Processing of Lipid Nanodispersions into Solid Powders by Spray Drying
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Lipid Nanodispersions
2.3. Loading of Lipid Nanodispersions with API
2.4. Spray Drying of Lipid Nanodispersions
2.5. Characterization of Lipid Dispersions and Powders
2.5.1. Particle Size Analysis
2.5.2. Viscosity Measurement
2.5.3. X-ray Diffraction
2.5.4. Differential Scanning Calorimetry
2.5.5. Residual Moisture
2.5.6. Determination of API Content
2.5.7. Scanning Electron Microscopy
3. Results and Discussion
3.1. Impact of Matrix Material on the Redispersibility of a Tristearin Nanosuspension
3.1.1. Use of Different Matrix Materials
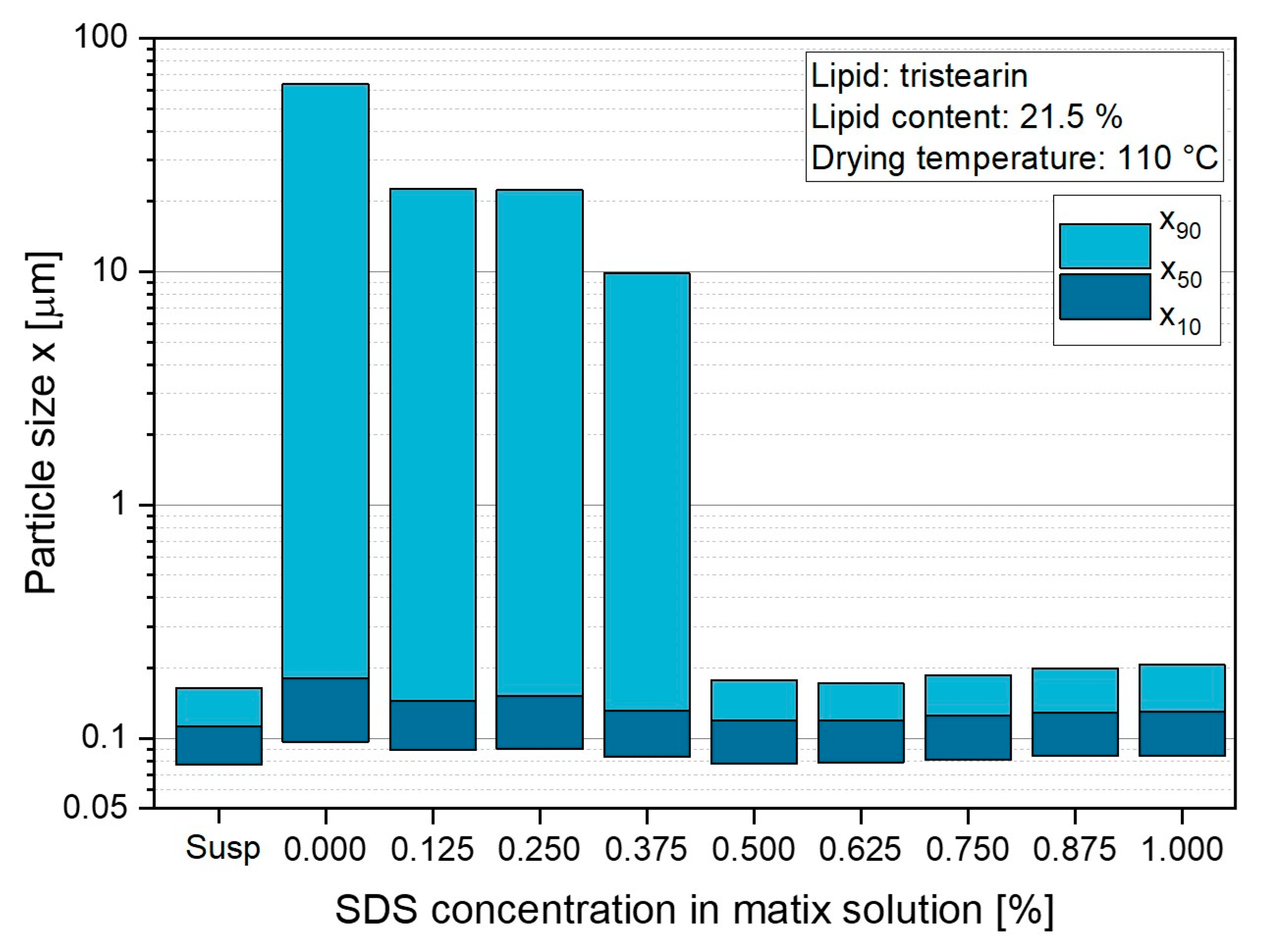
3.1.2. Variation in Surfactant Content in Lactose Matrix
3.2. Influence of Particle Stabilization on Drying Properties of Tristearin Nanosuspensions
3.3. Embedding of Different Lipid Nanosuspensions and -Emulsions in Lactose/SDS Matrix
3.4. Investigation of Drug-Loaded Lipid Nanodispersions
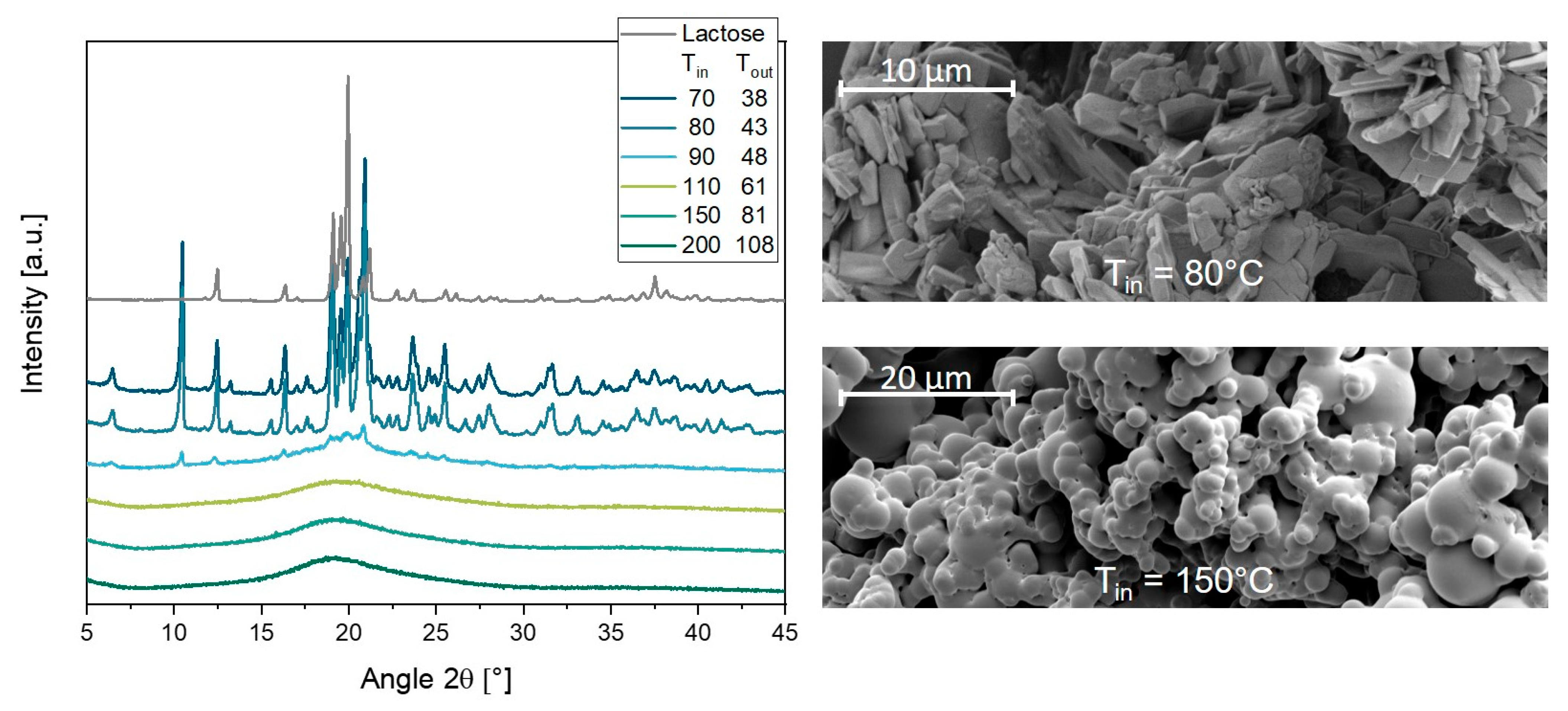
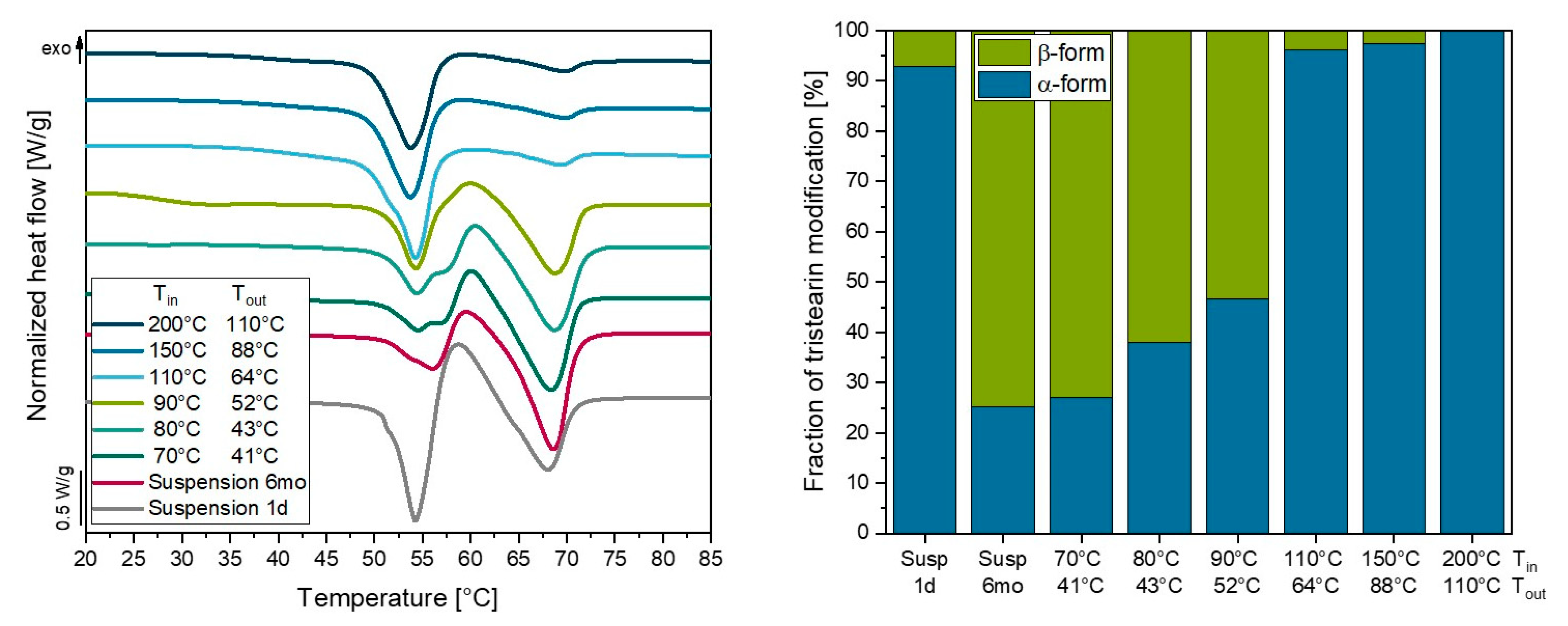
3.5. Influence of Drying Temperature on Lipid Nanoparticles
3.5.1. Particle Sizes and Process Evaluation
3.5.2. Melting of Lipid Particles during Spray Drying
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lipp, R. The innovator pipeline: Bioavailability challenges and advanced oral drug delivery opportunities. Am. Pharm. Rev. 2013, 16, 10–16. [Google Scholar]
- Bergström, C.A.S.; Charman, W.N.; Porter, C.J.H. Computational prediction of formulation strategies for beyond-rule-of-5 compounds. Adv. Drug Deliv. Rev. 2016, 101, 6–21. [Google Scholar] [CrossRef]
