Topical Semisolid Products—Understanding the Impact of Metamorphosis on Skin Penetration and Physicochemical Properties
Abstract
:1. Introduction
2. Evaporation
3. Supersaturation
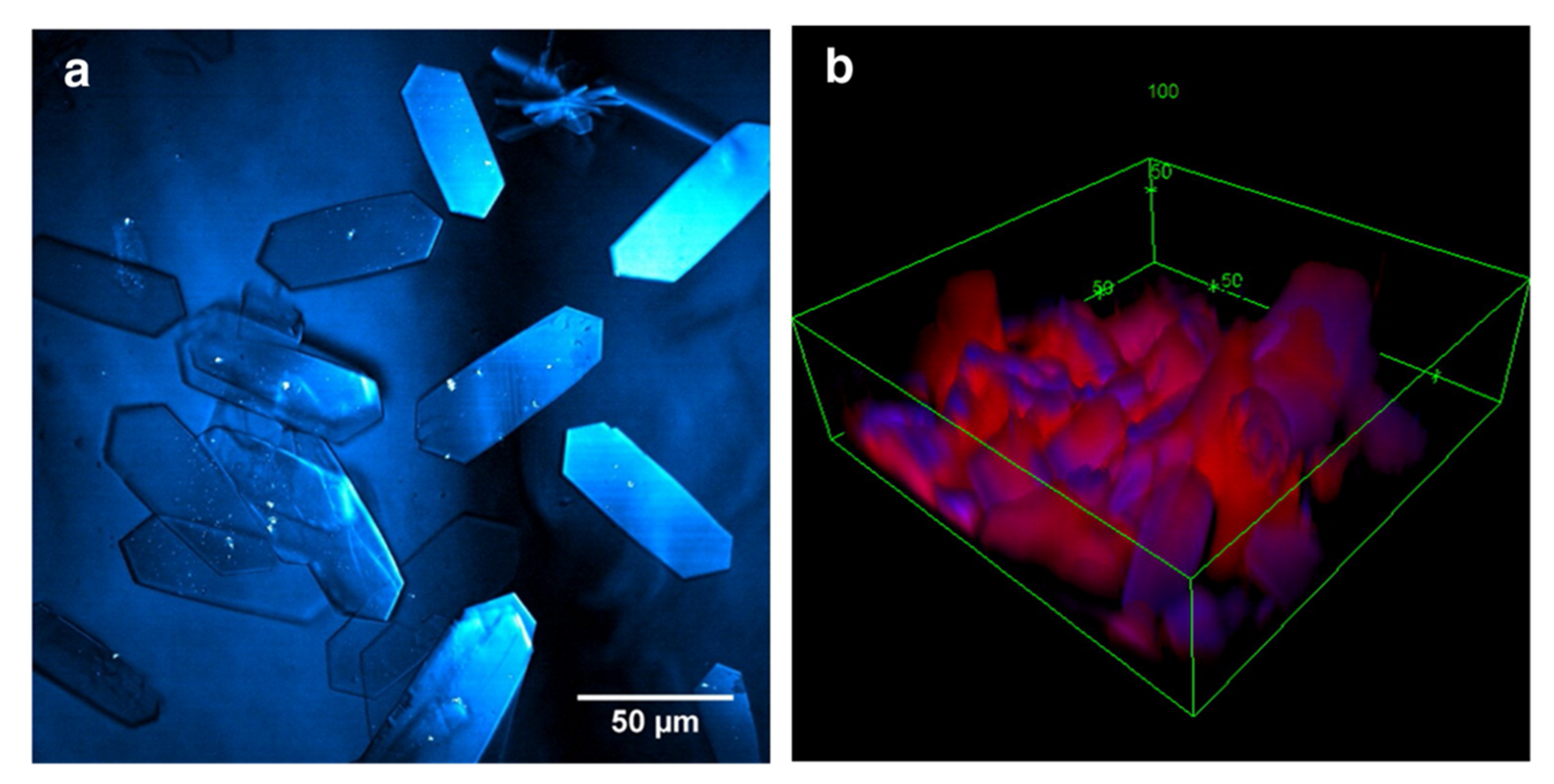
4. Crystallization
5. Viscosity
6. Thermodynamic Activity
7. Microstructural Change
8. Skin Permeation Studies
9. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Imran, M.; Iqubal, M.K.; Imtiyaz, K.; Saleem, S.; Mittal, S.; Rizvi, M.M.A.; Ali, J.; Baboota, S. Topical Nanostructured Lipid Carrier Gel of Quercetin and Resveratrol: Formulation, Optimization, in Vitro and Ex Vivo Study for the Treatment of Skin Cancer. Int. J. Pharm. 2020, 587, 119705. [Google Scholar] [CrossRef] [PubMed]
- Tapfumaneyi, P.; Imran, M.; Mohammed, Y.; Roberts, M.S. Recent Advances and Future Prospective of Topical and Transdermal Delivery Systems. Front. Drug Deliv. 2022, 2, 25. [Google Scholar] [CrossRef]
- Chang, R.-K.; Raw, A.; Lionberger, R.; Yu, L. Generic Development of Topical Dermatologic Products, Part II: Quality by Design for Topical Semisolid Products. AAPS J. 2013, 15, 674–683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Durand, C.; Alhammad, A.; Willett, K.C. Practical Considerations for Optimal Transdermal Drug Delivery. Am. J. Health-Syst. Pharm. 2012, 69, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Marwah, H.; Garg, T.; Goyal, A.K.; Rath, G. Permeation Enhancer Strategies in Transdermal Drug Delivery. Null 2016, 23, 564–578. [Google Scholar] [CrossRef]
