Rotary Jet Spinning (RJS): A Key Process to Produce Biopolymeric Wound Dressings
Abstract
1. Introduction
2. Spinning Techniques
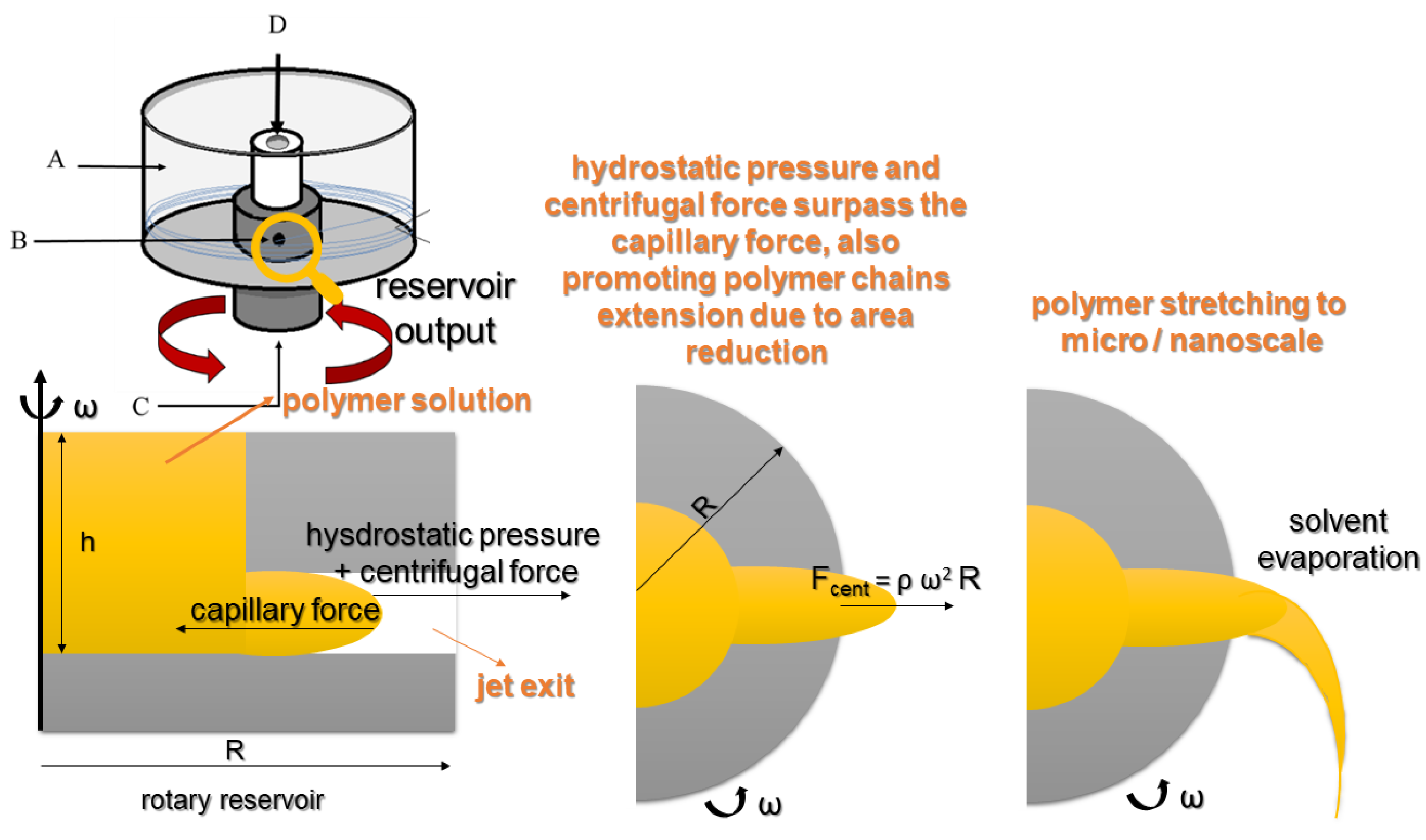
3. Fundamentals of the Rotary Jet Spinning (RJS)
3.1. Melt RJS
3.2. Immersion RJS
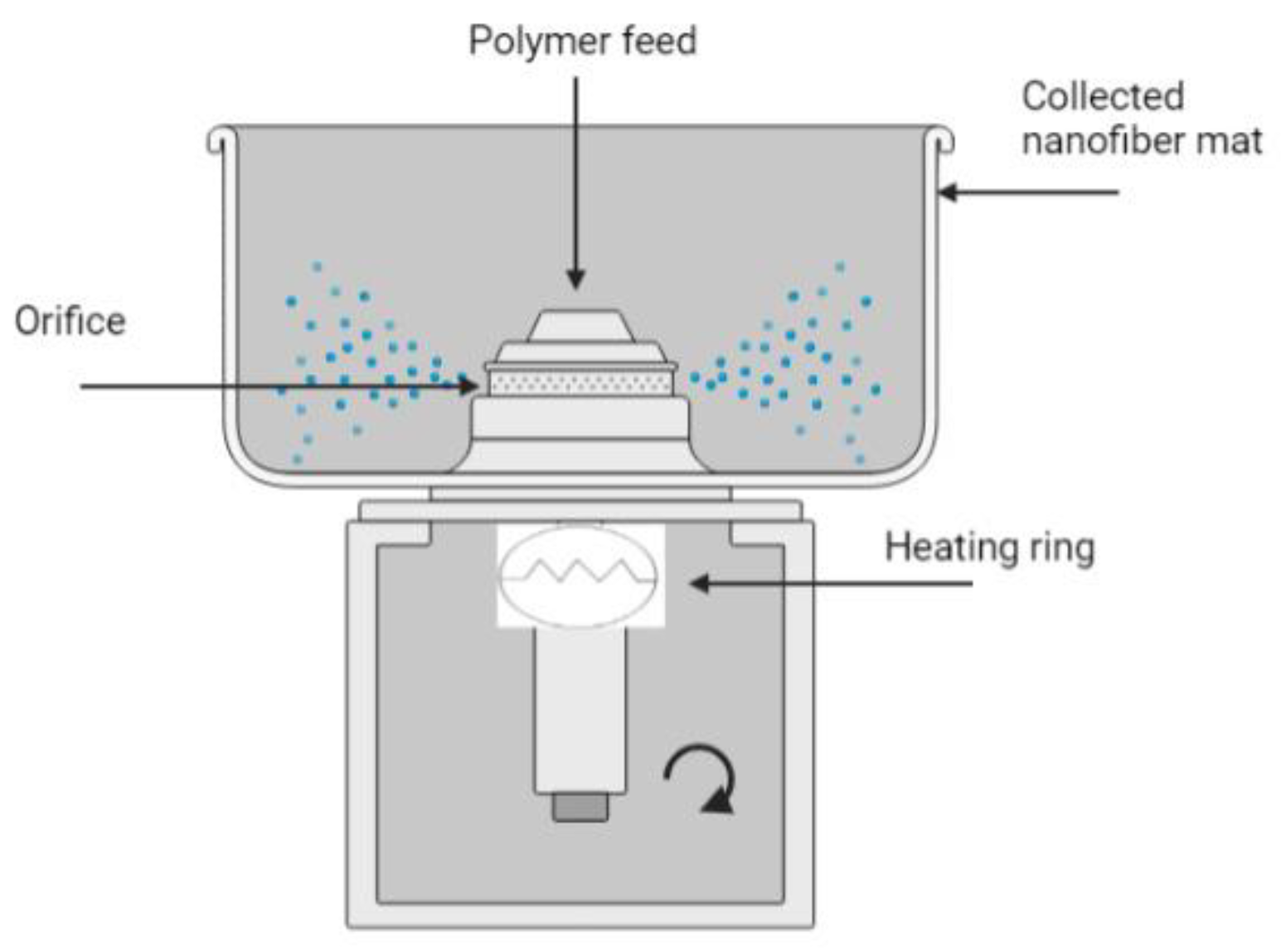
3.3. Nozzle-Less RJS
4. Parameters and Factors Influencing Rotary Jet Spinning
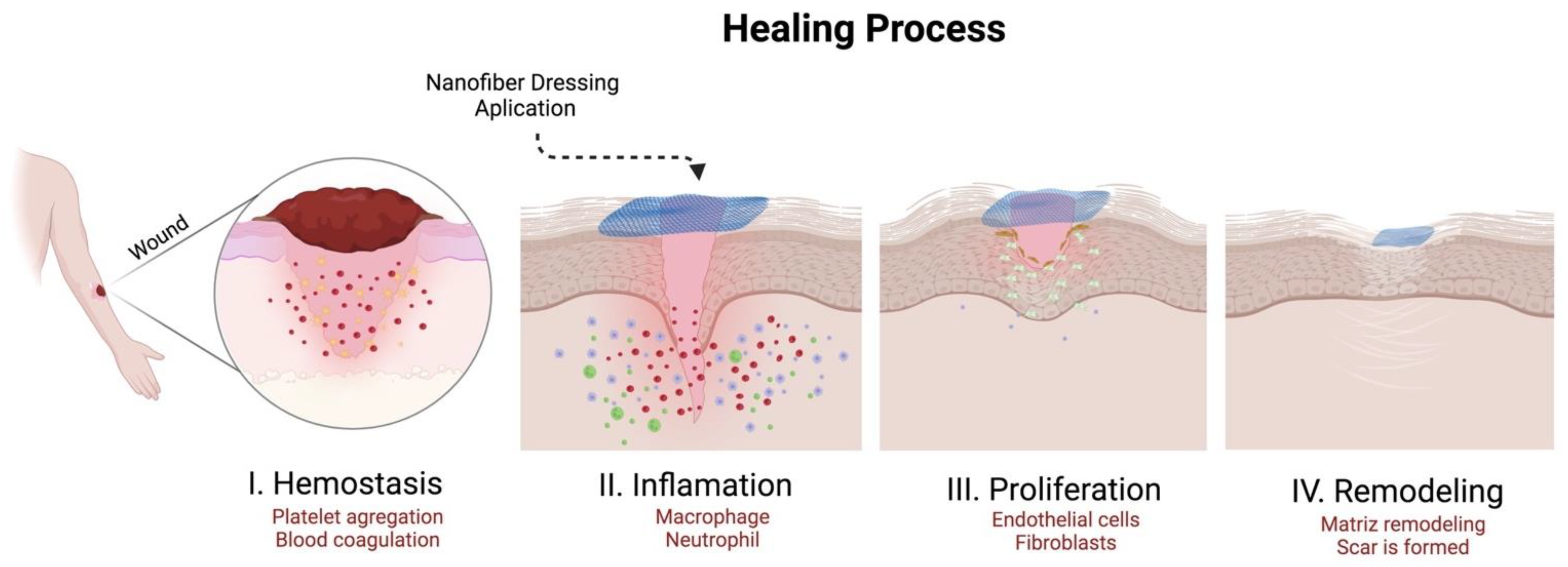
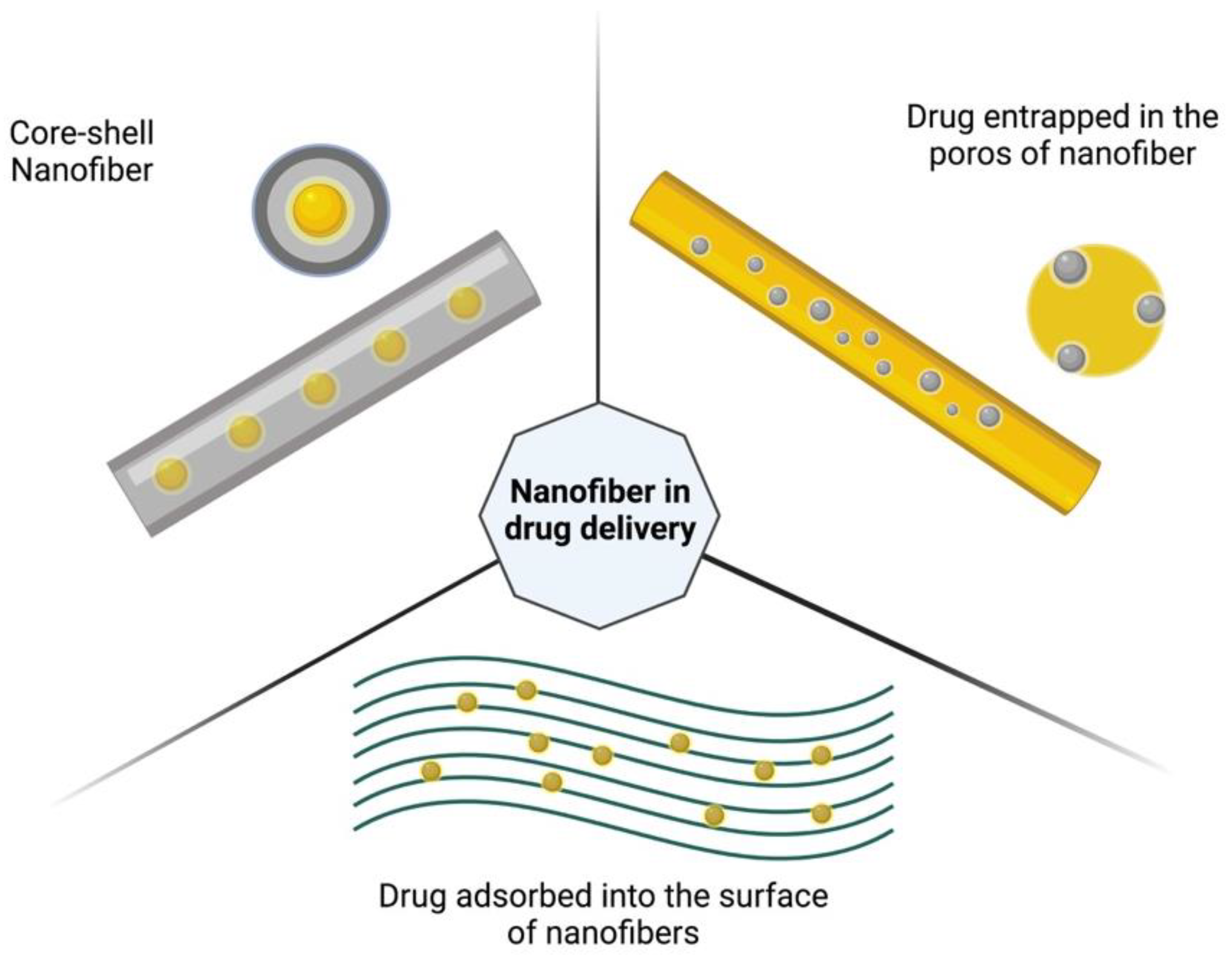
5. Biomedical Applications of RJS-Nanofibers
6. Other Applications: Filters and Batteries
7. Conclusions and Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Rahimnejad, M.; Derakhshanfar, S.; Zhong, W. Biomaterials and Tissue Engineering for Scar Management in Wound Care. Burn. Trauma 2017, 5, 4. [Google Scholar] [CrossRef] [PubMed]
- Metcalfe, A.D.; Ferguson, M.W.J. Tissue Engineering of Replacement Skin: The Crossroads of Biomaterials, Wound Healing, Embryonic Development, Stem Cells and Regeneration. J. R. Soc. Interface 2007, 4, 413–437. [Google Scholar] [CrossRef] [PubMed]
- Khan, R.; Morsi, Y.; Zhu, T.; Ahmad, A.; Xie, X.; Yu, F. Electrospinning: An Emerging Technology to Construct Polymer-Based Nano Fibrous Scaffolds for Diabetic Wound Healing. Front. Mater. Sci. 2021, 15, 10–35. [Google Scholar] [CrossRef]
- Packer, C.; Ali, S.; Manna, B. Diabetic Ulcer. Available online: https://www.ncbi.nlm.nih.gov/books/NBK499887/ (accessed on 9 May 2022).
