Gut Dysbiosis and Diabetic Foot Ulcer: Role of Probiotics
Abstract
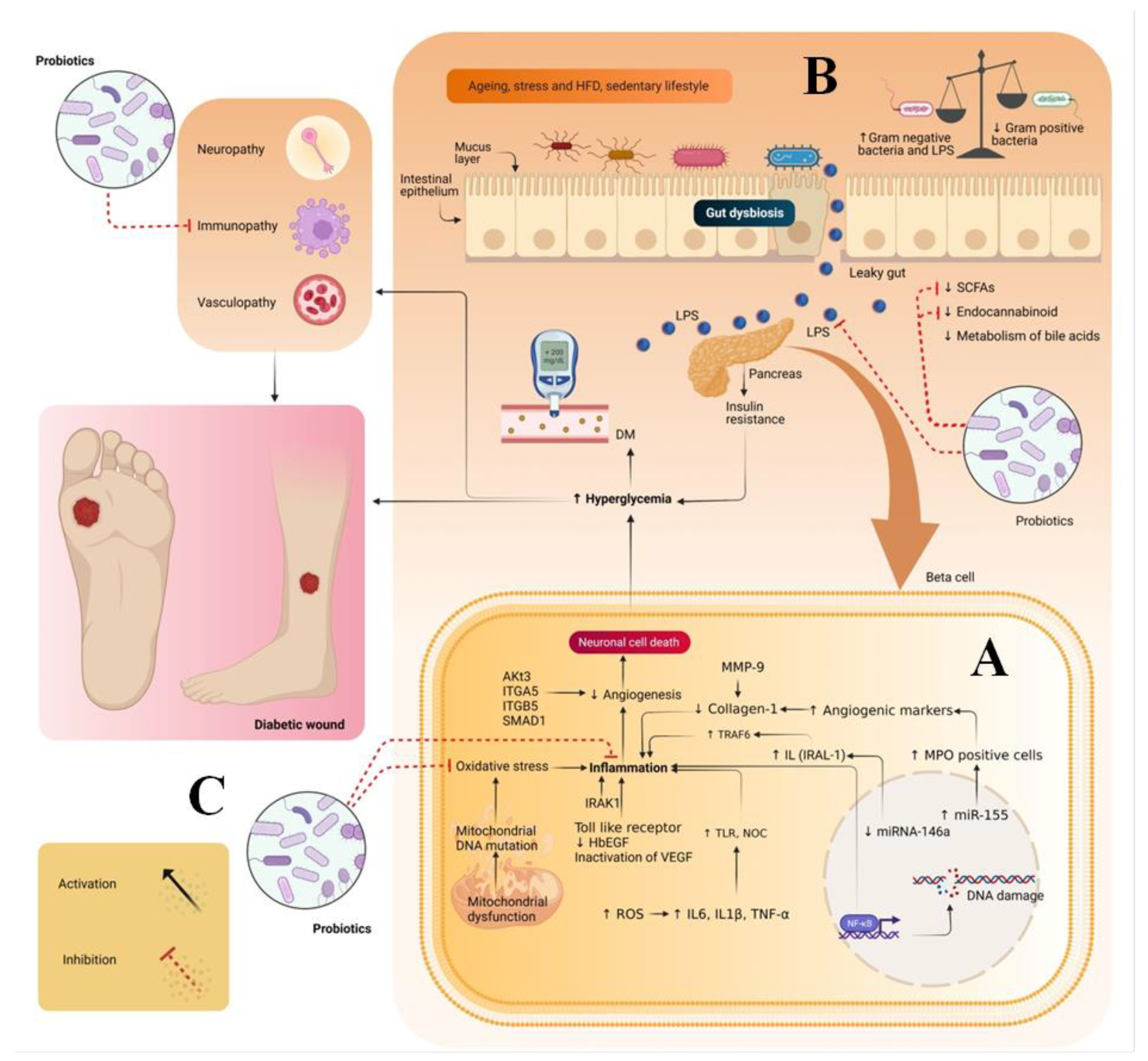
1. Introduction
2. Pathogenesis of Diabetic Wounds
3. Gut Dysbiosis and DW
4. Sources of Probiotics
5. Therapeutic Potential of Probiotics in Treating DW
6. Techniques Used for the Stabilization of Probiotics
- Freeze drying—Advantages: (i) Easy and convenient; (ii) Does not require freezing conditions. Disadvantages: Lengthy and expensive.
- Spray drying—Advantages: (i) Fast drying process; (ii) Powdered material obtained directly; (iii) Simple and easy to alter drying conditions; (iv) High production efficiency. Disadvantages: (i) Costly; (ii) An excessive amount of air is needed to increase the power consumption; (i) Equipment is complex; (ii) C overs large area.
- Fluidized bed dryer—Advantages: (i) High thermal efficiency; (ii) Handling time is short; (iii) It is possible to the materials in a shorter time. Disadvantages: (i) Chance of attrition of materials; (ii) Many organic powders develop electrostatic charge during drying.
- Extrusion—Advantages: (i) Low cost; (ii) Flexible. Disadvantages: (i) Size variances; (ii) Product limitation
- Microencapsulation—Advantages: (i) Protects materials from external stress; (ii) It is possible to prepare sustained and controlled release formulations. Disadvantages: (i) High cost; (ii) Non uniform coating effect the release profile of the active moiety in the body.
