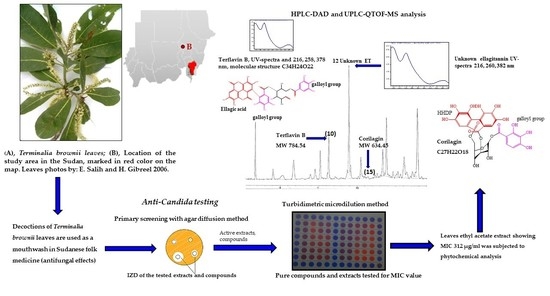
Anti-Candida Activity of Extracts Containing Ellagitannins, Triterpenes and Flavonoids of Terminalia brownii, a Medicinal Plant Growing in Semi-Arid and Savannah Woodland in Sudan
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Identification and Authentication
2.2. Plant Extract Preparations
2.2.1. Decoctions and Macerations (Extracts Prepared from Milli-Q Water)
2.2.2. Hot and Cold Methanol Extracts
2.2.3. Size Exclusion Chromatography (SEC) Using Sephadex LH-20
2.2.4. Sequential Extraction and Liquid–Liquid Fractionation
2.3. Candida Strains and Assays Used for Screening Antifungal Activity
2.3.1. Candida Strains
2.3.2. Agar Diffusion Assays
2.3.3. Minimum Inhibitory Concentration (MIC) Assay Using a Turbidimetric Microplate Method
2.4. Thin-Layer Chromatography (TLC) and DPPH Assay
2.5. High-Performance Liquid Chromatography (HPLC-DAD)
2.6. Mass Spectrometry (UHPLC/QTOF-MS)
3. Results
3.1. Effects of the Extracts against Yeasts
3.2. Effects of Pure Compounds Present in the T. brownii Extracts against Candida spp. Growth
3.3. Detection of Compounds with Antioxidant Effects
3.4. Phytochemistry of an Ethyl Acetate Extract of the Leaves
4. Discussion
4.1. Anti-Candida Effects of T. brownii Extracts in Relation to Other Research on Anti-Candida Effects of Terminalia Species
4.2. Antioxidant Potential of a Terminalia brownii Ethyl Acetate Extract of Leaves
4.3. Identified Compounds in a Leaf Ethyl Acetate Extract of T. brownii and Their Suggested Influence on the Growth of Candida spp.
4.3.1. Ellagitannins and Ellagic Acid Derivatives
4.3.2. Gallotannins
4.3.3. Gallic Acid and Protocatechuic Acid
4.3.4. Flavonoids
4.3.5. Monoterpenoids
4.3.6. Triterpenes, Sterols and Fatty Acids
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Walker, G.M. Yeasts. In Desk Encyclopedia of Microbiology; Academic Press: Cambridge, MA, USA; Elsevier: Amsterdam, The Netherlands, 2009; pp. 1174–1187. [Google Scholar]
- Hoffman, C.S.; Wood, V.; Fantes, P.A. An ancient yeast for young geneticists: A primer on the Schizosaccharomyces pombe model system. Genetics 2015, 201, 403–423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sims, C.R.; Ostrosky-Zeichner, L.; Rex, J.H. Invasive candidiasis in immunocompromised hospitalized patients. Arch. Med. Res. 2005, 36, 660–671. [Google Scholar]
- Shoham, S.; Nucci, M.; Walsh, T.J. Mucocutaneous and deeply invasive candidiasis. In Tropical Infectious Diseases; Elsevier Inc.: Amsterdam, The Netherlands, 2011; pp. 589–596. [Google Scholar]
- Keighley, C.; Chen, S.C.; Marriott, D.; Pope, A.; Chapman, B.; Kennedy, K.; Bak, N.; Underwood, N.; Wilson, H.L.; McDonald, K.; et al. Candidaemia and a risk predictive model for overall mortality: A prospective multicentre study. BMC Infect. Dis. 2019, 19, 1–10. [Google Scholar]
- Rautemaa-Richardson, R.; Richardson, M.D. Systemic fungal infections. Medicine 2017, 45, 757–762. [Google Scholar]
- Al Aboody, M.S.; Mickymaray, S. Anti-fungal efficacy and mechanisms of flavonoids. Antibiotics 2020, 9, 45. [Google Scholar]
- Jeanmonod, R.; Jeanmonod, D. Vaginal Candidiasis. National library of medicine, National center for biotechnology information (NIH). 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK459317/ (accessed on 8 September 2022).
- Rafat, Z.; Hashemi, S.J.; Ahamdikia, K.; Ghazvini, R.D.; Bazvandi, F. Study of skin and nail Candida species as a normal flora based on age groups in healthy persons in Tehran-Iran. J. Mycol. Méd. 2017, 27, 501–505. [Google Scholar] [PubMed]
- Chung, L.M.; Liang, J.A.; Lin, C.L.; Sun, L.M.; Kao, C.H. Cancer risk in patients with candidiasis: A nationwide population-based cohort study. Oncotarget 2017, 8, 63562. [Google Scholar]
- Vila, T.; Sultan, A.S.; Montelongo-Jauregui, D.; Jabra-Rizk, M.A. Oral candidiasis: A disease of opportunity. J. Fungi 2020, 6, 15. [Google Scholar]
- Sardi, J.C.O.; Scorzoni, L.; Bernardi, T.; Fusco-Almeida, A.M.; Giannini, M.M. Candida species: Current epidemiology, pathogenicity, biofilm formation, natural antifungal products and new therapeutic options. J. Med. Microbiol. 2013, 62, 10–24. [Google Scholar] [PubMed]
- Ciurea, C.N.; Kosovski, I.B.; Mare, A.D.; Toma, F.; Pintea-Simon, I.A.; Man, A. Candida and candidiasis—Opportunism versus pathogenicity: A review of the virulence traits. Microorganisms 2020, 8, 857. [Google Scholar]
- Turner, S.A.; Butler, G. The Candida pathogenic species complex. Cold Spring Harb. Perspect. Med. 2014, 4, a019778. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bongomin, F.; Gago, S.; Oladele, R.O.; Denning, D.W. Global and multi-national prevalence of fungal diseases—Estimate precision. J. Fungi 2017, 3, 57. [Google Scholar]
- Sun, L.; Wu, J.; Zhang, L.; Luo, M.; Sun, D. Synthesis and antifungal activities of some novel pyrimidine derivatives. Molecules 2011, 16, 5618–5628. [Google Scholar]
- Salih, E.Y.A. Ethnobotany, Phytochemistry and Antimicrobial Activity of Combretum, Terminalia and Anogeissus species (Combretaceae) Growing Naturally in Sudan. Tropical Forestry Reports. Doctoral Thesis, University of Helsinki, Helsinki, Finland, 2019; 193p. [Google Scholar]
- Silva, F.D.S.; Landell, M.F.; Paulino, G.V.B.; Coutinho, H.D.M.; Albuquerque, U.P. Antifungal activity of selected plant extracts based on an ethnodirected study. Acta Bot. Bras. 2020, 34, 442–448. [Google Scholar] [CrossRef]
- El Ghazali, G.B.; El Tohami, M.S.; El Egami, A.B.; Abdalla, W.S.; Mohammed, M.G. Medicinal Plants of the Sudan. Part IV. Medicinal Plants of Northern Kordofan; Omdurman Islamic University Press: Khartoum, Sudan, 1997. [Google Scholar]
- El Ghazali, G.B.; Abdalla, W.E.; Khalid, H.E.; Khalafalla, M.M.; Hamad, A.A. Medicinal Plants of the Sudan. Part V. Medicinal Plants of Ingassana Area; Sudan Currency Printing Press: Khartoum, Sudan, 2003. [Google Scholar]
- Mbwambo, Z.H.; Moshi, M.J.; Masimba, P.J.; Kapingu, M.C.; Nondo, R.S. Antimicrobial activity and brine shrimp toxicity of extracts of Terminalia brownii roots and stem. BMC Complement. Altern. Med. 2007, 7, 1–5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Machumi, F.; Midiwo, J.O.; Jacob, M.R.; Khan, S.I.; Tekwani, B.L.; Zhang, J.; Walker, L.A.; Muhammad, I. Phytochemical, antimicrobial and antiplasmodial investigations of Terminalia brownii. Nat. Prod. Commun. 2013, 8, 1934578X1300800619. [Google Scholar]