- Mohammed, Y.; Holmes, A.; Kwok, P.C.L.; Kumeria, T.; Namjoshi, S.; Imran, M.; Matteucci, L.; Ali, M.; Tai, W.; Benson, H.A.E.; et al. Advances and Future Perspectives in Epithelial Drug Delivery. Adv. Drug Deliv. Rev. 2022, 186, 114293. [Google Scholar] [CrossRef] [PubMed]
- Roberts, M.S.; Cheruvu, H.S.; Mangion, S.E.; Alinaghi, A.; Benson, H.A.E.; Mohammed, Y.; Holmes, A.; van der Hoek, J.; Pastore, M.; Grice, J.E. Topical Drug Delivery: History, Percutaneous Absorption, and Product Development. Adv. Drug Deliv. Rev. 2021, 177, 113929. [Google Scholar] [CrossRef]
- Verdier-Sévrain, S.; Bonté, F. Skin Hydration: A Review on Its Molecular Mechanisms. J. Cosmet. Dermatol. 2007, 6, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Prausnitz, M.R.; Mitragotri, S.; Langer, R. Current Status and Future Potential of Transdermal Drug Delivery. Nat. Rev. Drug Discov. 2004, 3, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Raney, S.G.; Franz, T.J.; Lehman, P.A.; Lionberger, R.; Chen, M.-L. Pharmacokinetics-Based Approaches for Bioequivalence Evaluation of Topical Dermatological Drug Products. Clin. Pharm. 2015, 54, 1095–1106. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food and Drug Administration. 21 CFR § 320.22—Criteria for Waiver of Evidence of in Vivo Bioavailability or Bioequivalence. Available online: https://www.ecfr.gov/current/title-21/chapter-I/subchapter-D/part-320/subpart-B/section-320.22 (accessed on 29 March 2022).