- Bunjes, H. Lipid nanoparticles for the delivery of poorly water-soluble drugs. J. Pharm. Pharmacol. 2010, 62, 1637–1645. [Google Scholar] [CrossRef] [PubMed]
- Mehnert, W.; Mäder, K. Solid lipid nanoparticles. Production, characterization and applications. Adv. Drug Deliv. Rev. 2012, 64, 83–101. [Google Scholar] [CrossRef]
- Lim, S.B.; Banerjee, A.; Önyüksel, H. Improvement of drug safety by the use of lipid-based nanocarriers. J. Control. Release 2012, 163, 34–45. [Google Scholar] [CrossRef]
- Stewart, K.D.; Johnston, J.A.; Matza, L.S.; Curtis, S.E.; Havel, H.A.; Sweetana, S.A.; Gelhorn, H.L. Preference for pharmaceutical formulation and treatment process attributes. Patient Prefer. Adher. 2016, 10, 1385–1399. [Google Scholar] [CrossRef]
- Scioli Montoto, S.; Muraca, G.; Esperanza Ruiz, M. Solid lipid nanoparticles for drug delivery: Pharmacological and biopharmaceutical aspects. Front. Mol. Biosci. 2020, 7, 587997. [Google Scholar] [CrossRef]
- Mahmoudian, M.; Dizaj, S.M.; Salatin, S.; Löbenberg, R.; Saadat, M.; Islambulchilar, Z.; Valizadeh, H.; Zakeri-Milani, P. Oral delivery of solid lipid nanoparticles: Underlining the physicochemical characteristics and physiological condition affecting the lipolysis rate. Expert Opin. Drug Deliv. 2021, 18, 1707–1722. [Google Scholar] [CrossRef]
- Vinarov, Z.; Abrahamsson, B.; Artursson, P.; Batchelor, H.; Berben, P.; Bernkop-Schnürch, A.; Butler, J.; Ceulemans, J.; Davies, N.; Dupont, D.; et al. Current challenges and future perspectives in oral absorption research: An option of the UNGAP network. Adv. Drug Deliv. Rev. 2021, 171, 289–331. [Google Scholar] [CrossRef]
- Hansen, T.; Holm, P.; Schultz, K. Process characteristics and compaction of spray-dried emulsions containing a drug dissolved in lipid. Int. J. Pharm. 2004, 287, 55–66. [Google Scholar] [CrossRef]