- Mechesso, A.F.; Asrade, B.; Hailu, H.; Toma, A. In vitro antimicrobial activity of three medicinal plants of Ethiopia against some selected bacterial isolates. Afr. J. Microbiol. Res. 2016, 10, 1779–1783. [Google Scholar]
- Salih, E.Y.A.; Kanninen, M.; Sipi, M.; Luukkanen, O.; Hiltunen, R.; Vuorela, H.; Julkunen-Tiitto, R.; Fyhrquist, P. Tannins, flavonoids and stilbenes in extracts of African savanna woodland trees Terminalia brownii, Terminalia laxiflora and Anogeissus leiocarpus showing promising antibacterial potential. S. Afr. J. Bot. 2017, 108, 370–386. [Google Scholar] [CrossRef]
- Salih, E.Y.; Julkunen-Tiitto, R.; Lampi, A.M.; Kanninen, M.; Luukkanen, O.; Sipi, M.; Lehtonen, M.; Vuorela, H.; Fyhrquist, P. Terminalia laxiflora and Terminalia brownii contain a broad spectrum of antimycobacterial compounds including ellagitannins, ellagic acid derivatives, triterpenes, fatty acids and fatty alcohols. J. Ethnopharmacol. 2018, 227, 82–96. [Google Scholar] [PubMed]
- Musa, S.M.; Fathelrhman, E.A.; Elsheikh, A.E.; Lubna, A.A.; Abdel, L.E.M.; Sakina, M.Y. Ethnobotanical study of medicinal plants in the Blue Nile State, South-eastern Sudan. J. Med. Plants Res. 2011, 5, 4287–4297. [Google Scholar]
- Muddathir, A.M.; Mitsunaga, T. Evaluation of anti-acne activity of selected Sudanese medicinal plants. J. Wood Sci. 2013, 59, 73–79. [Google Scholar]
- Darbyshire, I.; Kordofami, M. The plants of Sudan and South Sudan, an Annoted Checklist; Kew Publishing: London, UK, 2015. [Google Scholar]
- Ahmed, I.S.; Almagboul, A.Z. Isolation of gallic acid and flavonoids from antimicrobial extracts of Terminalia brownii leaves. J. Eng. Appl. Sci. 2020, 7, 29–33. [Google Scholar]
- Hagerman, E.A. Tannin Handbook; Miami University Oxford: Oxford, OH, USA, 2002. [Google Scholar]
- Salih, E.Y.; Julkunen-Tiitto, R.; Luukkanen, O.; Sipi, M.; Fahmi, M.K.; Fyhrquist, P. Potential anti-tuberculosis activity of the extracts and their active components of Anogeissus leiocarpa (Dc.) guill. and perr. with special emphasis on polyphenols. Antibiotics 2020, 9, 364. [Google Scholar]
- Salih, E.Y.; Julkunen-Tiitto, R.; Luukkanen, O.; Fahmi, M.K.; Fyhrquist, P. Hydrolyzable tannins (ellagitannins), flavonoids, pentacyclic triterpenes and their glycosides in antimycobacterial extracts of the ethnopharmacologically selected Sudanese medicinal plant Combretum hartmannianum Schweinf. Biomed Pharm. 2021, 144, 112264. [Google Scholar]
- Fyhrquist, P.; Mwasumbi, L.; Hæggström, C.A.; Vuorela, H.; Hiltunen, R.; Vuorela, P. Antifungal activity of selected species of Terminalia, Pteleopsis and Combretum (Combretaceae) collected in Tanzania. Pharm. Biol. 2004, 42, 308–317. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically; Approved Standard—9th ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2012. [Google Scholar]
- Fyhrquist, P.; Laakso, I.; Marco, S.G.; Julkunen-Tiitto, R.; Hiltunen, R. Antimycobacterial activity of ellagitannin and ellagic acid derivate rich crude extracts and fractions of five selected species of Terminalia used for treatment of infectious diseases in African traditional medicine. S. Afr. J. Bot. 2014, 90, 1–16. [Google Scholar]
- Fyhrquist, P.; Salih, E.Y.; Helenius, S.; Laakso, I.; Julkunen-Tiitto, R. HPLC-DAD and UHPLC/QTOF-MS Analysis of Polyphenols in Extracts of the African Species Combretum padoides, C. zeyheri and C. psidioides Related to Their Antimycobacterial Activity. Antibiotics 2020, 9, 459. [Google Scholar] [CrossRef]
- Taulavuori, K.; Julkunen-Tiitto, R.; Hyöky, V.; Taulavuori, E. Blue mood for superfood. Nat. Prod. Commun. 2013, 8, 1–2. [Google Scholar]
- Salih, E.Y.; Fyhrquist, P.; Abdalla, A.M.; Abdelgadir, A.Y.; Kanninen, M.; Sipi, M.; Luukkanen, O.; Fahmi, M.K.; Elamin, M.H.; Ali, H.A. LC-MS/MS tandem mass spectrometry for analysis of phenolic compounds and pentacyclic triterpenes in antifungal extracts of Terminalia brownii (Fresen). Antibiotics 2017, 6, 37. [Google Scholar]
- Pfundstein, B.; Desouky, S.K.E.; Hull, W.E.; Haubner, R.; Erben, G.; Owen, R.W. Polyphenolic compounds in the fruits of Egyptian medicinal plants (Terminalia bellerica, Terminalia chebula and Terminalia horrida): Characterization, quantitation and determination of antioxidant capacities. Phytochemistry 2010, 71, 1132–1148. [Google Scholar]
- Hooi Poay, T.; Sui Kiong, L.; Cheng Hock, C. Characterisation of galloylated cyanogenic glucosides and hydrolysable tannins from leaves of Phyllagathis rotundifolia by LC-ESI-MS/MS. Phytochem. Anal. 2011, 22, 516–525. [Google Scholar] [PubMed]
- Li, C.; Seeram, N.P. Ultra-fast liquid chromatography coupled with electrospray ionization time-of-flight mass spectrometry for the rapid phenolic profiling of red maple (Acer rubrum) leaves. Separ. Sci. 2018, 41, 2331–2346. [Google Scholar]
- Martini, N.D.; Katerere, D.R.P.; Eloff, J.N. Biological activity of five antibacterial flavonoids from Combretum erythrophyllum (Combretaceae). J. Ethnopharmacol. 2004, 93, 207–212. [Google Scholar] [CrossRef]
- Masoko, P.; Eloff, J.N. Screening of twenty-four South African Combretum and six Terminalia species (Combretaceae) for antioxidant activities. Afr. J. Tradit. Complem. 2007, 4, 231–239. [Google Scholar]
- Muzzarelli, R.A.A. Genipin-crosslinked chitosan hydrogels as biomedical and pharmaceutical aids. Carbohyd. Polym. 2009, 77, 1–9. [Google Scholar]
- Butler, M.F.; Ng, Y.F.; Pudney, P.D. Mechanism and kinetics of the crosslinking reaction between biopolymers containing primary amine groups and genipin. Polym. Sci. Ser. A+ Polym Chem.-UK 2003, 41, 3941–3953. [Google Scholar] [CrossRef]
- Salih, E.Y.A. Phytochemical and Antifungal Analysis of Extracts of Terminalia brownii (Fresen.) Shaf. 2009. Electronic Thesis Book, University of Khartoum. Master’s Thesis, University of Khartoum, Khartoum, Sudan. Available online: http://khartoumspace.uofk.edu/bitstream/handle/123456789/9338/Phytochemical%20and%20Antifungal%20Analysis%20of%20Extracts%20of%20Terminalia%20brownie%20(Fresen.%20Mus.%20Senckenb)%20Shaf.pdf?sequence=1 (accessed on 20 May 2020).
