver Fibrosis. Hepatology 2017, 65, 694–709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, Y.P.; Jiang, Y.Y.; Jin, X.; Chen, P.; Heng, Y.Q.; Cai, L.L.; Zhang, W.J.; Li, L.H.; Jia, L.J. Neddylation inhibition activates the protective autophagy through NF-kappa B-catalase-ATF3 Axis in human esophageal cancer cells. Cell Commun. Signal. 2020, 18, 72. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, S.; Pavlick, A.C.; Boasberg, P.; Thompson, J.A.; Mulligan, G.; Pickard, M.D.; Faessel, H.; Dezube, B.J.; Hamid, O. A phase I study of the investigational NEDD8-activating enzyme inhibitor pevonedistat (TAK-924/MLN4924) in patients with metastatic melanoma. Investig. New Drug 2016, 34, 439–449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swords, R.T.; Erba, H.P.; DeAngelo, D.J.; Bixby, D.L.; Altman, J.K.; Maris, M.; Hua, Z.; Blakemore, S.J.; Faessel, H.; Sedarati, F.; et al. Pevonedistat (MLN4924), a First-in-Class NEDD8-activating enzyme inhibitor, in patients with acute myeloid leukaemia and myelodysplastic syndromes: A phase 1 study. Br. J. Haematol. 2015, 169, 534–543. [Google Scholar] [CrossRef] [Green Version]
- Shah, J.J.; Jakubowiak, A.J.; O’Connor, O.A.; Orlowski, R.Z.; Harvey, R.D.; Smith, M.R.; Lebovic, D.; Diefenbach, C.; Kelly, K.; Hua, Z.; et al. Phase I Study of the Novel Investigational NEDD8-Activating Enzyme Inhibitor Pevonedistat (MLN4924) in Patients with Relapsed/Refractory Multiple Myeloma or Lymphoma. Clin. Cancer Res. 2016, 22, 34–43. [Google Scholar] [CrossRef] [Green Version]
- Mao, H.; Sun, Y. Neddylation-Independent Activities of MLN4924. Adv. Exp. Med. Biol. 2020, 1217, 363–372. [Google Scholar] [CrossRef]
- Zhou, Q.; Zheng, Y.; Sun, Y. Neddylation regulation of mitochondrial structure and functions. Cell Biosci. 2021, 11, 55. [Google Scholar] [CrossRef]
- Luo, Z.; Yu, G.; Lee, H.W.; Li, L.; Wang, L.; Yang, D.; Pan, Y.; Ding, C.; Qian, J.; Wu, L.; et al. The Nedd8-activating enzyme inhibitor MLN4924 induces autophagy and apoptosis to suppress liver cancer cell growth. Cancer Res. 2012, 72, 3360–3371. [Google Scholar] [CrossRef] [Green Version]
- Nawrocki, S.T.; Griffin, P.; Kelly, K.R.; Carew, J.S. MLN4924: A novel first-in-class inhibitor of NEDD8-activating enzyme for cancer therapy. Expert Opin. Investig. Drugs 2012, 21, 1563–1573. [Google Scholar] [CrossRef]
- Zhou, L.S.; Zhang, W.J.; Sun, Y.; Jia, L.J. Protein neddylation and its alterations in human cancers for targeted therapy. Cell Signal. 2018, 44, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Dubiel, D.; Bintig, W.; Kahne, T.; Dubiel, W.; Naumann, M. Cul3 neddylation is crucial for gradual lipid droplet formation during adipogenesis. Biochim. Biophys. Acta-Mol. Cell Res. 2017, 1864, 1405–1412. [Google Scholar] [CrossRef] [PubMed]
- Park, H.S.; Ju, U.I.; Park, J.W.; Song, J.Y.; Shin, D.H.; Lee, K.H.; Jeong, L.S.; Yu, J.; Lee, H.W.; Cho, J.Y.; et al. PPARgamma neddylation essential for adipogenesis is a potential target for treating obesity. Cell Death Differ. 2016, 23, 1296–1311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montaigne, D.; Butruille, L.; Staels, B. PPAR control of metabolism and cardiovascular functions. Nat. Rev. Cardiol. 2021, 18, 809–823. [Google Scholar] [CrossRef]