- Christensen, K.L.; Pedersen, G.P.; Kristensen, H.G. Technical optimisation of redispersible dry emulsions. Int. J. Pharm. 2001, 212, 195–202. [Google Scholar] [CrossRef]
- Christensen, K.L.; Pedersen, G.P.; Kristensen, H.G. Preparation of redispersible dry emulsions by spray drying. Int. J. Pharm. 2001, 212, 187–194. [Google Scholar] [CrossRef]
- Choi, Y.K.; Poudel, B.K.; Marasini, N.; Yang, K.Y.; Kim, J.W.; Kim, J.O.; Choi, H.-G.; Yong, C.S. Enhanced solubility and oral bioavailability of intraconazole by combining membrane emulsification and spray drying technique. Int. J. Pharm. 2012, 434, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Dollo, G.; Le Corre, P.; Guerin, A.; Chevanne, F.; Burgot, J.L.; Leverge, R. Spray-dried redispersible oil-in-water emulsion to improve oral bioavailability of poorly soluble drugs. Eur. J. Pharm. Sci. 2003, 19, 273–280. [Google Scholar] [CrossRef]
- Masters, K. Understanding and applying spray dryers in chemical processing. Powder Bulk Eng. 1990, 22, 36–44. [Google Scholar]
- Ziaee, A.; Albadarin, A.B.; Padrela, L.; Femmer, T.; O’Reilly, E. Spray drying of pharmaceuticals and biopharmaceuticals: Critical parameters and experimental process optimization approaches. Eur. J. Pharm. Sci. 2019, 127, 300–318. [Google Scholar] [CrossRef]
- Pinto, J.T.; Faulhammer, E.; Dieplinger, J.; Dekner, M.; Makert, C.; Nieder, M.; Paudel, A. Progress in spray-drying of protein pharmaceuticals: Literature analysis of trends in formulation and process attributes. Dry. Technol. 2021, 39, 1415–1446. [Google Scholar] [CrossRef]