- Chen, L.; Cheng, L.; Gao, W.; Chen, D.; Wang, C.; Ran, X. Telemedicine in Chronic Wound Management: Systematic Review And Meta-Analysis. JMIR Mhealth Uhealth 2020, 8, e15574. [Google Scholar] [CrossRef] [PubMed]
- Nicholas, M.N.; Jeschke, M.G.; Amini-Nik, S. Methodologies in Creating Skin Substitutes. Cell. Mol. Life Sci. C 2016, 73, 3453–3472. [Google Scholar] [CrossRef]
- Kalva, S.N.; Augustine, R.; Al Mamun, A.; Dalvi, Y.B.; Vijay, N.; Hasan, A. Active Agents Loaded Extracellular Matrix Mimetic Electrospun Membranes for Wound Healing Applications. J. Drug Deliv. Sci. Technol. 2021, 63, 102500. [Google Scholar] [CrossRef]
- Brown, M.S.; Ashley, B.; Koh, A. Wearable Technology for Chronic Wound Monitoring: Current Dressings, Advancements, and Future Prospects. Front. Bioeng. Biotechnol. 2018, 6, 47. [Google Scholar] [CrossRef]
- Gonçalves, M.M.; Carneiro, J.; Justus, B.; Espinoza, J.T.; Budel, J.M.; Farago, P.V.; Paula, J.P. de Preparation and Characterization of a Novel Antimicrobial Film Dressing for Wound Healing Application. Braz. J. Pharm. Sci. 2020, 56. [Google Scholar] [CrossRef]
- Markets and Markets Wound Dressings Market by Type (Traditional, Advanced (Alginate, Collagen, Hydrogel, Foam, Hydrocolloid, Film)), Wound Type (Traumatic, Surgical, Diabetic Foot, Venous Leg Ulcer & Burns), End User (Hospital, ASCs, Homecare)—Global Forecast to 2025. Available online: https://www.marketsandmarkets.com/Market-Reports/wound-dressings-market-123903496.html (accessed on 7 February 2022).
- Okur, M.E.; Karantas, I.D.; Şenyiğit, Z.; Üstündağ Okur, N.; Siafaka, P.I. Recent Trends on Wound Management: New Therapeutic Choices Based on Polymeric Carriers. Asian J. Pharm. Sci. 2020, 15, 661–684. [Google Scholar] [CrossRef]
- Afsharian, Y.P.; Rahimnejad, M. Bioactive Electrospun Scaffolds for Wound Healing Applications: A Comprehensive Review. Polym. Test. 2021, 93, 106952. [Google Scholar] [CrossRef]
- Vida, T.A.; Motta, A.C.; Santos, A.R.; Cardoso, G.B.C.; De Brito, C.C.; De Carvalho Zavaglia, C.A. Fibrous PCL/PLLA Scaffolds Obtained by Rotary Jet Spinning and Electrospinning. Mater. Res. 2017, 20, 910–916. [Google Scholar] [CrossRef]
- Rogalski, J.J. Rotary Jet Spinning of Polymer Fibres; Queen Mary Univesity of London: London, UK, 2018. [Google Scholar]
- Ciocca, B.E.; Munhoz, A.L.J.; Cardoso, G.B.C.; Rodrigues, A.A.; Pattaro, A.F.; Kaasi, A.; Filho, R.M.; Rodigues, A.A.; Pattaro, A.F.; Kaasi, A.; et al. Viability Assays of PLLA Fibrous Membranes Produced by Rotary Jet Spinning for Application in Tissue Engineering. Braz. Arch. Biol. Technol. 2019, 62, 1–10. [Google Scholar] [CrossRef]
- Mîndru, T.B.B.; Ignat, L.; Mîndru, I.B.B.; Pinteala, M. Morphological Aspects of Polymer Fiber Mats Obtained by Air Flow Rotary-Jet Spinning. Fibers Polym. 2013, 14, 1526–1534. [Google Scholar] [CrossRef]
- Badrossamay, M.R.; McIlwee, H.A.; Goss, J.A.; Parker, K.K. Nanofiber Assembly by Rotary Jet-Spinning. Nano Lett. 2010, 10, 2257–2261. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, M.; Vaught, L.; Law, C.; Meyer, J.L.; Elhajjar, R. Electrospinning Processing Techniques for the Manufacturing of Composite Dielectric Elastomer Fibers. Materials 2021, 14, 6288. [Google Scholar] [CrossRef]
- Rogalski, J.J.; Bastiaansen, C.W.M.; Peijs, T. PA6 Nanofibre Production: A Comparison between Rotary Jet Spinning and Electrospinning. Fibers 2018, 6, 37. [Google Scholar] [CrossRef]
- Chen, B.; Wang, J.; Lai, Z.; Zhang, Z.; Wu, Z. Modeling of Spinning Jet Behavior and Evaluation on Fiber Morphology for Centrifugal Spinning. J. Text. Inst. 2021, 113, 1–12. [Google Scholar] [CrossRef]
- Dos Santos, D.M.; Correa, D.S.; Medeiros, E.S.; Oliveira, J.E.; Mattoso, L.H.C. Advances in Functional Polymer Nanofibers: From Spinning Fabrication Techniques to Recent Biomedical Applications. ACS Appl. Mater. Interfaces 2020, 12, 45673–45701. [Google Scholar] [CrossRef]
- Sebe, I.; Kállai-Szabó, B.; Oldal, I.; Zsidai, L.; Zelkó, R. Development of Laboratory-Scale High-Speed Rotary Devices for a Potential Pharmaceutical Microfibre Drug Delivery Platform. Int. J. Pharm. 2020, 588, 119740. [Google Scholar] [CrossRef]
- Chen, C.; Dirican, M.; Zhang, X. Centrifugal Spinning-High Rate Production of Nanofibers. In Electrospinning: Nanofabrication and Applications; Elsevier Inc.: Amsterdam, The Netherlands, 2019; pp. 321–338. ISBN 9780323512701. [Google Scholar]
- Kwak, B.E.; Yoo, H.J.; Lee, E.; Kim, D.H. Large-Scale Centrifugal Multispinning Production of Polymer Micro- and Nano Fi Bers for Mask Filter Application with a Potential of Cospinning Mixed Multicomponent Fibers. ACS Macro Lett. 2021, 10, 382–388. [Google Scholar] [CrossRef]
- Zhao, Z.; Ma, C.; Chen, F.; Xu, G.; Pang, R.; Qian, X.; Shao, J.; Hu, X. Water Caltrop Shell-Derived Nitrogen-Doped Porous Carbons with High CO2 Adsorption Capacity. Biomass Bioenergy 2021, 145, 105969. [Google Scholar] [CrossRef]
- Li, W.-J.; Laurencin, C.T.; Caterson, E.J.; Tuan, R.S.; Ko, F.K. Electrospun Nanofibrous Structure: A Novel Scaffold for Tissue Engineering. J. Biomed. Mater. Res. 2002, 60, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Rogalski, J.J.; Botto, L.; Bastiaansen, C.W.M.; Peijs, T. A Study of Rheological Limitations in Rotary Jet Spinning of Polymer Nanofibers through Modeling and Experimentation. J. Appl. Polym. Sci. 2020, 137, 48963. [Google Scholar] [CrossRef]
- Sinatra, N.R.; Lind, J.U.; Parker, K.K. Fabricating Multi-Material Nanofabrics Using Rotary Jet Spinning. In Proceedings of the 2017 IEEE 17th International Conference on Nanotechnology, NANO 2017, Pittsburg, PA, USA, 25–28 July 2017; pp. 715–719. [Google Scholar] [CrossRef]
- Wu, Y.; Li, C.; Fan, F.; Liang, J.; Yang, Z.; Wei, X.; Chen, S. PVAm Nanofibers Fabricated by Rotary Jet Wet Spinning and Applied to Bisphenol A Recognition. ACS Omega 2019, 4, 21361–21369. [Google Scholar] [CrossRef] [PubMed]
- Holloway, S.; Harding, K.G. Wound Dressings. Surg. Oxf. Int. Ed. 2022, 40, 25–32. [Google Scholar] [CrossRef]
- Barbosa, K.A.; Rodrigues, I.C.P.; Tamborlin, L.; Luchessi, A.D.; Lopes, É.S.N.; Gabriel, L.P. Rotary Jet-Spun Curcumin-Loaded Poly L-Lactic Acid Membranes for Wound-Healing Applications. J. Mater. Res. Technol. 2022, 18, 3273–3282. [Google Scholar] [CrossRef]
- Xia, L.; Lu, L.; Liang, Y.; Cheng, B. Fabrication of Centrifugally Spun Prepared Poly(Lactic Acid)/Gelatin/Ciprofloxacin Nanofibers for Antimicrobial Wound Dressing. RSC Adv. 2019, 9, 35328–35335. [Google Scholar] [CrossRef]
- Li, Z.; Mei, S.; Dong, Y.; She, F.; Li, P.; Li, Y.; Kong, L. Multi-Functional Core-Shell Nanofibers for Wound Healing. Nanomaterials 2021, 11, 1546. [Google Scholar] [CrossRef]
- Ahn, S.; Chantre, C.O.; Gannon, A.R.; Lind, J.U.; Campbell, P.H.; Grevesse, T.; O’Connor, B.B.; Parker, K.K. Soy Protein/Cellulose Nanofiber Scaffolds Mimicking Skin Extracellular Matrix for Enhanced Wound Healing. Adv. Healthc. Mater. 2018, 7, 1701175. [Google Scholar] [CrossRef]