- Tontonoz, P.; Spiegelman, B.M. Fat and Beyond: The Diverse Biology of PPARγ. Annu. Rev. Biochem. 2008, 77, 289–312. [Google Scholar] [CrossRef]
- Skat-Rordam, J.; Hojland Ipsen, D.; Lykkesfeldt, J.; Tveden-Nyborg, P. A role of peroxisome proliferator-activated receptor gamma in non-alcoholic fatty liver disease. Basic Clin. Pharm. Toxicol. 2019, 124, 528–537. [Google Scholar] [CrossRef]
- Gai, W.; Peng, Z.; Liu, C.H.; Zhang, L.; Jiang, H. Advances in Cancer Treatment by Targeting the Neddylation Pathway. Front. Cell Dev. Biol. 2021, 9, 653882. [Google Scholar] [CrossRef]
- Watt, M.J.; Miotto, P.M.; De Nardo, W.; Montgomery, M.K. The Liver as an Endocrine Organ-Linking NAFLD and Insulin Resistance. Endocr. Rev. 2019, 40, 1367–1393. [Google Scholar] [CrossRef]
- Fujii, H.; Kawada, N.; Japan Study Group of NAFLD (JSG-NAFLD). The Role of Insulin Resistance and Diabetes in Nonalcoholic Fatty Liver Disease. Int. J. Mol. Sci. 2020, 21, 3863. [Google Scholar] [CrossRef]
- Sasaki, T.; Moro, K.; Kubota, T.; Kubota, N.; Kato, T.; Ohno, H.; Nakae, S.; Saito, H.; Koyasu, S. Innate Lymphoid Cells in the Induction of Obesity. Cell Rep. 2019, 28, 202–217.e7. [Google Scholar] [CrossRef]
- Shamsi, F.; Wang, C.H.; Tseng, Y.H. The evolving view of thermogenic adipocytes—Ontogeny, niche and function. Nat. Rev. Endocrinol. 2021, 17, 726–744. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Nie, T.; Li, K.; Wu, W.; Long, Q.; Feng, T.; Mao, L.; Gao, Y.; Liu, Q.; Gao, X.; et al. Hepatic CPT1A Facilitates Liver–Adipose Cross Talk via Induction of FGF21 in Mice. Diabetes 2022, 71, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Ross, T.T.; Overton, J.D.; Houmard, K.F.; Kinsey, S.T. Beta-GPA treatment leads to elevated basal metabolic rate and enhanced hypoxic exercise tolerance in mice. Physiol. Rep. 2017, 5, e13192. [Google Scholar] [CrossRef]
- Vamecq, J.; Roels, F.; Vandenbranden, C.; Draye, J.P. Peroxisomal Proliferation in Heart and Liver of Mice Receiving Chlorpromazine, Ethyl 2(5(4-Chlorophenyl)Pentyl) Oxiran-2-Carboxylic Acid or High-Fat Diet—A Biochemical and Morphometrical Comparative-Study. Pediatr. Res. 1987, 22, 748–754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Xie, C.; Zhai, Z.; Deng, Z.Y.; De Jonge, H.R.; Wu, X.; Ruan, Z. Uridine attenuates obesity, ameliorates hepatic lipid accumulation and modifies the gut microbiota composition in mice fed with a high-fat diet. Food Funct. 2021, 12, 1829–1840. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Liang, X.; Liu, Y.; Zheng, M. Neddylation: A Versatile Pathway Takes on Chronic Liver Diseases. Front. Med. 2020, 7, 586881. [Google Scholar] [CrossRef]
- Lachiondo-Ortega, S.; Mercado-Gomez, M.; Serrano-Macia, M.; Lopitz-Otsoa, F.; Salas-Villalobos, T.B.; Varela-Rey, M.; Delgado, T.C.; Martinez-Chantar, M.L. Ubiquitin-Like Post-Translational Modifications (Ubl-PTMs): Small Peptides with Huge Impact in Liver Fibrosis. Cells 2019, 8, 1575. [Google Scholar] [CrossRef] [Green Version]
- Soucy, T.A.; Smith, P.G.; Milhollen, M.A.; Berger, A.J.; Gavin, J.M.; Adhikari, S.; Brownell, J.E.; Burke, K.E.; Cardin, D.P.; Critchley, S.; et al. An inhibitor of NEDD8-activating enzyme as a new approach to treat cancer. Nature 2009, 458, 732–736. [Google Scholar] [CrossRef]