7. Market Status of Probiotics
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Awasthi, A.; Gulati, M.; Kumar, B.; Kaur, J.; Vishwas, S.; Khursheed, R.; Porwal, O.; Alam, A.; Kr, A.; Corrie, L.; et al. Review Article Recent Progress in Development of Dressings Used for Diabetic Wounds with Special Emphasis on Scaffolds. BioMed Res. Int. 2022, 2022, 1659338. [Google Scholar] [CrossRef] [PubMed]
- Awasthi, A.; Singh, S.K.; Kumar, B.; Gulati, M.; Kumar, R.; Wadhwa, S.; Khursheed, R.; Corrie, L.; KR, A.; Kumar, R.; et al. Treatment Strategies Against Diabetic Foot Ulcer: Success so Far and the Road Ahead. Curr. Diabetes Rev. 2020, 17, 421–436. [Google Scholar] [CrossRef] [PubMed]
- Awasthi, A.; Vishwas, S.; Gulati, M.; Corrie, L.; Kaur, J.; Khursheed, R.; Alam, A.; Alkhayl, F.F.A.; Khan, F.R.; Nagarethinam, S.; et al. Expanding Arsenal against Diabetic Wounds Using Nanomedicines and Nanomaterials: Success so Far and Bottlenecks. J. Drug Deliv. Sci. Technol. 2022, 74, 103534. [Google Scholar] [CrossRef]
- Yahfoufi, N.; Mallet, J.F.; Graham, E.; Matar, C. Role of Probiotics and Prebiotics in Immunomodulation. Curr. Opin. Food Sci. 2018, 20, 82–91. [Google Scholar] [CrossRef]
- Swain, M.R.; Anandharaj, M.; Ray, R.C.; Parveen Rani, R. Fermented Fruits and Vegetables of Asia: A Potential Source of Probiotics. Biotechnol. Res. Int. 2014, 2014, 250424. [Google Scholar] [CrossRef]
- James, A.; Wang, Y. Characterization, Health Benefits and Applications of Fruits and Vegetable Probiotics. CYTA—J. Food 2019, 17, 770–780. [Google Scholar] [CrossRef]
- Patel, A.R. Probiotic Fruit and Vegetable Juices- Recent Advances and Future Perspective. Int. Food Res. J. 2017, 24, 1850–1857. [Google Scholar]
- Plaza-Diaz, J.; Ruiz-Ojeda, F.J.; Gil-Campos, M.; Gil, A. Mechanisms of Action of Probiotics. Adv. Nutr. 2019, 10, S49–S66. [Google Scholar] [CrossRef]
- Tavakoli, M.; Habibi Najafi, M.B.; Mohebbi, M. Effect of the Milk Fat Content and Starter Culture Selection on Proteolysis and Antioxidant Activity of Probiotic Yogurt. Heliyon 2019, 5, e01204. [Google Scholar] [CrossRef]
- Sharma, S.; Singh, A.; Sharma, S.; Kant, A.; Sevda, S.; Taherzadeh, M.J.; Garlapati, V.K. Functional Foods as a Formulation Ingredients in Beverages: Technological Advancements and Constraints. Bioengineered 2021, 12, 11055–11075. [Google Scholar] [CrossRef]
- Azad, M.A.K.; Sarker, M.; Wan, D. Immunomodulatory Effects of Probiotics on Cytokine Profiles. Biomed Res. Int. 2018, 2018, 8063647. [Google Scholar] [CrossRef]
- Pegah, A.; Abbasi-Oshaghi, E.; Khodadadi, I.; Mirzaei, F.; Tayebinia, H. Probiotic and Resveratrol Normalize GLP-1 Levels and Oxidative Stress in the Intestine of Diabetic Rats. Metab. Open 2021, 10, 100093. [Google Scholar] [CrossRef]
- Denkova, R.; Goranov, B.; Teneva, D.; Kostov, G. Antimicrobial Activity of Probiotic Microorganisms: Mechanisms of Interaction and Methods of Examination, 1st ed.; Z.-Mendez Villas: Polvdiv, Bulgaria, 2017; pp. 201–212. [Google Scholar]
- Oh, B.T.; Jeong, S.Y.; Velmurugan, P.; Park, J.H.; Jeong, D.Y. Probiotic-Mediated Blueberry (Vaccinium Corymbosum L.) Fruit Fermentation to Yield Functionalized Products for Augmented Antibacterial and Antioxidant Activity. J. Biosci. Bioeng. 2017, 124, 542–550. [Google Scholar] [CrossRef]
- Helmy, S.A. Chemical Composition and Antimicrobial Activity of Some Essential Oils and Their Major Constituents. Int. J. Acad. Res. 2012, 4, 124–137. [Google Scholar] [CrossRef]
- Li, A.; Wang, Y.; Li, Z.; Qamar, H.; Mehmood, K.; Zhang, L.; Liu, J.; Zhang, H.; Li, J. Probiotics Isolated from Yaks Improves the Growth Performance, Antioxidant Activity, and Cytokines Related to Immunity and Inflammation in Mice. Microb. Cell Fact. 2019, 18, 112. [Google Scholar] [CrossRef]
- Abdhul, K.; Ganesh, M.; Shanmughapriya, S.; Kanagavel, M.; Anbarasu, K.; Natarajaseenivasan, K. Antioxidant activity of exopolysaccharide from probiotic strain Enterococcus faecium (BDU7) from Ngari. Int. J. Biol. Macromol. 2014, 70, 450–454. [Google Scholar] [CrossRef]
- Shori, A.B.; Aljohani, G.S.; Al-zahrani, A.J.; Al-sulbi, O.S.; Baba, A.S. Viability of Probiotics and Antioxidant Activity of Cashew Milk-Based Yogurt Fermented with Selected Strains of Probiotic lactobacillus Spp. LWT 2022, 153, 112482. [Google Scholar] [CrossRef]
- Das, D.; Goyal, A. Antioxidant Activity and γ-Aminobutyric Acid (GABA) Producing Ability of Probiotic Lactobacillus plantarum DM5 Isolated from Marcha of Sikkim. LWT—Food Sci. Technol. 2015, 61, 263–268. [Google Scholar] [CrossRef]
- Uugantsetseg, E.; Batjargal, B. Antioxidant Activity of Probiotic Lactic Acid Bacteria Isolated from Mongolian Airag. Mong. J. Chem. 2014, 15, 73–78. [Google Scholar] [CrossRef]
- Mäkelä, S.M.; Forssten, S.D.; Kailajärvi, M.; Langén, V.L.; Scheinin, M.; Tiihonen, K.; Ouwehand, A.C. Effects of Bifidobacterium animalis Ssp. Lactis 420 on Gastrointestinal Inflammation Induced by a Nonsteroidal Anti-Inflammatory Drug: A Randomized, Placebo-Controlled, Double-Blind Clinical Trial. Br. J. Clin. Pharmacol. 2021, 87, 4625–4635. [Google Scholar] [CrossRef]
- Shadnoush, M.; Hosseini, R.S.; Khalilnezhad, A.; Navai, L.; Goudarzi, H.; Vaezjalali, M. Effects of Probiotics on Gut Microbiota in Patients with Inflammatory Bowel Disease: A Double-Blind, Placebo-Controlled Clinical Trial. Korean J. Gastroenterol. 2015, 65, 215–221. [Google Scholar] [CrossRef]
- Babadi, M.; Khorshidi, A.; Aghadavood, E.; Samimi, M.; Kavossian, E.; Bahmani, F.; Mafi, A.; Shafabakhsh, R.; Satari, M.; Asemi, Z. The Effects of Probiotic Supplementation on Genetic and Metabolic Profiles in Patients with Gestational Diabetes Mellitus: A Randomized, Double-Blind, Placebo-Controlled Trial. Probiotics Antimicrob. Proteins 2019, 11, 1227–1235. [Google Scholar] [CrossRef]