- Merchiers, J.; Martínez Narváez, C.D.V.; Slykas, C.; Buntinx, M.; Deferme, W.; D’Haen, J.; Peeters, R.; Sharma, V.; Reddy, N.K. Centrifugally Spun Poly(Ethylene Oxide) Fibers Rival the Properties of Electrospun Fibers. J. Polym. Sci. 2021, 59, 2754–2762. [Google Scholar] [CrossRef]
- Rogalski, J.J.; Bastiaansen, C.W.M.; Peijs, T. Rotary Jet Spinning Review–a Potential High Yield Future for Polymer Nanofibers. Nanocomposites 2017, 3, 97–121. [Google Scholar] [CrossRef]
- Xu, H.; Chen, H.; Li, X.; Liu, C.; Yang, B. A Comparative Study of Jet Formation in Nozzle- and Nozzle-Less Centrifugal Spinning Systems. J. Polym. Sci. Part B Polym. Phys. 2014, 52, 1547–1559. [Google Scholar] [CrossRef]
- Haider, A.; Haider, S.; Kummara, M.R.; Kamal, T.; Alghyamah, A.-A.A.; Iftikhar, F.J.; Bano, B.; Khan, N.; Afridi, M.A.; Han, S.S.; et al. Advances in the Scaffolds Fabrication Techniques Using Biocompatible Polymers and Their Biomedical Application: A Techinical and Statistical Review. J. Saudi Chem. Soc. 2020, 24, 186–215. [Google Scholar] [CrossRef]
- Machado-Paula, M.M.; Corat, M.A.F.; Lancellotti, M.; Mi, G.; Marciano, F.R.; Vega, M.L.; Hidalgo, A.A.; Webster, T.J.; Lobo, A.O. A Comparison between Electrospinning and Rotary-Jet Spinning to Produce PCL Fibers with Low Bacteria Colonization. Mater. Sci. Eng. C 2020, 111, 110706. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.M.; Duan, Y.S.; Xu, Q.; Zhang, B. A Review on Nanofiber Fabrication with the Effect of High-Speed Centrifugal Force Field. J. Eng. Fiber. Fabr. 2019, 14, 1–11. [Google Scholar] [CrossRef]
- Balla, E.; Daniilidis, V.; Karlioti, G.; Kalamas, T.; Stefanidou, M.; Bikiaris, N.D.; Vlachopoulos, A.; Koumentakou, I.; Bikiaris, D.N. Poly(Lactic Acid): A Versatile Biobased Polymer for the Future with Multifunctional Properties—From Monomer Synthesis, Polymerization Techniques and Molecular Weight Increase to PLA Applications. Polymers 2021, 13, 1822. [Google Scholar] [CrossRef]
- Partheniadis, I.; Nikolakakis, I.; Laidmäe, I.; Heinämäki, J. A Mini-Review: Needleless Electrospinning of Nanofibers for Pharmaceutical and Biomedical Applications. Processes 2020, 8, 673. [Google Scholar] [CrossRef]
- Osorio-Arciniega, R.; García-Hipólito, M.; Alvarez-Fregoso, O.; Alvarez-Perez, M.A. Composite Fiber Spun Mat Synthesis and In Vitro Biocompatibility for Guide Tissue Engineering. Molecules 2021, 26, 7597. [Google Scholar] [CrossRef]
- Zhang, X.; Lu, Y. Centrifugal Spinning: An Alternative Approach to Fabricate Nanofibers at High Speed and Low Cost. Polym. Rev. 2014, 54, 677–701. [Google Scholar] [CrossRef]
- Yoon, J.; Yang, H.-S.; Lee, B.-S.; Yu, W.-R. Recent Progress in Coaxial Electrospinning: New Parameters, Various Structures, and Wide Applications. Adv. Mater. 2018, 30, 1704765. [Google Scholar] [CrossRef]
- Vass, P.; Szabó, E.; Domokos, A.; Hirsch, E.; Galata, D.; Farkas, B.; Démuth, B.; Andersen, S.K.; Vigh, T.; Verreck, G.; et al. Scale-Up of Electrospinning Technology: Applications in the Pharmaceutical Industry. WIREs Nanomed. Nanobiotechnol. 2020, 12, e1611. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.S.; Ang, B.C.; Andriyana, A.; Afifi, A.M. A Review on Fabrication of Nanofibers via Electrospinning and Their Applications. SN Appl. Sci. 2019, 1, 1248. [Google Scholar] [CrossRef]
- Juncos Bombin, A.D.; Dunne, N.J.; McCarthy, H.O. Electrospinning of Natural Polymers for the Production of Nanofibres for Wound Healing Applications. Mater. Sci. Eng. C 2020, 114, 110994. [Google Scholar] [CrossRef] [PubMed]
- Luraghi, A.; Peri, F.; Moroni, L. Electrospinning for Drug Delivery Applications: A Review. J. Control. Release 2021, 334, 463–484. [Google Scholar] [CrossRef] [PubMed]
- Rahmati, M.; Mills, D.K.; Urbanska, A.M.; Saeb, M.R.; Venugopal, J.R.; Ramakrishna, S.; Mozafari, M. Electrospinning for Tissue Engineering Applications. Prog. Mater. Sci. 2021, 117, 100721. [Google Scholar] [CrossRef]
- Alghoraibi, I.; Alomari, S. Different Methods for Nanofiber Design and Fabrication. In Handbook of Nanofibers; Barhoum, A., Bechelany, M., Makhlouf, A., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 1–46. ISBN 978-3-319-42789-8. [Google Scholar]
- Ambekar, R.S.; Kandasubramanian, B. Advancements in Nanofibers for Wound Dressing: A Review. Eur. Polym. J. 2019, 117, 304–336. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, J.; Su, Y.; Wang, H.; Wang, X.-X.; Huang, L.-P.; Yu, M.; Ramakrishna, S.; Long, Y.-Z. Recent Progress and Challenges in Solution Blow Spinning. Mater. Horiz. 2021, 8, 426–446. [Google Scholar] [CrossRef]
- Wei, Z. Research Process of Polymer Nanofibers Prepared by Melt Spinning. IOP Conf. Ser. Mater. Sci. Eng. 2018, 452, 22002. [Google Scholar] [CrossRef]
- Zamwar, K.P.; Tonge, S.; Raut, J.S.; Parshive, P.S.; Bharsakale, R.R.; Jain, N. A Review on Synthesis, Advantages and Disadvantages of Nanofibers. Mukt Shabd J. 2020, 9, 928–933. [Google Scholar]
- Anstey, A.; Chang, E.; Kim, E.S.; Rizvi, A.; Kakroodi, A.R.; Park, C.B.; Lee, P.C. Nanofibrillated Polymer Systems: Design, Application, and Current State of the Art. Prog. Polym. Sci. 2021, 113, 101346. [Google Scholar] [CrossRef]
- La Mantia, F.P.; Ceraulo, M.; Testa, P.; Morreale, M. Biodegradable Polymers for the Production of Nets for Agricultural Product Packaging. Materials 2021, 14, 323. [Google Scholar] [CrossRef] [PubMed]
- Atıcı, B.; Ünlü, C.H.; Yanilmaz, M. A Review on Centrifugally Spun Fibers and Their Applications. Polym. Rev. 2022, 62, 1–64. [Google Scholar] [CrossRef]
- de Andrade Pinto, S.A.; de Nadai Dias, F.J.; Cardoso, G.B.C.; dos Santos Junior, A.R.; de Aro, A.A.; Pino, D.S.; Meneghetti, D.H.; Vitti, R.P.; dos Santos, G.M.T.; de Carvalho Zavaglia, C.A. Polycaprolactone/Beta-Tricalcium Phosphate Scaffolds Obtained via Rotary Jet-Spinning: In Vitro and In Vivo Evaluation. Cells Tissues Organs 2021, 4, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Priyanto, A.; Hapidin, D.A.; Suciati, T.; Khairurrijal, K. Current Developments on Rotary Forcespun Nanofibers and Prospects for Edible Applications. Food Eng. Rev. 2022, 14, 435–461. [Google Scholar] [CrossRef]
- Liaw, C.-Y.; Huynh, S.; Gedeon, C.; Ji, S.; D’souza, C.; Abaci, A.; Guvendiren, M. Airbrushed Nanofibrous Membranes to Control Stem Cell Infiltration in 3D-Printed Scaffolds. AIChE J. 2021, 67, e17475. [Google Scholar] [CrossRef]
- Pimenta, F.A.; Carbonari, R.C.; Malmonge, S.M. Nanofibrous Tubular Scaffolds for Tissue Engineering of Small-Diameter Vascular Grafts—Development Using SBS Fabrication Technique and Mechanical Performance. Res. Biomed. Eng. 2022, 38, 797–811. [Google Scholar] [CrossRef]
- Ayati, S.S.; Karevan, M.; Stefanek, E.; Bhia, M.; Akbari, M. Nanofibers Fabrication by Blown-Centrifugal Spinning. Macromol. Mater. Eng. 2022, 307, 2100368. [Google Scholar] [CrossRef]
- van Eck, N.J.; Waltman, L. Software Survey: VOSviewer, a Computer Program for Bibliometric Mapping. Scientometrics 2010, 84, 523–538. [Google Scholar] [CrossRef]
- Centre for Science and Technology Studies, Leiden University, The Netherlands. VosViewer (Visualizing Landscapes). Available online: https://www.vosviewer.com/ (accessed on 1 September 2022).