- Opiyo, S.O.A.; Manguro, L.O.; Owuor, P.O.; Ochieng, C.M.; Ateka, E.; Lemmen, P. Antimicrobial compounds from Terminalia brownii against sweet potato pathogens. Nat. Prod. J. 2011, 1, 116–120. [Google Scholar]
- Basha, N.S.; Ogbaghebriel, A.; Yemane, K.; Zenebe, M. Isolation and screening of endophytic fungi from Eritrean traditional medicinal plant Terminalia brownii leaves for antimicrobial activity. Int. J. Green Pharm. (IJGP) 2012, 6, 40. [Google Scholar]
- Salih, E.; Kanninen, M.; Julkunen-Tiitto, R.; Sipi, M.; Luukkanen, O.; Hiltunen, R.; Vuorela, H.; Fyhrqvist, P.J. Polyphenol-rich extracts of Terminalia laxiflora and Terminalia brownii give promising anti-Candida activity. 2016. In Proceedings of the XXVIIIth International Conference on Polyphenols, Vienna, Austria, 11–15 July 2016; Available online: https://www.researchgate.net/publication/307914949_Polyphenol-rich_extracts_of_Terminalia_laxiflora_and_Terminalia_brownii_give_promising_anti-Candida_activity (accessed on 4 November 2022).
- Weldegergis, A.; Medhanie, S.; Yamane, B.; Mehari Andebrhan, M.; Semwal, K.C.; Gangwar, S.K. Analysis of antibacterial and antifungal activity of Terminalia brownii upon Escherichia coli and Candida albicans. Int. J. Sci. Nat. 2018, 9, 73–78. [Google Scholar]
- Taei, M.; Chadeganipour, M.; Mohammadi, R. An alarming rise of non-albicans Candida species and uncommon yeasts in the clinical samples; a combination of various molecular techniques for identification of etiologic agents. BMC Res. Notes 2019, 12, 1–7. [Google Scholar]
- Saivaraj, S.; Chandramohan, G. Antimicrobial activity of natural dyes obtained from Terminalia arjuna (Roxb.) Wight & Arn barks. World Sci. News 2018, 98, 221–227. [Google Scholar]
- Gonçalves, L.M.; Madeira, P.L.B.; Diniz, R.S.; Nonato, R.F.; Siqueira, F.S.F.D.; de Sousa, E.M.; Farias, D.C.S.; Rocha, F.M.G.; Rocha, C.H.L.; Lago, A.D.N.; et al. Effect of Terminalia catappa Linn. on Biofilms of Candida albicans and Candida glabrata and on Changes in Color and Roughness of Acrylic Resin. Evid-Based Compl. Alt. 2019, 2019. [Google Scholar]
- Vidya, A.G.; Vijayan, A.; Jyothis, L.J.; Nair, R.; Suja, K.P. Evaluation of antifungal efficacy of some medicinal plants on Candida spp. causing vulvovaginitis. Indian J. Exp. Biol. 2019, 57, 297–301. [Google Scholar]
- Ansari, M.A.; Kalam, A.; Al-Sehemi, A.G.; Alomary, M.N.; AlYahya, S.; Aziz, M.K.; Srivastava, S.; Alghamdi, S.; Akhtar, S.; Almalki, H.D.; et al. Counteraction of biofilm formation and antimicrobial potential of Terminalia catappa functionalized silver nanoparticles against Candida albicans and multidrug-resistant Gram-negative and Gram-positive bacteria. Antibiotics 2021, 10, 725. [Google Scholar] [PubMed]
- Das, G.; Kim, D.Y.; Fan, C.; Gutiérrez-Grijalva, E.P.; Heredia, J.B.; Nissapatorn, V.; Mitsuwan, W.; Pereira, M.L.; Nawaz, M.; Siyadatpanah, A.; et al. Plants of the genus Terminalia: An insight on its biological potentials, pre-clinical and clinical studies. Front. Pharmacol. 2020, 11, 561248. [Google Scholar]
- Yousuf, S.; Ahmad, A.; Khan, A.; Manzoor, N.; Khan, L.A. Effect of diallyldisulphide on an antioxidant enzyme system in Candida species. Can J. Microbiol. 2010, 56, 816–821. [Google Scholar] [CrossRef] [PubMed]
- Sintayehu, B.; Kakoti, B.B.; Kataki, M.S.; Gebrelibanos, M.; Periyasamy, G.; Aynalem, A.; Zeru, H.; Asres, K. Hepatoprotective, Antioxidant and Anticancer Activities of Terminalia brownii Fresen Leaf Extract. Ethiop. pharm. J. 2017, 33, 29–38. [Google Scholar] [CrossRef]
- Abd-elfattah, M.; Maina, N.; Kareru, P.G.; El-Shemy, H.A. Antioxidant Potential of Eight selected Kenyan Medicinal plants. Egypt J. Chem. 2022. [CrossRef]
- Han, Q.; Song, J.; Qiao, C.; Wong, L.; Xu, H. Preparative isolation of hydrolysable tannins chebulagic acid and chebulinic acid from Terminalia chebula by high-speed counter-current chromatography. J. Sep. Sci. 2006, 29, 1653–1657. [Google Scholar]
- Feng, X.H.; Xu, H.Y.; Wang, J.Y.; Duan, S.; Wang, Y.C.; Ma, C.M. In vivo hepatoprotective activity and the underlying mechanism of chebulinic acid from Terminalia chebula fruit. Phytomedicine 2021, 83, 153479. [Google Scholar]
- Sharma, K.K.; Katiyar, P. Chebulinic Acid for Alternative Treatment of Vulvovaginal Candidiasis by Targeting Agglutinin-Like Sequence Protein 3 in Candida albicans: In silico Approach. Indian J. Pharm. Sci. 2021, 83, 838–847. [Google Scholar] [CrossRef]
- Yoshida, T.; Hatano, T.; Ito, H.; Okuda, T. Structural diversity and antimicrobial activities of ellagitannins. In Chemistry and Biology of Ellagitannins: An Underestimated Class of Bioactive Plant Polyphenols; World Scientific: Singapore, 2009; pp. 55–93. [Google Scholar]
- Latté, K.P.; Kolodziej, H. Antifungal effects of hydrolysable tannins and related compounds on dermatophytes, mould fungi and yeasts. Z. Für Nat. C 2000, 55, 467–472. [Google Scholar]