- Buhse, L.; Kolinski, R.; Westenberger, B.; Wokovich, A.; Spencer, J.; Chen, C.W.; Turujman, S.; Gautam-Basak, M.; Kang, G.J.; Kibbe, A.; et al. Topical Drug Classification. Int. J. Pharm. 2005, 295, 101–112. [Google Scholar] [CrossRef]
- Hunter, A.M.; Grigson, C.; Wade, A. Influence of Topically Applied Menthol Cooling Gel on Soft Tissue Thermodynamics and Arterial and Cutaneous Blood Flow At Rest. Int. J. Sports Phys. 2018, 13, 483–492. [Google Scholar] [CrossRef] [Green Version]
- Surber, C.; Knie, U. Metamorphosis of Vehicles: Mechanisms and Opportunities. In Current Problems in Dermatology; Surber, C., Abels, C., Maibach, H., Karger, A.G.S., Eds.; Karger: Basel, Switzerland, 2018; Volume 54, pp. 152–165. ISBN 978-3-318-06384-4. [Google Scholar]
- Namjoshi, S.; Dabbaghi, M.; Roberts, M.S.; Grice, J.E.; Mohammed, Y. Quality by Design: Development of the Quality Target Product Profile (QTPP) for Semisolid Topical Products. Pharmaceutics 2020, 12, 287. [Google Scholar] [CrossRef] [Green Version]
- Mohammed, Y.; Namjoshi, S.; Telaprolu, K.; Crowe, A.; Jung, N.; Grice, J.; Windbergs, M.; Benson, H.; Raney, S.G.; Roberts, M.S. A Novel Method to Selectively Differentiate between the Loss of Water and Other Volatiles from Topical Semisolid Products; CRS: Mount Laurel, NJ, USA, 2017. [Google Scholar]
- Santos, P.; Watkinson, A.C.; Hadgraft, J.; Lane, M.E. Enhanced Permeation of Fentanyl from Supersaturated Solutions in a Model Membrane. Int. J. Pharm. 2011, 407, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Santos, P.; Watkinson, A.C.; Hadgraft, J.; Lane, M.E. Influence of Penetration Enhancer on Drug Permeation from Volatile Formulations. Int. J. Pharm. 2012, 439, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Pellett, M.A.; Castellano, S.; Hadgraft, J.; Davis, A.F. The Penetration of Supersaturated Solutions of Piroxicam across Silicone Membranes and Human Skin in Vitro. J. Control. Release 1997, 46, 205–214. [Google Scholar] [CrossRef]
- Pellett, M.A.; Roberts, M.S.; Hadgraft, J. Supersaturated Solutions Evaluated with an in Vitro Stratum Corneum Tape Stripping Technique. Int. J. Pharm. 1997, 151, 91–98. [Google Scholar] [CrossRef]
- Poulsen, B.J.; Young, E.; Coquilla, V.; Katz, M. Effect of Topical Vehicle Composition on the In Vitro Release of Fluocinolone Acetonide and Its Acetate Ester. J. Pharm. Sci. 1968, 57, 928–933. [Google Scholar] [CrossRef] [PubMed]
- Coldman, M.F.; Poulsen, B.J.; Higuchi, T. Enhancement of Percutaneous Absorption by the Use of Volatile: Nonvolatile Systems as Vehicles. J. Pharm. Sci. 1969, 58, 1098–1102. [Google Scholar] [CrossRef]
- Theeuwes, F.; Gale, R.M.; Baker, R.W. Transference: A Comprehensive Parameter Governing Permeation of Solutes through Membranes. J. Membr. Sci. 1976, 1, 3–16. [Google Scholar] [CrossRef]
- Barrett, C.W.; Hadgraft, J.W.; Caron, G.A.; Sarkany, I. The Effect of Particle Size and Vehicle on the Percutaneous Absorption of Fluocinolone Acetonide. Br. J. Dermatol. 1965, 77, 576–578. [Google Scholar] [CrossRef] [PubMed]
- Chia-Ming, C.; Flynn, G.L.; Weiner, N.D.; Szpunar, G.J. Bioavailability Assessment of Topical Delivery Systems: Effect of Vehicle Evaporation upon in Vitro Delivery of Minoxidil from Solution Formulations. Int. J. Pharm. 1989, 55, 229–236. [Google Scholar] [CrossRef] [Green Version]
- Iervolino, M.; Cappello, B.; Raghavan, S.L.; Hadgraft, J. Penetration Enhancement of Ibuprofen from Supersaturated Solutions through Human Skin. Int. J. Pharm. 2001, 212, 131–141. [Google Scholar] [CrossRef]
