Label-Free Assessment of Mannitol Accumulation Following Osmotic Blood–Brain Barrier Opening Using Chemical Exchange Saturation Transfer Magnetic Resonance Imaging
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Animals
2.3. 1H-NMR
2.4. In Vitro MRI
2.5. In Vivo MRI
2.6. Data Processing
2.7. Statistical Analysis
3. Results
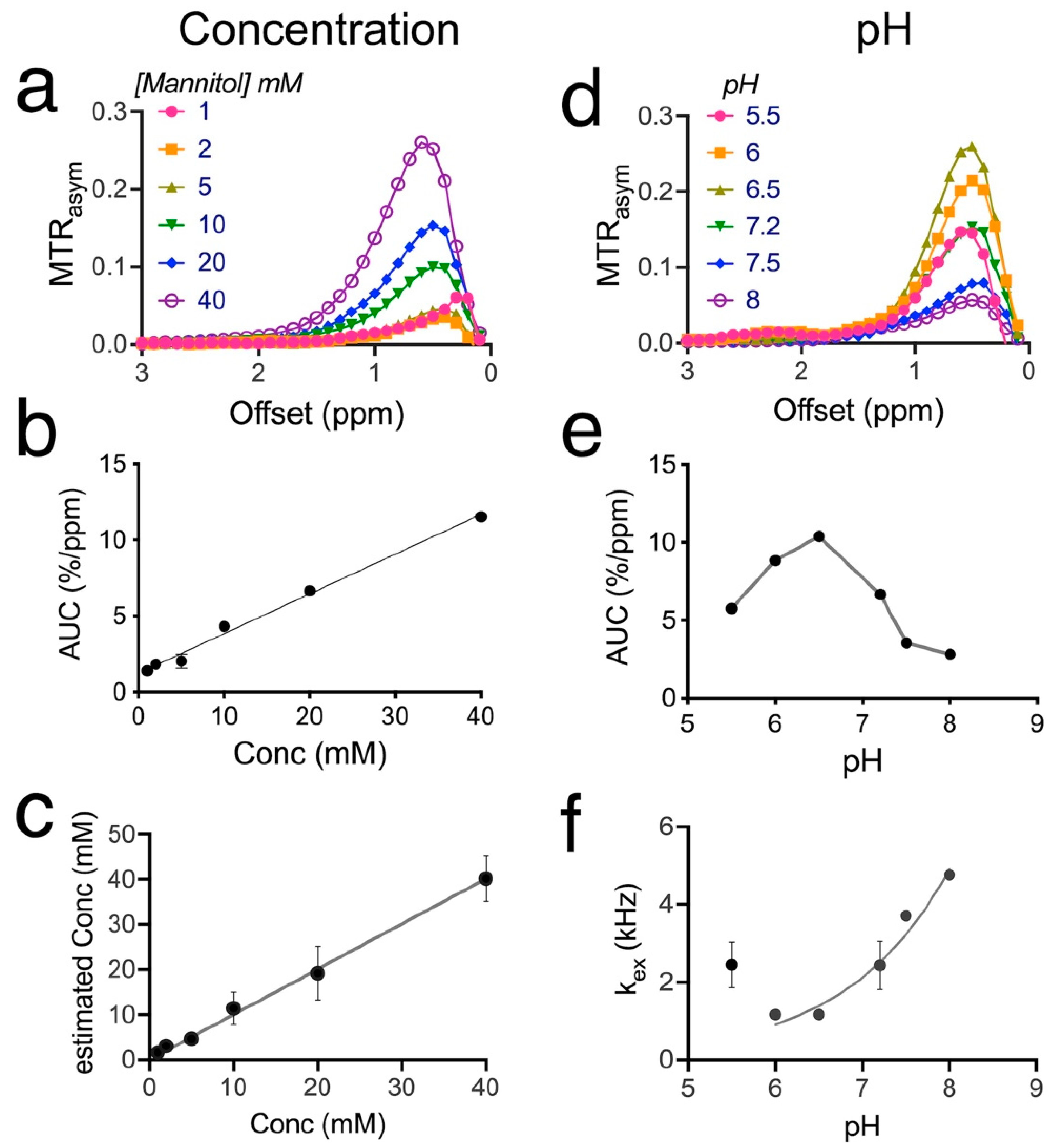
3.1. CEST Characteristics of mannitol
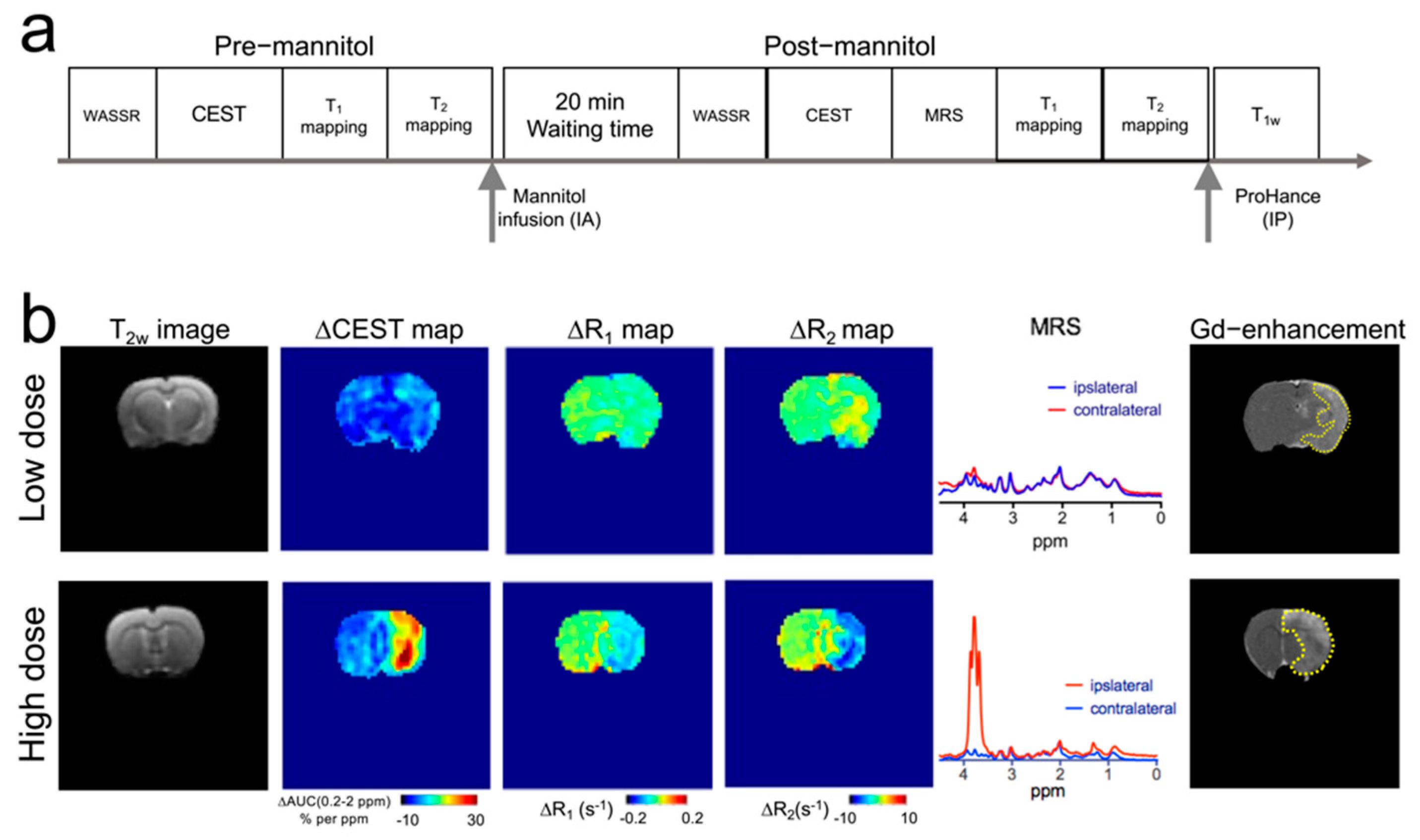
3.2. Mannitol Excess Causes Extravasation and Brain Accumulation of Mannitol
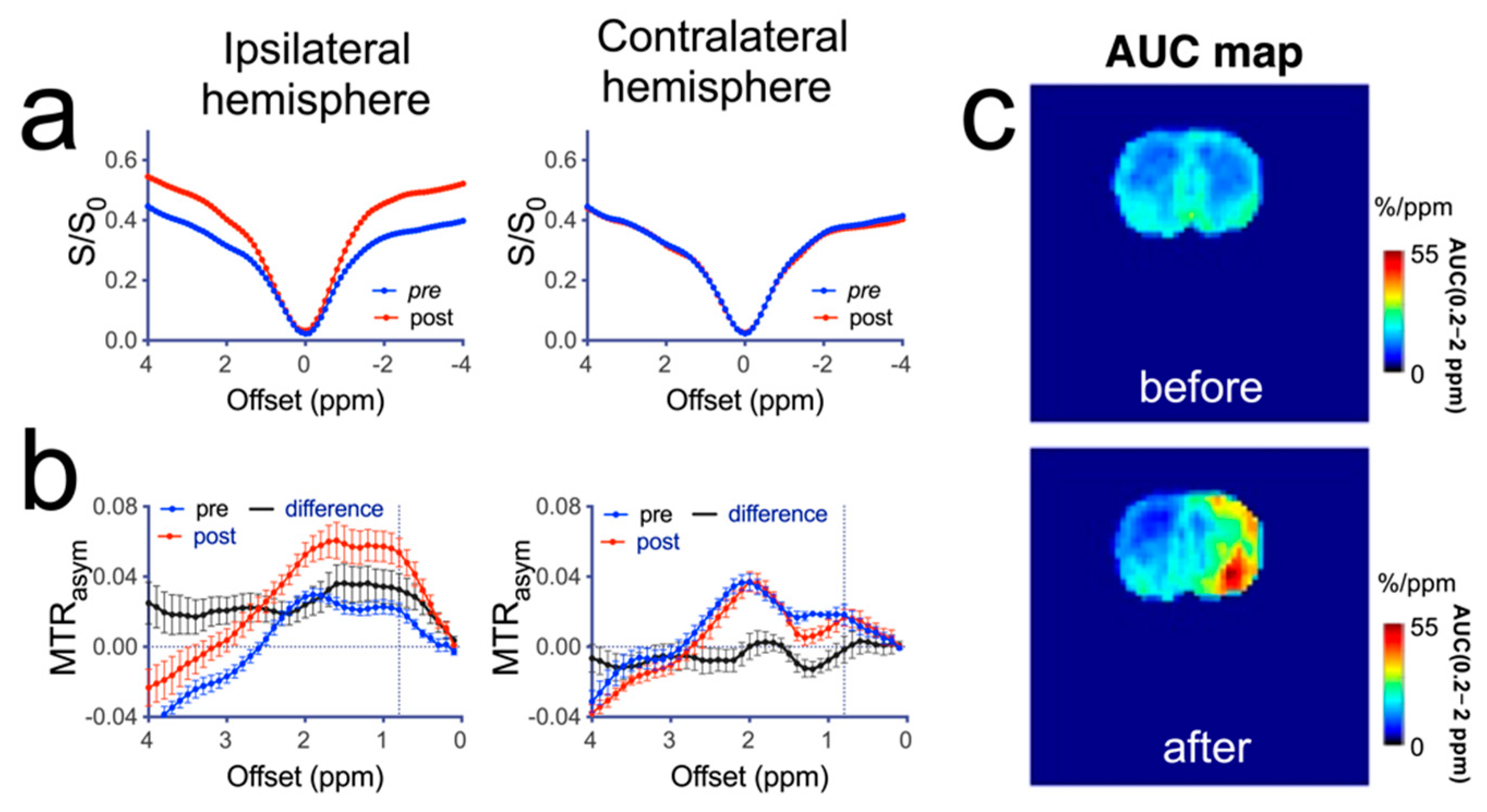
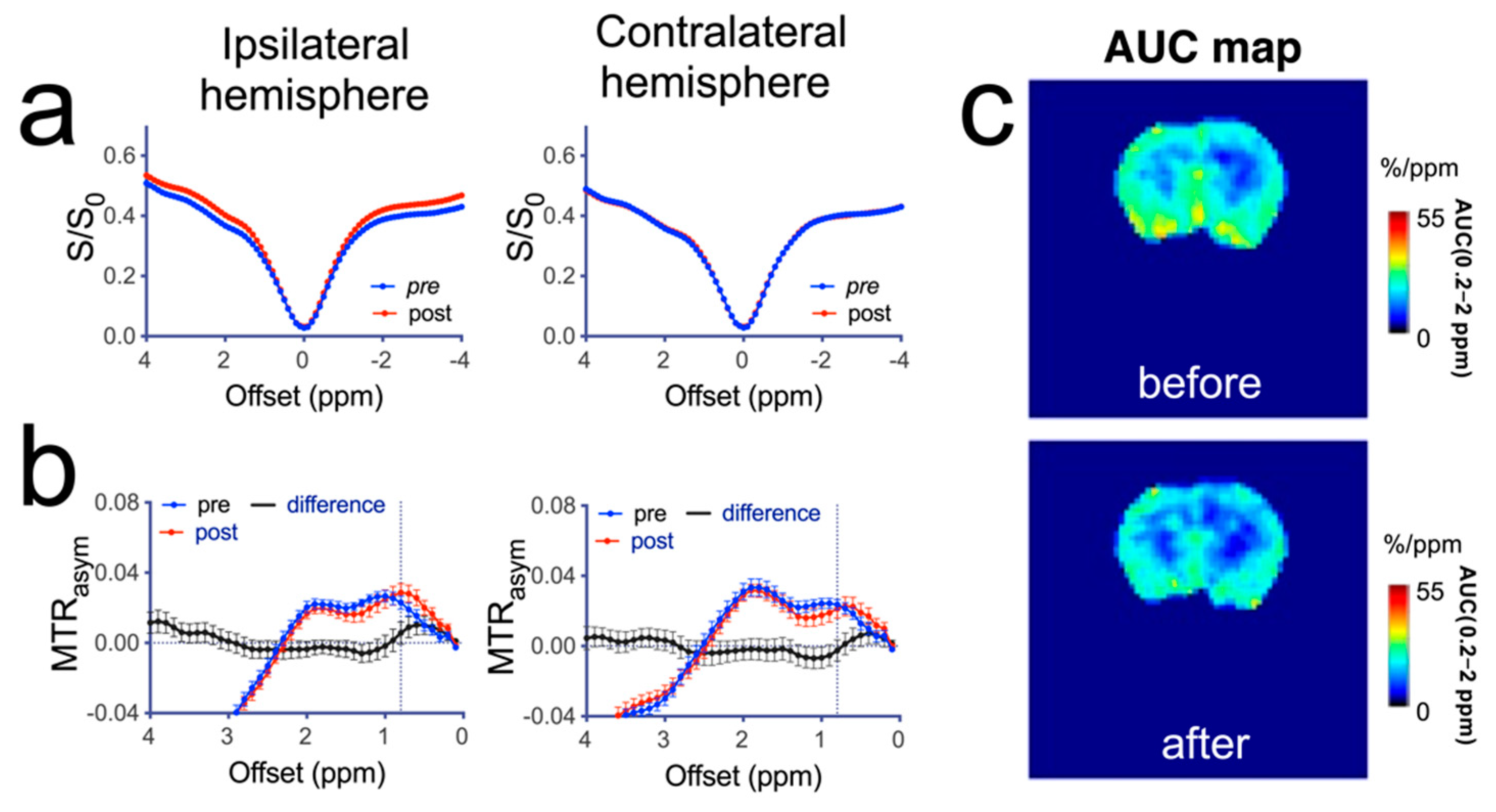
3.3. MRI Manifestations following Mannitol Accumulation in the Brain
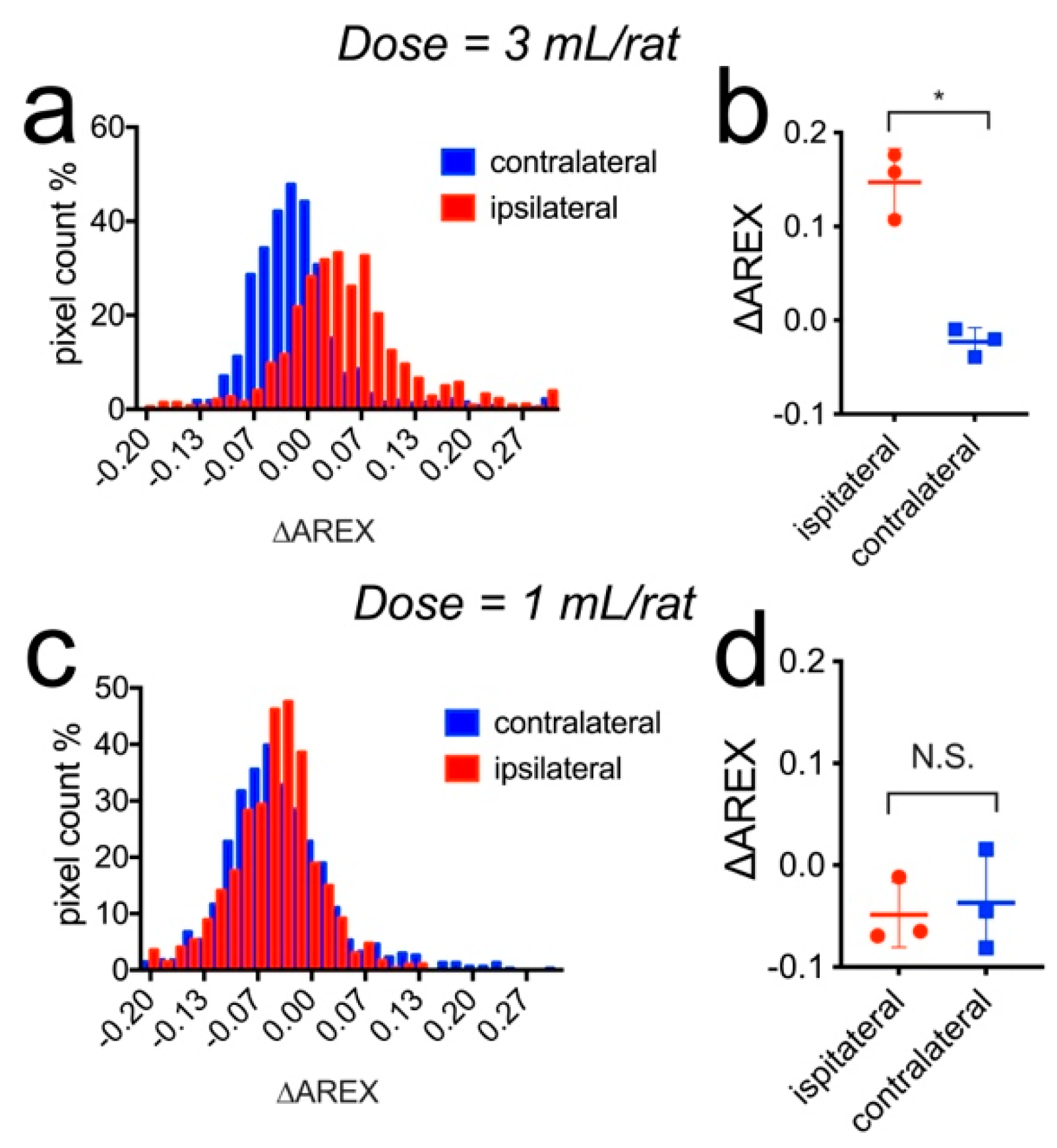
3.4. CEST MRI Detection of Mannitol in the Brain
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pardridge, W.M. Blood-brain barrier delivery. Drug Discov. Today 2007, 12, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Grossman, S.A.; Romo, C.G.; Rudek, M.A.; Supko, J.; Fisher, J.; Nabors, L.B.; Wen, P.Y.; Peereboom, D.M.; Ellingson, B.M.; Elmquist, W.; et al. Baseline requirements for novel agents being considered for phase II/III brain cancer efficacy trials: Conclusions from the Adult Brain Tumor Consortium’s first workshop on CNS drug delivery. Neuro Oncol. 2020, 22, 1422–1424. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Huang, H.; Huang, Y.; Sun, H.; Xu, H. Hypertonic saline or mannitol for treating elevated intracranial pressure in traumatic brain injury: A meta-analysis of randomized controlled trials. Neurosurg. Rev. 2019, 42, 499–509. [Google Scholar] [CrossRef]
- Videen, T.O.; Zazulia, A.R.; Manno, E.M.; Derdeyn, C.P.; Adams, R.E.; Diringer, M.N.; Powers, W.J. Mannitol bolus preferentially shrinks non-infarcted brain in patients with ischemic stroke. Neurology 2001, 57, 2120–2122. [Google Scholar] [CrossRef] [PubMed]
- Esquenazi, Y.; Lo, V.P.; Lee, K. Critical Care Management of Cerebral Edema in Brain Tumors. J. Intensive Care Med. 2017, 32, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Witherspoon, B.; Ashby, N.E. The use of mannitol and hypertonic saline therapies in patients with elevated intracranial pressure: A review of the evidence. Nurs. Clin. 2017, 52, 249–260. [Google Scholar] [CrossRef]
- Cook, A.M.; Morgan Jones, G.; Hawryluk, G.W.J.; Mailloux, P.; McLaughlin, D.; Papangelou, A.; Samuel, S.; Tokumaru, S.; Venkatasubramanian, C.; Zacko, C.; et al. Guidelines for the Acute Treatment of Cerebral Edema in Neurocritical Care Patients. Neurocrit. Care 2020, 32, 647–666. [Google Scholar] [CrossRef] [Green Version]
- Rapoport, S.I.; Hori, M.; Klatzo, I. Testing of a hypothesis for osmotic opening of the blood-brain barrier. Am. J. Physiol. 1972, 223, 323–331. [Google Scholar] [CrossRef] [Green Version]