- Silva Junior, I.F.; Raimondi, M.; Zacchino, S.; Cechinel Filho, V.; Noldin, V.F.; Rao, V.S.; Lima, J.; Martins, D.T. Evaluation of the antifungal activity and mode of action of Lafoensia pacari A. St.-Hil., Lythraceae, stem-bark extracts, fractions and ellagic acid. Rev. Bras. Farmacogn. 2010, 20, 422–428. [Google Scholar]
- Sampaio, A.D.G.; Gontijo, A.V.L.; Lima, G.D.M.G.; de Oliveira, M.A.C.; Lepesqueur, L.S.S.; Koga-Ito, C.Y. Ellagic acid–cyclodextrin complexes for the treatment of oral candidiasis. Molecules 2021, 26, 505. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.J.; Abula, A.; Abulizi, A.; Wang, C.; Dou, Q.; Maimaiti, Y.; Abudouaini, A.; Huo, S.X.; Aibai, S. Ellagic Acid Inhibits Trichophyton rubrum Growth via Affecting Ergosterol Biosynthesis and Apoptotic Induction. Evid-Based Compl. Alt. 2020. [Google Scholar] [CrossRef]
- Mysyakina, I.S.; Funtikova, N.S. The role of sterols in morphogenetic processes and dimorphism in fungi. Microbiology 2007, 76, 1–13. [Google Scholar]
- Parente-Rocha, J.A.; Bailão, A.M.; Amaral, A.C.; Taborda, C.P.; Paccez, J.D.; Borges, C.L.; Pereira, M. Antifungal resistance, metabolic routes as drug targets, and new antifungal agents: An overview about endemic dimorphic fungi. Mediat. Inflamm. 2017, 2017, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Engels, C.; Schieber, A.; Gaänzle, M.G. Inhibitory spectra and modes of antimicrobial action of gallotannins from mango kernels (Mangifera indica L.). Appl. Environ. Microb. 2011, 77, 2215–2223. [Google Scholar]
- Puupponen-Pimiä, R.; Nohynek, L.; Alakomi, H.-L.; Oksman-Caldentey, K.-M. The action of berry phenolics against human intestinal pathogens. Biofactors 2005, 23, 243–251. [Google Scholar]
- Nohynek, L.J.; Alakomi, H.-L.; Kähkönen, M.P.; Heinonen, M.; Helander, I.M.; Oksman-Caldentey, K.-M.; Puupponen-Pimiä, R.H. Berry phenolics: Antimicrobial properties and mechanisms of action against severe human pathogens. Nutr. Cancer 2006, 54, 18–32. [Google Scholar] [PubMed]
- Seneviratne, C.J.; Rosa, E.A. Antifungal drug discovery: New theories and new therapies. Front. Microbiol. 2016, 7, 728. [Google Scholar]
- Fifere, A.; Turin-Moleavin, I.A.; Rosca, I. Does Protocatechuic Acid Affect the Activity of Commonly Used Antibiotics and Antifungals? Life 2022, 12, 1010. [Google Scholar]
- Kimani, B.G.; Kerekes, E.B.; Szebenyi, C.; Krisch, J.; Vágvölgyi, C.; Papp, T.; Takó, M. In vitro activity of selected phenolic compounds against planktonic and biofilm cells of food-contaminating yeasts. Foods 2021, 10, 1652. [Google Scholar]
- Künstler, A.; Gullner, G.; Ádám, A.L.; Kolozsváriné Nagy, J.; Király, L. The versatile roles of sulfur-containing biomolecules in plant defense—A road to disease resistance. Plants 2020, 9, 1705. [Google Scholar]
- Alam, W.; Khan, H.; Shah, M.A.; Cauli, O.; Saso, L. Kaempferol as a dietary anti-inflammatory agent: Current therapeutic standing. Molecules 2020, 25, 4073. [Google Scholar]
- Traynor, A.M.; Sheridan, K.J.; Jones, G.W.; Calera, J.A.; Doyle, S. Involvement of sulfur in the biosynthesis of essential metabolites in pathogenic fungi of animals, particularly Aspergillus spp.: Molecular and therapeutic implications. Front. Microbiol. 2019, 2859. [Google Scholar]
- Amich, J. Sulfur Metabolism as a Promising Source of New Antifungal Targets. J. Fungi 2022, 8, 295. [Google Scholar] [CrossRef]
- Bagiu, R.V.; Vlaicu, B.; Butnariu, M. Chemical composition and in vitro antifungal activity screening of the Allium ursinum L. (Liliaceae). Int. J. Mol. Sci. 2012, 13, 1426–1436. [Google Scholar]
- Nascimento, J.E.T.D.; Rodrigues, A.L.M.; Lisboa, D.S.D.; Liberato, H.R.; Falcão, M.J.C.; da Silva, C.R.; Nobre Júnior, H.V.; Braz Filho, R.; Paula Junior, V.F.D.; Alves, D.R.; et al. Chemical composition and antifungal in vitro and in silico, antioxidant, and anticholinesterase activities of extracts and constituents of Ouratea fieldingiana (DC.) Baill. Evid-Based Compl Alt. 2018, 2018. [Google Scholar]
- Steele, C.; Leigh, J.; Swoboda, R.; Ozenci, H.; Fidel Jr, P.L. Potential role for a carbohydrate moiety in anti-Candida activity of human oral epithelial cells. Infect. Immun. 2001, 69, 7091–7099. [Google Scholar] [PubMed] [Green Version]
- Ortega, J.T.; Suárez, A.I.; Serrano, M.L.; Baptista, J.; Pujol, F.H.; Rangel, H.R. The role of the glycosyl moiety of myricetin derivatives in anti-HIV-1 activity in vitro. AIDS Res. Ther. 2017, 14, 1–6. [Google Scholar]
- Sudheeran, P.K.; Ovadia, R.; Galsarker, O.; Maoz, I.; Sela, N.; Maurer, D.; Feygenberg, O.; Oren Shamir, M.; Alkan, N. Glycosylated flavonoids: Fruit’s concealed antifungal arsenal. New Phytol. 2020, 225, 1788–1798. [Google Scholar] [CrossRef] [PubMed]
- Salazar-Aranda, R.; Granados-Guzmán, G.; Pérez-Meseguer, J.; González, G.M.; Waksman de Torres, N. Activity of polyphenolic compounds against Candida glabrata. Molecules 2015, 20, 17903–17912. [Google Scholar]
- Martins, C.D.M.; de Morais, S.A.; Martins, M.M.; Cunha, L.; da Silva, C.V.; Martins, C.H.; Leandro, L.F.; de Oliveira, A.; de Aquino, F.J.; Nascimento, E.A.D.; et al. Chemical composition, antifungal, and cytotoxicity Activities of Inga laurina (Sw.) willd leaves. Sci. World J. 2019, 2019. [Google Scholar]