- Casacio, C.A.; Madsen, L.S.; Terrasson, A.; Waleed, M.; Barnscheidt, K.; Hage, B.; Taylor, M.A.; Bowen, W.P. Quantum-Enhanced Nonlinear Microscopy. Nature 2021, 594, 201–206. [Google Scholar] [CrossRef]
- Belsey, N.A.; Garrett, N.L.; Contreras-Rojas, L.R.; Pickup-Gerlaugh, A.J.; Price, G.J.; Moger, J.; Guy, R.H. Evaluation of Drug Delivery to Intact and Porated Skin by Coherent Raman Scattering and Fluorescence Microscopies. J. Control. Release 2014, 174, 37–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saar, B.G.; Contreras-Rojas, L.R.; Xie, X.S.; Guy, R.H. Imaging Drug Delivery to Skin with Stimulated Raman Scattering Microscopy. Mol. Pharm. 2011, 8, 969–975. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saar, B.G.; Freudiger, C.W.; Reichman, J.; Stanley, C.M.; Holtom, G.R.; Xie, X.S. Video-Rate Molecular Imaging in Vivo with Stimulated Raman Scattering. Science 2010, 330, 1368–1370. [Google Scholar] [CrossRef] [Green Version]
- Cross, S.E.; Roberts, M.S.; Jiang, R.; Benson, H.A.E. Can Increasing the Viscosity of Formulations Be Used to Reduce the Human Skin Penetration of the Sunscreen Oxybenzone? J. Investig. Dermatol. 2001, 117, 147–150. [Google Scholar] [CrossRef] [Green Version]
- Binder, L.; Mazál, J.; Petz, R.; Klang, V.; Valenta, C. The Role of Viscosity on Skin Penetration from Cellulose Ether-Based Hydrogels. Ski. Res. Technol. 2019, 25, 725–734. [Google Scholar] [CrossRef] [Green Version]
- Connors, K.A. Thermodynamics of Pharmaceutical Systems: An Introduction for Students of Pharmacy; Wiley-Interscience: Hoboken, NJ, USA, 2002; ISBN 978-0-471-20241-7. [Google Scholar]
- Higuchi, T. Physical Chemical Analysis of Percutaneous Absorption Process from Creams and Ointments. J. Soc. Cosmet. Chem. 1960, 11, 85–97. [Google Scholar]
- Barry, B.W.; Harrison, S.M.; Dugard, P.H. Correlation of Thermodynamic Activity and Vapour Diffusion through Human Skin for the Model Compound, Benzyl Alcohol. J. Pharm. Pharmacol. 2011, 37, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Barry, B.W.; Harrison, S.M.; Dugard, P.H. Vapour and Liquid Diffusion of Model Penetrants through Human Skin; Correlation with Thermodynamic Activity. J. Pharm. Pharmacol. 2011, 37, 226–236. [Google Scholar] [CrossRef] [PubMed]
- Kokate, A.; Li, X.; Singh, P.; Jasti, B.R. Effect of Thermodynamic Activities of the Unionized and Ionized Species on Drug Flux across Buccal Mucosa. J. Pharm. Sci. 2008, 97, 4294–4306. [Google Scholar] [CrossRef] [PubMed]
- Roberts, M.S.; Anderson, R.A.; Swarbrick, J. Permeability of Human Epidermis to Phenolic Compounds. J. Pharm. Pharmacol. 1977, 29, 677–683. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Grice, J.E.; Li, P.; Jepps, O.G.; Wang, G.-J.; Roberts, M.S. Skin Solubility Determines Maximum Transepidermal Flux for Similar Size Molecules. Pharm. Res. 2009, 26, 1974–1985. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, P.; Roberts, M.S. Maximum Transepidermal Flux for Similar Size Phenolic Compounds Is Enhanced by Solvent Uptake into the Skin. J. Control. Release 2011, 154, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Anissimov, Y.G.; Roberts, M.S. Diffusion Modeling of Percutaneous Absorption Kinetics: 2. Finite Vehicle Volume and Solvent Deposited Solids. J. Pharm. Sci. 2001, 90, 504–520. [Google Scholar] [CrossRef]