- Ragab, D.M.; Elgindy, N.A. Recent Advances in Nanofibers Fabrication and Their Potential Applications in Wound Healing and Regenerative Medicine. Adv. Mater. Lett. 2018, 9, 665–676. [Google Scholar] [CrossRef]
- Rodchanasuripron, W.; Seadan, M.; Suttiruengwong, S. Properties of Non-Woven Polylactic Acid Fibers Prepared by the Rotational Jet Spinning Method. Mater. Today Sustain. 2020, 10, 100046. [Google Scholar] [CrossRef]
- Stojanovska, E.; Kurtulus, M.; Abdelgawad, A.; Candan, Z.; Kilic, A. Developing Lignin-Based Bio-Nanofibers by Centrifugal Spinning Technique. Int. J. Biol. Macromol. 2018, 113, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Ozisik, R.; Kotha, S.P.; Underhill, P.T. Highly Efficient Fabrication of Polymer Nanofiber Assembly by Centrifugal Jet Spinning: Process and Characterization. Macromolecules 2015, 48, 2593–2602. [Google Scholar] [CrossRef]
- Banerjee, D.; Sahani, V.R.; Singh, P.; Londhe, P. V Review—Approaches of Nanofibers Manufacturing with Bio-Medical Applications. Int. J. Eng. Technol. 2021, 8, 1363–1368. [Google Scholar]
- Barhoum, A.; Pal, K.; Rahier, H.; Uludag, H.; Soo, I.; Bechelany, M.; Kim, I.S.; Bechelany, M. Nanofibers as New-Generation Materials: From Spinning and Nano-Spinning Fabrication Techniques to Emerging Applications. Appl. Mater. Today 2019, 17, 1–35. [Google Scholar] [CrossRef]
- Shanmuganathan, K.; Fang, Y.; Chou, D.Y.; Sparks, S.; Hibbert, J.; Ellison, C.J. Solventless High Throughput Manufacturing of Poly(Butylene Terephthalate) Nanofibers. ACS Macro Lett. 2012, 1, 960–964. [Google Scholar] [CrossRef] [PubMed]
- O’Haire, T.; Rigout, M.; Russell, S.J.; Carr, C.M. Influence of Nanotube Dispersion and Spinning Conditions on Nanofibre Nanocomposites of Polypropylene and Multi-Walled Carbon Nanotubes Produced through ForcespinningTM. J. Thermoplast. Compos. Mater. 2014, 27, 205–214. [Google Scholar] [CrossRef]
- Andjani, D.; Sriyanti, I.; Fauzi, A.; Edikresnha, D.; Munir, M.M. Khairurrijal Fabrication of Polyvinylpyrrolidone Fibers by Means of Rotary Forcespinning Method. IOP Conf. Ser. Mater. Sci. Eng. 2018, 367, 012044. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, Z.; Lu, B.; Mei, S.; Xu, Q.; Liu, F. Research on Parametric Model for Polycaprolactone Nanofiber Produced by Centrifugal Spinning. J. Braz. Soc. Mech. Sci. Eng. 2018, 40, 186. [Google Scholar] [CrossRef]
- Hamanishi, N.; Kono, M.; Miyaki, T.; Suwa, S.; Rekimoto, J. Fibritary: Rotary Jet-Spinning for Personal Fiber Fabrication. Conf. Hum. Factors Comput. Syst. Proc. 2019, LBW1511, 1–6. [Google Scholar] [CrossRef]
- Zander, N.E.; Gillan, M.; Sweetser, D. Composite Fibers from Recycled Plastics Using Melt Centrifugal Spinning. Materials 2017, 10, 1044. [Google Scholar] [CrossRef]
- Gonzalez, G.M.; MacQueen, L.A.; Lind, J.U.; Fitzgibbons, S.A.; Chantre, C.O.; Huggler, I.; Golecki, H.M.; Goss, J.A.; Parker, K.K. Production of Synthetic, Para-Aramid and Biopolymer Nanofibers by Immersion Rotary Jet-Spinning. Macromol. Mater. Eng. 2017, 302, 1600365. [Google Scholar] [CrossRef]
- MacQueen, L.A.; Alver, C.G.; Chantre, C.O.; Ahn, S.; Cera, L.; Gonzalez, G.M.; O’Connor, B.B.; Drennan, D.J.; Peters, M.M.; Motta, S.E.; et al. Muscle Tissue Engineering in Fibrous Gelatin: Implications for Meat Analogs. npj Sci. Food 2019, 3, 20. [Google Scholar] [CrossRef] [PubMed]
- Muniz, N.O.; Vechietti, F.A.; Anesi, G.R.; Alberto, L.; Santos, L.; Guinea, G.V.; Dos Santos, L.A.L. Blend-Based Fibers Produced via Centrifugal Spinning and Electrospinning Processes: Physical and Rheological Properties. J. Mater. Res. 2020, 35, 2905–2916. [Google Scholar] [CrossRef]
- Ravishankar, P.; Khang, A.; Laredo, M.; Balachandran, K. Using Dimensionless Numbers to Predict Centrifugal Jet-Spun Nanofiber Morphology. Hindawi J. Mater. 2019, 2019, 4639658. [Google Scholar] [CrossRef]
- Wojasinski, M.; Ciach, T. Production of Nano- and Microfibers from Synthetic and Natural Polymers—Nanofibers Technology. In Synthetic Nano- and Microfibers; Wagterveld, R.M., Marijnissen, J.C.M., Gradon, L., Pobrano, A.M., Eds.; Wetsus: Leeuwarden, The Netherlands, 2020; pp. 19–33. ISBN 9781716632426. [Google Scholar]
- Golecki, H.M.I.; Yuan, H.; Glavin, C.; Potter, B.; Badrossamay, M.R.; Goss, J.A.; Phillips, M.D.; Parker, K.K. Effect of Solvent Evaporation on Fiber Morphology in Rotary Jet Spinning. Langmuir 2014, 30, 13369–13374. [Google Scholar] [CrossRef]
- Huttunen, M.; Kellomäki, M. A Simple and High Production Rate Manufactoring Method for Submicron Polymer Fibres. J. Tissue Eng. Renerative Med. 2011, 5, 239–243. [Google Scholar] [CrossRef]
- Krifa, M.; Yuan, W. Morphology and Pore Size Distribution of Electrospun and Centrifugal Forcespun Nylon 6 Nanofiber Membranes. Text. Res. J. 2016, 86, 1294–1306. [Google Scholar] [CrossRef]
- Liang, C.; Hu, C.; Yan, K.; Thomas, H.; Zhu, X. Hydrophilic Nonwovens by ForcespinningTM of Isotactic Polypropylene Blended with Amphiphilic Surfactants. Fibers Polym. 2016, 17, 1646–1656. [Google Scholar] [CrossRef]
- Abir, S.S.H.; Hasan, M.T.; Alcoutlabi, M.; Lozano, K. The Effect of Solvent and Molecular Weight on the Morphology of Centrifugally Spun Poly(Vinylpyrrolidone) Nanofibers. Fibers Polym. 2021, 22, 2394–2403. [Google Scholar] [CrossRef]
- Dos Reis Paganotto, G.F.; De Barros, G.D.; Marques, V.G.; Takimi, A.S. Production of Recycled EPS Fibers by Centrifugal Spinning. Rev. Mater. 2021, 26. [Google Scholar] [CrossRef]
- Hou, T.; Li, X.; Lu, Y.; Yang, B. Highly Porous Fibers Prepared by Centrifugal Spinning. Mater. Des. 2017, 114, 303–311. [Google Scholar] [CrossRef]
- Obregon, N.; Agubra, V.; Pokhrel, M.; Campos, H.; Flores, D.; De la Garza, D.; Mao, Y.; Macossay, J.; Alcoutlabi, M. Effect of Polymer Concentration, Rotational Speed, and Solvent Mixture on Fiber Formation Using Forcespinning®. Fibers 2016, 4, 20. [Google Scholar] [CrossRef]
- de Souza, L.; Alavarse, A.C.; da Vinci, M.A.; Bonvent, J.J. The Synergistic Effect of Polymer Composition, Solvent Volatility, and Collector Distance on Pullulan and PVA Fiber Production by Rotary Jet Spinning. Fibers Polym. 2021, 22, 942–956. [Google Scholar] [CrossRef]
- Li, X.; Lu, Y.; Hou, T.; Zhou, J.; Wang, A.; Zhang, X.; Yang, B. Jet Evolution and Fiber Formation Mechanism of Amylopectin Rich Starches in Centrifugal Spinning System. J. Appl. Polym. Sci. 2021, 138, 50275. [Google Scholar] [CrossRef]