- Smiljkovic, M.; Stanisavljevic, D.; Stojkovic, D.; Petrovic, I.; Vicentic, J.M.; Popovic, J.; Grdadolnik, S.G.; Markovic, D.; Sankovic-Babice, S.; Glamoclija, J.; et al. Apigenin-7-O-glucoside versus apigenin: Insight into the modes of anticandidal and cytotoxic actions. Excli. J. 2017, 16, 795. [Google Scholar] [PubMed]
- Ivanov, M.; Kannan, A.; Stojković, D.S.; Glamočlija, J.; Calhelha, R.C.; Ferreira, I.C.; Sanglard, D.; Soković, M. Flavones, flavonols, and glycosylated derivatives—Impact on Candida albicans growth and virulence, expression of cdr1 and erg11, cytotoxicity. Pharmaceuticals 2020, 14, 27. [Google Scholar] [PubMed]
- Ivanov, M.; Ćirić, A.; Stojković, D. Emerging Antifungal Targets and Strategies. Int. J. Mol. Sci. 2022, 23, 2756. [Google Scholar] [PubMed]
- Venkatalakshmi, P.; Vadivel, V.; Brindha, P. Identification of flavonoids in different parts of Terminalia catappa L. Using LC-ESI-MS/MS and investigation of their anticancer effect in EAC cell line model. J. Pharm. Sci. Res. 2016, 8, 176. [Google Scholar]
- Lin, Y.L.; Kuo, Y.H.; Shiao, M.S.; Chen, C.C.; Ou, J.C. Flavonoid glycosides from Terminalia catappa L. J. Chin. Chem. Soc.-Taip. 2000, 47, 253–256. [Google Scholar]
- Dwevedi, A.; Dwivedi, R.; Sharma, Y.K. Exploration of phytochemicals found in Terminalia sp. and their antiretroviral activities. Pharmacogn. Rev. 2016, 10, 73. [Google Scholar]
- Ambika; Singh, P.P.; Chauhan, S.M.S. Activity-guided isolation of antioxidants from the leaves of Terminalia arjuna. Nat. Prod. Res. 2014, 28, 760–763. [Google Scholar]
- Sharma, P. Production of flavonoids from Terminalia arjuna (ROXB.) in vivo and in vitro tissue cultures. Int. J. ChemTech Res. 2014, 6, 881–885. [Google Scholar]
- Kimutai, M.B.; Were, M.L.; Ambrose, K.K.; Mibey, R. Extraction and Analysis of Spectral Properties and ChroMophoric Characterization of Natural Dye Extract from Barks of Terminalia brownii Fresen (Combretaceae). Am. J. Appl. Chem. 2019, 7, 116–122. [Google Scholar] [CrossRef] [Green Version]
- Koudouna, E.; Huertas-Bello, M.; Rodriguez, C.N.; Henao, S.C.; Navarrete, M.L.; Avila, M.Y. Genipin in an ex vivo corneal model of bacterial and fungal keratitis. Transl. Vis. Sci. Technol. 2021, 10, 31. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Nam, Y.R.; Woo, J.; Kim, W.K.; Nam, J.H. Gardenia jasminoides extract and its constituent, genipin, inhibit activation of CD3/CD28 co-stimulated CD4+ T cells via ORAI1 channel. Korean J. Physiol. Pha. Off. J. Korean Physiol. Soc. Korean Soc. Pharmacol. 2020, 24, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.Y.; Parton, L.E.; Ye, C.P.; Krauss, S.; Shen, R.; Lin, C.T.; Porco Jr, J.A.; Lowell, B.B. Genipin inhibits UCP2-mediated proton leak and acutely reverses obesity-and high glucose-induced β cell dysfunction in isolated pancreatic islets. Cell Metab. 2006, 3, 417–427. [Google Scholar] [PubMed] [Green Version]
- Alorbu, C.; Cai, L. Fungal resistance and leachability of genipin-crosslinked chitosan treated wood. Int. Biodeterior. Biodegrad. 2022, 169, 105378. [Google Scholar] [CrossRef]
- Muhamad, S.H.A.; On, S.; Sanusi, S.N.; Hashim, A.A.; Zai, M.A. November. Antioxidant activity of Camphor leaves extract based on variation solvent. J. Phys. Conf. Ser. 2019, 1349, 012102. [Google Scholar]
- Dawé, A.; Talom, B.; Kapche, G.D.W.F.; Siddiqui, K.; Yakai, F.; Talla, E.; Shaiq, M.A.; Lubna, I.; Ngadjui, B.T. Termiglaucescin, a new polyhydroxy triterpene glucoside from Terminalia glaucescens with antioxidant and anti-inflammatory potential. Z. Für Nat. C 2017, 72, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Anokwuru, C.P.; Tankeu, S.; van Vuuren, S.; Viljoen, A.; Ramaite, I.D.; Taglialatela-Scafati, O.; Combrinck, S. Unravelling the antibacterial activity of Terminalia sericea root bark through a metabolomic approach. Molecules 2020, 25, 3683. [Google Scholar]
- Akbar, S.; Ishtiaq, S.; Jahangir, M.; Elhady, S.S.; Bogari, H.A.; Alahdal, A.M.; Ashour, M.L.; Youssef, F.S. Evaluation of The Antioxidant, Antimicrobial, and Anticancer Activities of Dicliptera bupleuroides Isolated Compounds Using In Vitro and In Silico Studies. Molecules 2021, 26, 7196. [Google Scholar] [PubMed]
- Yinusa, I.; Adeiza, H.A.; Balkees, W.A. Phytochemical, proximate and antimicrobial analysis of the fruit of Sarcocephalus latifolius (Smith Bruce). Lapai. J. Appl. Nat. Sci. 2016, 1, 38–46. [Google Scholar]
- Liu, M.; Katerere, D.R.; Gray, A.I.; Seidel, V. Phytochemical and antifungal studies on Terminalia mollis and Terminalia brachystemma. Fitoterapia 2009, 80, 369–373. [Google Scholar]
- Mailafiya, M.M.; Yusuf, A.J.; Abdullahi, M.I.; Aleku, G.A.; Ibrahim, I.A.; Yahaya, M.; Abubakar, H.; Sanusi, A.; Adamu, H.W.; Alebiosu, C.O. Antimicrobial activity of stigmasterol from the stem bark of Neocarya macrophylla. J. Med. Plants Econ. Dev. 2018, 2, 1–5. [Google Scholar]