- Burkhardt, J.-K.; Riina, H.; Shin, B.J.; Christos, P.; Kesavabhotla, K.; Hofstetter, C.P.; Tsiouris, A.J.; Boockvar, J.A. Intra-arterial delivery of bevacizumab after blood-brain barrier disruption for the treatment of recurrent glioblastoma: Progression-free survival and overall survival. World Neurosurg. 2012, 77, 130–134. [Google Scholar] [CrossRef] [Green Version]
- Neuwelt, E.A.; Frenkel, E.P.; Diehl, J.T.; Maravilla, K.R.; Vu, L.H.; Clark, W.K.; Rapoport, S.I.; Barnett, P.A.; Hill, S.A.; Lewis, S.E.; et al. Osmotic blood-brain barrier disruption: A new means of increasing chemotherapeutic agent delivery. Trans. Am. Neurol. Assoc. 1979, 104, 256–260. [Google Scholar]
- Doolittle, N.D.; Miner, M.E.; Hall, W.A.; Siegal, T.; Hanson, E.J.; Osztie, E.; McAllister, L.D.; Bubalo, J.S.; Kraemer, D.F.; Fortin, D.; et al. Safety and efficacy of a multicenter study using intraarterial chemotherapy in conjunction with osmotic opening of the blood-brain barrier for the treatment of patients with malignant brain tumors. Cancer 2000, 88, 637–647. [Google Scholar] [CrossRef]
- Charest, G.; Sanche, L.; Fortin, D.; Mathieu, D.; Paquette, B. Optimization of the route of platinum drugs administration to optimize the concomitant treatment with radiotherapy for glioblastoma implanted in the Fischer rat brain. J. Neurooncol. 2013, 115, 365–373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lesniak, W.G.; Chu, C.; Jablonska, A.; Du, Y.; Pomper, M.G.; Walczak, P.; Janowski, M. A Distinct Advantage to Intraarterial Delivery of (89)Zr-Bevacizumab in PET Imaging of Mice With and Without Osmotic Opening of the Blood-Brain Barrier. J. Nucl. Med. 2019, 60, 617–622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lesniak, W.G.; Chu, C.; Jablonska, A.; Behnam Azad, B.; Zwaenepoel, O.; Zawadzki, M.; Lisok, A.; Pomper, M.G.; Walczak, P.; Gettemans, J.; et al. PET imaging of distinct brain uptake of a nanobody and similarly-sized PAMAM dendrimers after intra-arterial administration. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 1940–1951. [Google Scholar] [CrossRef] [PubMed]
- Hersh, D.S.; Wadajkar, A.S.; Roberts, N.; Perez, J.G.; Connolly, N.P.; Frenkel, V.; Winkles, J.A.; Woodworth, G.F.; Kim, A.J. Evolving Drug Delivery Strategies to Overcome the Blood Brain Barrier. Curr. Pharm. Des. 2016, 22, 1177–1193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, L.; Liu, J.; Chu, C.; Han, Z.; Yadav, N.; Xu, J.; Bai, R.; Staedtke, V.; Pearl, M.; Walczak, P.; et al. Deuterium oxide as a contrast medium for real-time MRI-guided endovascular neurointervention. Theranostics 2021, 11, 6240–6250. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.; Jablonska, A.; Lesniak, W.G.; Thomas, A.M.; Lan, X.; Linville, R.M.; Li, S.; Searson, P.C.; Liu, G.; Pearl, M.; et al. Optimization of osmotic blood-brain barrier opening to enable intravital microscopy studies on drug delivery in mouse cortex. J. Control. Release 2020, 317, 312–321. [Google Scholar] [CrossRef]