- Fauzi, A.; Zahra, F.; Miftahul Munir, M.; Khairurrijal, K. Fabrication and Characterization of Rotary Forcespun Styrofoam Fibers. IOP Conf. Ser. Mater. Sci. Eng. 2019, 515, 012039. [Google Scholar] [CrossRef]
- Padron, S.; Fuentes, A.; Caruntu, D.; Lozano, K. Experimental Study of Nanofiber Production through Forcespinning. J. Appl. Phys. 2013, 113, 24318. [Google Scholar] [CrossRef]
- McEachin, Z.; Lozano, K. Production and Charactherization of Polycaprolactone Nanofibers via ForcespinningTM Technology. J. Appl. Polym. Sci. 2012, 126, 473–479. [Google Scholar] [CrossRef]
- Ardi, A.; Fauzi, A.; Rajak, A.; Khairurrijal, K. The Effect of Rotational Speed of Rotary Forcespinning to the Morphology of Polyvinylpyrrolidone (PVP) Fibers with Garlic Extract. Mater. Today Proc. 2021, 44, 3403–3407. [Google Scholar] [CrossRef]
- Fang, Y.; Dulaney, A.D.; Gadley, J.; Maia, J.M.; Ellison, C.J. Manipulating Characteristic Timescales and Fiber Morphology in Simultaneous Centrifugal Spinning and Photopolymerization. Polymer 2015, 73, 42–51. [Google Scholar] [CrossRef]
- Chang, W.-M.; Wang, C.-C.; Chen, C.-Y. The Combination of Electrospinning and Forcespinning: Effects on a Viscoelastic Jet and a Single Nanofiber. Chem. Eng. J. 2014, 244, 540–551. [Google Scholar] [CrossRef]
- Laurencin, C.T.; Nair, L.S. Nanotechnology and Regenerative Engineering: The Scaffold, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar]
- Jiménez, A.; Peltzer, M.; Ruseckaite, R. Poly(Lactic Acid) Science and Technology; Polymer Chemistry Series; Jiménez, A., Peltzer, M., Ruseckaite, R., Eds.; The Royal Society of Chemistry: Cambridge, UK, 2015; ISBN 978-1-84973-879-8. [Google Scholar]
- Loordhuswamy, A.M.; Krishnaswamy, V.R.; Korrapati, P.S.; Thinakaran, S.; Rengaswami, G.D.V. Fabrication of Highly Aligned Fibrous Scaffolds for Tissue Regeneration by Centrifugal Spinning Technology. Mater. Sci. Eng. C 2014, 42, 799–807. [Google Scholar] [CrossRef] [PubMed]
- Chantre, C.O.; Gonzalez, G.M.; Ahn, S.; Cera, L.; Campbell, P.H.; Hoerstrup, S.P.; Parker, K.K. Porous Biomimetic Hyaluronic Acid and Extracellular Matrix Protein Nanofiber Scaffolds for Accelerated Cutaneous Tissue Repair. ACS Appl. Mater. Interfaces 2019, 11, 45498–45510. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Mei, S.; Dong, Y.; She, F.; Kong, L. High Efficiency Fabrication of Chitosan Composite Nanofibers with Uniform Morphology via Centrifugal Spinning. Polymers 2019, 11, 1550. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Mei, S.; Dong, Y.; She, F.; Li, C.; Li, Y.; Kong, L. Oxidized Chitosan-Tobramycin (OCS-TOB) Submicro-Fibers for Biomedical Applications. Pharmaceutics 2022, 14, 1197. [Google Scholar] [CrossRef]
- Vasconcellos, L.M.R.; de Elias, C.M.V.; Minhoto, G.B.; Abdala, J.M.A.; Andrade, T.M.; de Araujo, J.C.R.; Gusmão, S.B.S.; Viana, B.C.; Marciano, F.R.; Lobo, A.O. Rotary-Jet Spun Polycaprolactone/Nano-Hydroxyapatite Scaffolds Modified by Simulated Body Fluid Influenced the Flexural Mode of the Neoformed Bone. J. Mater. Sci. Mater. Med. 2020, 31, 72. [Google Scholar] [CrossRef] [PubMed]
- Guner, M.B.; Dalgic, A.D.; Tezcaner, A.; Yilanci, S.; Keskin, D. A Dual-Phase Scaffold Produced by Rotary Jet Spinning and Electrospinning for Tendon Tissue Engineering. Biomed. Mater. 2020, 15, 065014. [Google Scholar] [CrossRef]
- Giorno, L.P.; Rodrigues, L.R.; Jr, A.R.S. Fibrous PCL Scaffolds as Tissue Substitutes. Int. J. Adv. Med. Biotechnol. 2020, 3, 40–45. [Google Scholar] [CrossRef]
- Andrade, T.M.; Mello, D.C.R.; Elias, C.M.V.; Abdala, J.M.A.; Silva, E.; Vasconcellos, L.M.R.; Tim, C.R.; Marciano, F.R.; Lobo, A.O. In Vitro and in Vivo Evaluation of Rotary-Jet-Spun Poly(ε-Caprolactone) with High Loading of Nano-Hydroxyapatite. J. Mater. Sci. Mater. Med. 2019, 30, 19. [Google Scholar] [CrossRef]
- Capulli, A.K.; Emmert, M.Y.; Pasqualini, F.S.; Kehl, D.; Caliskan, E.; Lind, J.U.; Sheehy, S.P.; Park, S.J.; Ahn, S.; Weber, B.; et al. JetValve: Rapid Manufacturing of Biohybrid Scaffolds for Biomimetic Heart Valve Replacement. Biomaterials 2017, 133, 229–241. [Google Scholar] [CrossRef]
- Chanes-Cuevas, O.A.; Arellano-sánchez, U.; Álvarez-gayosso, C.A. Synthesis of PLA/SBA-15 Composite Scaffolds for Bone Tissue Engineering 2. Experimental Section. Mater. Res. 2020, 23, 1–13. [Google Scholar] [CrossRef]
- Zamproni, L.N.; Grinet, M.A.V.M.; Mundim, M.T.V.V.; Reis, M.B.C.; Galindo, L.T.; Marciano, F.R.; Lobo, A.O.; Porcionatto, M. Rotary Jet-Spun Porous Microfibers as Scaffolds for Stem Cells Delivery to Central Nervous System Injury. Nanomed. Nanotechnol. Biol. Med. 2019, 15, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Pereira Rodrigues, I.C.; Tamborlin, L.; Rodrigues, A.A.; Jardini, A.L.; Ducati Luchessi, A.; Maciel Filho, R.; Najar Lopes, É.S.; Pellizzer Gabriel, L. Polyurethane Fibrous Membranes Tailored by Rotary Jet Spinning for Tissue Engineering Applications. J. Appl. Polym. Sci. 2020, 137, 48455. [Google Scholar] [CrossRef]
- Rodrigues, I.C.P.; Pereira, K.D.; Woigt, L.F.; Jardini, A.L.; Luchessi, A.D.; Lopes, É.S.N.; Webster, T.J.; Gabriel, L.P. A Novel Technique to Produce Tubular Scaffolds Based on Collagen and Elastin. Artif. Organs 2021, 45, E113–E122. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.; Liu, Q.; Zimmerman, J.F.; Lee, K.Y.; Jin, Q.; Peters, M.M.; Rosnach, M.; Choi, S.; Kim, S.L.; Ardoña, H.A.M.; et al. Recreating the Heart’s Helical Structure-Function Relationship with Focused Rotary Jet Spinning. Science 2022, 377, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Rosa, J.C.; Bonvent, J.J.; Santos, A.R.J. Poly (ε-Caprolactone)/Poly (Lactic Acid) Fibers Produced by Rotary Jet Spinning for Skin Dressing with Antimicrobial Activity. J. Biomater. Appl. 2022, 36, 1641–1651. [Google Scholar] [CrossRef]
- Kim, H.S.; Sun, X.; Lee, J.-H.; Kim, H.-W.; Fu, X.; Leong, K.W. Advanced Drug Delivery Systems and Artificial Skin Grafts for Skin Wound Healing. Adv. Drug Deliv. Rev. 2019, 146, 209–239. [Google Scholar] [CrossRef]
- Akombaetwa, N.; Bwanga, A.; Makoni, P.A.; Witika, B.A. Applications of Electrospun Drug-Eluting Nanofibers in Wound Healing: Current and Future Perspectives. Polymers 2022, 14, 2931. [Google Scholar] [CrossRef]
- Morie, A.; Garg, T.; Goyal, A.K.; Rath, G. Nanofibers as Novel Drug Carrier—An Overview. Artif. Cells Nanomed. Biotechnol. 2016, 44, 135–143. [Google Scholar] [CrossRef]
- Goyal, R.; Macri, L.K.; Kaplan, H.M.; Kohn, J. Nanoparticles and Nanofibers for Topical Drug Delivery. J. Control. Release 2016, 240, 77–92. [Google Scholar] [CrossRef]