- Pudipeddi, M.; Serajuddin, A.T.M. Trends in Solubility of Polymorphs. J. Pharm. Sci. 2005, 94, 929–939. [Google Scholar] [CrossRef] [PubMed]
- Toll, R.; Jacobi, U.; Richter, H.; Lademann, J.; Schaefer, H.; Blume-Peytavi, U. Penetration Profile of Microspheres in Follicular Targeting of Terminal Hair Follicles. J. Investig. Dermatol. 2004, 123, 168–176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kahraman, E.; Güngör, S.; Ozsoy, Y. Potential Enhancement and Targeting Strategies of Polymeric and Lipid-Based Nanocarriers in Dermal Drug Delivery. Ther. Deliv. 2017, 8, 967–985. [Google Scholar] [CrossRef]
- Subongkot, T.; Sirirak, T. Development and Skin Penetration Pathway Evaluation of Microemulsions for Enhancing the Dermal Delivery of Celecoxib. Colloids Surf. B Biointerfaces 2020, 193, 111103. [Google Scholar] [CrossRef]
- Lademann, J.; Richter, H.; Schaefer, U.F.; Blume-Peytavi, U.; Teichmann, A.; Otberg, N.; Sterry, W. Hair Follicles—A Long-Term Reservoir for Drug Delivery. Ski. Pharm. Physiol. 2006, 19, 232–236. [Google Scholar] [CrossRef]
- Simões, A.; Veiga, F.; Vitorino, C. Progressing Towards the Sustainable Development of Cream Formulations. Pharmaceutics 2020, 12, 647. [Google Scholar] [CrossRef] [PubMed]
- Patel, H.K.; Barot, B.S.; Parejiya, P.B.; Shelat, P.K.; Shukla, A. Topical Delivery of Clobetasol Propionate Loaded Microemulsion Based Gel for Effective Treatment of Vitiligo: Ex Vivo Permeation and Skin Irritation Studies. Colloids Surf. B Biointerfaces 2013, 102, 86–94. [Google Scholar] [CrossRef]
- Jung, N.; Namjoshi, S.; Mohammed, Y.; Grice, J.E.; Benson, H.A.E.; Raney, S.G.; Roberts, M.S.; Windbergs, M. Application of Confocal Raman Microscopy for the Characterization of Topical Semisolid Formulations and Their Penetration into Human Skin Ex Vivo. Pharm. Res. 2022, 39, 935–948. [Google Scholar] [CrossRef] [PubMed]
- Kreilgaard, M. Dermal Pharmacokinetics of Microemulsion Formulations Determined by In Vivo Microdialysis. Pharm. Res. 2001, 18, 367–373. [Google Scholar] [CrossRef]
- Lademann, J.; Jacobi, U.; Surber, C.; Weigmann, H.-J.; Fluhr, J.W. The Tape Stripping Procedure—Evaluation of Some Critical Parameters. Eur. J. Pharm. Biopharm. 2009, 72, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Santos, L.L.; Swofford, N.J.; Santiago, B.G. In Vitro Permeation Test (IVPT) for Pharmacokinetic Assessment of Topical Dermatological Formulations. Curr. Protoc. Pharmacol. 2020, 91, e79. [Google Scholar] [CrossRef]
- Caspers, P.J.; Bruining, H.A.; Puppels, G.J.; Lucassen, G.W.; Carter, E.A. In Vivo Confocal Raman Microspectroscopy of the Skin: Noninvasive Determination of Molecular Concentration Profiles. J. Investig. Dermatol. 2001, 116, 434–442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sinkó, B.; Garrigues, T.M.; Balogh, G.T.; Nagy, Z.K.; Tsinman, O.; Avdeef, A.; Takács-Novák, K. Skin–PAMPA: A New Method for Fast Prediction of Skin Penetration. Eur. J. Pharm. Sci. 2012, 45, 698–707. [Google Scholar] [CrossRef]
- Engesland, A.; Skar, M.; Hansen, T.; Škalko-basnet, N.; Flaten, G.E. New Applications of Phospholipid Vesicle-Based Permeation Assay: Permeation Model Mimicking Skin Barrier. J. Pharm. Sci. 2013, 102, 1588–1600. [Google Scholar] [CrossRef] [PubMed]
- Organisation for Economic Cooperation and Development. OECD Guideline for Testing of Chemicals No. 428: Skin Absorption: In Vitro Methods; OECD: Paris, France, 2004. [Google Scholar]