- Irshad, M.D.; Ahmad, A.; Zafaryab, M.D.; Ahmad, F.; Manzoor, N.; Singh, M.; Rizvi, M.M.A. Composition of Cassia fistula oil and its antifungal activity by disrupting ergosterol biosynthesis. Nat. Prod. Commun. 2013, 8, 1934578X1300800233. [Google Scholar]
 percentage of growth inhibition;
percentage of growth inhibition;  percentage of growth.
percentage of growth.
 percentage of growth inhibition;
percentage of growth inhibition;  percentage of growth.
percentage of growth.



| T. brownii Extracts | C. parapsilosis ATCC 7330 | C. tropicalis ATCC 750 | C. albicans ATCC 10231 | C. glabrata ATCC 2001 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Average | SEM | AI | Average | SEM | AI | Average | SEM | AI | Average | SEM | AI | |
| Leaf extracts | ||||||||||||
| L hex | 15.67 | 0.33 | 0.55 | 27.00 | 0.58 | 1.03 | NA | 17.33 | 0.33 | 0.47 | ||
| L Dic | 16.33 | 0.33 | 0.58 | 16.00 | 0.00 | 0.61 | 16.33 | 0.33 | 0.55 | 20.33 | 0.33 | 0.55 |
| L acet | 32.93 | 0.07 | 1.16 | 29.83 | 0.09 | 1.13 | 28.00 | 0.00 | 0.94 | 38.60 | 0.31 | 1.04 |
| L ethyl | 31.00 | 0.00 | 1.09 | 24.50 | 0.29 | 0.93 | 28.00 | 0.00 | 0.94 | 22.60 | 0.21 | 0.61 |
| L aqu | 29.00 | 0.00 | 1.02 | 18.50 | 0.50 | 0.70 | 23.67 | 0.33 | 0.79 | 34.83 | 0.17 | 0.94 |
| L HH2O | 30.90 | 0.10 | 1.09 | 26.33 | 0.33 | 1.00 | 26.10 | 0.21 | 0.64 | 32.67 | 0.17 | 0.88 |
| L H2O* | 32.00 | 0.00 | 1.13 | 18.67 | 0.17 | 0.71 | 19.93 | 0.07 | 0.67 | 35.00 | 0.00 | 0.95 |
| L Me* | 30.23 | 0.23 | 1.07 | 28.80 | 0.06 | 1.09 | 28.27 | 0.27 | 0.95 | 30.67 | 0.33 | 0.83 |
| L MeSox | 31.80 | 0.06 | 1.12 | 30.90 | 0.06 | 1.17 | 29.00 | 0.00 | 0.97 | 32.00 | 0.00 | 0.86 |
| Stem bark extracts | ||||||||||||
| B hex | 20.00 | 0.00 | 0.71 | 23.50 | 0.29 | 0.89 | 19.33 | 0.33 | 0.65 | 25.33 | 0.33 | 0.68 |
| B Dic | 21.67 | 0.33 | 0.76 | 20.00 | 0.00 | 0.76 | 25.00 | 0.00 | 0.84 | 25.00 | 0.00 | 0.68 |
| B ethyl | 29.50 | 0.50 | 1.04 | 28.50 | 0.25 | 1.08 | 26.00 | 0.00 | 0.87 | 28.10 | 0.10 | 0.76 |
| B aqu | 26.00 | 0.00 | 0.92 | 20.50 | 0.29 | 0.78 | 20.57 | 0.30 | 0.69 | 21.67 | 0.33 | 0.59 |
| B HH2O | 28.33 | 0.33 | 1.00 | 26.00 | 0.00 | 0.99 | 23.43 | 0.30 | 0.79 | 28.00 | 0.00 | 0.76 |
| B H2O* | 27.20 | 0.20 | 0.96 | 19.50 | 0.29 | 0.74 | 23.67 | 0.33 | 0.79 | 22.67 | 0.33 | 0.61 |
| B Me* | 30.63 | 0.15 | 1.08 | 27.67 | 0.17 | 1.05 | 26.80 | 0.20 | 0.90 | 26.93 | 0.07 | 0.73 |
| B MeSox | 30.93 | 0.07 | 1.09 | 29.00 | 0.00 | 1.10 | 28.33 | 0.33 | 0.95 | 30.30 | 0.15 | 0.82 |
| Stem wood extracts | ||||||||||||
| W hex | 26.50 | 0.29 | 0.94 | 27.83 | 0.17 | 1.06 | 19.33 | 0.44 | 0.65 | 30.00 | 0.00 | 0.81 |
| W Dic | 25.00 | 0.00 | 0.88 | 24.33 | 0.33 | 0.92 | 26.67 | 0.33 | 0.89 | 25.67 | 0.33 | 0.69 |
| W ethyl | 34.67 | 0.24 | 1.22 | 15.00 | 0.00 | 0.57 | 22.50 | 0.25 | 0.75 | 32.60 | 0.30 | 0.88 |
| W aqu | NT | 19.33 | 0.33 | 0.73 | NT | 0.00 | NT | |||||
| W HH2O | 27.67 | 0.17 | 0.98 | 24.50 | 0.29 | 0.93 | 21.17 | 0.09 | 0.71 | 29.50 | 0.29 | 0.80 |
| W H2O* | 26.00 | 0.00 | 0.92 | 27.00 | 0.00 | 1.03 | 25.00 | 0.00 | 0.84 | 35.50 | 0.29 | 0.96 |
| W Me* | 18.67 | 0.17 | 0.66 | 18.33 | 0.33 | 0.70 | 22.33 | 0.33 | 0.75 | 15.33 | 0.17 | 0.41 |
| W MeSox | 25.00 | 0.00 | 0.88 | 20.67 | 0.67 | 0.78 | 24.00 | 0.00 | 0.80 | 34.67 | 0.33 | 0.94 |
| Root extracts | ||||||||||||
| R hex | 20.67 | 0.33 | 0.73 | 26.00 | 058 | 0.99 | NA | NA | 22.67 | 0.33 | 0.61 | |
| R Dic | 21.00 | 0.00 | 0.74 | 23.00 | 0.00 | 0.87 | 21.67 | 0.33 | 0.73 | 21.00 | 0.00 | 0.57 |
| R acet | 20.50 | 0.29 | 0.72 | 29.67 | 0.33 | 1.13 | 28.00 | 0.00 | 0.94 | 40.67 | 0.33 | 1.10 |
| R ethyl | 21.50 | 0.29 | 0.76 | 30.33 | 0.33 | 1.15 | 26.33 | 0.33 | 0.88 | 29.97 | 0.03 | 0.81 |
| R aqu | 29.00 | 0.00 | 1.02 | 28.67 | 0.33 | 1.09 | 24.67 | 0.33 | 0.83 | 38.17 | 0.17 | 1.03 |
| R HH2O | 28.67 | 0.33 | 1.01 | 26.00 | 0.00 | 0.99 | 29.50 | 0.29 | 0.99 | 29.30 | 0.35 | 0.79 |
| R H2O* | 25.93 | 0.07 | 0.92 | 24.73 | 0.15 | 0.94 | 24.10 | 0.10 | 0.81 | 20.33 | 0.33 | 0.55 |
| R Me* | 21.50 | 0.29 | 0.76 | 30.00 | 0.00 | 1.14 | 26.33 | 0.33 | 0.88 | 40.00 | 0.00 | 1.08 |
| R MeSox | 31.57 | 0.13 | 1.11 | 31.63 | 0.19 | 1.20 | 29.93 | 0.03 | 1.00 | 32.00 | 0.00 | 0.86 |
| R Ethanol wash | 32.00 | 0.00 | 1.13 | 38.17 | 0.17 | 1.03 | 25.33 | 0.33 | 0.85 | 40.00 | 0.00 | 1.08 |
| R Acetone wash | 28.00 | 0.00 | 0.99 | 29.00 | 0.00 | 1.02 | NT | NT | ||||
| Root bark extracts | ||||||||||||
| Rb hex | 18.67 | 0.33 | 0.66 | 29.00 | 0 | 1.10 | NA | NA | 24.00 | 0.00 | 0.65 | |
| RbDic | 19.07 | 0.28 | 0.67 | 19.95 | 0.04 | 0.76 | 19.00 | 0.00 | 0.64 | 23.00 | 0.00 | 0.62 |
| Rb acet | 31.00 | 0.00 | 1.09 | 22.67 | 0.33 | 0.86 | 27.53 | 0.29 | 0.92 | 33.00 | 0.00 | 0.89 |
| Rb ethyl | 31.00 | 0.00 | 1.09 | 31.00 | 0 | 1.18 | 29.90 | 0.10 | 1.00 | 35.50 | 0.29 | 0.96 |
| Rb aqu | 18.00 | 0.00 | 0.64 | 20.67 | 0.33 | 0.78 | 19.73 | 0.15 | 0.66 | 17.67 | 0.17 | 0.48 |
| Rb HH2O | 24.00 | 0.00 | 0.85 | 24.67 | 0.17 | 0.94 | 26.00 | 0.00 | 0.87 | 23.00 | 0.00 | 0.62 |
| Rb H2O* | 22.33 | 0.33 | 0.79 | 25.00 | 0.00 | 0.95 | NT | 19.67 | 0.17 | 0.53 | ||