- Chu, C.; Liu, G.; Janowski, M.; Bulte, J.W.M.; Li, S.; Pearl, M.; Walczak, P. Real-Time MRI Guidance for Reproducible Hyperosmolar Opening of the Blood-Brain Barrier in Mice. Front. Neurol. 2018, 9, 921. [Google Scholar] [CrossRef]
- Janowski, M.; Walczak, P.; Pearl, M.S. Predicting and optimizing the territory of blood-brain barrier opening by superselective intra-arterial cerebral infusion under dynamic susceptibility contrast MRI guidance. J. Cereb. Blood Flow Metab. 2016, 36, 569–575. [Google Scholar] [CrossRef] [Green Version]
- Zawadzki, M.; Walecki, J.; Kostkiewicz, B.; Kostyra, K.; Pearl, M.S.; Solaiyappan, M.; Walczak, P.; Janowski, M. Real-time MRI guidance for intra-arterial drug delivery in a patient with a brain tumor: Technical note. BMJ Case Rep. 2019, 12. [Google Scholar] [CrossRef]
- Zawadzki, M.; Walecki, J.; Kostkiewicz, B.; Kostyra, K.; Walczak, P.; Janowski, M. Follow-up of intra-arterial delivery of bevacizumab for treatment of butterfly glioblastoma in patient with first-in-human, real-time MRI-guided intra-arterial neurointervention. J. Neurointerv. Surg. 2021, 13, 1037–1039. [Google Scholar] [CrossRef] [PubMed]
- Node, Y.; Nakazawa, S. Clinical study of mannitol and glycerol on raised intracranial pressure and on their rebound phenomenon. Adv. Neurol. 1990, 52, 359–363. [Google Scholar] [PubMed]
- Grande, P.O.; Romner, B. Osmotherapy in brain edema: A questionable therapy. J. Neurosurg. Anesthesiol. 2012, 24, 407–412. [Google Scholar] [CrossRef]
- Cho, J.; Kim, Y.H.; Han, H.S.; Park, J. Accumulated mannitol and aggravated cerebral edema in a rat model of middle cerebral artery infarction. J. Korean Neurosurg. Soc. 2007, 42, 337–341. [Google Scholar] [CrossRef] [Green Version]
- Kaufmann, A.M.; Cardoso, E.R. Aggravation of vasogenic cerebral edema by multiple-dose mannitol. J. Neurosurg. 1992, 77, 584–589. [Google Scholar] [CrossRef]
- Palma, L.; Bruni, G.; Fiaschi, A.I.; Mariottini, A. Passage of mannitol into the brain around gliomas: A potential cause of rebound phenomenon. A study on 21 patients. J. Neurosurg. Sci. 2006, 50, 63–66. [Google Scholar] [PubMed]
- Garcia-Morales, E.J.; Cariappa, R.; Parvin, C.A.; Scott, M.G.; Diringer, M.N. Osmole gap in neurologic-neurosurgical intensive care unit: Its normal value, calculation, and relationship with mannitol serum concentrations. Crit. Care Med. 2004, 32, 986–991. [Google Scholar] [CrossRef] [PubMed]
- Peeling, J.; Sutherland, G. High-resolution 1H NMR spectroscopy studies of extracts of human cerebral neoplasms. Magn. Reson. Med. 1992, 24, 123–136. [Google Scholar] [CrossRef]
- Sankar, T.; Assina, R.; Karis, J.P.; Theodore, N.; Preul, M.C. Neurosurgical implications of mannitol accumulation within a meningioma and its peritumoral region demonstrated by magnetic resonance spectroscopy: Case report. J. Neurosurg. 2008, 108, 1010–1013. [Google Scholar] [CrossRef] [Green Version]