- Zaharuddin, L.; Mokhtar, N.M.; Muhammad Nawawi, K.N.; Raja Ali, R.A. A Randomized Double-Blind Placebo-Controlled Trial of Probiotics in Post-Surgical Colorectal Cancer. BMC Gastroenterol. 2019, 19, 131. [Google Scholar] [CrossRef]
- Trial, A.A.R.P. Nutrients Effects of 12-Week Ingestion of Yogurt Containing Lactobacillus Plantarum OLL2712 on Glucose Metabolism and Chronic Inflammation in Prediabetic Adults: A Randomized Placebo-Controlled Trial. Nutrients. 2020, 12, 374. [Google Scholar] [CrossRef]
- Choi, M.; Lee, Y.; Lee, N.K.; Bae, C.H.; Park, D.C.; Paik, H.D.; Park, E. Immunomodulatory Effects by Bifidobacterium longum KACC 91563 in Mouse Splenocytes and Macrophages. J. Microbiol. Biotechnol. 2019, 29, 1739–1744. [Google Scholar] [CrossRef]
- Enani, S.M.; Childs, C.E.; Przemska, A.; Maidens, C.; Dong, H.; Rowland, I.; Tuohy, K.; Todd, S.; Gosney, M.; Yaqoob, P. Effects of a Novel Probiotic, Bifidobacterium longum Bv. Infantis CCUG 52486 with Prebiotic on the B-Cell Response to Influenza Vaccination. Proc. Nutr. Soc. 2014, 73, 52486. [Google Scholar] [CrossRef]
- Dong, H.; Rowland, I.; Thomas, L.V.; Yaqoob, P. Immunomodulatory Effects of a Probiotic Drink Containing Lactobacillus casei Shirota in Healthy Older Volunteers. Eur. J. Nutr. 2013, 52, 1853–1863. [Google Scholar] [CrossRef]
- Villena, J.; Salva, S.; Agüero, G.; Alvarez, S. Immunomodulatory and Protective Effect of Probiotic Lactobacillus casei against Candida Albicans Infection in Malnourished Mice. Microbiol. Immunol. 2011, 55, 434–445. [Google Scholar] [CrossRef]
- D’ambrosio, S.; Ventrone, M.; Fusco, A.; Casillo, A.; Dabous, A.; Cammarota, M.; Corsaro, M.M.; Donnarumma, G.; Schiraldi, C.; Cimini, D. Limosilactobacillus fermentum from Buffalo Milk Is Suitable for Potential Biotechnological Process Development and Inhibits Helicobacter Pylori in a Gastric Epithelial Cell Model. Biotechnol. Rep. 2022, 34, e00732. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, X.; Guo, J.; He, Q.; Li, H.; Song, Y.; Zhang, H. Lactobacillus casei Reduces Susceptibility to Type 2 Diabetes via Microbiota-Mediated Body Chloride Ion Influx. Sci. Rep. 2014, 4, 5654. [Google Scholar] [CrossRef]
- Sharma, P.; Bhardwaj, P.; Singh, R. Administration of Lactobacillus casei and Bifidobacterium Bifidum Ameliorated Hyperglycemia, Dyslipidemia, and Oxidative Stress in Diabetic Rats. Int. J. Prev. Med. 2016, 7, 102. [Google Scholar] [CrossRef]
- Asgharzadeh, F.; Tanomand, A.; Ashoori, M.R.; Asgharzadeh, A.; Zarghami, N. Investigating the Effects of Lactobacillus casei on Some Biochemical Parameters in Diabetic Mice. J. Endocrinol. Metab. Diabetes S. Afr. 2017, 22, 47–50. [Google Scholar] [CrossRef]
- Wang, G.; Li, X.; Zhao, J.; Zhang, H.; Chen, W. Lactobacillus casei CCFM419 Attenuates Type 2 Diabetes via a Gut Microbiota Dependent Mechanism. Food Funct. 2017, 8, 3155–3164. [Google Scholar] [CrossRef]
- Niibo, M.; Shirouchi, B.; Umegatani, M.; Morita, Y.; Ogawa, A.; Sakai, F.; Kadooka, Y.; Sato, M. Probiotic Lactobacillus gasseri SBT2055 Improves Insulin Secretion in a Diabetic Rat Model. J. Dairy Sci. 2019, 102, 997–1006. [Google Scholar] [CrossRef]
- Li, X.; Wang, N.; Yin, B.; Fang, D.; Jiang, T.; Fang, S.; Zhao, J.; Zhang, H.; Wang, G.; Chen, W. Effects of Lactobacillus plantarum CCFM0236 on Hyperglycaemia and Insulin Resistance in High-Fat and Streptozotocin-Induced Type 2 Diabetic Mice. J. Appl. Microbiol. 2016, 121, 1727–1736. [Google Scholar] [CrossRef]
- Lee, E.; Jung, S.R.; Lee, S.Y.; Lee, N.K.; Paik, H.D.; Lim, S. Il Lactobacillus plantarum Strain Ln4 Attenuates Diet-Induced Obesity, Insulin Resistance, and Changes in Hepatic MRNA Levels Associated with Glucose and Lipid Metabolism. Nutrients 2018, 10, 643. [Google Scholar] [CrossRef]
- Balakumar, M.; Prabhu, D.; Sathishkumar, C.; Prabu, P.; Rokana, N.; Kumar, R.; Raghavan, S.; Soundarajan, A.; Grover, S.; Batish, V.K.; et al. Improvement in Glucose Tolerance and Insulin Sensitivity by Probiotic Strains of Indian Gut Origin in High-Fat Diet-Fed C57BL/6J Mice. Eur. J. Nutr. 2018, 57, 279–295. [Google Scholar] [CrossRef]
- Bagarolli, R.A.; Tobar, N.; Oliveira, A.G.; Araújo, T.G.; Carvalho, B.M.; Rocha, G.Z.; Vecina, J.F.; Calisto, K.; Guadagnini, D.; Prada, P.O.; et al. Probiotics Modulate Gut Microbiota and Improve Insulin Sensitivity in DIO Mice. J. Nutr. Biochem. 2017, 50, 16–25. [Google Scholar] [CrossRef]
- Peral, M.C.; Rachid, M.M.; Gobbato, N.M.; Huaman Martinez, M.A.; Valdez, J.C. Interleukin-8 Production by Polymorphonuclear Leukocytes from Patients with Chronic Infected Leg Ulcers Treated with Lactobacillus plantarum. Clin. Microbiol. Infect. 2010, 16, 281–286. [Google Scholar] [CrossRef]
- Ahmadi Majd, S.; Khorasgani, M.R.; Talebi, A. Study of Diabetic Cutaneous Wound Healing in Rats Treated with Lactobacillus casei and Its Exopolysaccharide. Int. J. Adv. Biotechnol. Res. 2016, 7, 2083–2092. [Google Scholar]
- Mohseni, S.; Bayani, M.; Bahmani, F.; Tajabadi-Ebrahimi, M.; Bayani, M.A.; Jafari, P.; Asemi, Z. The Beneficial Effects of Probiotic Administration on Wound Healing and Metabolic Status in Patients with Diabetic Foot Ulcer: A Randomized, Double-Blind, Placebo-Controlled Trial. Diabetes. Metab. Res. Rev. 2018, 34, e2970. [Google Scholar] [CrossRef]
- González, M.P.B.; Quiñones-Gutiérrez, Y. Antibiosis of Cefotaxime/Clindamycin and Lactobacillus acidophilus on Related Bacteria to Diabetic Foot Ulcer. Food Nutr. Sci. 2018, 9, 277–289. [Google Scholar] [CrossRef]
- Salaran, M.; Oryan, A.; Nikahval, B.; Kamali, A.; Ghaemi, M.; Abbasi-Teshnizi, F.; Azizzadeh, M. Topical Application of Lactobacillus plantarum on Burn Wound Healing in Diabetic Rats. Iran. J. Vet. Surg. 2019, 14, 60–72. [Google Scholar] [CrossRef]