- Yang, D.; Zhao, Y.; Liu, J.; Sun, Y.; Jia, L. Protective autophagy induced by RBX1/ROC1 knockdown or CRL inactivation via modulating the DEPTOR-MTOR axis. Autophagy 2012, 8, 1856–1858. [Google Scholar] [CrossRef] [Green Version]
- Duda, D.M.; Borg, L.A.; Scott, D.C.; Hunt, H.W.; Hammel, M.; Schulman, B.A. Structural insights into NEDD8 activation of cullin-RING ligases: Conformational control of conjugation. Cell 2008, 134, 995–1006. [Google Scholar] [CrossRef]
- Barbier-Torres, L.; Delgado, T.C.; Garcia-Rodriguez, J.L.; Zubiete-Franco, I.; Fernandez-Ramos, D.; Buque, X.; Cano, A.; Gutierrez-de Juan, V.; Fernandez-Dominguez, I.; Lopitz-Otsoa, F.; et al. Stabilization of LKB1 and Akt by neddylation regulates energy metabolism in liver cancer. Oncotarget 2015, 6, 2509–2523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simoes, I.C.M.; Fontes, A.; Pinton, P.; Zischka, H.; Wieckowski, M.R. Mitochondria in non-alcoholic fatty liver disease. Int. J. Biochem. Cell Biol. 2018, 95, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Xie, L.; Liu, Z.; Li, C.; Liang, Y. MLN4924 Exerts a Neuroprotective Effect against Oxidative Stress via Sirt1 in Spinal Cord Ischemia-Reperfusion Injury. Oxid. Med. Cell. Longev. 2019, 2019, 7283639. [Google Scholar] [CrossRef] [PubMed]
- Pick, E. The necessity of NEDD8/Rub1 for vitality and its association with mitochondria-derived oxidative stress. Redox Biol. 2020, 37, 101765. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Viscarra, J.; Kim, S.J.; Sul, H.S. Transcriptional regulation of hepatic lipogenesis. Nat. Rev. Mol. Cell Biol. 2015, 16, 678–689. [Google Scholar] [CrossRef] [Green Version]
- Ju, U.I.; Jeong, D.W.; Seo, J.; Park, J.B.; Park, J.W.; Suh, K.S.; Kim, J.B.; Chun, Y.S. Neddylation of sterol regulatory element-binding protein 1c is a potential therapeutic target for nonalcoholic fatty liver treatment. Cell Death Dis. 2020, 11, 283. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Q.; Li, H.; Li, Y.; Tan, M.; Fan, S.; Cao, C.; Meng, F.; Zhu, L.; Zhao, L.; Guan, M.X.; et al. Inhibiting neddylation modification alters mitochondrial morphology and reprograms energy metabolism in cancer cells. JCI Insight 2019, 4, e121582. [Google Scholar] [CrossRef] [Green Version]
- Chan, D.C. Mitochondrial Dynamics and Its Involvement in Disease. Annu. Rev. Pathol. 2020, 15, 235–259. [Google Scholar] [CrossRef] [Green Version]
- Bhatti, J.S.; Bhatti, G.K.; Reddy, P.H. Mitochondrial dysfunction and oxidative stress in metabolic disorders—A step towards mitochondria based therapeutic strategies. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 1066–1077. [Google Scholar] [CrossRef]
- Roca-Portoles, A.; Tait, S.W.G. Mitochondrial quality control: From molecule to organelle. Cell. Mol. Life Sci. 2021, 78, 3853–3866. [Google Scholar] [CrossRef]
- Lahera, V.; de Las Heras, N.; Lopez-Farre, A.; Manucha, W.; Ferder, L. Role of Mitochondrial Dysfunction in Hypertension and Obesity. Curr. Hypertens. Rep. 2017, 19, 11. [Google Scholar] [CrossRef] [PubMed]
- Morio, B.; Panthu, B.; Bassot, A.; Rieusset, J. Role of mitochondria in liver metabolic health and diseases. Cell Calcium 2021, 94, 102336. [Google Scholar] [CrossRef]