- Salminen, H.; Ankenbrand, J.; Zeeb, B.; Badolato Bönisch, G.; Schäfer, C.; Kohlus, R.; Weiss, J. Influence of spray drying on the stability of food-grade solid lipid nanoparticles. Food Res. Int. 2019, 119, 741–750. [Google Scholar] [CrossRef]
- Freitas, C.; Müller, R.H. Spray-drying of solid lipid nanoparticles (SLNTM). Eur. J. Pharm. Biopharm. 1998, 46, 145–151. [Google Scholar] [CrossRef]
- Glaubitt, K.; Ricci, M.; Giovagnoli, S. Exploring the nano spray-drying technology as an innovative manufacturing method for solid lipid nanoparticle dry powders. AAPS PharmSciTech 2019, 20, 19. [Google Scholar] [CrossRef]
- Helgason, T.; Awad, T.S.; Kristbersson, K.; McClements, D.J.; Weiss, J. Effect of surfactant surface coverage on formation of solid lipid nanoparticles (SLN). J. Colloid Interface Sci. 2009, 334, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Bunjes, H.; Koch, M.H.J.; Westesen, K. Effects of surfactants on the crystallization and polymorphism of lipid nanoparticles. Prog. Colloid Polym. Sci. 2002, 121, 7–10. [Google Scholar]
- Jenning, V.; Schäfer-Korting, M.; Gohla, S. Vitamin A-loaded solid lipid nanoparticles for topical use: Drug release properties. J. Control. Release 2000, 66, 115–126. [Google Scholar] [CrossRef]
- Westesen, K.; Bunjes, H.; Koch, M.H.J. Physicochemical characterization of lipid nanoparticles and evaluation of their drug loading capacity and sustained release potential. J. Control. Release 1997, 48, 223–236. [Google Scholar] [CrossRef]
- Bunjes, H.; Westesen, K.; Koch, M.H.J. Crystallization tendency and polymorphic transitions in triglyceride nanoparticles. Int. J. Pharm. 1996, 129, 159–173. [Google Scholar] [CrossRef]
- Rosenblatt, K.M.; Bunjes, H. Evaluation of the drug loading capacity of different lipid nanoparticle dispersions by passive drug loading. Eur. J. Pharm. Biopharm. 2017, 117, 49–59. [Google Scholar] [CrossRef]