- Venosi, S.; Ceccarelli, G.; De Angelis, M.; Laghi, L.; Bianchi, L.; Martinelli, O.; Maruca, D.; Cavallari, E.N.; Toscanella, F.; Vassalini, P.; et al. Infected Chronic Ischemic Wound Topically Treated with a Multi-Strain Probiotic Formulation: A Novel Tailored Treatment Strategy. J. Transl. Med. 2019, 17, 364. [Google Scholar] [CrossRef]
- Chuang, Y.C.; Cheng, M.C.; Lee, C.C.; Chiou, T.Y.; Tsai, T.Y. Effect of Ethanol Extract from Lactobacillus plantarum TWK10-Fermented Soymilk on Wound Healing in Streptozotocin-Induced Diabetic Rat. AMB Express 2019, 9, 163. [Google Scholar] [CrossRef]
- Kumari, D.; Khan, H.; Jiskani, A.R.; Rafique, M.; Asif, M.; Kumar, V.; Maqsood, S. Neovascularization: Topical Effects of Streptococcus thermophilus and Low Level Laser Therapy in Treatment of Diabetic Wound in Rats. Int. J. Res. Med. Sci. 2019, 7, 3357–3361. [Google Scholar] [CrossRef]
- Campos, L.F.; Tagliari, E.; Casagrande, T.A.C.; de Noronha, L.; Campos, A.C.L.; Matias, J.E.F. Effects of Probiotics Supplementation on Skin Wound Healing in Diabetic Rats. Arq. Bras. Cir. Dig. 2020, 33, 1–6. [Google Scholar] [CrossRef]
- Layus, B.I.; Gerez, C.L.; Rodriguez, A.V. Antibacterial Activity of Lactobacillus plantarum CRL 759 Against Methicillin-Resistant Staphylococcus Aureus and Pseudomonas Aeruginosa. Arab. J. Sci. Eng. 2020, 45, 4503–4510. [Google Scholar] [CrossRef]
- Mohtashami, M.; Mohamadi, M.; Azimi-Nezhad, M.; Saeidi, J.; Nia, F.F.; Ghasemi, A. Lactobacillus bulgaricus and Lactobacillus plantarum Improve Diabetic Wound Healing through Modulating Inflammatory Factors. Biotechnol. Appl. Biochem. 2021, 68, 1421–1431. [Google Scholar] [CrossRef]
- Kulkarni, S.; Haq, S.F.; Samant, S.; Sukumaran, S. Adaptation of Lactobacillus acidophilus to Thermal Stress Yields a Thermotolerant Variant Which Also Exhibits Improved Survival at PH 2. Probiotics Antimicrob. Proteins 2018, 10, 717–727. [Google Scholar] [CrossRef]
- Seyoum, Y.; Humblot, C.; Baxter, B.A.; Nealon, N.J.; Weber, A.M.; Ryan, E.P. Metabolomics of Rice Bran Differentially Impacted by Fermentation with Six Probiotics Demonstrates Key Nutrient Changes for Enhancing Gut Health. Front. Nutr. 2022, 8, 795334. [Google Scholar] [CrossRef]
- Rokka, S.; Rantamäki, P. Protecting Probiotic Bacteria by Microencapsulation: Challenges for Industrial Applications. Eur. Food Res. Technol. 2010, 231, 1–12. [Google Scholar] [CrossRef]
- Shah, N.P. Probiotic Bacteria: Selective Enumeration and Survival in Dairy Foods. J. Dairy Sci. 2000, 83, 894–907. [Google Scholar] [CrossRef]
- Krisch, J.; Kerekes, E.B.; Takó, M.; Vágvölgyi, C. Cell Immobilization for the Dairy Industry. In Microbial Fermentation and Enzyme Technology; Thatoi, H., Mohapatra, P.K.D., Mohapatra, S., Mondal, K.C., Eds.; CRC Press: Boca Raton, FL, USA, 2020; pp. 115–127. [Google Scholar] [CrossRef]
- Bampi, G.B.; Backes, G.T.; Cansian, R.L.; de Matos, F.E.; Ansolin, I.M.A.; Poleto, B.C.; Corezzolla, L.R.; Favaro-Trindade, C.S. Spray Chilling Microencapsulation of Lactobacillus acidophilus and Bifidobacterium animalis Subsp. Lactis and Its Use in the Preparation of Savory Probiotic Cereal Bars. Food Bioprocess Technol. 2016, 9, 1422–1428. [Google Scholar] [CrossRef]
- Rajagopal, V.; Ramaiyan, B. Encapsulation “The Future of Probiotics”—A Review. Adv. Biol. Res. 2021, 3, 96–103. [Google Scholar]
- Nag, A.; Das, S. Improving Ambient Temperature Stability of Probiotics with Stress Adaptation and Fluidized Bed Drying. J. Funct. Foods 2013, 5, 170–177. [Google Scholar] [CrossRef]
- Htwe, M.M.; Teanpaisan, R.; Khongkow, P.; Amnuaikit, T. Liposomes of Probiotic’s Lyophilized Cell Free Supernatant; A Potential Cosmeceutical Product. Pharmazie 2019, 74, 462–466. [Google Scholar] [CrossRef]
- Arepally, D.; Reddy, R.S.; Goswami, T.K.; Coorey, R. A Review on Probiotic Microencapsulation and Recent Advances of Their Application in Bakery Products. Food Bioprocess Technol. 2022, 15, 1677–1699. [Google Scholar] [CrossRef]
- Liliana, S.C.; Vladimir, V.C. Probiotic Encapsulation. Afr. J. Microbiol. Res. 2013, 7, 4743–4753. [Google Scholar] [CrossRef]
- Trilokia, M.; Campus Chatha, M.; Julie Bandral, I.D.; Ankita Chib, I.; Preeti Choudhary Agromet Observer, I.; Corresponding Author, I.; Bandral, J.D.; Chib, A.; Choudhary, P. Microencapsulation for Food: An Overview. Pharma Innov. J. 2022, 11, 1174–1180. [Google Scholar]
- Nedovic, V.; Kalusevic, A.; Manojlovic, V.; Levic, S.; Bugarski, B. An Overview of Encapsulation Technologies for Food Applications. Procedia Food Sci. 2011, 1, 1806–1815. [Google Scholar] [CrossRef]
- Oberoi, K.; Tolun, A.; Sharma, K.; Sharma, S. Microencapsulation: An Overview for the Survival of Probiotic Bacteria. J. Microbiol. Biotechnol. Food Sci. 2019, 9, 280–287. [Google Scholar] [CrossRef]
- Mutukumira, A.N.; Ang, J.; Lee, S.J. Microencapsulation of Probiotic Bacteria. Microbiol. Monographs 2015, 4, 63–80. [Google Scholar] [CrossRef]
- Šipailienė, A.; Petraitytė, S. Encapsulation of Probiotics: Proper Selection of the Probiotic Strain and the Influence of Encapsulation Technology and Materials on the Viability of Encapsulated Microorganisms. Probiotics Antimicrob. Proteins 2018, 10, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Damodharan, K.; Palaniyandi, S.A.; Yang, S.H.; Suh, J.W. Co-Encapsulation of Lactic Acid Bacteria and Prebiotic with Alginate-Fenugreek Gum-Locust Bean Gum Matrix: Viability of Encapsulated Bacteria under Simulated Gastrointestinal Condition and during Storage Time. Biotechnol. Bioprocess Eng. 2017, 22, 265–271. [Google Scholar] [CrossRef]
- Santillo, A.; Albenzio, M.; Bevilacqua, A.; Corbo, M.R.; Sevi, A. Encapsulation of Probiotic Bacteria in Lamb Rennet Paste: Effects on the Quality of Pecorino Cheese. J. Dairy Sci. 2012, 95, 3489–3500. [Google Scholar] [CrossRef] [PubMed]
- Moreno, J.S.; Dima, P.; Chronakis, I.S.; Mendes, A.C. Electrosprayed Ethyl Cellulose Core-Shell Microcapsules for the Encapsulation of Probiotics. Pharmaceutics 2022, 14, 7. [Google Scholar] [CrossRef]