- Franz, T.J. Percutaneous Absorption on the Relevance of in Vitro Data. J. Investig. Derm. 1975, 64, 190–195. [Google Scholar] [CrossRef] [Green Version]
- Sil, B.C.; Alvarez, M.P.; Zhang, Y.; Kung, C.-P.; Hossain, M.; Iliopoulos, F.; Luo, L.; Crowther, J.M.; Moore, D.J.; Hadgraft, J.; et al. 3D-Printed Franz Type Diffusion Cells. Int. J. Cosmet. Sci. 2018, 40, 604–609. [Google Scholar] [CrossRef] [Green Version]
- Tiboni, M.; Curzi, G.; Aluigi, A.; Casettari, L. An Easy 3D Printing Approach to Manufacture Vertical Diffusion Cells for in Vitro Release and Permeation Studies. J. Drug Deliv. Sci. Technol. 2021, 65, 102661. [Google Scholar] [CrossRef]
- Wagner, H.; Lehr, C.-M.; Schaefer, U.F.; Kostka, K.-H. Human Skin Penetration of Flufenamic Acid: In Vivo/In Vitro Correlation (Deeper Skin Layers) for Skin Samples from the Same Subject. J. Investig. Dermatol. 2002, 118, 540–544. [Google Scholar] [CrossRef] [Green Version]
- Wagner, H.; Kostka, K.-H.; Lehr, C.-M.; Schaefer, U.F. Drug Distribution in Human Skin Using Two Different In Vitro Test Systems: Comparison with In Vivo Data. Pharm. Res. 2000, 17, 1475–1481. [Google Scholar] [CrossRef]
- Finnin, B.; Walters, K.A.; Franz, T.J. In Vitro Skin Permeation Methodology. In Topical and Transdermal Drug Delivery; Benson, H.A.E., Watkinson, A.C., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2012; pp. 85–108. ISBN 978-1-118-14050-5. [Google Scholar]
- Bronaugh, R.L.; Stewart, R.F. Methods for In Vitro Percutaneous Absorption Studies IV: The Flow-through Diffusion Cell. J. Pharm. Sci. 1985, 74, 64–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanojo, H.; Roemelé, P.E.H.; van Veen, G.H.; Stieltjes, H.; Junginger, H.E.; Boddé, H.E. New Design of a Flow-through Permeation Cell for Studying in Vitro Permeation Studies across Biological Membranes. J. Control. Release 1997, 45, 41–47. [Google Scholar] [CrossRef]
- Arora, S.; Clarke, J.; Tsakalozou, E.; Ghosh, P.; Alam, K.; Grice, J.E.; Roberts, M.S.; Jamei, M.; Polak, S. Mechanistic Modeling of In Vitro Skin Permeation and Extrapolation to In Vivo for Topically Applied Metronidazole Drug Products Using a Physiologically Based Pharmacokinetic Model. Mol. Pharm. 2022, 19, 3139–3152. [Google Scholar] [CrossRef]
- Chen, M.; Liu, X.; Fahr, A. Skin Penetration and Deposition of Carboxyfluorescein and Temoporfin from Different Lipid Vesicular Systems: In Vitro Study with Finite and Infinite Dosage Application. Int. J. Pharm. 2011, 408, 223–234. [Google Scholar] [CrossRef] [PubMed]
- Cristofoli, M.; Kung, C.-P.; Hadgraft, J.; Lane, M.E.; Sil, B.C. Ion Pairs for Transdermal and Dermal Drug Delivery: A Review. Pharmaceutics 2021, 13, 909. [Google Scholar] [CrossRef] [PubMed]
- Valenta, C.; Siman, U.; Kratzel, M.; Hadgraft, J. The Dermal Delivery of Lignocaine: Influence of Ion Pairing. Int. J. Pharm. 2000, 197, 77–85. [Google Scholar] [CrossRef]
- Sarveiya, V.; Templeton, J.F.; Benson, H.A.E. Ion-Pairs of Ibuprofen: Increased Membrane Diffusion. J. Pharm. Pharmacol. 2004, 56, 717–724. [Google Scholar] [CrossRef] [PubMed]


| APIs | Dosage Form | Qualitative and Quantitative Assessment (Q1 & Q2) | Comparative Physicochemical Characterization (Q3) | In Vitro Release Testing (IVRT) | In Vitro Permeation Testing (IVPT) | Additional In Vivo Pharmacokinetic Study | Year |
|---|---|---|---|---|---|---|---|
| Acyclovir | Ointment | + | + | + | 2019 | ||
| Acyclovir | Cream | + | + | + | + | 2016 | |
| Bexarotene | Gel | + | + | + | 2019 | ||
| Clindamycin phosphate | Gel | + | + | + | 2020 | ||