- Naini, V.; Byron, P.R.; Phillips, E.M. Physicochemical stability of crystalline sugars and their spray-dried forms: Dependence upon relative humidity and suitability for use in powder inhalers. Drug Dev. Ind. Pharm. 1998, 24, 895–909. [Google Scholar] [CrossRef]
- Roos, Y. Melting and glass transition of low molecular weight carbohydrates. Carbohydr. Res. 1993, 238, 39–48. [Google Scholar] [CrossRef]
- Maury, M.; Murphy, K.; Kumar, S.; Shi, L.; Lee, G. Effects of process variables on the powder yield of spray-dried trehalose on a laboratory spray-drier. Eur. J. Pharm. Biopharm. 2005, 59, 565–573. [Google Scholar] [CrossRef]
- Steiner, D.; Emmendörffer, J.F.; Bunjes, H. Orodispersible films: A delivery platform for solid lipid nanoparticles? Pharmaceutics 2021, 13, 2162. [Google Scholar] [CrossRef]
- Braem, C.; Blaschke, T.; Panek-Minkin, G.; Herrmann, W.; Schlupp, P.; Paepenmüller, T.; Müller-Goymann, C.; Mehnert, W.; Bittl, R.; Schäfer-Korting, M.; et al. Interaction of drug molecules with carrier systems as studied by parelectric spectroscopy and electron spin resonance. J. Control. Release 2007, 119, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Berton-Carabin, C.C.; Coupland, J.N.; Elias, R.J. Effect of the lipophilicity of model ingredients on their location and reactivity in emulsions and solid lipid nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2013, 431, 9–17. [Google Scholar] [CrossRef]
- Joseph, S.; Rappolt, M.; Schoenitz, M.; Huzhalska, V.; Augustin, W.; Scholl, S.; Bunjes, H. Stability of the metastable α-polymorph in solid triglyceride drug-carrier nanoparticles. Langmuir 2015, 31, 6663–6674. [Google Scholar] [CrossRef] [PubMed]