- Doblado, L.; Lueck, C.; Rey, C.; Samhan-Arias, A.K.; Prieto, I.; Stacchiotti, A.; Monsalve, M. Mitophagy in Human Diseases. Int. J. Mol. Sci. 2021, 22, 3903. [Google Scholar] [CrossRef] [PubMed]
- Chouchani, E.T.; Kazak, L.; Spiegelman, B.M. New Advances in Adaptive Thermogenesis: UCP1 and Beyond. Cell Metab. 2019, 29, 27–37. [Google Scholar] [CrossRef]
- Heinonen, S.; Jokinen, R.; Rissanen, A.; Pietilainen, K.H. White adipose tissue mitochondrial metabolism in health and in obesity. Obes. Rev. 2020, 21, e12958. [Google Scholar] [CrossRef] [PubMed]
- Benador, I.Y.; Veliova, M.; Liesa, M.; Shirihai, O.S. Mitochondria Bound to Lipid Droplets: Where Mitochondrial Dynamics Regulate Lipid Storage and Utilization. Cell Metab. 2019, 29, 827–835. [Google Scholar] [CrossRef] [Green Version]
- Benador, I.Y.; Veliova, M.; Mahdaviani, K.; Petcherski, A.; Wikstrom, J.D.; Assali, E.A.; Acin-Perez, R.; Shum, M.; Oliveira, M.F.; Cinti, S.; et al. Mitochondria Bound to Lipid Droplets Have Unique Bioenergetics, Composition, and Dynamics that Support Lipid Droplet Expansion. Cell Metab. 2018, 27, 869–885.e6. [Google Scholar] [CrossRef] [Green Version]
- Fucho, R.; Casals, N.; Serra, D.; Herrero, L. Ceramides and mitochondrial fatty acid oxidation in obesity. FASEB J. 2017, 31, 1263–1272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vamecq, J. Chlorpromazine and Carnitine-Dependency of Rat-Liver Peroxisomal Beta-Oxidation of Long-Chain Fatty-Acids. Biochem. J. 1987, 241, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Jacobson, K.A. Adipocyte purinergic receptors activated by uracil nucleotides as obesity and type 2 diabetes targets. Curr. Opin. Pharm. 2022, 63, 102190. [Google Scholar] [CrossRef]
- Deng, Y.; Wang, Z.V.; Gordillo, R.; An, Y.; Zhang, C.; Liang, Q.; Yoshino, J.; Cautivo, K.M.; De Brabander, J.; Elmquist, J.K.; et al. An adipo-biliary-uridine axis that regulates energy homeostasis. Science 2017, 355, eaaf5375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jain, S.; Jacobson, K.A. Purinergic signaling in diabetes and metabolism. Biochem. Pharm. 2021, 187, 114393. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Jacobson, K.A. Purinergic Signaling in Liver Pathophysiology. Front. Endocrinol. 2021, 12, 718429. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Wang, Z.V.; Gordillo, R.; Zhu, Y.; Ali, A.; Zhang, C.; Wang, X.; Shao, M.; Zhang, Z.; Iyengar, P.; et al. Adipocyte Xbp1s overexpression drives uridine production and reduces obesity. Mol. Metab. 2018, 11, 1–17. [Google Scholar] [CrossRef] [PubMed]
| HMDB ID | log2fold | p-Value | Up/Down | m/z | Description |
|---|---|---|---|---|---|
| HMDB0001904 | −22.012587 | 0.048762 | Down | 244.0920 | 3-Nitrotyrosine |
| HMDB0012243 | −7.299020 | 0.000100 | Down | 395.1660 | Kinetin-7-N-glucoside |
| HMDB0014592 | −4.649321 | 0.042829 | Down | 352.2106 | Dipivefrin |
| HMDB0013222 | −3.586083 | 0.027941 | Down | 132.0766 | Beta-Guanidinopropionic acid |
| HMDB0001212 | −3.212516 | 0.034643 | Down | 217.0474 | Hydantoin-5-propionic acid |
| HMDB0038968 | −2.893064 | 0.015947 | Down | 281.0355 | 1-Propenyl 1-(1-propenylsulfinyl)propyl disulfide |