- Maioriello, A.V.; Chaljub, G.; Nauta, H.J.; Lacroix, M. Chemical shift imaging of mannitol in acute cerebral ischemia. Case report. J. Neurosurg. 2002, 97, 687–691. [Google Scholar] [CrossRef]
- Ward, K.M.; Aletras, A.H.; Balaban, R.S. A new class of contrast agents for MRI based on proton chemical exchange dependent saturation transfer (CEST). J. Magn. Reson. 2000, 143, 79–87. [Google Scholar] [CrossRef] [PubMed]
- van Zijl, P.C.; Yadav, N.N. Chemical exchange saturation transfer (CEST): What is in a name and what isn’t? Magn. Reson. Med. 2011, 65, 927–948. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Zijl, P.C.; Jones, C.K.; Ren, J.; Malloy, C.R.; Sherry, A.D. MRI detection of glycogen in vivo by using chemical exchange saturation transfer imaging (glycoCEST). Proc. Natl. Acad. Sci. USA 2007, 104, 4359–4364. [Google Scholar] [CrossRef] [Green Version]
- Chan, K.W.; McMahon, M.T.; Kato, Y.; Liu, G.; Bulte, J.W.; Bhujwalla, Z.M.; Artemov, D.; van Zijl, P.C. Natural D-glucose as a biodegradable MRI contrast agent for detecting cancer. Magn. Reson. Med. 2012, 68, 1764–1773. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walker-Samuel, S.; Ramasawmy, R.; Torrealdea, F.; Rega, M.; Rajkumar, V.; Johnson, S.P.; Richardson, S.; Goncalves, M.; Parkes, H.G.; Arstad, E.; et al. In vivo imaging of glucose uptake and metabolism in tumors. Nat. Med. 2013, 19, 1067–1072. [Google Scholar] [CrossRef] [Green Version]
- Jin, T.; Mehrens, H.; Wang, P.; Kim, S.G. Glucose metabolism-weighted imaging with chemical exchange-sensitive MRI of 2-deoxyglucose (2DG) in brain: Sensitivity and biological sources. NeuroImage 2016, 143, 82–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nasrallah, F.A.; Pages, G.; Kuchel, P.W.; Golay, X.; Chuang, K.H. Imaging brain deoxyglucose uptake and metabolism by glucoCEST MRI. J. Cereb. Blood Flow Metab. 2013, 33, 1270–1278. [Google Scholar] [CrossRef] [Green Version]
- Rivlin, M.; Horev, J.; Tsarfaty, I.; Navon, G. Molecular imaging of tumors and metastases using chemical exchange saturation transfer (CEST) MRI. Sci. Rep. 2013, 3, 3045. [Google Scholar] [CrossRef] [Green Version]
- Rivlin, M.; Navon, G. CEST MRI of 3-O-methyl-D-glucose on different breast cancer models. Magn. Reson. Med. 2018, 79, 1061–1069. [Google Scholar] [CrossRef]
- Rivlin, M.; Tsarfaty, I.; Navon, G. Functional molecular imaging of tumors by chemical exchange saturation transfer MRI of 3-O-Methyl-D-glucose. Magn. Reson. Med. 2014, 72, 1375–1380. [Google Scholar] [CrossRef]