- Corzo, C.; Fuchsbichler, A.; Savencu, I.; Urich, J.A.; Zimmer, A.; Lochmann, D.; Reyer, S.; Salar-Behzadi, S. Lipid-microparticles for pulmonary delivery of active pharmaceutical ingredients: Impact of lipid crystallization on spray-dring processability. Int. J. Pharm. 2021, 610, 121259. [Google Scholar] [CrossRef] [PubMed]
- Synder, H.E.; Lechuga-Ballesteros, D. Spray-drying: Theory and pharmaceutical applications. In Pharmaceutical Dosage Forms: Tablets; Informa Healthcare; Augsburger, L., Hoag, S., Eds.; CRC Press: Boca Raton, FL, USA, 2008; pp. 227–260. [Google Scholar]






| Particle Formulation | ||
|---|---|---|
| Formulation | PVA/SDS | HPMC/SDS |
| Lipid Nanosuspension | 14.6 mPas | 103.0 mPas |
| Drying Formulation with Lipid Content: | ||
| 21.5 wt.% | 1.5 mPas | 2.5 mPas |
| 31.5 wt.% | 1.8 mPas | 5.4 mPas |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Steiner, D.; Schumann, L.V.; Bunjes, H. Processing of Lipid Nanodispersions into Solid Powders by Spray Drying. Pharmaceutics 2022, 14, 2464. https://doi.org/10.3390/pharmaceutics14112464
Steiner D, Schumann LV, Bunjes H. Processing of Lipid Nanodispersions into Solid Powders by Spray Drying. Pharmaceutics. 2022; 14(11):2464. https://doi.org/10.3390/pharmaceutics14112464
Chicago/Turabian StyleSteiner, Denise, Leonie V. Schumann, and Heike Bunjes. 2022. "Processing of Lipid Nanodispersions into Solid Powders by Spray Drying" Pharmaceutics 14, no. 11: 2464. https://doi.org/10.3390/pharmaceutics14112464
APA StyleSteiner, D., Schumann, L. V., & Bunjes, H. (2022). Processing of Lipid Nanodispersions into Solid Powders by Spray Drying. Pharmaceutics, 14(11), 2464. https://doi.org/10.3390/pharmaceutics14112464