| HMDB0036650 | −2.275692 | 0.019872 | Down | 325.0318 | Sinalbin A |
| HMDB0015269 | −2.131924 | 0.014434 | Down | 427.1073 | Sulfinpyrazone |
| HMDB0034364 | −1.790339 | 0.000586 | Down | 304.0628 | Cepharadione A |
| HMDB0003040 | −1.719765 | 0.023093 | Down | 267.0731 | Arabinosylhypoxanthine |
| HMDB0041468 | −1.685299 | 0.000997 | Down | 302.0658 | 1,3,5-Trihydroxy-10-methylacridone |
| HMDB0034364 | −1.607278 | 0.001931 | Down | 304.0629 | Cepharadione A |
| HMDB0000296 | −1.520245 | 0.011211 | Down | 243.0616 | Uridine |
| HMDB0014340 | −1.508761 | 0.000539 | Down | 312.0947 | Vidarabine |
| HMDB0032852 | −1.483694 | 0.010759 | Down | 241.0825 | 2-(Ethylamino)-4,5-dihydroxybenzamide |
| HMDB0038968 | −1.446022 | 0.027435 | Down | 281.0356 | 1-Propenyl 1-(1-propenylsulfinyl)propyl disulfide |
| HMDB0040795 | 1.033134 | 0.000000 | Up | 347.1854 | 7′-O-Methylmarmin |
| HMDB0057488 | 1.279045 | 0.010018 | Up | 1373.9427 | CL(16:1(9Z)/16:1(9Z)/18:1(11Z)/16:1(9Z)) |
| HMDB0040795 | 1.291746 | 0.000001 | Up | 347.1855 | 7′-O-Methylmarmin |
| HMDB0004886 | 1.389978 | 0.001551 | Up | 1158.7890 | Trihexosylceramide (d18:1/24:0) |
| HMDB0014902 | 1.420620 | 0.000000 | Up | 444.1687 | Mometasone |
| HMDB0009422 | 1.451045 | 0.016616 | Up | 810.5281 | PE(20:4(8Z,11Z,14Z,17Z)/18:1(9Z)) |
| HMDB0015621 | 1.562168 | 0.000135 | Up | 328.1063 | Sulfadimethoxine |
| HMDB0012389 | 1.584051 | 0.002586 | Up | 812.5430 | PS(18:1(9Z)/18:0) |
| HMDB0009090 | 1.610539 | 0.019622 | Up | 788.5444 | PE(18:2(9Z,12Z)/18:0) |
| HMDB0010660 | 1.839512 | 0.004755 | Up | 762.5259 | PG(18:3(6Z,9Z,12Z)/16:0) |
| HMDB0010661 | 1.893689 | 0.006705 | Up | 760.5106 | PG(18:3(6Z,9Z,12Z)/16:1(9Z)) |
| HMDB0010662 | 1.894613 | 0.005903 | Up | 790.5562 | PG(18:3(6Z,9Z,12Z)/18:0) |
| HMDB0012378 | 2.180602 | 0.002500 | Up | 814.5566 | PS(18:0/18:0) |
| HMDB0014761 | 2.250722 | 0.000000 | Up | 460.1635 | Fluphenazine |
| HMDB0012356 | 2.319175 | 0.006175 | Up | 786.5241 | PS(16:0/18:0) |
| HMDB0010624 | 2.361055 | 0.002972 | Up | 816.5743 | PG(18:1(11Z)/20:3(8Z,11Z,14Z)) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ge, M.; Huang, L.; Ma, Y.; Sun, S.; Wu, L.; Xu, W.; Yang, D. MLN4924 Treatment Diminishes Excessive Lipid Storage in High-Fat Diet-Induced Non-Alcoholic Fatty Liver Disease (NAFLD) by Stimulating Hepatic Mitochondrial Fatty Acid Oxidation and Lipid Metabolites. Pharmaceutics 2022, 14, 2460. https://doi.org/10.3390/pharmaceutics14112460
Ge M, Huang L, Ma Y, Sun S, Wu L, Xu W, Yang D. MLN4924 Treatment Diminishes Excessive Lipid Storage in High-Fat Diet-Induced Non-Alcoholic Fatty Liver Disease (NAFLD) by Stimulating Hepatic Mitochondrial Fatty Acid Oxidation and Lipid Metabolites. Pharmaceutics. 2022; 14(11):2460. https://doi.org/10.3390/pharmaceutics14112460
Chicago/Turabian StyleGe, Mengxiao, Linlin Huang, Yinjun Ma, Shuangyi Sun, Lijun Wu, Wei Xu, and Dongqin Yang. 2022. "MLN4924 Treatment Diminishes Excessive Lipid Storage in High-Fat Diet-Induced Non-Alcoholic Fatty Liver Disease (NAFLD) by Stimulating Hepatic Mitochondrial Fatty Acid Oxidation and Lipid Metabolites" Pharmaceutics 14, no. 11: 2460. https://doi.org/10.3390/pharmaceutics14112460