- Rivlin, M.; Navon, G. Glucosamine and N-acetyl glucosamine as new CEST MRI agents for molecular imaging of tumors. Sci. Rep. 2016, 6, 32648. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Zhang, S.; Fujiwara, K.; Zhang, J.; Li, Y.; Liu, J.; van Zijl, P.C.M.; Lu, Z.R.; Zheng, L.; Liu, G. Extradomain-B Fibronectin-Targeted Dextran-Based Chemical Exchange Saturation Transfer Magnetic Resonance Imaging Probe for Detecting Pancreatic Cancer. Bioconjug. Chem. 2019, 30, 1425–1433. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Liu, D.; Li, Y.; Xu, X.; Xu, J.; Yadav, N.N.; Zhou, S.; van Zijl, P.C.M.; Liu, G. CEST MRI monitoring of tumor response to vascular disrupting therapy using high molecular weight dextrans. Magn. Reson. Med. 2019, 82, 1471–1479. [Google Scholar] [CrossRef]
- Li, Y.; Qiao, Y.; Chen, H.; Bai, R.; Staedtke, V.; Han, Z.; Xu, J.; Chan, K.W.Y.; Yadav, N.; Bulte, J.W.M.; et al. Characterization of tumor vascular permeability using natural dextrans and CEST MRI. Magn. Reson. Med. 2018, 79, 1001–1009. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Banerjee, S.R.; Yang, X.; Yadav, N.; Lisok, A.; Jablonska, A.; Xu, J.; Li, Y.; Pomper, M.G.; van Zijl, P. A dextran-based probe for the targeted magnetic resonance imaging of tumours expressing prostate-specific membrane antigen. Nat. Biomed Eng. 2017, 1, 977–982. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Liu, G. Sugar-based biopolymers as novel imaging agents for molecular magnetic resonance imaging. Wiley. Interdiscip. Rev. Nanomed. Nanobiotechnol. 2019, 11, e1551. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Bettegowda, C.; Qiao, Y.; Staedtke, V.; Chan, K.W.; Bai, R.; Li, Y.; Riggins, G.J.; Kinzler, K.W.; Bulte, J.W.; et al. Noninvasive imaging of infection after treatment with tumor-homing bacteria using Chemical Exchange Saturation Transfer (CEST) MRI. Magn. Reson. Med. 2013, 70, 1690–1698. [Google Scholar] [CrossRef] [Green Version]
- Herz, K.; Lindig, T.; Deshmane, A.; Schittenhelm, J.; Skardelly, M.; Bender, B.; Ernemann, U.; Scheffler, K.; Zaiss, M. T1rho-based dynamic glucose-enhanced (DGErho) MRI at 3 T: Method development and early clinical experience in the human brain. Magn. Reson. Med. 2019, 82, 1832–1847. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.; Sehgal, A.A.; Yadav, N.N.; Laterra, J.; Blair, L.; Blakeley, J.; Seidemo, A.; Coughlin, J.M.; Pomper, M.G.; Knutsson, L.; et al. d-glucose weighted chemical exchange saturation transfer (glucoCEST)-based dynamic glucose enhanced (DGE) MRI at 3T: Early experience in healthy volunteers and brain tumor patients. Magn. Reson. Med. 2020, 84, 247–262. [Google Scholar] [CrossRef]
- Walczak, P.; Wojtkiewicz, J.; Nowakowski, A.; Habich, A.; Holak, P.; Xu, J.; Adamiak, Z.; Chehade, M.; Pearl, M.S.; Gailloud, P.; et al. Real-time MRI for precise and predictable intra-arterial stem cell delivery to the central nervous system. J. Cereb. Blood Flow Metab. 2017, 37, 2346–2358. [Google Scholar] [CrossRef] [Green Version]