- Borooah, B. Overview of Materials and Techniques for Encapsulation of Natural Products: A Mini-Review. Int. J. Pharm. Sci. Res. 2022, 13, 621–627. [Google Scholar] [CrossRef]
- Calinoiu, L.F.; Ştefanescu, B.E.; Pop, I.D.; Muntean, L.; Vodnar, D.C. Chitosan Coating Applications in Probiotic Microencapsulation. Coatings 2019, 9, 194. [Google Scholar] [CrossRef]
- Giraffa, G.; Gatti, M.; Rossetti, L.; Senini, L.; Neviani, E. Molecular Diversity within Lactobacillus helveticus as Revealed by Genotypic Characterization. Appl. Environ. Microbiol. 2000, 66, 1259–1265. [Google Scholar] [CrossRef]
- Wang, A.; Lin, J.; Zhong, Q. Spray-Coating as a Novel Strategy to Supplement Broiler Feed Pellets with Probiotic Lactobacillus salivarius NRRL B-30514. LWT 2021, 137, 110419. [Google Scholar] [CrossRef]
- Muhammad, Z.R.R.R.Z.M.Z. Resistant Starch-Based Edible Coating Composites for Digestion and Physicochemical Characteristics. Coatings 2021, 11, 587. [Google Scholar] [CrossRef]
- Mirzamani, S.S.; Bassiri, A.R.; Tavakolipour, H.; Azizi, M.H.; Kargozari, M. Survival of Fluidized Bed Encapsulated Lactobacillus acidophilus under Simulated Gastro-Intestinal Conditions and Heat Treatment during Bread Baking. J. Food Meas. Charact. 2021, 15, 5477–5484. [Google Scholar] [CrossRef]
- Tavakolipour, H.; Kargozari, M. Fluidized Bed Microencapsulation of Lactobacillus sporogenes with Some Selected Hydrocolloids for Probiotic Bread Production. J. Food Biosci. Technol. 2021, 11, 23–34. [Google Scholar]
- Meng, J.; Wang, Y.Y.; Hao, Y.P.; Zhang, S.B.; Ding, C.H.; You, Y.Z. Coating Function and Stabilizing Effects of Surface Layer Protein from Lactobacillus acidophilus ATCC 4356 on Liposomes. Int. J. Biol. Macromol. 2021, 183, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Sultana, M.; Chan, E.S.; Pushpamalar, J.; Choo, W.S. Advances in Extrusion-Dripping Encapsulation of Probiotics and Omega-3 Rich Oils. Trends Food Sci. Technol. 2022, 123, 69–86. [Google Scholar] [CrossRef]
- Afzaal, M.; Saeed, F.; Hussain, S.; Mohamed, A.A.; Alamri, M.S.; Ahmad, A.; Ateeq, H.; Tufail, T.; Hussain, M. Survival and Storage Stability of Encapsulated Probiotic under Simulated Digestion Conditions and on Dried Apple Snacks. Food Sci. Nutr. 2020, 8, 5392–5401. [Google Scholar] [CrossRef]
- Bhagwat, A.; Bhushette, P.; Annapure, U.S. Spray Drying Studies of Probiotic Enterococcus Strains Encapsulated with Whey Protein and Maltodextrin. Beni-Suef Univ. J. Basic Appl. Sci. 2020, 9, 33. [Google Scholar] [CrossRef]
- Zhou, H.; Li, S.; Chen, Y.; Zhang, Q.; Bai, X.; Zhu, C.; Liu, H.; Wang, L.; Wu, C.; Pan, X.; et al. Evaluation of Streptococcus thermophilus IFFI 6038 Microcapsules Prepared Using an Ultra-Fine Particle Processing System. AAPS PharmSciTech 2018, 19, 1020–1028. [Google Scholar] [CrossRef]
- Moghaddas Kia, E.; Ghasempour, Z.; Ghanbari, S.; Pirmohammadi, R.; Ehsani, A. Development of Probiotic Yogurt by Incorporation of Milk Protein Concentrate (MPC) and microencapsulated Lactobacillus paracasei in Gellan-Caseinate Mixture. Br. Food J. 2018, 120, 1516–1528. [Google Scholar] [CrossRef]
- Ester, B.; Noelia, B.; Laura, C.J.; Francesca, P.; Cristina, B.; Rosalba, L.; Marco, D.R. Probiotic Survival and in Vitro Digestion of L Salivarius Spp. Salivarius Encapsulated by High Homogenization Pressures and Incorporated into a Fruit Matrix. LTW 2019, 111, 883–888. [Google Scholar] [CrossRef]
- Thangrongthong, S.; Puttarat, N.; Ladda, B.; Itthisoponkul, T.; Pinket, W.; Kasemwong, K.; Taweechotipatr, M. Microencapsulation of Probiotic lactobacillus Brevis ST-69 Producing GABA Using Alginate Supplemented with Nanocrystalline Starch. Food Sci. Biotechnol. 2020, 29, 1475–1482. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Mukherjee, S.; Li, J.; Hou, W.; Pan, C.; Liu, J. Mucosal Immunity–Mediated Modulation of the Gut Microbiome by Oral Delivery of Probiotics into Peyer’s Patches. Sci. Adv. 2021, 7, abf0677. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.grandviewresearch.com/industry-analysis/probiotics-market (accessed on 10 August 2022).
- Available online: https://www.marketdataforecast.com/market-reports/north-america-probiotics-market (accessed on 10 August 2022).
- Available online: https://www.researchgate.net/figure/1-List-of-probiotic-products-available-in-the-market_tbl1_324371121 (accessed on 11 August 2022).
- Available online: https://www.livestrong.com/article/411592-ingredients-of-activia-yogurt/ (accessed on 11 August 2022).
- Available online: https://brainreference.com/adult-formula-cp-1-review/#:~:text=Adult%20Formula%20Cp-1%20contains%20the%20following%20ingredients%3A%20Adult,capsules.%203%29%20What%20Does%20Adult%20Formula%20Cp-1%20Do%3F (accessed on 11 August 2022).
- Available online: https://www.alignprobiotics.com/en-us/why-align/how-to-take-align-probiotic (accessed on 11 August 2022).
- Available online: https://celiac-disease.com/review-attune-probiotic-bars/ (accessed on 11 August 2022).
- Available online: https://biokplus.com/ (accessed on 11 August 2022).
- Available online: https://biokplus.com/products/bio-k-probiotic-capsules-daily-care-50b (accessed on 14 August 2022).
- Available online: https://in.iherb.com/pr/culturelle-probiotics-digestive-daily-probiotic-10-billion-cfus-30-once-daily-vegetarian-capsules/56049#:~:text=Take%20charge%20of%20your%20digestive%20health%20with%20Culturelle%C2%AE,balance%20your%20digestive%20system%2C%20plus%20the%20prebiotic%20inulin (accessed on 14 August 2022).
- Available online: https://oribalt.lt/en/25/gefilus (accessed on 14 August 2022).
- Available online: https://medical.gerber.com/faqs/infant-formula-faq#:~:text=Gerber%20%C2%AE%20Good%20Start%20%C2%AE%20Gentle%20formula%20has,the%20developing%20immune%20system%20in%20formula%20fed%20infants (accessed on 16 August 2022).