- Sebe, I.; Szabó, P.; Kállai-Szabó, B.; Zelkó, R. Incorporating Small Molecules or Biologics into Nanofibers for Optimized Drug Release: A Review. Int. J. Pharm. 2015, 494, 516–530. [Google Scholar] [CrossRef]
- Zupančič, Š. Core-Shell Nanofibers as Drug Delivery Systems. Acta Pharm. 2019, 69, 131–153. [Google Scholar] [CrossRef]
- Hrib, J.; Sirc, J.; Hobzova, R.; Hampejsova, Z.; Bosakova, Z.; Munzarova, M.; Michalek, J. Nanofibers for Drug Delivery—Incorporation and Release of Model Molecules, Influence of Molecular Weight and Polymer Structure. Beilstein J. Nanotechnol. 2015, 6, 1936–1945. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Wang, L.; Zhu, K. Coaxial Electrospinning for Encapsulation and Controlled Release of Fragile Water-Soluble Bioactive Agents. J. Control. Release 2014, 193, 296–303. [Google Scholar] [CrossRef]
- Odermatt, E.; Bargon, R.; Grafahrend, D.; Neumüller, D.; Reibel, D. Medical Device and Method for the Production Thereof. Patent Number US 10736985B2, 11 August 2020. [Google Scholar]
- Yoon, K.; Hsiao, B.S.; Chu, B. Functional Nanofibers for Environmental Applications. J. Mater. Chem. 2008, 18, 5326–5334. [Google Scholar] [CrossRef]
- Graham, K.; Ouyang, M.; Raether, T.; Grafe, T.; Mcdonald, B.; Knauf, P. Polymeric Nanofibers in Air Filtration Applications. In Proceedings of the Fifteenth Annual Technical Conference & Expo of the American Filtration & Separations Society, Galveston, TX, USA, 9–12 April 2002; pp. 9–12. [Google Scholar]
- Podgórski, A.; Bałazy, A.; Gradoń, L. Application of Nanofibers to Improve the Filtration Efficiency of the Most Penetrating Aerosol Particles in Fibrous Filters. Chem. Eng. Sci. 2006, 61, 6804–6815. [Google Scholar] [CrossRef]
- Arican, F.; Uzuner-Demir, A.; Polat, O.; Sancakli, A.; Ismar, E. Fabrication of Gelatin Nanofiber Webs via Centrifugal Spinning for N95 Respiratory Filters. Bull. Mater. Sci. 2022, 45, 93. [Google Scholar] [CrossRef]
- Jiang, J.; Shao, Z.; Wang, X.; Zhu, P.; Deng, S.; Li, W.; Zheng, G. Three-Dimensional Composite Electrospun Nanofibrous Membrane by Multi-Jet Electrospinning with Sheath Gas for High-Efficiency Antibiosis Air Filtration. Nanotechnology 2021, 32, 245707. [Google Scholar] [CrossRef]
- Mokhena, T.C.; Jacobs, V.; Luyt, A.S. A Review on Electrospun Bio-Based Polymers for Water Treatment. Express Polym. Lett. 2015, 9, 839–880. [Google Scholar] [CrossRef]
- Kumar, A.; Sinha-Ray, S. A Review on Biopolymer-Based Fibers via Electrospinning and Solution Blowing and Their Applications. Fibers 2018, 6, 45. [Google Scholar] [CrossRef]
- Wang, X.; Yeh, T.M.; Wang, Z.; Yang, R.; Wang, R.; Ma, H.; Hsiao, B.S.; Chu, B. Nanofiltration Membranes Prepared by Interfacial Polymerization on Thin-Film Nanofibrous Composite Scaffold. Polymer 2014, 55, 1358–1366. [Google Scholar] [CrossRef]
- Berson, S.; De Bettignies, R.; Bailly, S.; Guillerez, S. Poly(3-Hexylthiophene) Fibers for Photovoltaic Applications. Adv. Funct. Mater. 2007, 17, 1377–1384. [Google Scholar] [CrossRef]
- Yang, X.; Loos, J.; Veenstra, S.C.; Verhees, W.J.H.H.; Wienk, M.M.; Kroon, J.M.; Michels, M.A.J.J.; Janssen, R.A.J.J. Nanoscale Morphology of High-Performance Polymer Solar Cells. Nano Lett. 2005, 5, 579–583. [Google Scholar] [CrossRef] [PubMed]
- Shirakawa, H.; Louis, E.J.; MacDiarmid, A.G.; Chiang, C.K.; Heeger, A.J. Synthesis of Electrically Conducting Organic Polymers: Halogen Derivatives of Polyacetylene (CHx). J. Chem. Soc. Chem. Commun. 1977, 16, 578–580. [Google Scholar] [CrossRef]
- Sariciftci, N.S.; Smilowitz, L.; Heeger, A.J.; Wudl, F. Photoinduced Electron Transfer from a Conducting Polymer to Buckminsterfullerene. Science 1992, 258, 1474–1476. [Google Scholar] [CrossRef] [PubMed]
- Nitti, P.; Gallo, N.; Natta, L.; Scalera, F.; Palazzo, B.; Sannino, A.; Gervaso, F. Influence of Nanofiber Orientation on Morphological and Mechanical Properties of Electrospun Chitosan Mats. J. Healthc. Eng. 2018, 2018, 3651480. [Google Scholar] [CrossRef] [PubMed]
- Vazquez, B.; Vazquez, H.; Lozano, K. Preparation and Characterization of Polyvinylidene Fluoride Nanofibrous Membranes by ForcespinningTM. Polym. Eng. Sci. 2012, 52, 2260–2265. [Google Scholar] [CrossRef]
- Zhou, B.; Pu, H.; Pan, H.; Wan, D. Proton Exchange Membranes Based on Semi-Interpenetrating Polymer Networks of Nafion and Poly(Vinylidene Fluoride) via Radiation Crosslinking. Int. J. Hydrogen Energy 2011, 36, 6809–6816. [Google Scholar] [CrossRef]
- Kepler, R.G.; Anderson, R.A. Ferroelectric Polymers. Adv. Phys. 1992, 41, 1–57. [Google Scholar] [CrossRef]
- Salimi, A.; Yousefi, A.A. Conformational Changes and Phase Transformation Mechanisms in PVDF Solution-Cast Films. J. Polym. Sci. Part B Polym. Phys. 2004, 42, 3487–3495. [Google Scholar] [CrossRef]




| Techniques | Advantages | Disadvantages | Applications | References |
|---|---|---|---|---|
| Electrospinning | Nanometric fiber diameters (100–1100 nm), large surface area, uniform and aligned fibers, high porosity, simple fabrication, superior mechanical properties, and ECM-like structure. | Requires a high voltage source and conductivity solution, uses toxic solvents, has low productivity, has difficulties in scaling and equipment handling. | Biomedical: regenerative medicine and drug delivery systems. Others: electrochemistry (Li-air battery separator), catalysis (sensors), photocatalysis (organic solar cells), and environmental (filters). | [44,45,46,47,48,49,50] |
| Melt Blowing | Long and continuous fibers, high productivity, solvent-free. | High temperatures, thermal degradation, larger fiber diameters, and polymers limitation due to viscosity control. | Textile area and filters. | [51,52,53,54,55,56] |
| Drawing | Simple process, high repeatability, produces unique, continuous, and long nanofibers. | It uses viscoelastic materials. Limited to laboratory scale, it is a discontinuous process. | Agriculture packaging. | [14,51,55,57] |
| Rotary Jet Spinning | Process easy to scale, good repeatability, fiber dimension control, free from high voltage, low cost, simple operation, eco-friendly. Numerous polymers can be processed, besides polymeric emulsions and suspensions, with high productivity. | Might require high temperatures. Larger diameter fibers. Fiber properties can be affected by the material’s characteristics and quality/configuration of RJS equipment. | Controlled drug release, wound dressings, tissue engineering, aerosol filtration, energy storage, edible films, nutraceuticals, food encapsulation, and packaging. | [14,31,51,53,58,59,60] |