- Liu, G.; Gilad, A.A.; Bulte, J.W.; van Zijl, P.C.; McMahon, M.T. High-throughput screening of chemical exchange saturation transfer MR contrast agents. Contrast Media Mol. Imaging 2010, 5, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Gillen, J.; Landman, B.A.; Zhou, J.; van Zijl, P.C. Water saturation shift referencing (WASSR) for chemical exchange saturation transfer (CEST) experiments. Magn. Reson. Med. 2009, 61, 1441–1450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Li, Y.; Slania, S.; Yadav, N.N.; Liu, J.; Wang, R.; Zhang, J.; Pomper, M.G.; van Zijl, P.C.; Yang, X.; et al. Phenols as Diamagnetic T2 -Exchange Magnetic Resonance Imaging Contrast Agents. Chemistry (Easton) 2018, 24, 1259–1263. [Google Scholar]
- Cloyd, J.C.; Snyder, B.D.; Cleeremans, B.; Bundlie, S.R.; Blomquist, C.H.; Lakatua, D.J. Mannitol pharmacokinetics and serum osmolality in dogs and humans. J. Pharmacol. Exp. Ther. 1986, 236, 301–306. [Google Scholar] [PubMed]
- Better, O.S.; Rubinstein, I.; Winaver, J.M.; Knochel, J.P. Mannitol therapy revisited (1940–1997). Kidney Int. 1997, 52, 886–894. [Google Scholar] [CrossRef] [Green Version]
- Obach, R.S.; Baxter, J.G.; Liston, T.E.; Silber, B.M.; Jones, B.C.; MacIntyre, F.; Rance, D.J.; Wastall, P. The prediction of human pharmacokinetic parameters from preclinical and in vitro metabolism data. J. Pharmacol. Exp. Ther. 1997, 283, 46–58. [Google Scholar]
- Chen, L.; Zeng, H.; Xu, X.; Yadav, N.N.; Cai, S.; Puts, N.A.; Barker, P.B.; Li, T.; Weiss, R.G.; van Zijl, P.C.M.; et al. Investigation of the contribution of total creatine to the CEST Z-spectrum of brain using a knockout mouse model. NMR Biomed. 2017, 30, e3834. [Google Scholar] [CrossRef]
- Miller, C.O.; Cao, J.; Chekmenev, E.Y.; Damon, B.M.; Cherrington, A.D.; Gore, J.C. Noninvasive measurements of glycogen in perfused mouse livers using chemical exchange saturation transfer NMR and comparison to (13)C NMR spectroscopy. Anal. Chem. 2015, 87, 5824–5830. [Google Scholar] [CrossRef]
- Zaiss, M.; Xu, J.; Goerke, S.; Khan, I.S.; Singer, R.J.; Gore, J.C.; Gochberg, D.F.; Bachert, P. Inverse Z-spectrum analysis for spillover-, MT-, and T1 -corrected steady-state pulsed CEST-MRI--application to pH-weighted MRI of acute stroke. NMR Biomed. 2014, 27, 240–252. [Google Scholar] [CrossRef] [Green Version]
- Lee, D.H.; Heo, H.Y.; Zhang, K.; Zhang, Y.; Jiang, S.; Zhao, X.; Zhou, J. Quantitative assessment of the effects of water proton concentration and water T1 changes on amide proton transfer (APT) and nuclear overhauser enhancement (NOE) MRI: The origin of the APT imaging signal in brain tumor. Magn. Reson. Med. 2017, 77, 855–863. [Google Scholar] [CrossRef] [Green Version]
- Wu, R.; Liu, C.M.; Liu, P.K.; Sun, P.Z. Improved measurement of labile proton concentration-weighted chemical exchange rate (k(ws)) with experimental factor-compensated and T(1) -normalized quantitative chemical exchange saturation transfer (CEST) MRI. Contrast Media Mol. Imaging 2012, 7, 384–389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zaiss, M. CEST Sources. 2014. Available online: http://www.cest-sources.org (accessed on 1 February 2022).