| Crisaborole | Ointment | + | + | + | + | + | 2019 |
| Dapsone | Gel | + | + | + | + | + | 2019 |
| Docosanol | Cream | + | + | + | 2017 | ||
| Doxepin hydrochloride | Cream | + | + | + | + | + | 2019 |
| Erythromycin | Gel | + | 2019 | ||||
| Fluocinolone acetonide | Cream | + | 2018 | ||||
| Gentamicin sulfate | Ointment | + | 2017 | ||||
| Gentamicin sulfate | Cream | + | 2017 | ||||
| Hydrocortisone | Cream | + | 2017 | ||||
| Ivermectin | Cream | + | + | + | + | + | 2019 |
| Lidocaine | Ointment | + | + | 2016 | |||
| Luliconazole | Cream | + | + | + | + | 2018 | |
| Metronidazole | Gel | + | + | + | 2019 | ||
| Metronidazole | Cream | + | + | + | + | 2019 | |
| Oxymetazoline hydrochloride | Cream | + | + | + | + | 2019 | |
| Penciclovir | Cream | + | + | + | + | 2018 | |
| Pimecrolimus | Cream | + | + | + | + | 2019 | |
| Silver sulfadiazine | Cream | + | + | + | 2017 | ||
| Tacrolimus | Ointment | + | + | + | + | 2018 | |
| Tretinoin | Gel | + | + | + | 2020 | ||
| Triamcinolone acetonide | Cream | + | 2017 |
| NO. | Available Techniques | Working Range | Advantages | Disadvantage |
|---|---|---|---|---|
| 1 | Dynamic Light Scattering | 10 nm–10 µm | Smaller sample volume | Need of sample dilution, unsuitable for viscous samples. |
| 2 | Laser Diffraction | 10 nm–1 mm | Reproducible, smaller sample volume, suitable for spherical particles. | Inaccurate results for irregularly shaped particles need sample dilution. |
| 3 | Morphologically Directed Raman Spectroscopy (MDRS) | 1 μm–1 mm | Spectroscopic interrogation of particles. | Shorter working range. |
| 4 | Optical microscopy (bright-field microscopy and polarized light microscopy) | 1 μm–1 mm | Rapid identification of drug crystals. | Shorter working range, sensitive to sample preparation. |
| NO. | Skin Models | Examples | Advantages | Disadvantages |
| 1 | Human skin models | Full-thickness human skin, dermatomed human skin, the epidermis. | Anatomically identical to human in vivo skin | Ethical considerations, Inconsistency between skin donors |
| 2 | Animal skin models | Rats skin, snakeskin, porcine skin, macaque skin. | Histologically similar to human skin | More permeable than ex vivo skin |
| 3 | Reconstructed human skin equivalents | Reconstructed full-thickness skin, the reconstructed epidermis (EpiSkin®, SkinEthic®, EpiDerm®). | Structurally close to human skin | More permeable than ex vivo skin |
| 4 | Synthetic membranes | Strat-M™, parallel artificial membrane permeability assay [54], phospholipid vesicle-based permeation assay [55]. | Reproducible and consistent results | Differences in lipid compositions to the human skin |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, X.; Imran, M.; Mohammed, Y. Topical Semisolid Products—Understanding the Impact of Metamorphosis on Skin Penetration and Physicochemical Properties. Pharmaceutics 2022, 14, 2487. https://doi.org/10.3390/pharmaceutics14112487
Jin X, Imran M, Mohammed Y. Topical Semisolid Products—Understanding the Impact of Metamorphosis on Skin Penetration and Physicochemical Properties. Pharmaceutics. 2022; 14(11):2487. https://doi.org/10.3390/pharmaceutics14112487
Chicago/Turabian StyleJin, Xuping, Mohammad Imran, and Yousuf Mohammed. 2022. "Topical Semisolid Products—Understanding the Impact of Metamorphosis on Skin Penetration and Physicochemical Properties" Pharmaceutics 14, no. 11: 2487. https://doi.org/10.3390/pharmaceutics14112487