| Air Brushing | Uncharged solution, fibers diameter controlled by air pressure and nozzle diameter, coating various shapes, fast deposition rates. | Highly viscous polymer solutions are difficult to produce fibers, require compressed air, and solvent evaporation depends on the solvent itself. | Scaffolds, tissue engineering, filtration. | [61,62,63] |
| RJS Type | Sub-Type | Characteristics | References |
|---|---|---|---|
| Traditional RJS | Melt Spinning |
| [84] |
| [85,86] | |||
| Polymer Solution Spinning |
| [87] [75,83,87,88,89,90,91] [75,83,88,92] [74,88,92,93,94] [74,88] [91] [74,88] [75,83,90,91] [27] [83,94,95] [92] [94] [75,91] | |
| Hybrid RJS | Electrostatic-Centrifugal Spinning |
| [96] |
| Photo-Centrifugal Spinning |
| [97] |
| Polymers Used | Applications | Characteristics | References |
|---|---|---|---|
| Biological ECM 1/HA 2 | Tissue engineering | These scaffolds of porous nanofibers produced by iRJS 3 have tunable properties, as they are composed of biological molecules (HA, fibrinogen, collagen, gelatin, and chondroitin sulfate) that biomimics the ECM to speed up tissue regeneration | [102] |
| CS 4/PEO 5 | Tissue engineering | Fabrication of continuous, ultrafine, and uniform beads-free nanofibers with high CS content for enhanced antimicrobial and biocompatibility | [103] |
| OCS 6/TOB 7 | Tissue engineering | OCS grafted with an antibiotic (TOB) was processed with PEO in a RJS equipment, such polymer improved the spinnability, with the formulation 1:3 OCS-TOB/PEO showing the best antibacterial activity | [104] |
| PCL 8 | Bone regeneration | PCL scaffolds combined with nHAp 9 produced via RJS were used in bone structures. The results showed that the PCL/nHAp scaffolds had a positive influence on the flexural mode of the newformed bone | [105] |
| PCL | Tissue engineering | This study demonstrates that RJS-spun fibers have a unique morphology compared to electrospun fibers, are non-cytotoxic when in contact with mammalian cells, and reduce bacterial colonization without the need for further incorporation of antibiotics or prior chemical treatment | [39] |
| PCL/Gelatin | Tendon tissue engineering | Dual-phase fibers have been developed involving RJS and WES 10 techniques. The fiber core is formed by gelatin, presenting adequate mechanical strength, and also helping the tendon osteogeneses | [106] |
| PCL/Gelatin | Tissue engineering | RJS proved to be effective to produce non-toxic PCL-gelatin fibers that possibly allow their use as scaffolds | [107] |
| PCL/nHAp | Orthopedic applications | Scaffolds with PCL/nHAp showed reduced bacterial proliferation in bones (in vitro and in vivo) since the structures obtained presented superhydrophobic behavior | [108] |
| PCL/β-TCP 11 | Bone grafting | PCL and β-TCP were solubilized in chloroform, and further spun at 3500 rpm, where formulations M5 and M10% promoted better collagen and osteoclasts production | [59] |
| P4HB 12/Gelatin | Scaffolds for heart valve replacement | The hybrid fibers (core—gelatin, exterior—P4HB) produced a biomimetic fibrous matrix, such as heart valves, improving the regeneration of the fibrous tissue | [109] |
| PLA 13 | Bone tissue engineering | PLA/SBA 14-15fiber improved polymer matrix biocompatibility and osteoblast cells’ adhesion | [110] |
| PLA | Tissue engineering | Polymeric roughened microfibers (PRM 15), with high porosity, produced by RJS, improved the mesenchymal stem cells’ adhesion and tissue incorporation, reducing the stroke lesion area | [111] |
| PLLA 16 | Tissue engineering | Fibrous PLLA membranes produced by the RJS technique had non-toxic behavior, presenting biocompatibility and bioadhesion, which makes them adequate support for fibroblastic and osteoblastic cells’ proliferation | [15] |
| PU 17 | Tissue engineering | The PU fibrous structures produced by RJS, both aligned and random, showed compatibility with the cultured osteoblastic cell line, which allows its application in tissue engineering | [112] |
| PU | Tissue engineering | PU scaffolds combined with collagen and elastin showed an absence of solvent in the fibers, besides hydrophilic behavior, which possibly allows their application as tubular scaffolds for regeneration of vascular systems | [113] |
| PVP 18 | Biomedical | The compact equipment easily controlled the operating parameters, producing aligned and homogeneous PVP fibers suitable for drug delivery systems | [22] |
| PCL | Tissue engineering | Fabrication of scaffolds with micro and nanofibers of polycaprolactone and gelatin for the cultivation of cardiomyocytes for a biofabrication of ventricles | [114] |
| PLA/PCL | Biomedical | Dressing fibers produced by RJS containing polymeric fibers incorporating VANC 19 were developed in order to evaluate the antimicrobial potential against Staphylococcus aureus | [115] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bahú, J.O.; Melo de Andrade, L.R.; Crivellin, S.; Khouri, N.G.; Sousa, S.O.; Fernandes, L.M.I.; Souza, S.D.A.; Concha, L.S.C.; Schiavon, M.I.R.B.; Benites, C.I.; et al. Rotary Jet Spinning (RJS): A Key Process to Produce Biopolymeric Wound Dressings. Pharmaceutics 2022, 14, 2500. https://doi.org/10.3390/pharmaceutics14112500
Bahú JO, Melo de Andrade LR, Crivellin S, Khouri NG, Sousa SO, Fernandes LMI, Souza SDA, Concha LSC, Schiavon MIRB, Benites CI, et al. Rotary Jet Spinning (RJS): A Key Process to Produce Biopolymeric Wound Dressings. Pharmaceutics. 2022; 14(11):2500. https://doi.org/10.3390/pharmaceutics14112500
Chicago/Turabian StyleBahú, Juliana O., Lucas R. Melo de Andrade, Sara Crivellin, Nadia G. Khouri, Sara O. Sousa, Luiza M. I. Fernandes, Samuel D. A. Souza, Luz S. Cárdenas Concha, Maria I. R. B. Schiavon, Cibelem I. Benites, and et al. 2022. "Rotary Jet Spinning (RJS): A Key Process to Produce Biopolymeric Wound Dressings" Pharmaceutics 14, no. 11: 2500. https://doi.org/10.3390/pharmaceutics14112500
APA StyleBahú, J. O., Melo de Andrade, L. R., Crivellin, S., Khouri, N. G., Sousa, S. O., Fernandes, L. M. I., Souza, S. D. A., Concha, L. S. C., Schiavon, M. I. R. B., Benites, C. I., Severino, P., Souto, E. B., & Concha, V. O. C. (2022). Rotary Jet Spinning (RJS): A Key Process to Produce Biopolymeric Wound Dressings. Pharmaceutics, 14(11), 2500. https://doi.org/10.3390/pharmaceutics14112500