- Available online: https://www.verywellhealth.com/goodbelly-probiotic-juice-drinks-1945005 (accessed on 16 August 2022).
- Available online: https://scottspharmacy1.com/probiotic-by-one-wellness-place/ (accessed on 16 August 2022).
- Available online: https://swansoneurope.com/en/swanson-ultimate-probiotic-formula.htmL (accessed on 16 August 2022).
- Available online: https://shop.vsl3.com/products/vsl-3%C2%AE-450-packets-30-count (accessed on 18 August 2022).
- Available online: https://oureverydaylife.com/facts-about-yoplait-yogurt-12564843.htmL (accessed on 19 August 2022).
- Available online: https://patents.google.com/patent/WO2019180748A1/en?q=probiotic+wound+healing&oq=probiotic+for+wound+healing (accessed on 19 August 2022).
- Available online: https://link.springer.com/chapter/10.1007/978-981-15-8214-1_15 (accessed on 19 August 2022).
- Available online: https://patents.google.com/patent/US20180280312A1/en?q=probiotic+wound&oq=probiotic+for+wound (accessed on 21 August 2022).
- Available online: https://patents.google.com/patent/RU2401116C2/en?q=probiotic+wound+healing&oq=probiotic+for+wound+healing (accessed on 21 August 2022).
- Available online: https://patents.google.com/patent/CN102946891A/en?q=probiotic+wound+healing&oq=probiotic+for+wound+healing&page=14 (accessed on 21 August 2022).
- Available online: https://www.bing.com/search?q=KR20090023626A&cvid=81dc8b1b619244cd921c08e1e1848cb2&aqs=edge..69i57.922j0j4&FORM=ANAB01&PC=U531 (accessed on 21 August 2022).
- Available online: https://patents.google.com/patent/KR20090023626A/en?q=probiotic+wound&oq=probiotic+for+wound&page=4 (accessed on 21 August 2022).
- Available online: https://patents.google.com/patent/EP2450062A1/en?q=probiotic+wound&oq=probiotic+for+wound (accessed on 24 August 2022).
- Available online: https://patents.google.com/patent/KR101885403B1/en?q=probiotic+foot+ulcer&oq=probiotic+for+foot+ulcer (accessed on 24 August 2022).
- Available online: https://patents.google.com/patent/EP2837292A1/en (accessed on 24 August 2022).
- Available online: https://patents.google.com/patent/US20030017192A1/en (accessed on 26 August 2022).
- Available online: https://patents.google.com/patent/KR102083002B1/en?q=probiotic+wounds&oq=probiotic+on+wounds (accessed on 3 September 2022).
- Available online: https://patents.google.com/patent/WO2020261055A1/en?q=probiotic+wound+healing&oq=probiotic+for+wound+healing (accessed on 3 September 2022).
- Available online: https://patents.google.com/patent/JP6944399B2/en?q=probiotic+wound+healing&oq=probiotic+for+wound+healing (accessed on 7 September 2022).
- Available online: https://patents.google.com/patent/WO2017099559A1/en (accessed on 7 September 2022).
- Available online: https://patents.google.com/patent/CN107438666B/en?q=probiotic+wound+healing&oq=probiotic+for+wound+healing (accessed on 10 September 2022).
- Available online: https://patents.google.com/patent/EP1607096B1/en (accessed on 13 September 2022).
- Available online: https://patents.google.com/patent/WO1996008261A1/en (accessed on 13 September 2022).
- Available online: https://patents.google.com/patent/US6468525B1/en (accessed on 15 September 2022).
- Available online: https://patents.google.com/patent/WO2002065840A3/en (accessed on 16 September 2022).
| Source | Fermented Product | Micro-Organism Isolated |
|---|---|---|
| Bamboo shoots | Soidon | Lactococcus lactis, Lactobacillus brevis and Leuconostoc fallax |
| Black mustard seeds | Hardline | Lactobacillus sanfranciscensis, Lactobacillus casei, Lactobacillus brevis, Lactobacillus acetotolerans, Lactobacillus paracasei and Lactobacillus pontis |
| Broccoli | Yan-tsai-shin | Leuconostoc Mesenteroides, Weissella cibaria, Lactobacillus plantarum, Enterococcus sulfurous and Weissella paramesenteroides, |
| Cabbage | Dhamuoi | Leuconostoc mesenteroides and Lactobacillus plantarum |
| Celery, cabbage, radish, and cucumber | Pascal | Lactobacillus brevis, Lactobacillus plantarum, Lactobacillus lactis, Leuconostoc mesenteroides, Lactobacillus fermentum, and Lactobacillus pentosus |
| Cherries | Cherries juice | Enterococcus gallinarum and Pediococcus pentosaceus |
| Chinese cabbage | Kimchi | Weissella koreensis, Lactobacillus lactis, Lactobacillus plantarum, Leuconostoc gasicomitatum, Lactobacillus brevis, Lactobacillus curvatus, Leuconostoc citreum, Pediococcus pentosaceus, Lactobacillus sakei, Weissella confusa, and Leuconostoc mesenteroides |
| Cucumber | Khalpi | Leuconostoc fallax, Lactobacillus brevis and Lactobacillus plantarum |
| Cucumber | Jiang-guais | Enterococcus casseliflavus, Weissella hellenica, Leuconostoc lactis, Lactobacillus Plantarum and Weissella cibaria |
| Cummingcordia | Pobuzihi | Weissella cibaria, Pediococcus pentosaceus, Lactobacills plantarum, Lactobacillus pobuzihii and Weissella paramesenteroides |
| Durian fruit | Tempoyak | Lactobacills durianis Lactobacillus brevis Leuconostoc mesenteroides Lactobacillus fermentum and Liquorilactobacillus mali |
| Field mustard | Nozawana-zuke | Leuconostoc and Lactobacillus |
| Fresh cabbage | Sauerkraut | Lactobacillus spp., Leuconostoc spp. and Pediococcus spp. |
| Fresh peaches | Yan-taozih | Weissella cibaria, Lactobacillus brevis, Weissella minor, Leuconostoc mesenteroides, Enterococcus faecalis, Lactobacillus lactis and Weissella paramesenteroides |
| Ginger | Yan-jiangis | Lactobacillus plantarum and Weissella cibaria |
| Grapes | Wine (red) | Lactobacillus Plantarum, Pediococcus parvulus, Oenococcus oeni and Lactobacillus casei |
| Green peppers and green tomatoes | Tursu | Pediococcus pentosaceus, Leuconostoc mesenteroides, Lactobacillus brevis and Lactobacillus plantarum |
| Maganesaag | Goyang | Lactobacillus Brevis, Pediococcus pentosaceus, Lactococcus lactis, yeasts Candida spp., Enterococcus faecium and Lactobacillus plantarum |
| Mustard leaves | Inziangsang | Pediococcus Lactobacillus plantarum and Lactobacillus brevis |