- Bie, C.; Li, Y.; Zhou, Y.; Bhujwalla, Z.M.; Song, X.; Liu, G.; van Zijl, P.C.M.; Yadav, N.N. Deep learning-based classification of preclinical breast cancer tumor models using chemical exchange saturation transfer magnetic resonance imaging. NMR Biomed. 2022, 35, e4626. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.M.; Mougin, O.E.; Carradus, A.J.; Geades, N.; Dury, R.; Morley, W.; Gowland, P.A. The z-spectrum from human blood at 7T. NeuroImage 2018, 167, 31–40. [Google Scholar] [CrossRef]
- van Zijl, P.C.M.; Lam, W.W.; Xu, J.; Knutsson, L.; Stanisz, G.J. Magnetization Transfer Contrast and Chemical Exchange Saturation Transfer MRI. Features and analysis of the field-dependent saturation spectrum. NeuroImage 2018, 168, 222–241. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Xu, J.; Chan, K.W.Y.; Liu, J.; Liu, H.; Li, Y.; Chen, L.; Liu, G.; van Zijl, P.C.M. GlucoCEST imaging with on-resonance variable delay multiple pulse (onVDMP) MRI. Magn. Reson. Med. 2019, 81, 47–56. [Google Scholar] [CrossRef] [Green Version]
- Wishart, D.S.; Feunang, Y.D.; Marcu, A.; Guo, A.C.; Liang, K.; Vazquez-Fresno, R.; Sajed, T.; Johnson, D.; Li, C.; Karu, N.; et al. HMDB 4.0: The human metabolome database for 2018. Nucleic Acids Res. 2018, 46, D608–D617. [Google Scholar] [CrossRef] [PubMed]
- Yadav, N.N.; Xu, J.; Bar-Shir, A.; Qin, Q.; Chan, K.W.; Grgac, K.; Li, W.; McMahon, M.T.; van Zijl, P.C. Natural D-glucose as a biodegradable MRI relaxation agent. Magn. Reson. Med. 2014, 72, 823–828. [Google Scholar] [CrossRef] [Green Version]
- Xin, L.; Tkac, I. A practical guide to in vivo proton magnetic resonance spectroscopy at high magnetic fields. Anal. Biochem. 2017, 529, 30–39. [Google Scholar] [CrossRef]
- Hall, C.; Lueshen, E.; Mosat, A.; Linninger, A.A. Interspecies scaling in pharmacokinetics: A novel whole-body physiologically based modeling framework to discover drug biodistribution mechanisms in vivo. J. Pharm. Sci. 2012, 101, 1221–1241. [Google Scholar] [CrossRef]
- Torre-Healy, A.; Marko, N.F.; Weil, R.J. Hyperosmolar therapy for intracranial hypertension. Neurocrit. Care 2012, 17, 117–130. [Google Scholar] [CrossRef]
- Goldwasser, P.; Fotino, S. Acute renal failure following massive mannitol infusion. Appropriate response of tubuloglomerular feedback? Arch. Intern. Med. 1984, 144, 2214–2216. [Google Scholar] [CrossRef] [PubMed]
- Whelan, T.V.; Bacon, M.E.; Madden, M.; Patel, T.G.; Handy, R. Acute renal failure associated with mannitol intoxication. Report of a case. Arch. Intern. Med. 1984, 144, 2053–2055. [Google Scholar] [CrossRef] [PubMed]
- Perez-Perez, A.J.; Pazos, B.; Sobrado, J.; Gonzalez, L.; Gandara, A. Acute renal failure following massive mannitol infusion. Am. J. Nephrol. 2002, 22, 573–575. [Google Scholar] [CrossRef]
- Joshi, S.; Meyers, P.M.; Ornstein, E. Intracarotid delivery of drugs: The potential and the pitfalls. Anesthesiology 2008, 109, 543–564. [Google Scholar] [CrossRef] [Green Version]
- Siegal, T.; Rubinstein, R.; Bokstein, F.; Schwartz, A.; Lossos, A.; Shalom, E.; Chisin, R.; Gomori, J.M. In vivo assessment of the window of barrier opening after osmotic blood-brain barrier disruption in humans. J. Neurosurg. 2000, 92, 599–605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beluomini, M.A.; da Silva, J.L.; Sedenho, G.C.; Stradiotto, N.R. D-mannitol sensor based on molecularly imprinted polymer on electrode modified with reduced graphene oxide decorated with gold nanoparticles. Talanta 2017, 165, 231–239. [Google Scholar] [CrossRef]
- Jin, T.; Mehrens, H.; Hendrich, K.S.; Kim, S.G. Mapping brain glucose uptake with chemical exchange-sensitive spin-lock magnetic resonance imaging. J. Cereb. Blood Flow Metab. 2014, 34, 1402–1410. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.; van Zijl, P.C.M.; Han, X.; Dong, C.M.; Cheng, G.W.Y.; Tse, K.H.; Knutsson, L.; Chen, L.; Lai, J.H.C.; Wu, E.X.; et al. Altered d-glucose in brain parenchyma and cerebrospinal fluid of early Alzheimer’s disease detected by dynamic glucose-enhanced MRI. Sci Adv. 2020, 6, eaba3884. [Google Scholar] [CrossRef]
- Bender, B.; Herz, K.; Deshmane, A.; Richter, V.; Tabatabai, G.; Schittenhelm, J.; Skardelly, M.; Scheffler, K.; Ernemann, U.; Kim, M.; et al. GLINT: GlucoCEST in neoplastic tumors at 3 T—Clinical results of GlucoCEST in gliomas. Magn. Reson. Mater. Phys. Biol. Med. 2021, 35, 11–85. [Google Scholar] [CrossRef]