| Rb Me* | 30.73 | 0.09 | 1.08 | 29.90 | 0.06 | 1.14 | 28.00 | 0.00 | 0.94 | 32.67 | 0.33 | 0.88 |
| Rb MeSox | 32.60 | 0.06 | 1.15 | 30.60 | 0.21 | 1.16 | 29.70 | 0.15 | 1.00 | 33.00 | 0.00 | 0.89 |
| Amphotericin B | 28.33 | 0.33 | 1.00 | 26.33 | 0.33 | 1.00 | 29.80 | 0.17 | 1.00 | 37.00 | 0.58 | 1.00 |
| T. brownii extracts | C. parapsilosis | C. tropicalis | C. albicans | C. glabrata |
|---|---|---|---|---|
| ATCC 7330 | ATCC 750 | ATCC 10231 | ATCC 2001 | |
| Leaf extracts | ||||
| L. hex | NT | 2500 * | NT | NT |
| L. acet | 312 * | 625 (IC 90) | 312 (IC 99) | 312 (IC 99.8) |
| L. ethyl | 312 (IC 93) | 1250 (IC89) | 625 (IC 94) | 2500 * |
| L. aqu | 2500 * | NT | 312 (95.5) | 625 (IC 97) |
| L. HH2O | 625 * | 2500 * | 625 (IC 89.8) | 2500 (IC 99.8) |
| L. H2O* | 625 * | NT | NT | 1250 (IC 97) |
| L. Me* | 625 * | 1250 (IC 93.2) | 1250 * | 625 (IC 95.7) |
| L. MeSox | 312 (91.4) | 625 (IC 96.2) | 1250 * | 625 (IC 98.1) |
| Stem bark extracts | ||||
| B. hex | NT | 5000 * | NT | 2500 * |
| B. Dic | 5000 * | NT | 5000 * | 2500 * |
| B. ethyl | 1250 (IC 98.6) | 1250 (IC 96.6) | 2500 (IC 89.6) | 1250 (IC 99.8) |
| B. aqu | 2500 * | NT | NT | NT |
| B. HH2O | 1250 * | 2500 * | NT | 1250 * |
| B. H2O* | 1250 * | NT | 5000 * | 5000 * |
| B. Me* | 1250 (IC 95.3) | 1250 * | 2500 (IC 90.6) | 312 (IC 92.4) |
| B. MeSox | 625 (IC 94) | 625 (IC 91.2) | 1250 (93.1) | 312 (IC 97.4) |
| Stem wood extracts | ||||
| W hex | 1250 * | 2500 (IC 89) | NT | 1250 (IC 94.1) |
| W Dic | 2500 * | 1250 * | 5000 * | 1250 * |
| W ethyl | 1250 (IC 91.2) | NT | NT | 625 (IC 97.7) |
| W aqu | NT | NT | NT | NT |
| W HH2O | 625 * | 5000 * | 1250 (IC 99) | 1250 (IC 95.4) |
| W H2O* | 1250 * | 2500 * | 1250 * | 625 * |
| W Me* | NT | NT | 2500 (IC 99) | NT |
| W MeSox | 1250 (IC 99.6) | 1250 (IC 98.3) | 312 (IC 96) | 625 (IC 98.3) |
| Root extracts | ||||
| R hex | 5000 (IC 90) | 2500 * | NT | 1250 (IC 98) |
| R Dic | 5000 * | 5000 * | NT | NT |
| R acet | 5000 * | 1250 (IC 93.5) | 1250 * | 312 (IC 96.5) |
| R ethyl | 1250 (IC 91.3) | 1250 (IC 97.5) | 625 (IC 99) | 625 (IC 96.5) |
| R aqu | 1250 * | 1250 * | 625 (IC 97) | 625 (IC 98) |
| R HH2O | 1250 * | 2500 * | 1250 * | 1250 * |
| R H2O* | 2500 * | NT | NT | NT |
| R Me* | 5000 * | 2500 * | 2500 * | 312 (IC 96.3) |
| R MeSox | 625 (IC 92.5) | 1250 (IC 90.8) | 312 (IC 89) | 312 (IC 95.5) |
| R Ethanol wash | 93.75 (IC 97) | 187.50 (IC 92.6) | 187.50 (IC 975) | NT |
| R Aceton wash | 375 (IC 90.5) | NT | NT | 375 (IC 99) |
| Root bark extracts | ||||
| Rb hex | NT | 1250 * | NT | 2500 (IC 93.2) |
| RbDic | NT | NT | NT | 2500 * |
| Rb acet | 1250 (IC 98) | 5000 * | 2500 * | 1250 (IC 94.7) |
| Rb ethyl | 1250 (IC 91.8) | 1250 (IC 90.6) | 1250 (IC 93.7) | 625 (IC 99) |
| Rb HH2O | 2500 * | 2500 (IC 99) | 5000 (IC 89) | 5000 (IC 89) |
| Rb H2O* | 5000 * | 2500 (IC 95.5) | NT | NT |
| Rb Me* | 1250 (IC 94.5) | 1250 (IC 96.9) | 5000 (IC 92.6) | 2500 * |
| Rb MeSox | 625 (IC 93.3) | 1250 (IC 98.6) | 2500 (IC 97.9) | 1250 (IC 96.7) |
| Pure compounds and amphotericin B | ||||
| Sitosterol | 125 (IC 96.9) | 1000 (IC 99.5) | 1000 (IC 93.5) | 250 (IC 96.0) |
| Stigmasterol | NT | NT | 1000 (IC 95.7) | 500 (IC 92.4) |
| Gallic acid | 1000 (IC 98.6) | 1000 (IC 97.9) | 1000 (IC 99.9) | 1000 (99.8) |
| Ellagic acid | 500 (IC 89.5) | NT | NT | 500 (IC 99.5) |
| Quercetin | NT | NT | 250 (IC 96.6) | 250 (95.5) |
| Apigenin | 125 (IC 95.5) | NT | 500 (IC 97.8) | 500 (IC 96.7) |
| Luteolin | 500 (IC 99) | 500 (IC 99.8) | 1000 (IC 95) | NT |
| Corilagin | >1000 | 500 (IC 99.4) | >1000 | NA > 1000 |
| Friedelin | 500 (IC 90) | 500 (IC 91.4) | NT | NT |
| Amphotericin B | 3.9 (IC 98.9) | 7.8 (IC 93.7) | 62.5 (IC 96.7) | 7.8 (IC 97.5) |
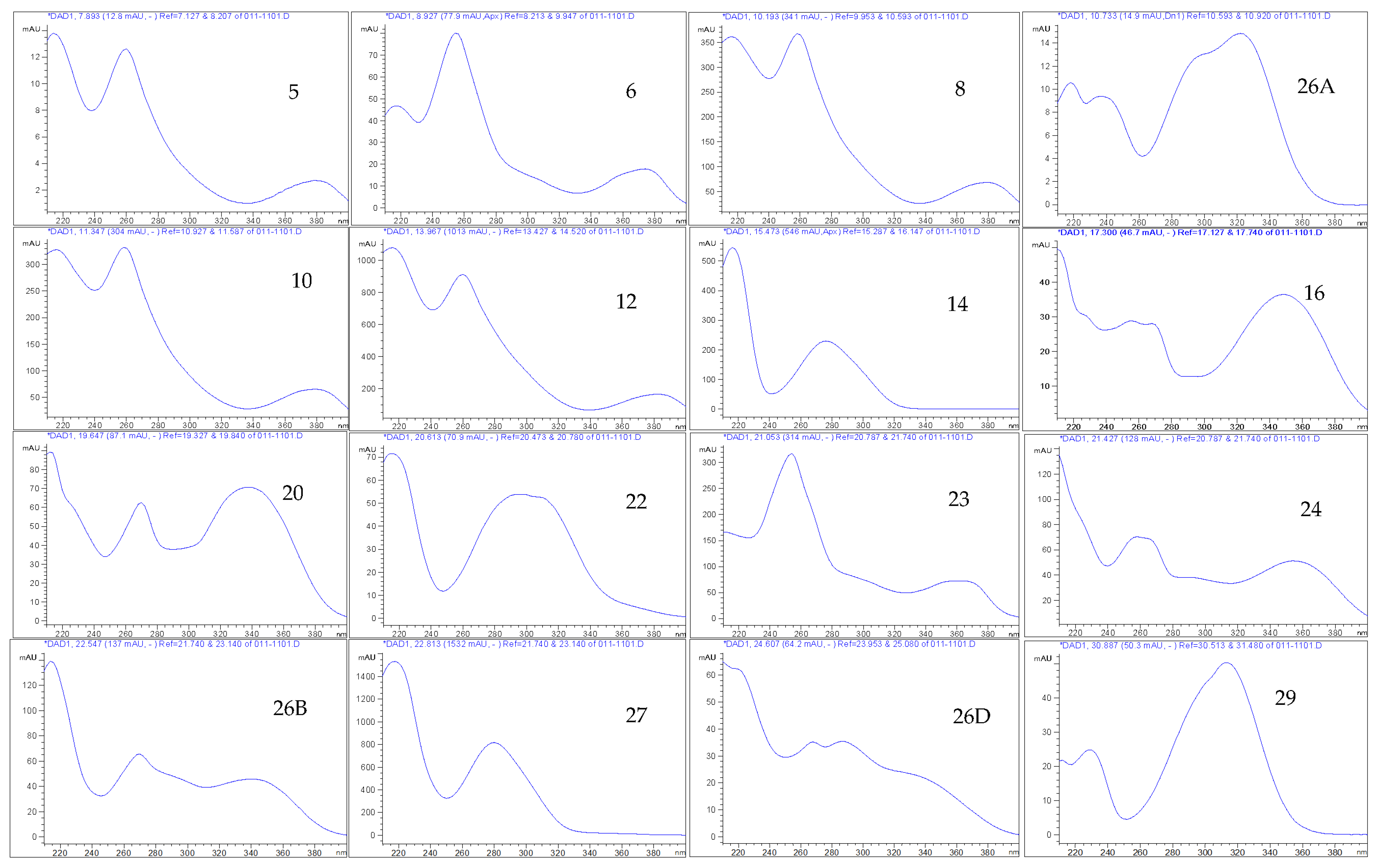
| HPLC-DAD and UHPLC/QTOF-MS | Molecular Formula | tR HPLC-DAD (min) | tR UHPLC/QTOF-MS (min) | [M-H]− (m/z) | Exact Mass (calc.) | UVλ Absorption Max. from HPLC-DAD | Peak Area (%) at 270 nm | PPM Value |
|---|---|---|---|---|---|---|---|---|
| Sample and Compounds | ||||||||
| Gallic acid (1) | C7H6O5 | 1.760 | 1.165 | 169.0146 | 170.0213 | 216, 272 | 5.55 | 6.47 |
| gallotanin | 2.074 | 218, 266 | 0.06 | |||||
| ellagitannin | 3.251 | 216, 260, 380 | 0.02 | |||||
| Protocatechuic acid (PCA) (2) | C7H6O4 | 4.076 | 2.347 | 153.0198 | 154.0264 | 216, 220, 260, 294 | 1.79 | 7.79 |
| gallotannin | 4.488 | 222, 288 | 0.10 | |||||
| Unknown compound (3) | 5.351 | 216 | 1.60 | |||||
| 1,3-di-galloyl-β-D-glucose (4) | C20H20O14 | 6.734 | 4.248 | 483.0796 | 484.0846 | 216, 272 | 4.65 | 5.78 |
| ellagitannin (5) | 7.896 | 214, 260, 378 | 0.41 | |||||
| gallo-ellagitannin | 8.633 | 222, 258, 376 | 0.29 | |||||
| Isomer of methyl-(S)-flavogallonate (6) | 8.925 | 8.52 | 483.0811 | 218, 254, 374 | 1.06 | |||
| Unknown compound (7) | 9.540 | 220 | 2.99 | |||||
| ellagitannin (8) | 10.199 | 3.363 | 541.0291 | 216, 258, 380 | 3.91 | |||
| ellagitannin | 10.490 | 218, 258, 364 | 0.13 | |||||
| Unknown flavonoid (26A) | 10.708 | 218, 234, 298, 322 | 0.12 | |||||