| Mustard cabbage leaf | Sayur asin | Lactobacillus confusus, Lactobacillus plantarum, Leuconostoc mesenteroides and Pediococcus pentosaceus |
| Rayosag, mustard leaves, cauliflower leaves, and cabbages | Gundruk | Pediococcus pentosaceus, Lactobacillus casei, Lactobacillus plantarum and Lactobacillus fermentum |
| Radish taproot | Sinki | Lactobacillus casei, Leuconostoc fallax, Lactobacillus brevis and Lactobacillus plantarum |
| Turnips | Shalgam juice | Lactobacillus paracasei, Pediococcus pentosaceus, Lactobacillus brevis and Lactobacillus buchneri |
| Wax gourd | Yan-Dong-Gua | Weissella cibaria and Weissella paramesenteroides |
| Probiotic Strain | Assay | Results | References |
|---|---|---|---|
| Antioxidant effect | |||
| Bacillus amyloliquefaciens, Starmerella bombicola, and Lactobacillus brevis | DPPH, ABTS |
| [14] |
| Bifidobacterium breve, Rhamnosus GG, Probionebacterium freudenreichii and Lactobacillus retueria, | DPPH, ABTS |
| [15] |
| BS1, BS2, BV | TAOC, MDA, SOD |
| [16] |
| Enterococcus faecium | DPPH, Superoxide, Hydroxyl scavenging assay |
| [17] |
| Lactobacillus acidophilus | DPPH |
| [9] |
| Lactobacillus plantarum, Lactobacillus rhamnosus, Lactobacillus casei, | DPPH |
| [18] |
| Lactobacillus plantarum DM5 | DPPH, Superoxide anion, Hydroxyl |
| [19] |
| Lactobacillus paracasei A-4, Lactobacillus plantarum A-7, Lactobacillus paracasei BL-12, Lactobacillus paracasei DU-8, Lactococcus lactis T-8 | DPPH |
| [20] |
| Anti-inflammatory | |||
| Probiotic strain | Design/ participants | Results | References |
| Bifidobacterium animalis ssp. lactis 420 (900 billion CFU/day) | Randomized/50 |
| [21] |
| Lactobacillus acidophilus La-5 and Bifidobacterium BB-12 (106 CFU/g each) | Randomized double-blind/210 |
| [22] |
| Lactobacillus acidophilus, Lactobacillus casei, Bifidobacterium bifidum, Lactobacillus fermentum (2 × 109 CFU/g each) | Randomized double-blind/48 |
| [23] |
| Lactobacillus acidophilus, Lactobacillus infantis, Bifidobacterium bifidum, Lactobacillus fermentum and Bifidobacterium longum (6 billion CFU each) | Randomized double-blind/ 52 |
| [24] |
| Lactobacillus plantarum OLL2712 (5 × 109 CFU) | Randomized/ 130 |
| [25] |
| Immunomodulatory effect | |||
| Probiotics strain | Animal model/other | Results | References |
| Bifidobacterium longum KACC 91563(100 billion CFU/g) | Male BALB/c mice |
| [26] |
| Bifidobacterium longum CCUG 52486 (5 × 108 CFU/day) | Human |
| [27] |
| Lactobacillus casei Shirota (1.3 × 1010 CFU/day) | Human |
| [28] |
| Lactobacillus casei; CRL 431 (109 cells/day) | Female BALB/c mice |
| [29] |
| Limosilactobacillus fermentum (109 CFU/mL) | Female Balb/c mice |
| [30] |
| Antidiabetic effect | |||
| Probiotic strain | Animal model | Results | References |
| Lactobacillus casei (4.0 × 109 CFU/rat/day) | Rat |
| [31] |
| Lactobacillus casei and Bifidio bifidum (1 × 107 cfu/mL) | Wistar rat |
| [32] |
| Lactobacillus.casei (109 CFU/mL) | Mice |
| [33] |
| Lactobacillus casei CCFM419 (109 CFU) | Mice |
| [34] |
| Lactobacillus. Gasseri (6 × 107 cfu/g) | Rat |
| [35] |
| Lactobacillus plantarum CCFM0236 (8 × 109 cfu/mL) | Mice |
| [36] |
| Lactobacillus.plantarum, strain Ln4 (5 × 108 cfu/day) | Male mice |
| [37] |
| Lactobacillus.plantarum MTCC5690 and Lactobacillus fermentum MTCC5689 (1.5 × 109 colonies/day) | C57BL/6J male mice |
| [38] |
| Lactobacillus.rhamnoss, Lactobacillus.acidophilus, Bifidio bifidumi (6 × 108 CFU each) | Mice |
| [39] |
| Probiotic Strains | Microencapsulation Technique | Parameters Test | Observation | References |
|---|---|---|---|---|
| LA and BL | Spray chilling | Viability count |
| [56] |
| LRIMC-501 | Spray chilling | Viability count |
| [72] |
| Ls | Spray coating using Sucrose | Viability count |
| [73] |
| LA | Spray coating using maize and potato | Viability count |
| [74] |
| LA | Fluidized bed coating | Thermal stability |
| [75] |
| LS | Fluidized bed coating | Thermal stability |
| [76] |
| LA | Liposome | Thermal stability |
| [77] |
| LP-PR01 | Extrusion-dripping technique | Thermal stability |
| [78] |
| LA-ATCC-4356 | Extrusion-dripping technique | Thermal stability |
| [79] |
| Enterococcus | Spray drying | Stability |
| [80] |
| ST IFFI 6038 | Extrusion | Viability count |
| [81] |
| LP | pH induced gelation | Viability count |
| [82] |
| Ls | Alginate coating by homogenization pressure | Viability count |
| [83] |
| LB-ST-69 | Matrix polymerization | Viability count |
| [84] |
| YEP | Co-extrusion | Viability count |
| [85] |
| Brand and Trade Name | Manufacturer | Country | Stains Isolated | Food Type | References |
|---|---|---|---|---|---|
| Aciforce | Biohorma | The Netherlands | Enterococcus faecium, Lactobacillus acidophilus, Bifidobacterium bifidum, Lactococcus lactis | Lyophilized products | |
| Activia | Danone | France | Bifidus actiregularis | Creamy yoghurt | |
| Actimel | Danone | France | Lactobacillus casei Immunitas | Probiotic yoghurt drink | |
| Bacilac | THT | Belgium | Lactobacillus acidophilus, Lactobacillus rhamnosus | Lyophilized product | |
| Bactisubtil | Synthelabo | Belgium | Bacillus sp. strain IP5832 | Lyophilized product | |
| Hellus | Tallinna Piimatööstuse AS | Estonia | Lactobacillus fermentum ME-3 | Dairy product | |
| Jovita Probiotisch | H & J Bruggen | Germany | Lactobacillus strain | Probiotic yoghurt | [88] |
| Proflora | Chefaro | Belgium | Lactobacillus delbrueckii subsp. bulgaricus, Lactobacillus acidophilus, Bifidobacterium, Streptococcus thermophilus | Lyophilized product | |
| Provie | Skanemejerier | Sweden | Lactobacillus plantarum | Fruit drink | |
| ProViva | Skanemejerier | Sweden | Lactobacillus plantarum | Fruit drink | |
| Rela | Ingman Foods | Finland | Lactobacillus reuteri | Cultured milk | |
| Revital Active | Olma | Czech Republic | Lactobacillus acidophilus | yoghurt drink | |
| Yakult | Yakult | Japan | Lactobacillus casei Shirota | Milk drink | |