- Li, Y.; Chen, H.; Xu, J.; Yadav, N.N.; Chan, K.W.; Luo, L.; McMahon, M.T.; Vogelstein, B.; van Zijl, P.C.; Zhou, S.; et al. CEST theranostics: Label-free MR imaging of anticancer drugs. Oncotarget 2016, 7, 6369–6378. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Han, Z.; Liu, G. Repurposing Clinical Agents for Chemical Exchange Saturation Transfer Magnetic Resonance Imaging: Current Status and Future Perspectives. Pharmaceuticals 2020, 14, 11. [Google Scholar] [CrossRef]
- Lock, L.L.; Li, Y.; Mao, X.; Chen, H.; Staedtke, V.; Bai, R.; Ma, W.; Lin, R.; Li, Y.; Liu, G.; et al. One-Component Supramolecular Filament Hydrogels as Theranostic Label-Free Magnetic Resonance Imaging Agents. ACS Nano 2017, 11, 797–805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, Y.; Zhang, J.; Qi, X.; Li, S.; Liu, G.; Siddhanta, S.; Barman, I.; Song, X.; McMahon, M.T.; Bulte, J.W.M. Furin-mediated intracellular self-assembly of olsalazine nanoparticles for enhanced magnetic resonance imaging and tumour therapy. Nat. Mater. 2019, 18, 1376–1383. [Google Scholar] [CrossRef] [PubMed]
- Ngen, E.J.; Bar-Shir, A.; Jablonska, A.; Liu, G.; Song, X.; Ansari, R.; Bulte, J.W.M.; Janowski, M.; Pearl, M.; Walczak, P.; et al. Imaging the DNA Alkylator Melphalan by CEST MRI: An Advanced Approach to Theranostics. Mol. Pharm. 2016, 13, 3043–3053. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Krishnamurthy, L.C.; Sun, P.Z. Brain pH Imaging and its Applications. Neuroscience 2021, 474, 51–62. [Google Scholar] [CrossRef]
- Randtke, E.A.; Chen, L.Q.; Pagel, M.D. The reciprocal linear QUEST analysis method facilitates the measurements of chemical exchange rates with CEST MRI. Contrast Media Mol Imaging 2014, 9, 252–258. [Google Scholar] [CrossRef] [Green Version]
- Zaiss, M.; Anemone, A.; Goerke, S.; Longo, D.L.; Herz, K.; Pohmann, R.; Aime, S.; Rivlin, M.; Navon, G.; Golay, X.; et al. Quantification of hydroxyl exchange of D-Glucose at physiological conditions for optimization of glucoCEST MRI at 3, 7 and 9.4 Tesla. NMR Biomed. 2019, 32, e4113. [Google Scholar] [CrossRef] [Green Version]
- Zaiss, M.; Angelovski, G.; Demetriou, E.; McMahon, M.T.; Golay, X.; Scheffler, K. QUESP and QUEST revisited—Fast and accurate quantitative CEST experiments. Magn. Reson. Med. 2018, 79, 1708–1721. [Google Scholar] [CrossRef]
- de Graaf, R.A.; Brown, P.B.; McIntyre, S.; Nixon, T.W.; Behar, K.L.; Rothman, D.L. High magnetic field water and metabolite proton T1 and T2 relaxation in rat brain in vivo. Magn. Reson. Med. 2006, 56, 386–394. [Google Scholar] [CrossRef]
- Stanisz, G.J.; Odrobina, E.E.; Pun, J.; Escaravage, M.; Graham, S.J.; Bronskill, M.J.; Henkelman, R.M. T1, T2 relaxation and magnetization transfer in tissue at 3T. Magn. Reson. Med. 2005, 54, 507–512. [Google Scholar] [CrossRef]
- Hills, B.; Cano, C.; Belton, P. Proton NMR relaxation studies of aqueous polysaccharide systems. Macromolecules 1991, 24, 2944–2950. [Google Scholar] [CrossRef]




| Hydroxyl | Amine/Guanidinium | Amide | NOE | MTC | |
|---|---|---|---|---|---|
| Proton concentration (mM) | 45 | 100 | 72 | 100 | 5500 |
| Exchange rate (Hz) | 1400 | 1100 | 30 | 16 | 15 |
| Offset (ppm) | 0.9 | 2 | 3.5 | −3.5 | −2.3 |
| T2 (ms) | 55 | 170 | 100 | 5 | 9.1 × 10−3 |
| T1 (s) | 1 | 1 | 1 | 1 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Chu, C.; Zhang, J.; Bie, C.; Chen, L.; Aafreen, S.; Xu, J.; Kamson, D.O.; van Zijl, P.C.M.; Walczak, P.; et al. Label-Free Assessment of Mannitol Accumulation Following Osmotic Blood–Brain Barrier Opening Using Chemical Exchange Saturation Transfer Magnetic Resonance Imaging. Pharmaceutics 2022, 14, 2529. https://doi.org/10.3390/pharmaceutics14112529
Liu J, Chu C, Zhang J, Bie C, Chen L, Aafreen S, Xu J, Kamson DO, van Zijl PCM, Walczak P, et al. Label-Free Assessment of Mannitol Accumulation Following Osmotic Blood–Brain Barrier Opening Using Chemical Exchange Saturation Transfer Magnetic Resonance Imaging. Pharmaceutics. 2022; 14(11):2529. https://doi.org/10.3390/pharmaceutics14112529
Chicago/Turabian StyleLiu, Jing, Chengyan Chu, Jia Zhang, Chongxue Bie, Lin Chen, Safiya Aafreen, Jiadi Xu, David O. Kamson, Peter C. M. van Zijl, Piotr Walczak, and et al. 2022. "Label-Free Assessment of Mannitol Accumulation Following Osmotic Blood–Brain Barrier Opening Using Chemical Exchange Saturation Transfer Magnetic Resonance Imaging" Pharmaceutics 14, no. 11: 2529. https://doi.org/10.3390/pharmaceutics14112529
APA StyleLiu, J., Chu, C., Zhang, J., Bie, C., Chen, L., Aafreen, S., Xu, J., Kamson, D. O., van Zijl, P. C. M., Walczak, P., Janowski, M., & Liu, G. (2022). Label-Free Assessment of Mannitol Accumulation Following Osmotic Blood–Brain Barrier Opening Using Chemical Exchange Saturation Transfer Magnetic Resonance Imaging. Pharmaceutics, 14(11), 2529. https://doi.org/10.3390/pharmaceutics14112529