| 1,6-di-galloyl-β-D-glucose (9) | C20H20O14 | 11.142 | 4.714 | 483.0808 | 484.0846 | 216, 272 | 0.68 | 8.26 |
| Terflavin B (10) | C34H24O22 | 11.303 | 3.546 | 783.0701 | 784.0750 | 216, 258, 378 | 5.37 | 3.70 |
| gallotannin | 11.481 | 216, 256 | 0.09 | |||||
| ellagitannin | 12.114 | 220, 256, 378 | 0.43 | |||||
| ellagitannin | 12.867 | 214, 260, 380 | 0.28 | |||||
| ellagtannin | 13.114 | 214, 260, 378 | 0.12 | |||||
| gallotannin | 13.300 | 218, 278 | 0.06 | |||||
| Methyl galate (11) (gallic acid based) (Methyl 3,4,5-trihydroxybenzoate) | C8H8O5 | 13.722 | 4.013 | 183.0304 | 184.0369 | 216, 280 | 1.52 | 7.06 |
| ellagitannin (unknown) (12) {main compound} | 13.922 | 4.529 | 617.0164 | 216, 260, 382 | 15.46 | |||
| 1,2,3-tri-O-galloyol-β-D-glucose (13) | C27H24O18 | 14.146 | 5.680 | 635.0889 | 636.0954 | 218, 272 | 1.98 | 2.04 |
| gallotannin | 15.154 | 216, 272 | 0.29 | |||||
| gallotannin (14) | 15.473 | 216, 276 | 3.32 | |||||
| gallotannin | 15.722 | 216, 258, 380 | 0.30 | |||||
| gallotannin | 15.891 | 218, 274 | 0.19 | |||||
| Corilagin (15) | C27H22O18 | 16.031 | 5.545 | 633.0756 | 634.0798 | 216, 266, 354 | 0.20 | 5.68 |
| gallotannin | 16.450 | 216, 292 | 0.28 | |||||
| gallotannin | 17.011 | 218, 278 | 0.13 | |||||
| Luteolin-7-O-glucoside (16) | C21H20O11 | 17.317 | 447.0945 | 448.0999 | 228, 256, 268, 350 | 0.46 | 5.36 | |
| gallotannin (17) | 17.611 | 216, 276 | 0.53 | |||||
| ellagitannin | 17.812 | 218, 256, 368 | 0.01 | |||||
| gallotannin | 18.013 | 220, 276 | 0.28 | |||||
| 3,4,6-tri-O-galloyol-β-D-glucose (18) | C27H24O18 | 18.312 | 5.661 | 635.0904 | 636.0954 | 224, 276 | 4.07 | 4.40 |
| galloyl ellagitannin | 18.563 | 218, 274, 382 | 0.38 | |||||
| gallotannin (19) (Di-O-galloyl derivative) (Maplexin D) | C20H20O13 | 18.785 | 6.094 | 467.0802 | 468.0897 | 218, 256 | 8.31 | −3.63 |
| gallotannin | 18.967 | 216, 278 | 0.41 | |||||
| galloyl ellagitannin | 19.225 | 216, 276, 342 | 0.26 | |||||
| Apigenin (20) | C15H10O5 | 19.651 | 269.0455 | 270.0525 | 212, 270, 338 | 0.98 | 2.96 | |
| 1,2,3,6-tetra-O-galloyl-β-D-glucose (21a) | C34H28O22 | 20.113 | 6.572 | 787.0872 | 788.1062 | 218, 279 | 0.88 | −14.21 |
| unknown gallotannin (21b) | 20.288 | 218, 274 | 1.04 | |||||
| Kaempferol-4′-sulfate (22) | C15H10O9S | 20.617 | 7.010 | 365.2928 | 3663 | 216, 298, 308 | 0.50 | 1.64 |
| Di-methyl ellagic acid glucoside (23) | C22H20O13 | 21.089 | 7.750 | 491.0843 | 492.0897 | 210, 254, 366 | 5.08 | 4.88 |
| Myricetin-3-rhamnoside (24) | C21H20O12 | 21.422 | 7.800 | 463.3798 | 464.3800 | 210, 258, 354 | 1.70 | 16.37 |
| gallotannin | 21.909 | 218, 282 | 0.41 | |||||
| gallotannin (25) | 22.158 | 216, 280 | 1.71 | |||||
| Unknown flavonoids (26B) | 22.557 | 214, 270, 340 | 1.19 | |||||
| Chebulinic acid (Eutannin) (27) or (1,3,6-tri-O-galloyl-2,4-O-chebuloyl-β-D-Glc) | C41H32O27 | 22.800 | 8.159 | 955.0790 | 95.608 | 218, 280, 381 | 13.33 | 7.11 |
| gallotannin (28) | 23.362 | 218, 282 | 0.33 | |||||
| Unknown flavonoids (26C) | 24.290 | 214, 264, 286, 350 | 0.31 | |||||
| Unknown flavonoids (26D) | 24.602 | 210, 222, 268, 286 | 1.50 | |||||
| gallotannin | 26.253 | 216, 282 | 0.14 | |||||
| gallotannin | 29.000 | 216, 280 | 0.17 | |||||
| Genipin (29) | C11H14O5 | 30.878 | 12.222 | 225.1504 | 225.9037 | 210, 233, 288 | 0.33 | |
| Sericic acid (30) | C30H48O6 | 35.440 | 13.937 | 503.3379 | 504.3438 | 210, 290, 328 | 0.03 | 3.77 |
| ellagic acid derivative (31) | 36.426 | 212, 250, 306, 370 | 0.04 | |||||
| Unknown compound (32) | 38.726 | 17.834 | 293.1759 | 0.05 | ||||
| Genkwanin (Apigenin 7-O-methyl ether) (33) | C16H12O5 | 39.012 | 18.417 | 283.192 | 284.1981 | 210, 218, 288 | 0.05 | 5.98 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salih, E.Y.A.; Julkunen-Tiitto, R.; Luukkanen, O.; Fyhrqvist, P. Anti-Candida Activity of Extracts Containing Ellagitannins, Triterpenes and Flavonoids of Terminalia brownii, a Medicinal Plant Growing in Semi-Arid and Savannah Woodland in Sudan. Pharmaceutics 2022, 14, 2469. https://doi.org/10.3390/pharmaceutics14112469
Salih EYA, Julkunen-Tiitto R, Luukkanen O, Fyhrqvist P. Anti-Candida Activity of Extracts Containing Ellagitannins, Triterpenes and Flavonoids of Terminalia brownii, a Medicinal Plant Growing in Semi-Arid and Savannah Woodland in Sudan. Pharmaceutics. 2022; 14(11):2469. https://doi.org/10.3390/pharmaceutics14112469
Chicago/Turabian StyleSalih, Enass Y. A., Riitta Julkunen-Tiitto, Olavi Luukkanen, and Pia Fyhrqvist. 2022. "Anti-Candida Activity of Extracts Containing Ellagitannins, Triterpenes and Flavonoids of Terminalia brownii, a Medicinal Plant Growing in Semi-Arid and Savannah Woodland in Sudan" Pharmaceutics 14, no. 11: 2469. https://doi.org/10.3390/pharmaceutics14112469
APA StyleSalih, E. Y. A., Julkunen-Tiitto, R., Luukkanen, O., & Fyhrqvist, P. (2022). Anti-Candida Activity of Extracts Containing Ellagitannins, Triterpenes and Flavonoids of Terminalia brownii, a Medicinal Plant Growing in Semi-Arid and Savannah Woodland in Sudan. Pharmaceutics, 14(11), 2469. https://doi.org/10.3390/pharmaceutics14112469