| Yosa | Bioferme | Finland | Bifidobacterium lactis, Lactobacillus acidophilus | Yoghurt-like oat product | |
| Vitamel | Campina | The Netherlands | Lactobacillus casei GG, Lactobacillus acidophilus, Bifidobacterium bifidum | Dairy products | |
| Vifit | Campina | The Netherlands | Lactobacillus strain | Yoghurt drink | |
| Activia | Danone | France | Bifidus actiregularis | Creamy yoghurt |
| Probiotic Name | Manufacturer | Strain | Colony Forming Units (CFUs) | Health Claims | References |
|---|---|---|---|---|---|
| Activa yogurt | Dannon Inc | Lactobacillus bulgaricus, Streptococcus thermophilus, Bifidobacterium regularis, Bifidobacterium animalis DN-173010 | 10 billion |
| [89] |
| Adult Formula CP-1 | Custom Probiotics Inc | Lactobacillus rhamnosus, Lactobacillus acidophilus, Bifidobacterium bifidum, Bifidobacterium lactis | 50 billion |
| [90] |
| Align capsules | Proctor & Gamble | Bifidobacterium. infantis 35624 | 1 billion |
| [91] |
| Attune nutrition bars | Attune Foods | Lactobacillus casei Lc-11, Bifidobacterium lactis HN019, Lactobacillus acidophilus NCFM | 6.1 billion |
| [92] |
| Bio-K+ cultured milk-based probiotic | Bio-K+ Int Inc. | Lactobacillus casei LBC804, Lactobacillus acidophilus CL1285 | 50 billion |
| [93] |
| Bio-K+ probiotic capsules | Bio-K+ Int Inc. | Lactobacillus casei LBC804, Lactobacillus acidophilus CL1285 | 50 billion |
| [94] |
| Culturelle capsules | Amerifit Nutrition, Inc | Lactobacillus rhamnosus GG | 10 billion |
| [95] |
| Gefilus juice | Valio Ltd. | Lactobacillus rhamnosus GG | 5 million |
| [96] |
| Gerber Good Start Protect Plus powdered infant milk formula | Nestle | Bifidobacterium lactis Bb-12 | 10 billion |
| [97] |
| Good Belly fruit drink | Next Foods | Lactobacillus plantarum 299v | 20 billion |
| [98] |
| OWP probiotics | One Wellness Place | Bifidobacterium breve, Bifidobacterium longum, Bifidobacterium infantis, Lactobacillus acidophilus, Lactobacillus plantarum, Lactobacillus rhamnosus | 15 billion |
| [99] |
| Ultimate Probiotic Formula | Swanson Health Products | Bifidobacterium longum, Bifidobacterium lactis, Lactobacillus plantarum, Lactobacillus casei, Lactobacillus sylvarius, Lactobacillus bulgaricus, Lactobacillus sporogenes + Prebiotic NutraFlora FOS | 60 billion |
| [100] |
| VSL#3 saket | Sigma-Tau Pharmaceuticals | Bifidobacterium breve, Bifidobacterium longum, Bifidobacterium infantis, Lactobacillus acidophilus, Streptococcus thermophilus, Lactobacillus casei | 450 billion |
| [101] |
| Yo-Plus yogurt | Yoplait Inc | Bifidobacterium animalis subsp Bb-12, Streptococcus thermophilus, Lactobacillus bulgaricus + Prebiotics | >5 billion |
| [102] |
| Probiotic Formulation Composition | Patent Number | Beneficial Claims | References |
|---|---|---|---|
| Therapeutic potential | |||
| A61K35/741—Probiotics | WO2019180748A1 |
| [103] |
| Bacillus circulans ATCC PTA-5614, 5615, 5616 | US 7361497 B2 |
| [104] |
| Bacillus strain, Saccharomyces cerevisiae, Saccharomyces boulardii, LAB | US20180280312A1 |
| [105] |
| Bacillus subtilis, Lactobacillus plantarum | RU2401116C2 |
| |
| Bifidobacterium strain AH1714 | CN102946891A |
| [106] |
| Enterococcus faecium | EP0508701A2 |
| [107] |
| Enterococcus mundtii | KR20090023626A |
| [108] |
| Lactobacillus acidophilus LPV 31 | EP2450062A1 |
| [109] |
| LAB | KR101885403B1 |
| [110] |
| Lactobacillus casei, Lactobacillus rhamnosus + tagatose | EP2837292 A1 |
| [111] |
| Lactobacillus genera, Bifidobacterium genera | US20030017192 A1 |
| [112] |
| Lactobacillus plantarum, Lactobacillus brevis | KR102083002B1 |
| [113] |
| Lactobacillus plantarum, Lactobacillus acidophilus | WO2020261055A1 |
| [114] |
| Lactobacillus plantarum, Lactobacillus acidophilus, Bifidobacterium longum | JP6944399B2 |
| [115] |
| Probiotic bacteria + sodium laureth sulfate + alkyl polyglycozide + cocamide DEA + glycerol + orange terpenes + fragrance + D-pantenol + ethyl hydroxy ethyl cellulose + orange terpenes + citric acid | WO2017099559A1 |
| [116] |
| Probiotic + valproic acid | US20190282523A1 |
| [117] |
| Recombinant probiotic | CN107438666B |
| [118] |
| Nutraceutical | |||
| Bacillus coagulans, clostridium, Bacillus subtilis or Lactobacillus sporogenes + arabinogalactan | EP1607096B1 |
| [119] |
| Bifidobacterium, Lactococcus and Staphylococcus, Saccharomyces, Clostridium, Lactobacillus, Enteroccus, Peptostreptococcus, Eubacterium, Streptococcus, | WO 1996008261 A1 |
| [120] |
| Bifidobacterium longum, Bfidobacterium bifidum, Lactobacillus salivarius, Lactobacillus acidophilus, Bifidobacterium infantis, L-glutamine, fructooligosaccharides and N-acetyl glucosamine | US6468525B1 |
| [121] |
| Probiotic food | WO2002065840A3 |
| [122] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Awasthi, A.; Corrie, L.; Vishwas, S.; Gulati, M.; Kumar, B.; Chellappan, D.K.; Gupta, G.; Eri, R.D.; Dua, K.; Singh, S.K. Gut Dysbiosis and Diabetic Foot Ulcer: Role of Probiotics. Pharmaceutics 2022, 14, 2543. https://doi.org/10.3390/pharmaceutics14112543
Awasthi A, Corrie L, Vishwas S, Gulati M, Kumar B, Chellappan DK, Gupta G, Eri RD, Dua K, Singh SK. Gut Dysbiosis and Diabetic Foot Ulcer: Role of Probiotics. Pharmaceutics. 2022; 14(11):2543. https://doi.org/10.3390/pharmaceutics14112543
Chicago/Turabian StyleAwasthi, Ankit, Leander Corrie, Sukriti Vishwas, Monica Gulati, Bimlesh Kumar, Dinesh Kumar Chellappan, Gaurav Gupta, Rajaraman D. Eri, Kamal Dua, and Sachin Kumar Singh. 2022. "Gut Dysbiosis and Diabetic Foot Ulcer: Role of Probiotics" Pharmaceutics 14, no. 11: 2543. https://doi.org/10.3390/pharmaceutics14112543
APA StyleAwasthi, A., Corrie, L., Vishwas, S., Gulati, M., Kumar, B., Chellappan, D. K., Gupta, G., Eri, R. D., Dua, K., & Singh, S. K. (2022). Gut Dysbiosis and Diabetic Foot Ulcer: Role of Probiotics. Pharmaceutics, 14(11), 2543. https://doi.org/10.3390/pharmaceutics14112543







